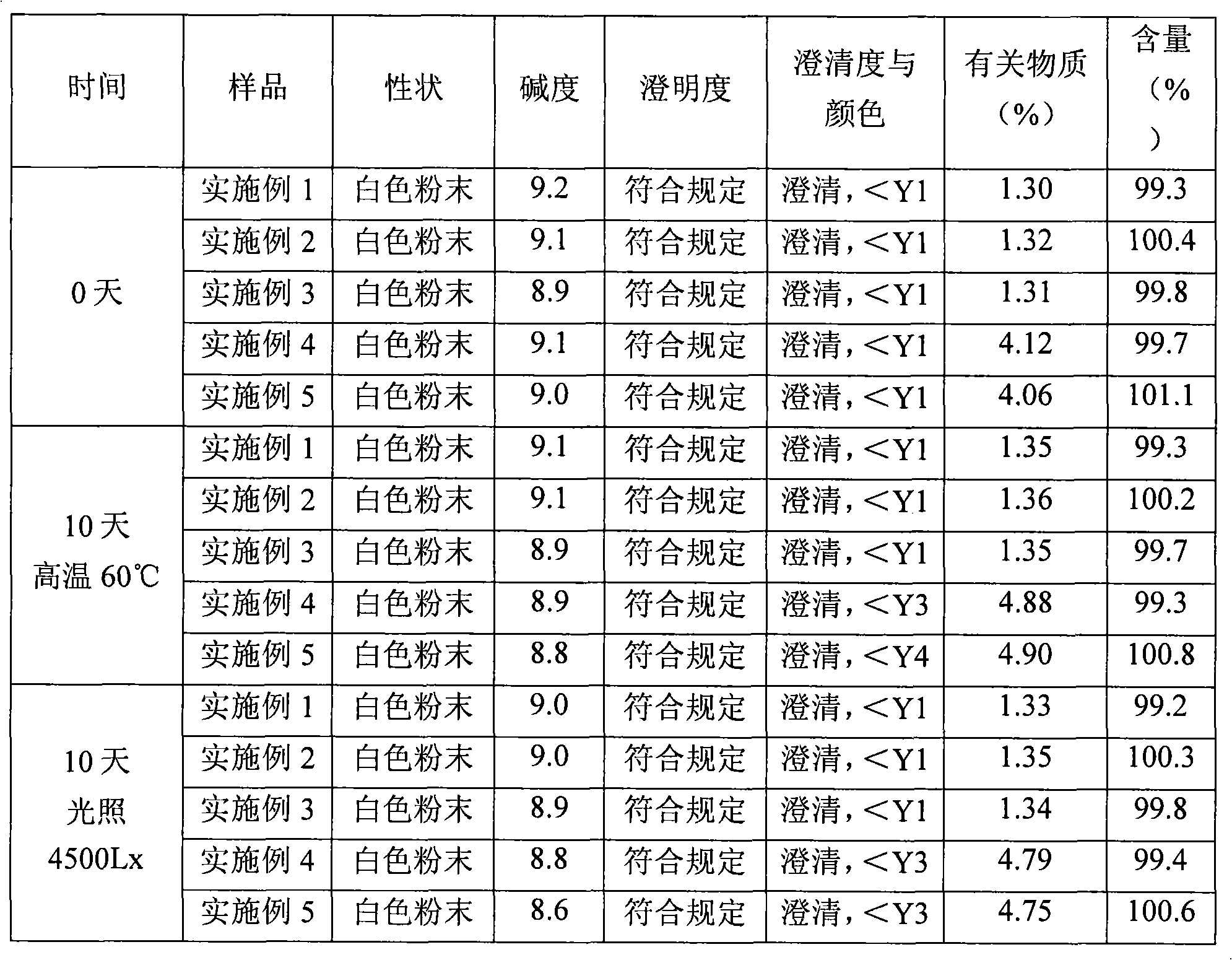
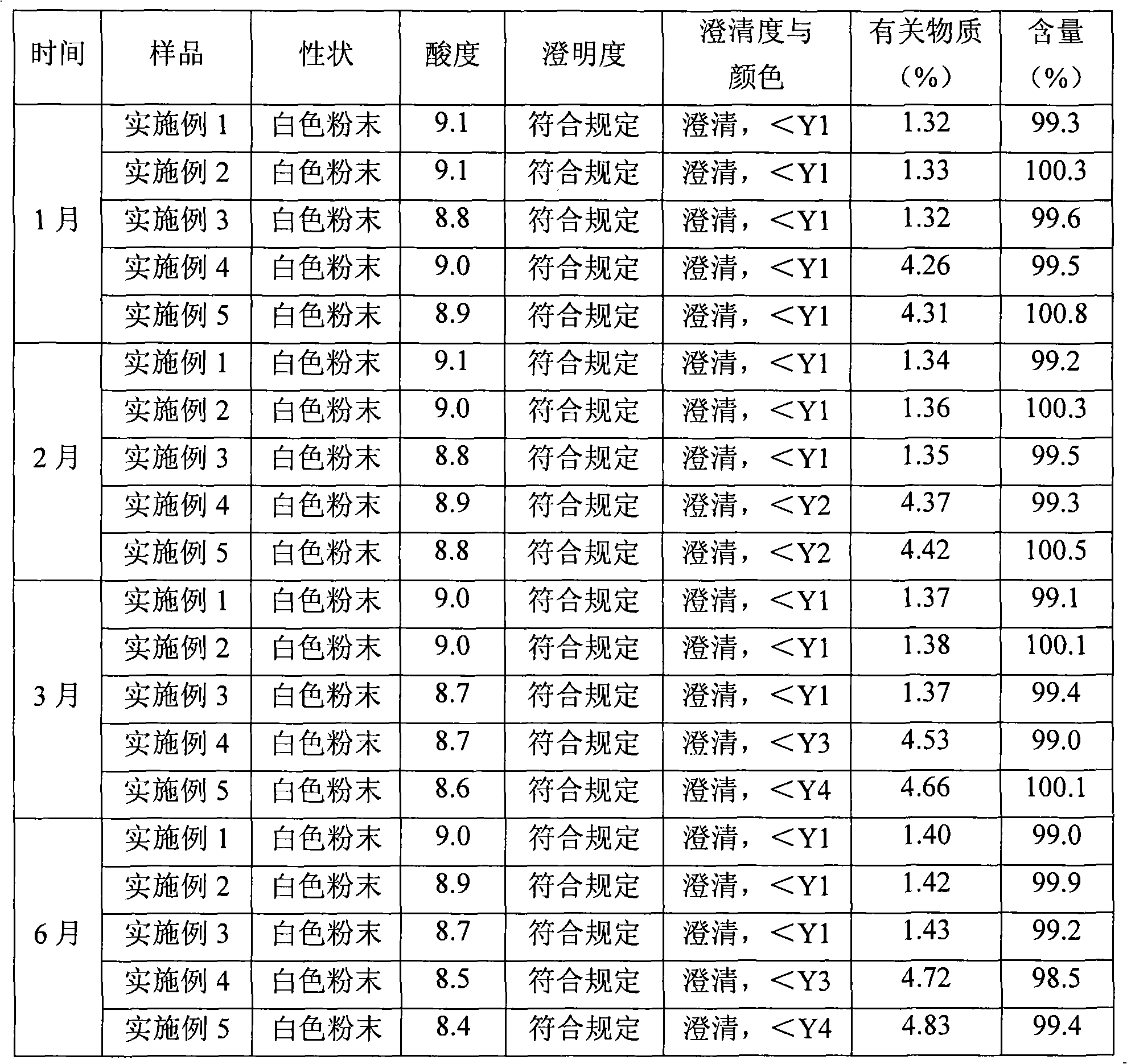
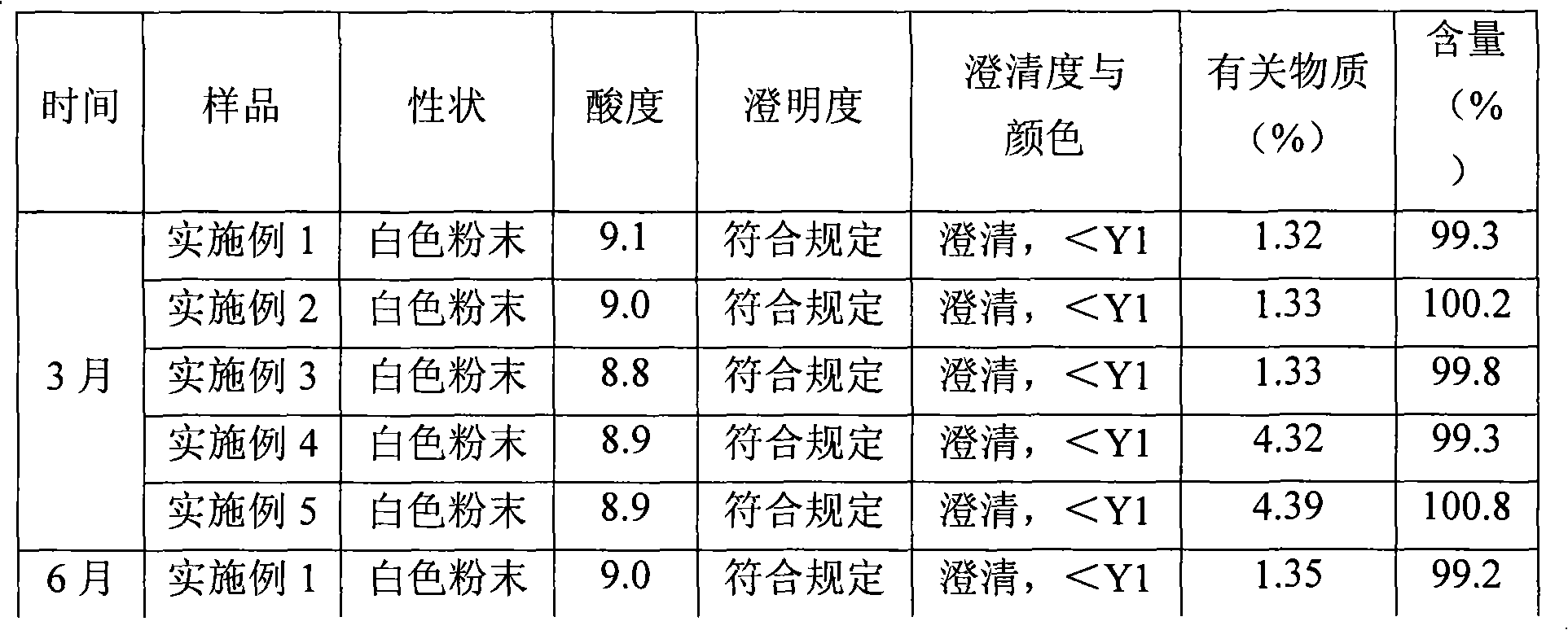
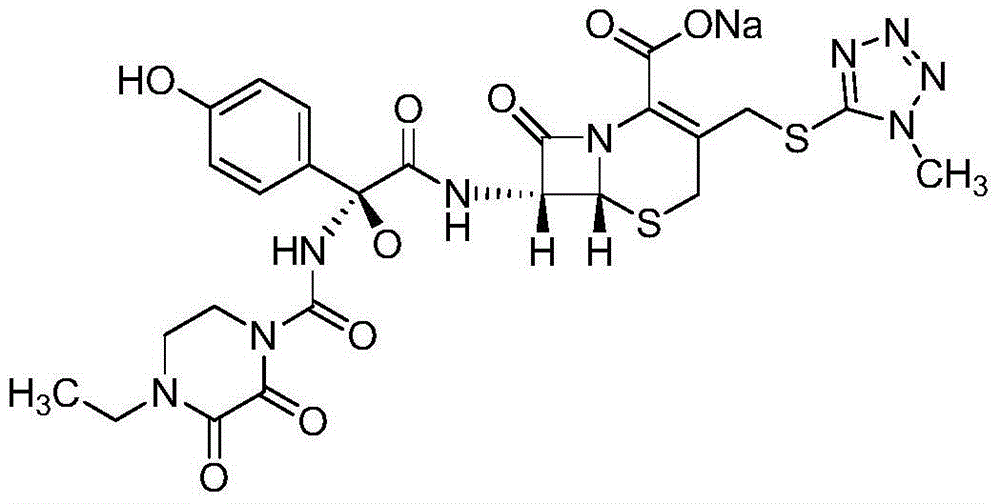
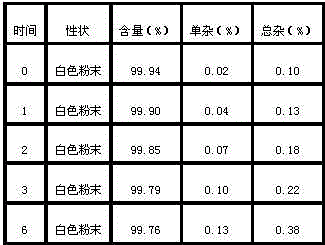
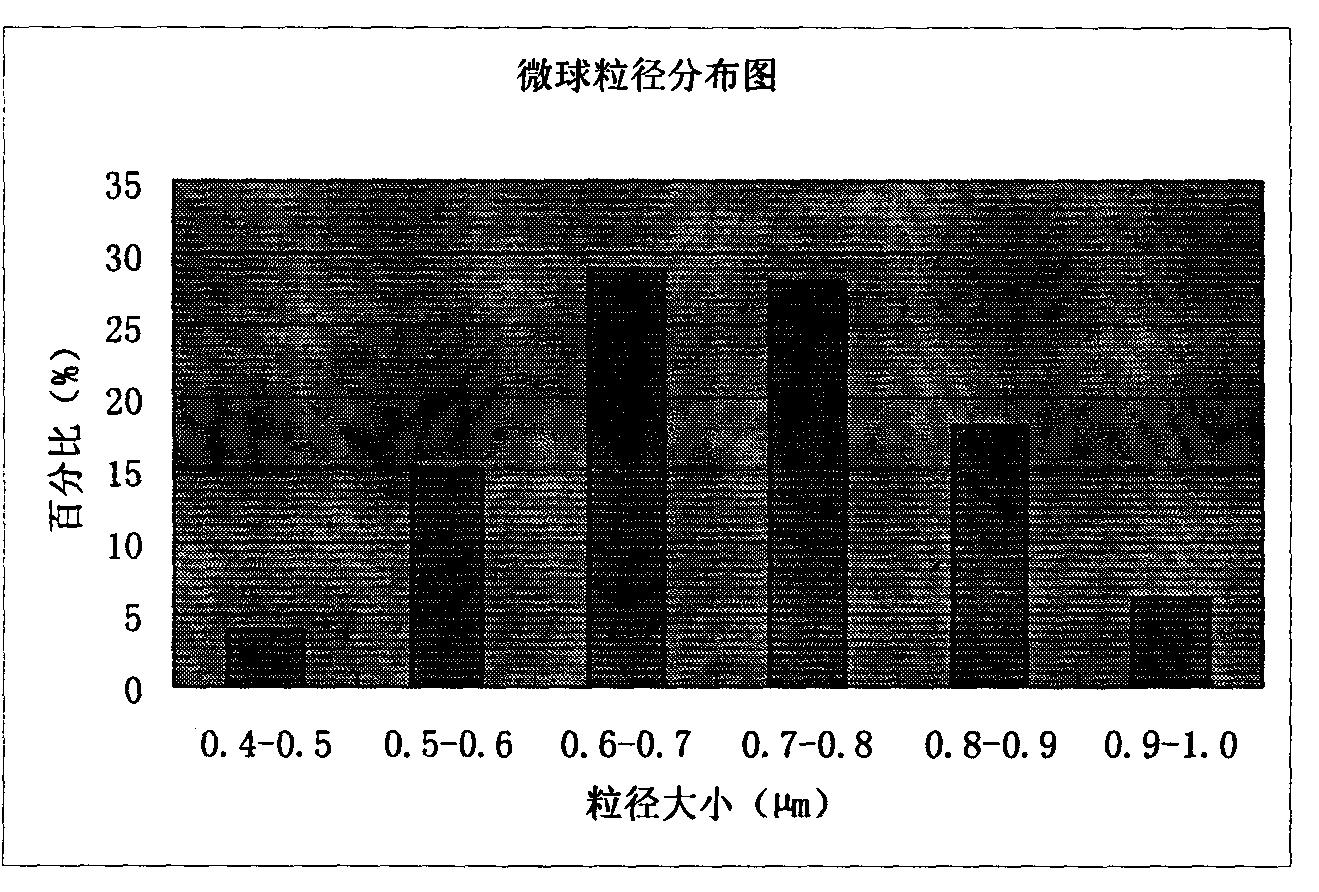
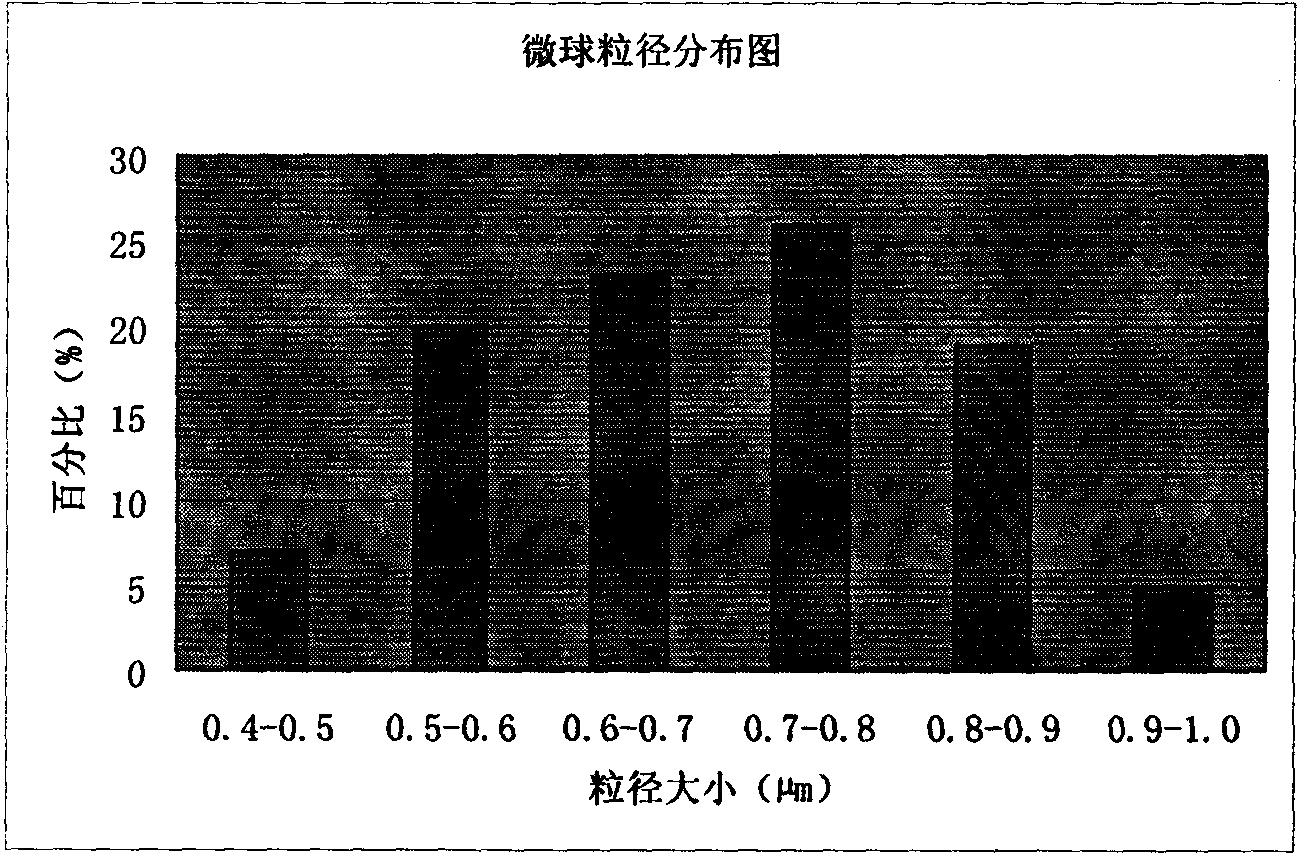
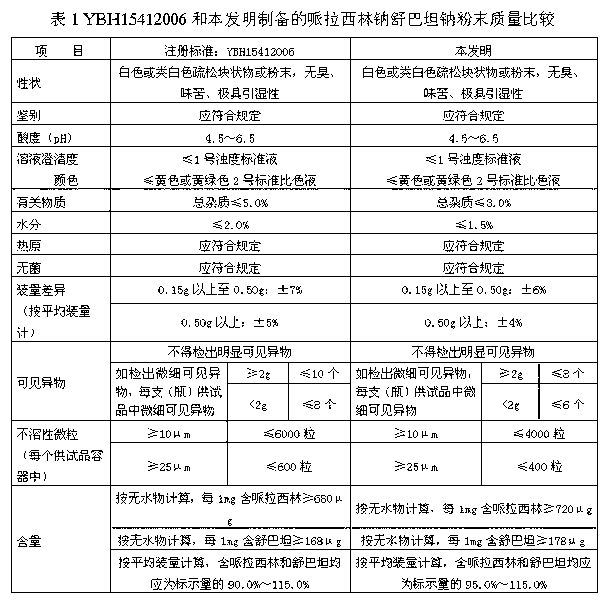
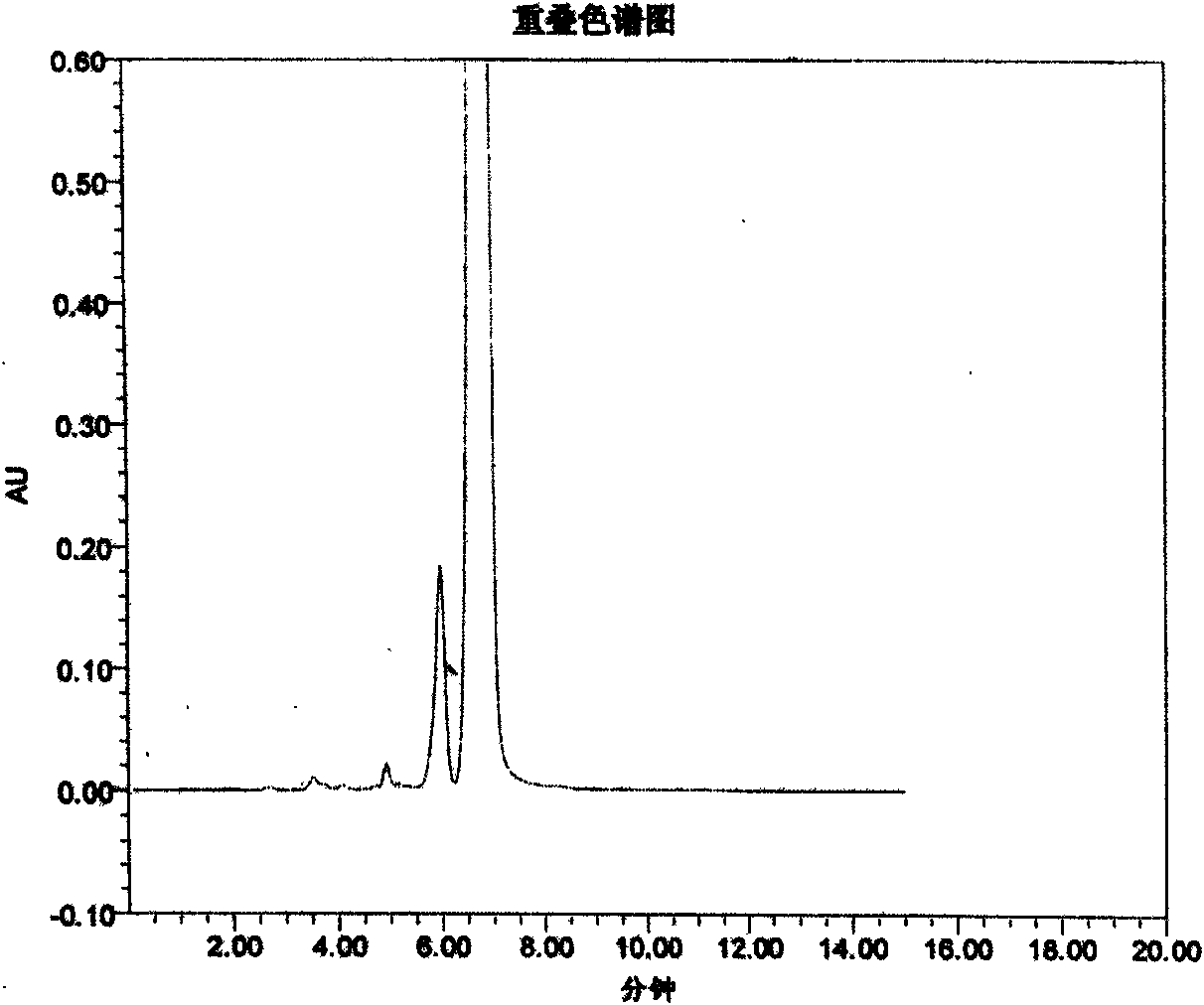
Patents
Literature
179 results about "Sulbactam Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
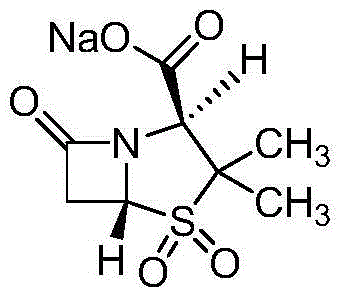
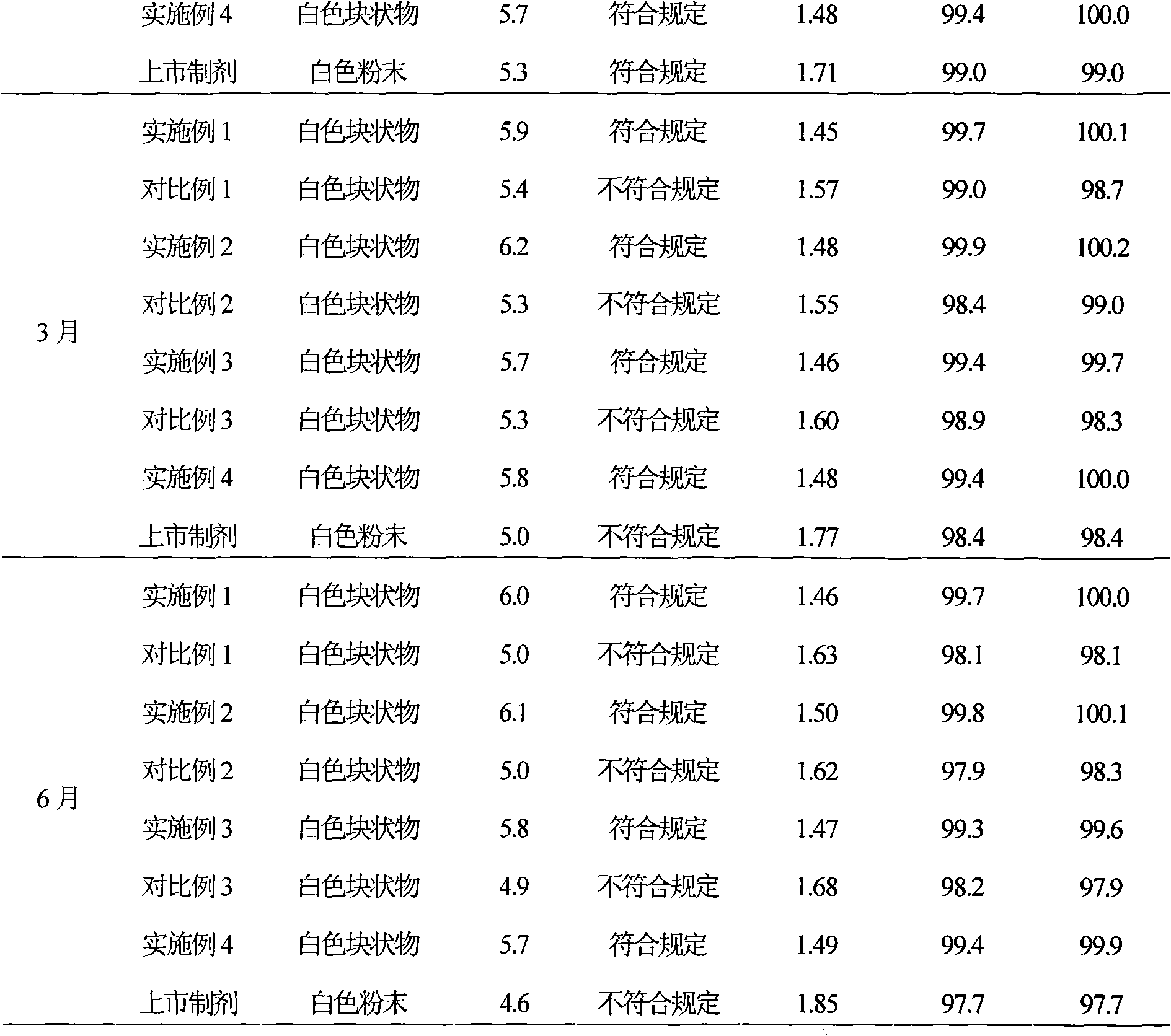
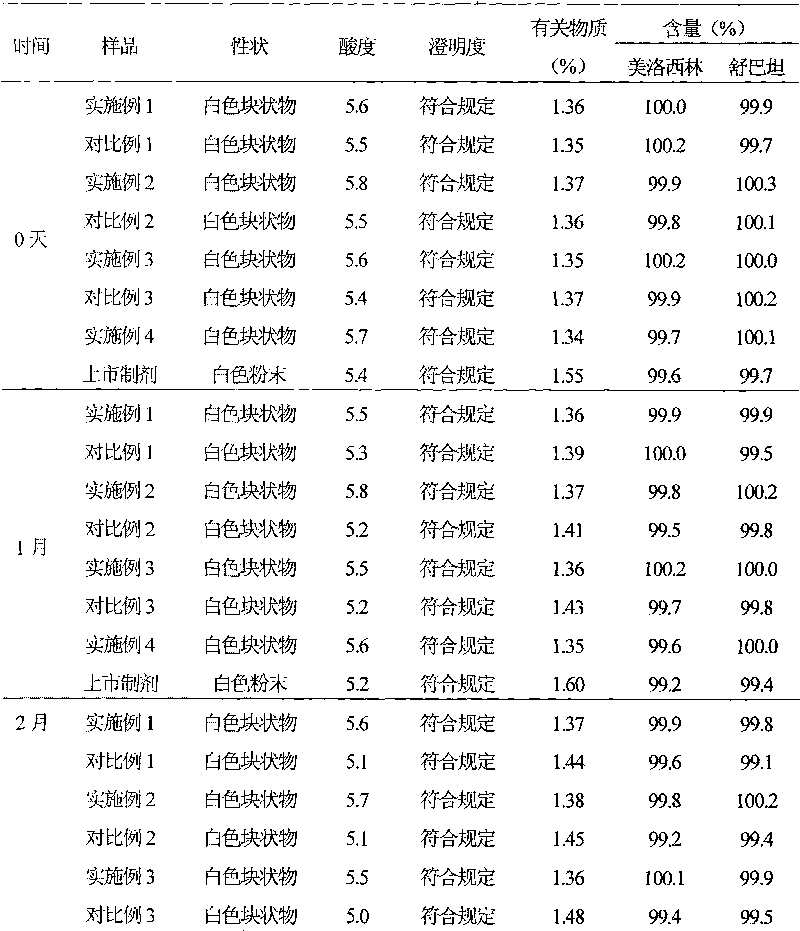
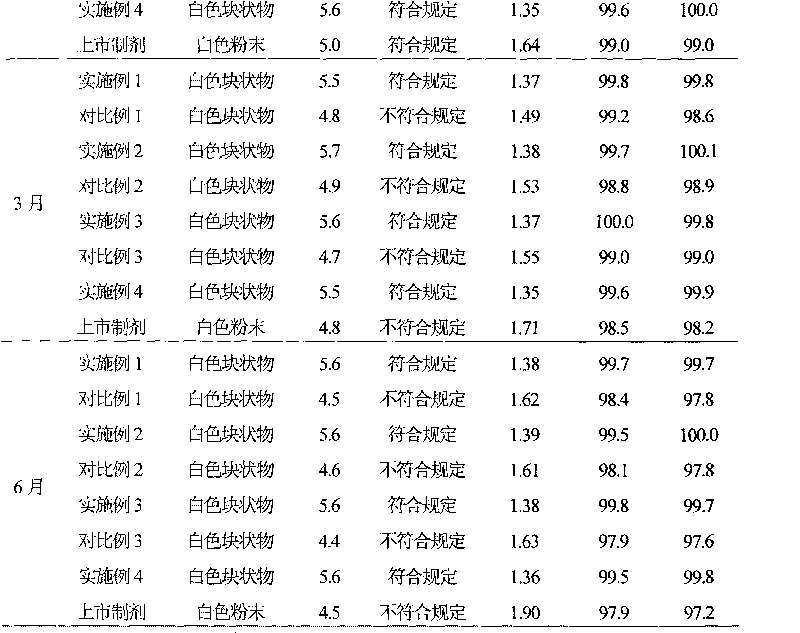
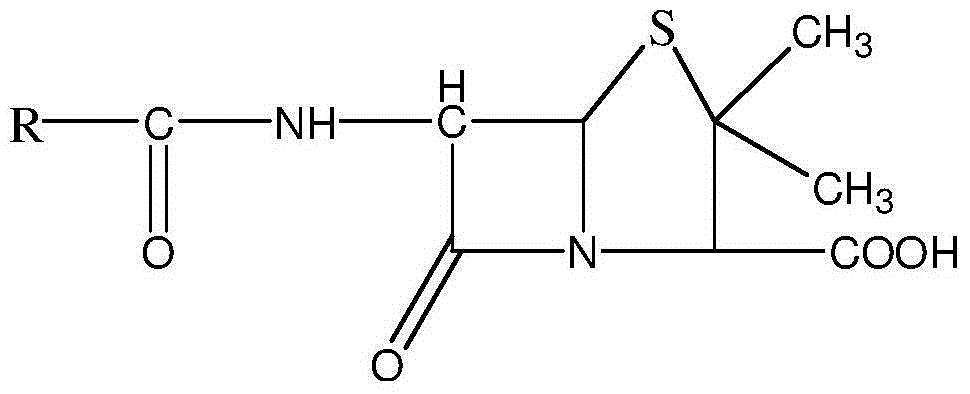
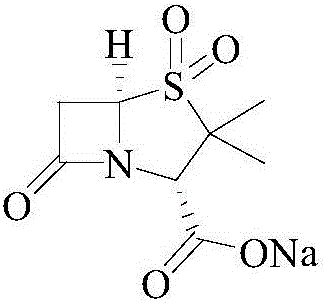
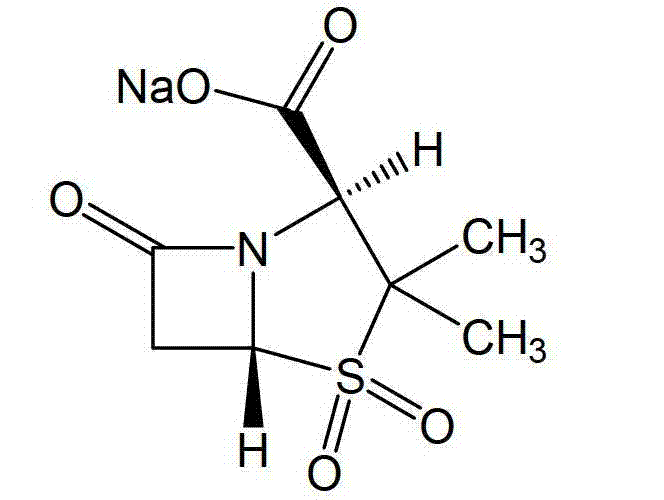
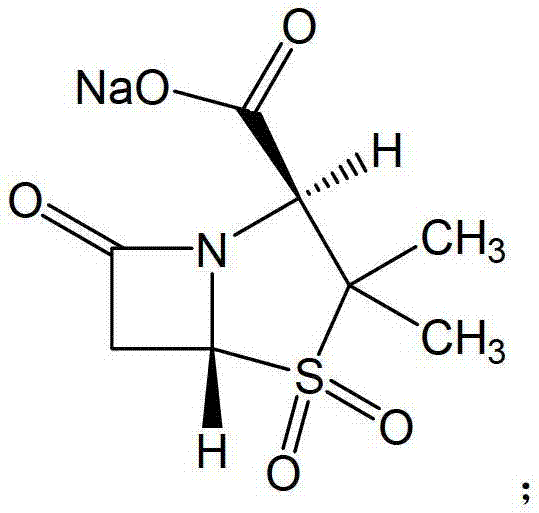
The sodium salt form of sulbactam, a beta-lactam with weak antibacterial property. Sulbactam sodium contains a beta-lactam ring and irreversibly binds to beta-lactamase at or near its active site, thereby blocking enzyme activity and preventing metabolism of other beta-lactam antibiotics. Combining this agent with a beta-lactamase sensitive antibiotic such as penicillins and cephalosporins against penicillinase-producing and beta-lactamase-producing organisms, results in a decreased turnover rate of the sensitive antibiotic and enhances its antibacterial property.
Amoxicillin sodium and sulbactam sodium for injection and preparation of freeze-dried injection thereof
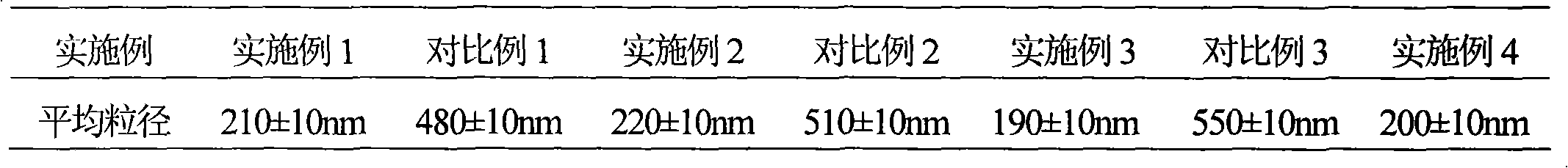
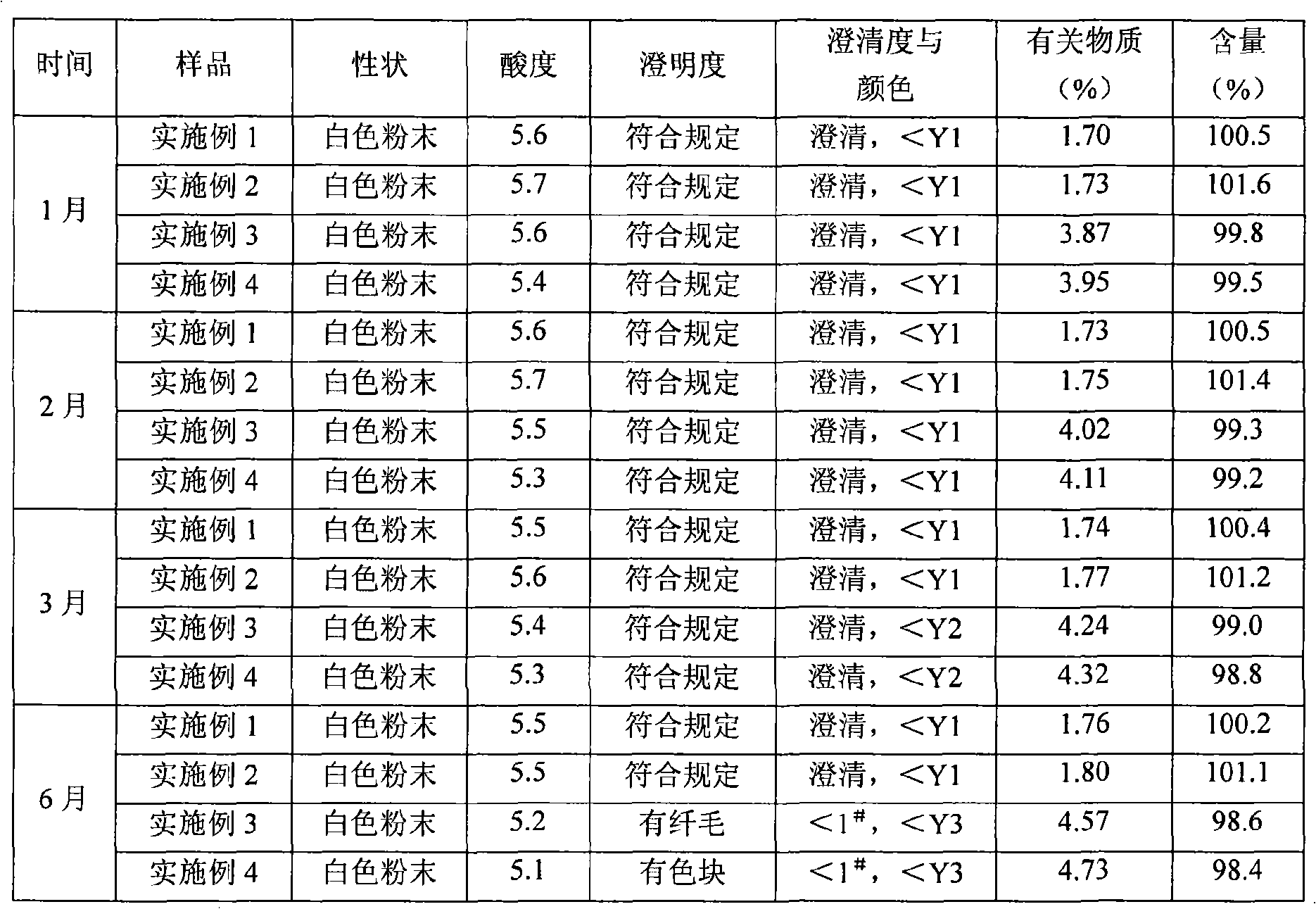
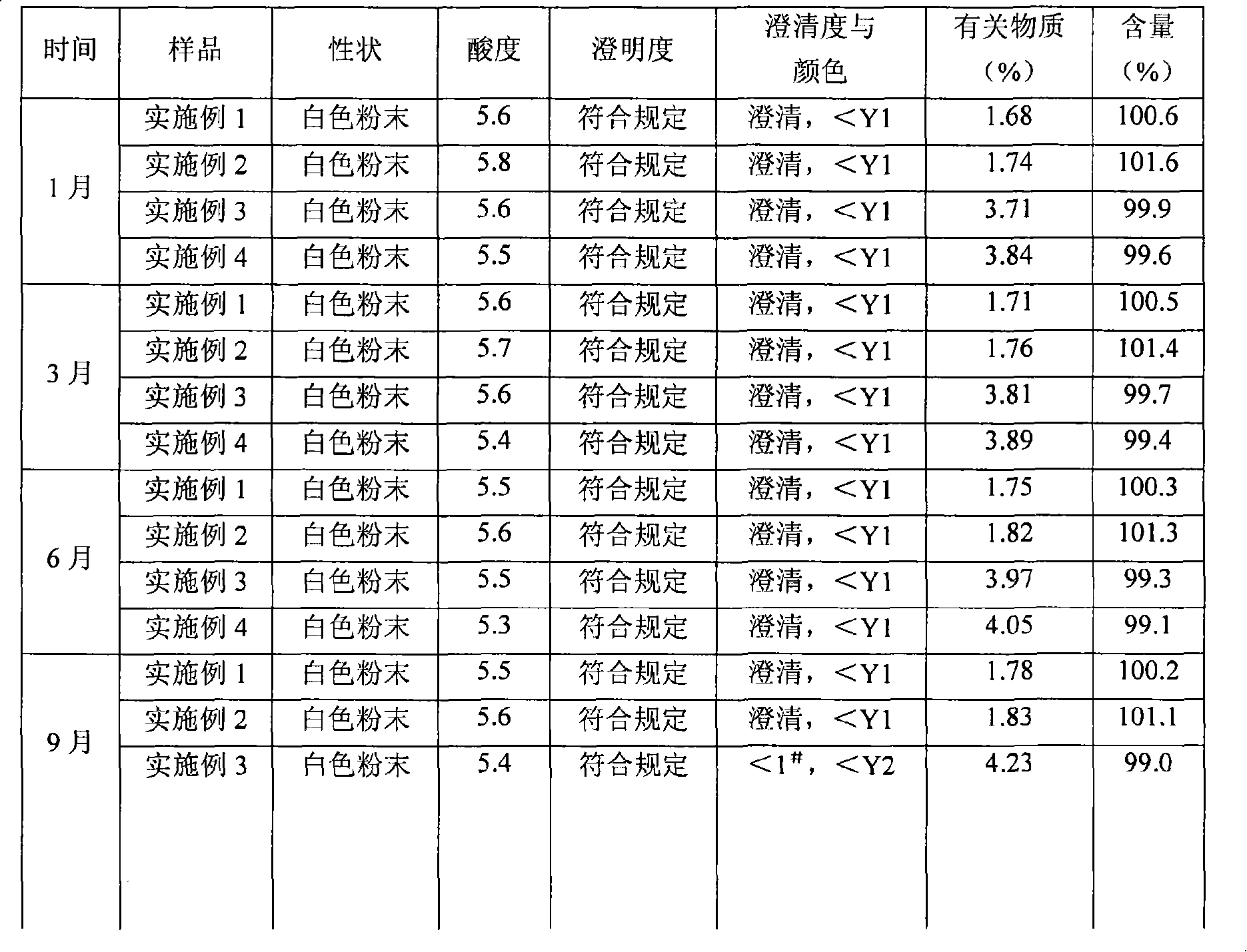
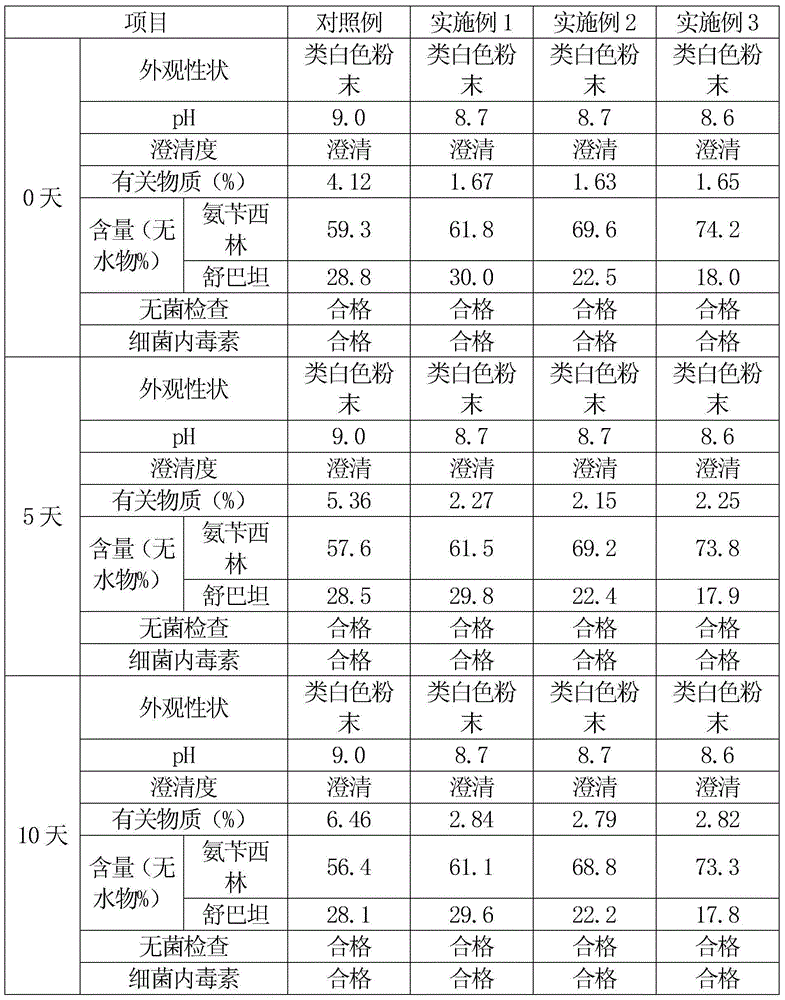
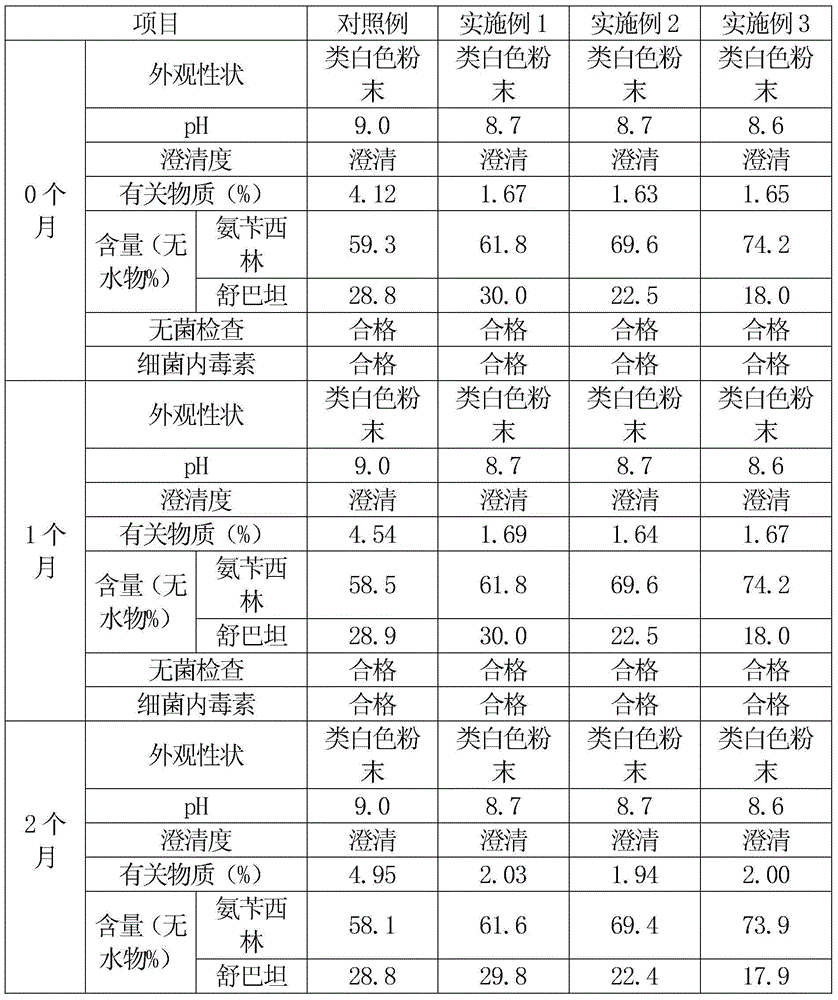
InactiveCN101322701AImprove stabilityAvoid lostAntibacterial agentsPharmaceutical delivery mechanismFreeze-dryingAmoxicillin Sodium
The invention discloses a preparation method of amoxicillin sodium and sulbactam sodium for injection and a freeze-dried powder injection thereof as well as a separating and purifying method of the amoxicillin sodium and sulbactam sodium for injection. By adopting high speed countercurrent chromatography, the invention prepares trichloromethane, ethyl acetate, methanol and water to form a solvent system with an immobile phase and a mobile phase and separates and purifies amoxicillin sodium and sulbactam sodium to obtain the amoxicillin sodium and sulbactam sodium for injection; the purity of the obtained product can reach more than 98% and the prepared injection has improved stability.
Owner:海南华旗药业销售有限公司
Preparation method of cefoperazone sodium and sulbactam sodium powder injection for injection
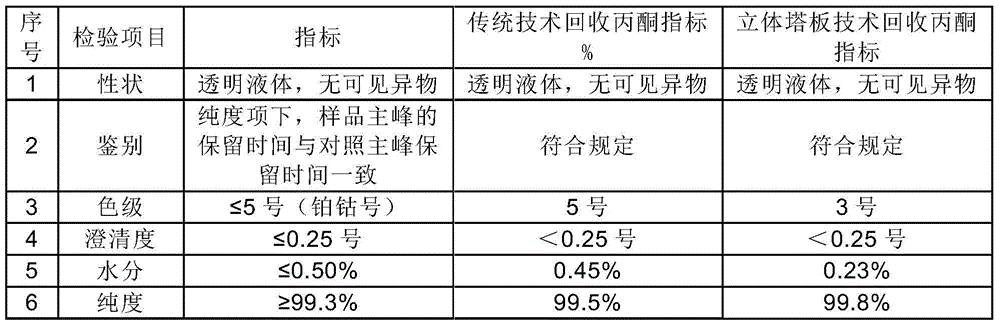
The invention discloses a preparation method of a cefoperazone sodium and sulbactam sodium powder injection for injection. A recovered solvent is prepared by a stereo mass transfer tower plate technology, and is directly applied to production of cefoperazone sodium and sulbactam sodium products; the quality indexes such as color grade, clarity and purity of the obtained cefoperazone sodium and sulbactam sodium powder injection product for injection are greatly improved; the cefoperazone sodium and sulbactam sodium powder injection is high in quality stability and few in impurity; and the preparation method is low in production cost and simple in process.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA +1
Medicinal composition consisting of ceftriaxone sodium and sulbactam sodium and preparation method thereof
ActiveCN102462684AStable contentEasy to storeAntibacterial agentsPowder deliverySolubilityCurative effect
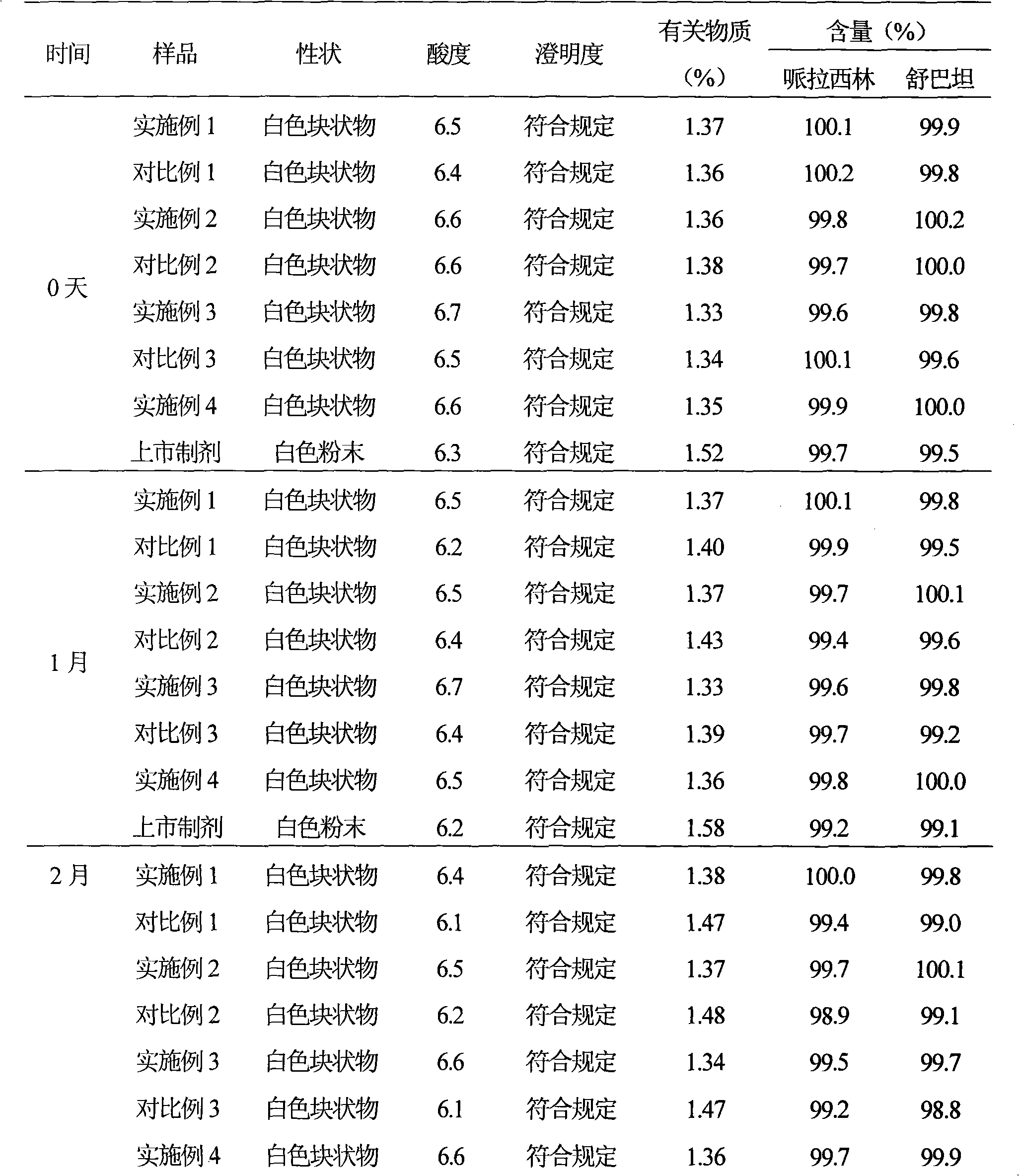
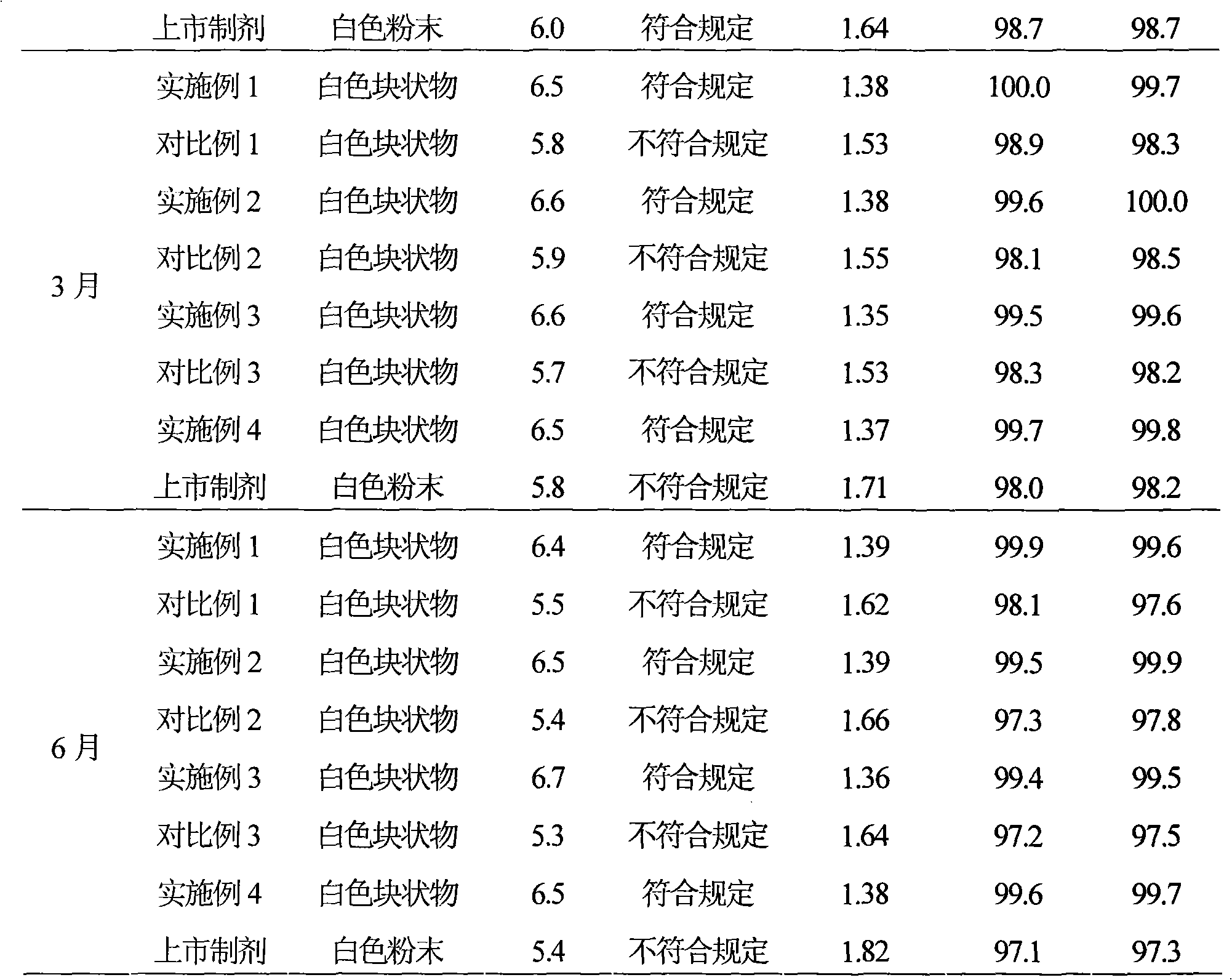
The invention aims to provide a medicinal composition consisting of ceftriaxone sodium and sulbactam sodium, which does not have any sensitization effect, has high stability and is efficient, and a preparation method thereof. The weight ratio of the ceftriaxone sodium to the sulbactam sodium to a stable conditioning agent component in the medicinal composition is (1-100):(0.25-100):(0.0005-9). The medicinal composition provided by the invention is in a good crystal form, and has stable and controllable quality at the temperature between 20 DEG C below zero and 60 DEG C in production and transportation processes and a good curative effect; and an effective period can be up to 36 months. During clinical administration, the medicinal composition is dissolved and diluted through the conventional transfusion, the ceftriaxone sodium and the sulbactam sodium in the medicinal composition have stable content, high solubility and indecomposability, insoluble crystals and sensitized high polymers are not produced, the influences of temperature and illumination are small, high stability is achieved, and the effective period is 36 months, so that a better curative effect is achieved. A preparation method of the medicinal composition provided by the invention is scientific and reasonable, is convenient to operate, and is suitable for large-scale industrial production.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Mezlocillin sodium compound and medicine composition thereof
The invention relates to a mezlocillin sodium compound which is determined by adopting X-ray powder diffraction and has characteristic peaks shown in 2theta of 8.9, 15.7, 16.5, 18.9, 19.8, 24.6, 26.4, 27.8, 29.0, 29.7, 31.8, 33.2, 34.7, 36.8, 37.5, 38.9 and 40.1 in a map. The invention also relates to a mezlocillin sodium compound and a medicine composition with a medicine active component of the mezlocillin sodium compound or the mezlocillin sodium compound and sulbactam sodium or tazobactam sodium. The medicine composition is a powder injection of the mezlocillin sodium compound, or a medicine mixture powder injection of the mezlocillin sodium compound and the sulbactam sodium or tazobactam sodium. The mezlocillin sodium compound has the advantages of difficulty in absorbing mixture, good flowability, high dissolving speed, kept extremely high stability, and greatly improved convenience and safety of the mezlocillin sodium.
Owner:HUNAN KELUN PHARMA
Suspensoid powder injection of piperacillin sodium sulbactam sodium medicine composition and novel application thereof
InactiveCN101632671AUnexpected effectImprove stabilityPowder deliveryDigestive systemFreeze-dryingPharmacology
The invention relates to suspensoid powder injection of a piperacillin sodium sulbactam medicine composition prepared by applying an emulsification suspensoid technology and freeze drying. The invention further relates to a novel application of the suspensoid powder injection in preparing a medicine treating the infection of the oral cavity.
Owner:HAINAN YONGTIAN PHARMA INST
Mezlocillin sodium and sulbactam sodium for injection and freeze-dried injection preparation thereof
InactiveCN101322685AImprove stabilityAvoid lostAntibacterial agentsPowder deliveryCountercurrent chromatographyFreeze-drying
The invention provides a preparation method of mezlocillin sodium-sulbactam sodium for injection and the freeze-dried powder injection thereof as well as a separating and purifying method of the mezlocillin sodium and sulbactam sodium for injection. By adopting high speed countercurrent chromatography, the invention prepares trichloromethane, methanol and water to form a solvent system with an immobile phase and a mobile phase and separates and purifies mezlocillin sodium and sulbactam sodium to obtain the mezlocillin sodium and sulbactam sodium for injection; the purity of the obtained product can reach more than 99% and the prepared injection has improved stability.
Owner:海南华旗药业销售有限公司
Liposome injection of amoxicillin sodium sulbactam sodium medicinal composition
InactiveCN101822669AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPowder deliveryDipalmitoylphosphatidylcholineFreeze-drying
The invention provides liposome injection of an amoxicillin sodium sulbactam sodium medicinal composition. The liposome injection consists of amoxicillin sodium, sulbactam sodium, liposome carriers, freeze-drying supporting agents and optional antioxidant, wherein the liposome carriers are dipalmitoyl phosphatidyl choline and deoxysodium cholate. The liposome injection has high stability; in the freeze-drying process, a phenomenon of breaking liposome caused by dehydration, fusion, ice crystal generation and the like does not occur; and the liposome can remain good encapsulation ratio after being hydrated and redissolved.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Cefuroxime sodium and sulbactam sodium composition for injection
InactiveCN1729987AImprove antibacterial propertiesImprove the bactericidal effectAntibacterial agentsHeterocyclic compound active ingredientsCefuroxime SodiumSulbactam Sodium
The invention provides a composition of cefuroxime sodium and sulbactam sodium for injection, which comprises cefuroxime sodium and sulbactam sodium by the weight ratio of 15:1. The composition has good antibiotic action and low cost of production.
Owner:刘全胜
Suspensoid powder injection of amoxicillin sodium sulbactam sodium medicine composition and novel application thereof
InactiveCN101632660AUnexpected effectImprove stabilityPowder deliveryPharmaceutical product form changeActive componentFreeze-drying
The invention relates to suspensoid powder injection of an amoxicillin sodium sulbactam sodium medicine composition and a preparation method thereof. The suspensoid powder injection contains Tween 80, cholesterol, deoxysodium cholate, a freeze-drying support agent and active components. The invention also relates to a novel application of the suspensoid powder injection in preparing a medicine treating prostatitis further.
Owner:HAINAN YONGTIAN PHARMA INST
Medicinal-composition suspension powder injection with mezlocillin sodium and sulbactam sodium, and novel application thereof
InactiveCN101703506AUnexpected effectImprove stabilityAntibacterial agentsPowder deliveryActive componentCholesterol
The invention belongs to the technical field of medicine, and discloses a medicinal-composition suspension powder injection taking mezlocillin sodium and sulbactam sodium as active components. The injection comprises 4 parts of mezlocillin sodium, 1 part of sulbactam sodium, 5 to 20 parts of Tween 80, 1 to 10 parts of cholesterol, 0.5 to 15 parts of deoxysodium cholate, and 2 to 30 parts of frozen-dried supporting agent. The invention further discloses novel application of the injection in the preparation of medicaments for preventing postoperative infection of appendicitis.
Owner:HAINAN YONGTIAN PHARMA INST
Beta- lactamase suppressing antibacterial compound drugs
InactiveCN1565457AHigh tissue contentWide distribution in the bodyAntibacterial agentsOrganic active ingredientsCompounding drugsCeftizoxime
The invention discloses a beta- lactamase suppressing antibacterial compound drugs, which comprises ceftizoxime, or cefodizime and beta-lactam enzyme inhibitor by the active acid weight ratio of 1-10:10-1, which are in the forms of alkali metal salts or free acid and assisting solvents, the beta-lactam enzyme inhibitor can be Tazobactam, or clavulanic acid, or tapazole or their derivatives.
Owner:张哲峰
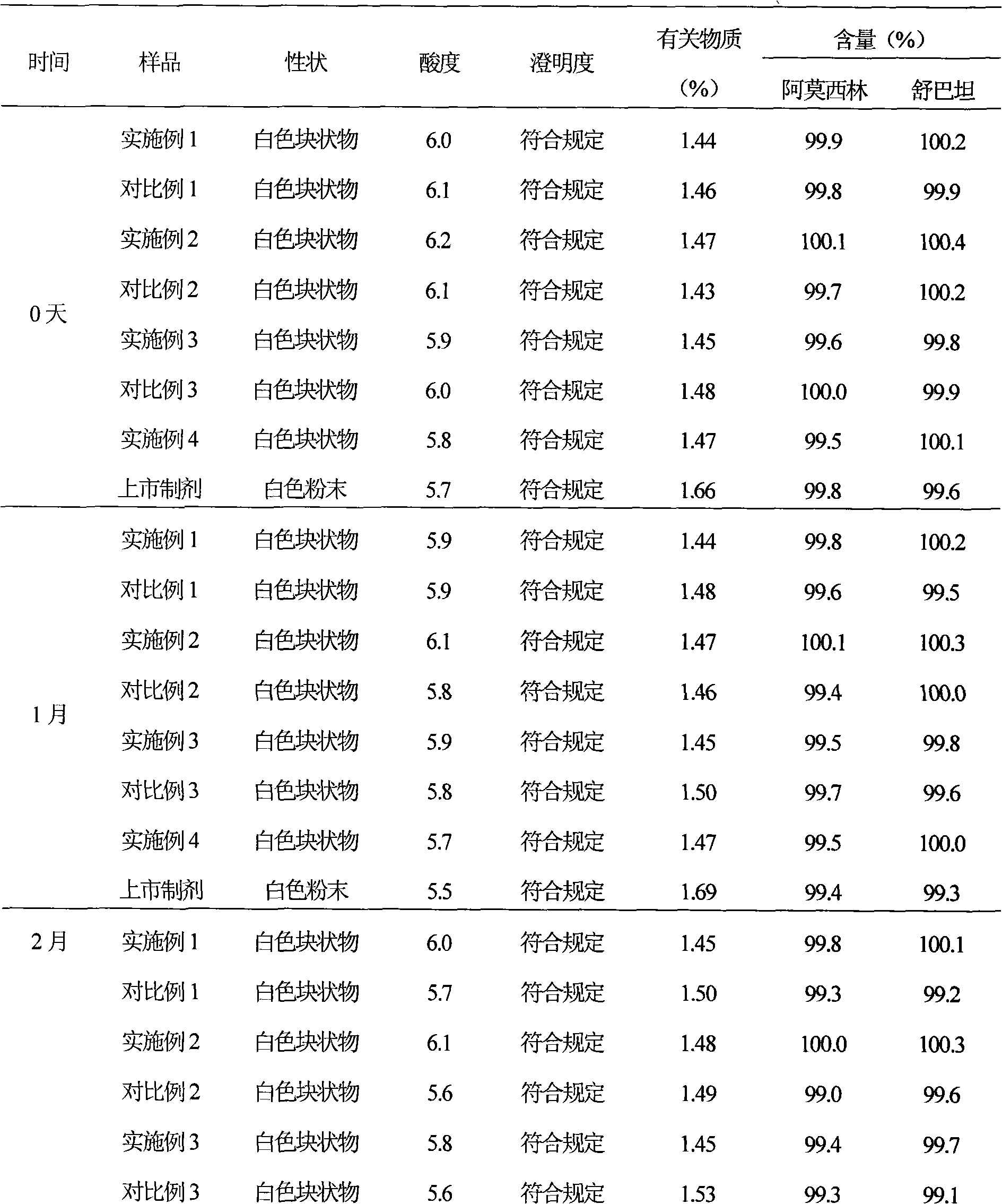
Ampicillin sodium sulbactam sodium preparation for injection and preparation method thereof
InactiveCN104644629ARaise the reaction temperatureHigh reaction yieldAntibacterial agentsPowder deliveryAmpicillin Sodium/ Sulbactam SodiumAmpicillin Sodium
The invention discloses an ampicillin sodium sulbactam sodium preparation for injection and a preparation method thereof. The ampicillin sodium sulbactam sodium preparation for injection is formed by mixing high-purity ampicillin sodium crystals with high-purity sulbactam sodium uniformly, wherein the weight ratio of ampicillin sodium to sulbactam sodium is (2-4):1. The ampicillin sodium sulbactam sodium preparation for injection has the advantages of high purity, less impurity, high stability, difficult irritability and the like.
Owner:NORTH CHINA PHARMA COMPANY +2
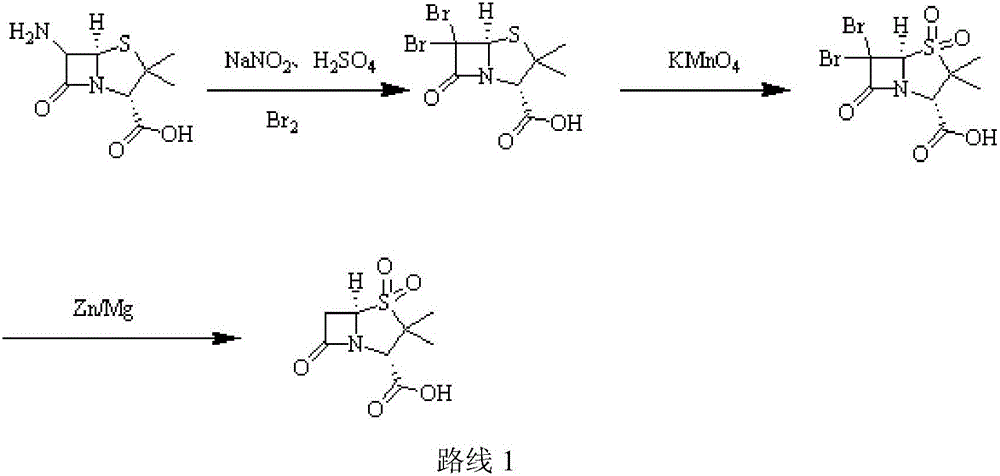
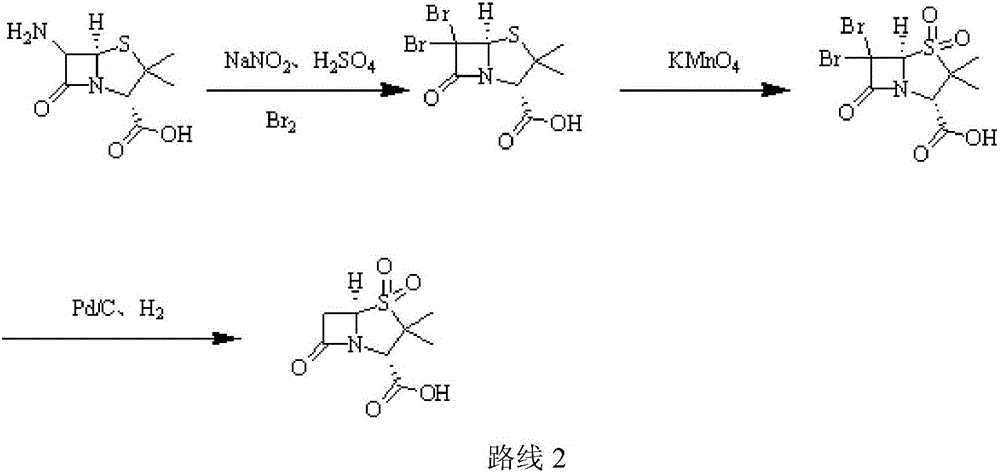
Sulbactam sodium preparation method
InactiveCN106699774AReduce pressure on environmental protectionEasy to operateOrganic chemistryStrong acidsReaction step
The invention relates to a sulbactam sodium preparation method belongs to the technical field of synthesis of beta-lactamase inhibitors. The sulbactam sodium preparation method comprises the steps that 6-amino penicillanic acid (6-APA) is used as a raw material, reacts in strong acid and a sodium nitrite water solution, then reacts under the effects of copper powder and hypophosphorous acid, and then a target product sulbactam sodium is prepared through oxidation and substitution reaction. The reaction process is simple in operation, the method includes few reaction steps, a by-product is reduced, the final reaction yield is high, the treatment difficulty and cost after wastewater production are reduced, the pressure of environmental protection is reduced for enterprises, the product production cost is reduced, and the sulbactam sodium preparation method is suitable for industrial production.
Owner:淄博鑫泉医药技术服务有限公司
Sulbactam sodium bacteriophage complex and the preparing method thereof
InactiveCN101062032AGuaranteed efficacyLong elimination half-lifeAntibacterial agentsPowder deliveryAntioxidantMass ratio
The invention discloses a diastole bar apron sodium antibiotics and preparing craft, which is characterized by the following: allocating with mass ratio as 100-400 antibiotics, 100-400 diastole bar apron sodium, 10-300 liposome and 10-300 polyvinyl pyrrolidon; keeping the drug-effect of common diastole bar apron sodium antibiotics compound; prolonging nullifying half-decay time of the antibiotics; adding antioxidant into composite drug; increasing stability of the drug. This invention can prolongs the effective time of the drug.
Owner:HUNAN KANGDU PHARMA
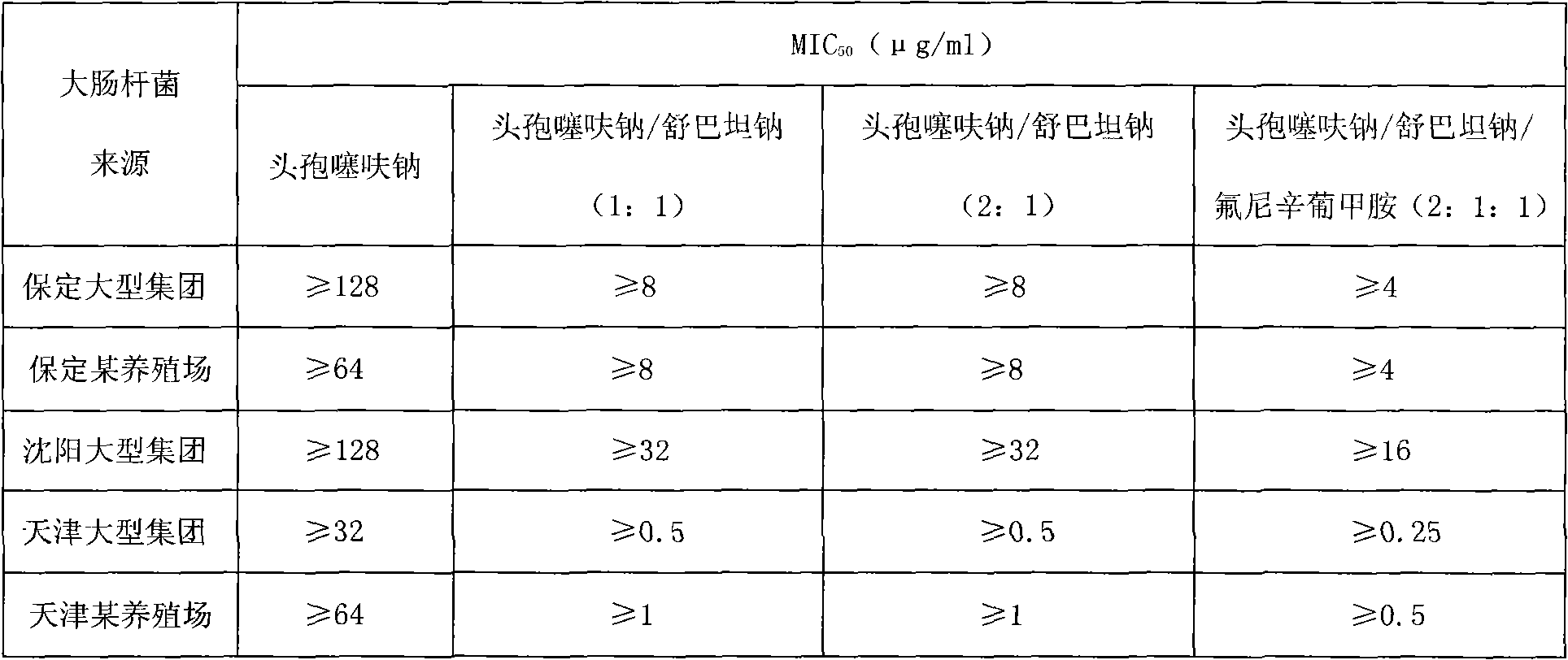
Compound preparation of ceftiofur sodium
InactiveCN101879172AAddressing drug resistanceImprove survival rateAntibacterial agentsHeterocyclic compound active ingredientsEscherichia coliCeftiofur sodium
The invention relates to a compound preparation of ceftiofur sodium, in particular to the application of a compound preparation of ceftiofur sodium in preparing medicines for treating colibacillus or salmonella infection of chicken and swine. The compound preparation of ceftiofur sodium comprises the following formula in parts by weight: 0.5-8 parts of ceftiofur sodium, 0.5-6 parts of fosfomycin sodium, 1-3 parts of sulbactam sodium and 4-7 parts of enorfloxacin sodium. The formula can be prepared into a premixed agent, a drink agent or an injection in the using process. The applicant of the invention, by summarizing of many years of tests and according to the antibacterial synergy principle, prepares the compound preparation of ceftiofur sodium which effectively solves the drug tolerance problem of the colibacillus and salmonella, simultaneously improves the action effect and the field of application and improves the survival rate of livestock and fowl.
Owner:杨建彬
Compound ceftiofur sodium freeze-dried power injection used for injection
ActiveCN102106857AHigh antibacterial activityDelay drug resistanceAntibacterial agentsPowder deliveryFLUNIXIN MEGLUMINECeftiofur sodium
The invention is a compound ceftiofur sodium freeze-dried power injection used for injection, and the compound ceftiofur sodium freeze-dried power injection provided by the invention is mainly composed of the following effective components in parts by weight: 2-4 parts of ceftiofur sodium, 1 parts of sulbactam sodium and 1 parts of flunixin meglumine; a vacuum freeze drying method is adopted to prepare the effective components into the compound ceftiofur sodium freeze-dried power injection used for injection. The compound ceftiofur sodium freeze-dried power injection has the advantages that the preparation cost can be lowered, and the drug resistance of bacteria on the ceftiofur sodium is relieved; and the broad antimicrobial spectrum is wide, the activity is strong, the drug administration times is less, and low stress and slight side effect are endured; and the negative effect of endotoxin can be effectively blocked.
Owner:QILU ANIMAL HEALTH PROD
Antibiotics medicine for injection
InactiveCN1432361AHigh antibacterial activityBroad spectrum antibacterialAntibacterial agentsOrganic active ingredientsEscherichia coliUpper urinary tract infection
The present invention relates to medicine technology and is improved mezlocillin sodium injection. The injection has one medicine weight ratio of mezlocillin sodium / Sulbactam sodium 1-16 to 1. It hasstrong antibacterial activity, wide antibacterial spectrum and thus high curative effect, and may be used in treating serious urinary tract infection, respiratory tract infection, intestinal tract, otorbinolaryngological infection, abdominal cavity infection, etc. caused by zymogenic staphylococcus, pneumococcus, enterococcus, purulent streptococcus, Hemophilus infuenzae, etc.
Owner:REYOUNG PHARMA
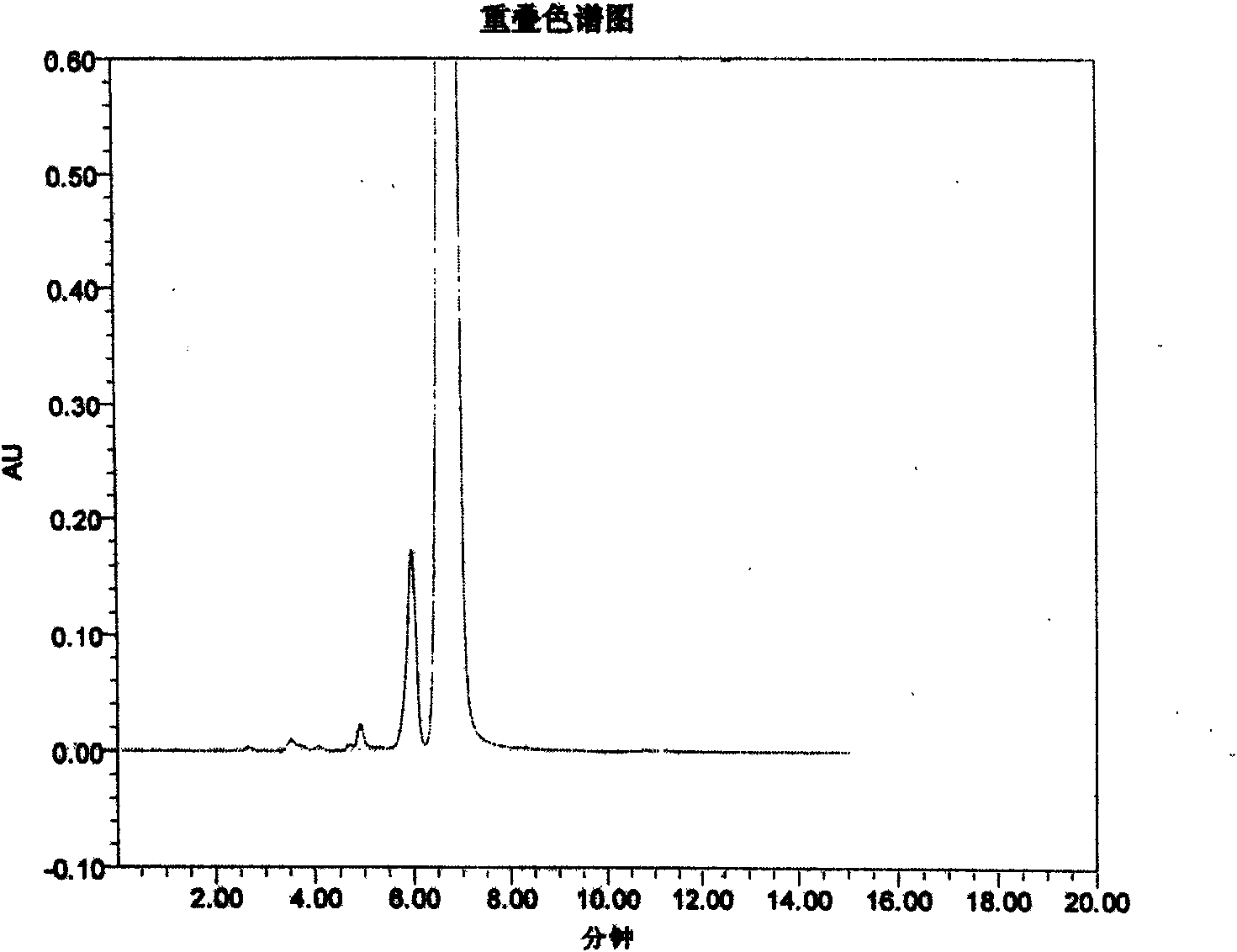
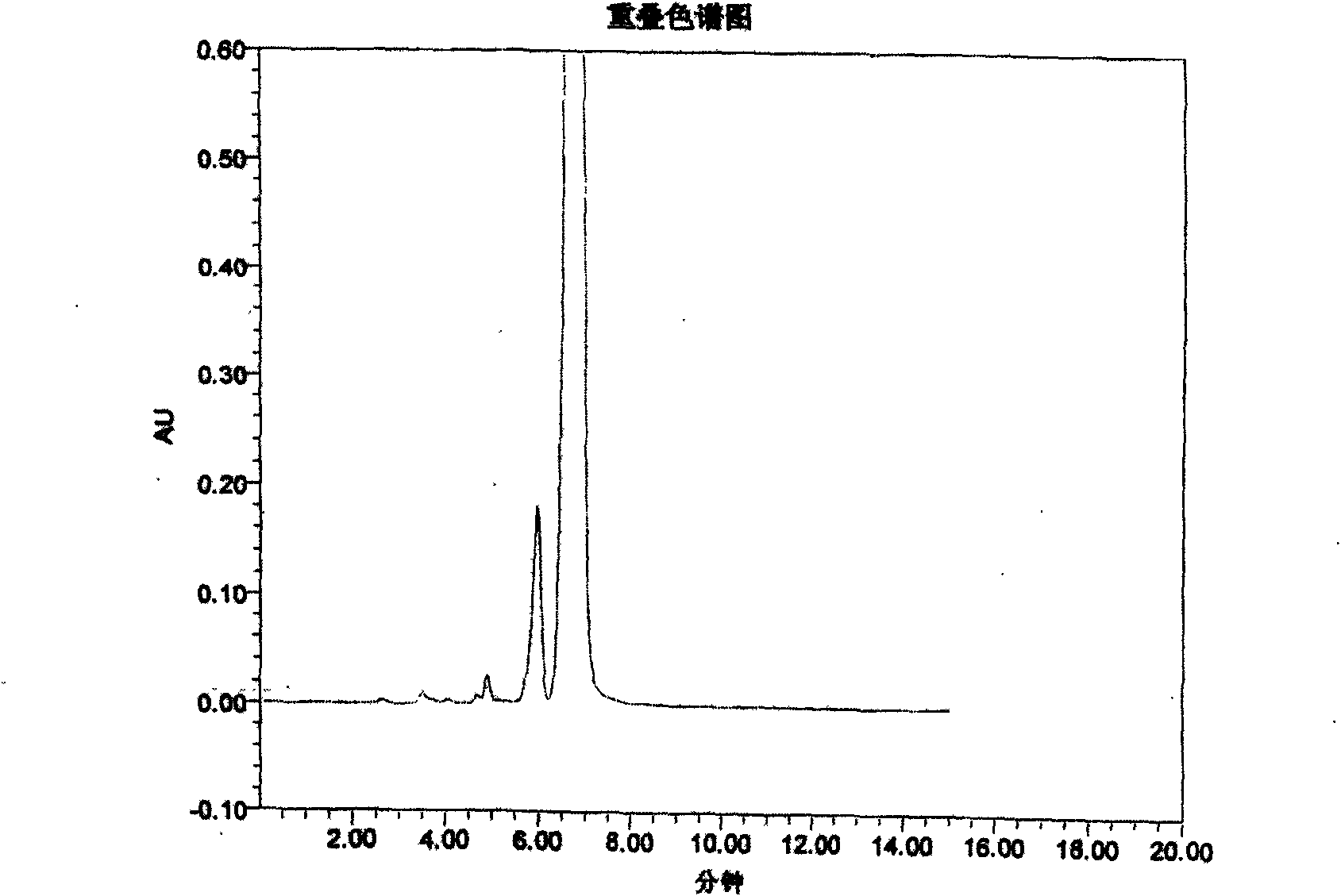
Novel method for detecting piperacillin sodium and sulbactam sodium for compound injection
InactiveCN101852780AComponent separationHeterocyclic compound active ingredientsImpuritySulbactam Sodium
The invention discloses a novel high performance liquid chromatography (HPLC) method, which can detect the content and relevant impurities of two single components in the compound preparation of piperacillin sodium and sulbactam sodium simultaneously, and the two components do not interfere and influence each other. The method has the advantages of simple operation, strong specificity, high sensitivity, wide linear range, and high stability, and can be used for detecting compound preparations and materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Eutectic of Piperacillin sodium and Sulbactam sodium, preparation method thereof, pharmaceutical composition containing eutectic and application of pharmaceutical composition
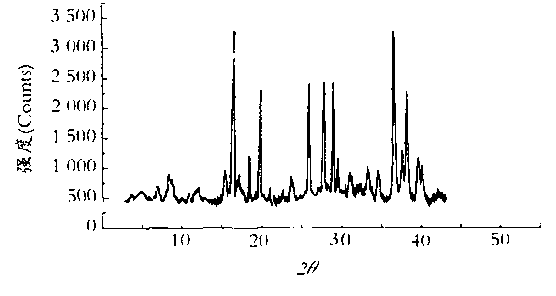
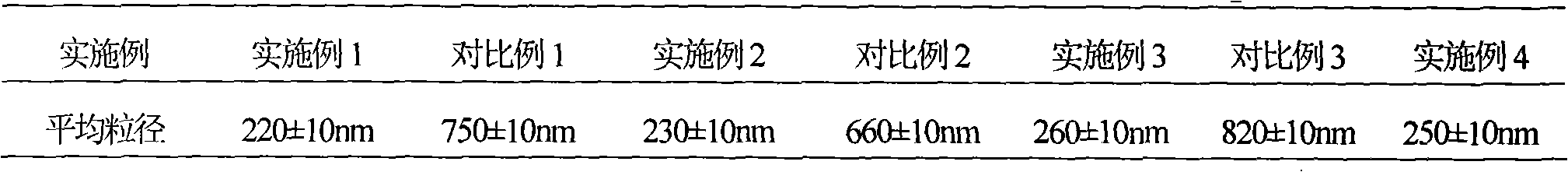
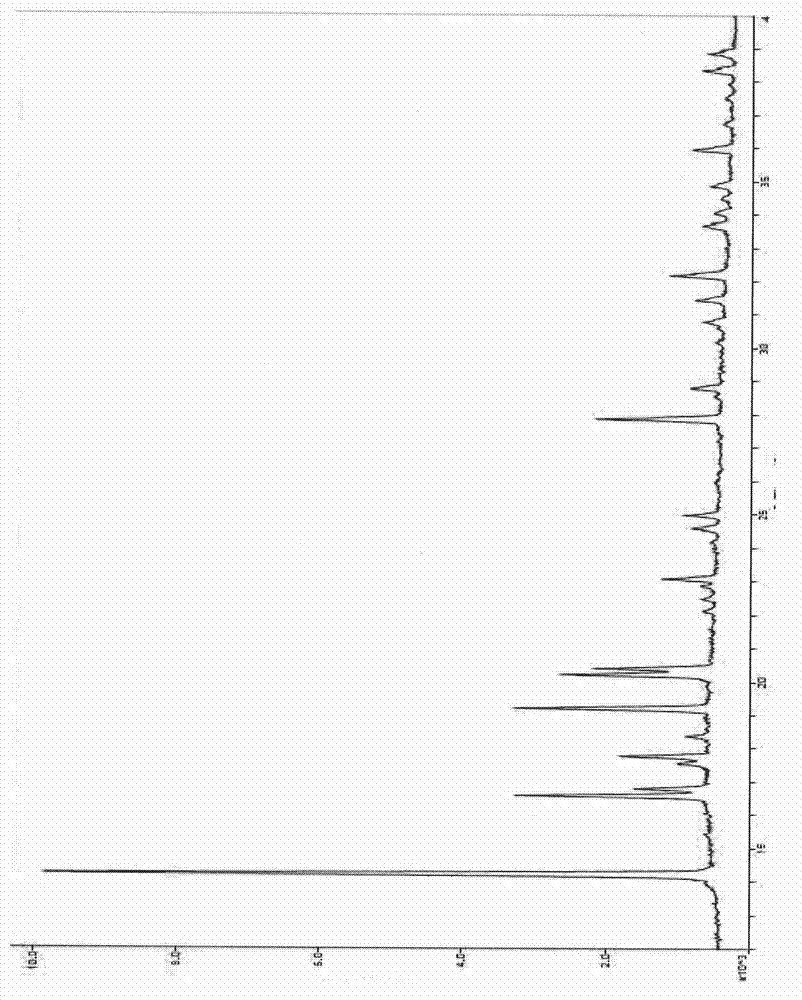
ActiveCN102898438ALittle difference in loadingSmall fluctuationAntibacterial agentsOrganic chemistrySolubilityX-ray
The invention relates to an eutectic of Piperacillin sodium and Sulbactam sodium, a preparation method thereof, a pharmaceutical composition containing the eutectic and an application of the pharmaceutical composition on treating infection of super bacteria producing NDM-1 and other drug-resistance bacteria. The eutectic of the Piperacillin sodium and the Sulbactam sodium comprises diffraction angles of 14.24 DEG, 16.58 DEG, 16.79 DEG, 17.77 DEG, 19.20 DEG, 20.21 DEG, 20.39 DEG, 23.06 DEG, 27.86 DEG and 32.16 DEG represented by 2[theta] in an X-ray powder diffraction analysis spectra. The eutectic provided by the invention is excellent in solubility, low in hygroscopicity, relatively small in powder volume, high in purity, low in content of related substances, and easy to filter and dry during industrial production. A compound preparation prepared by the eutectic has obviously improved stability; not only is the shelf life of the products prolonged, but also the product safety is improved, thereby reducing potential adverse risks of the drugs and further protecting the health of patients.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Cefoperazone sodium and sulbactam sodium combination and preparation method thereof
InactiveCN101284009AImprove stabilityGuaranteed qualifiedAntibacterial agentsPharmaceutical delivery mechanismActivated carbonFreeze-drying
The invention provides a cefoperazone sodium / sulbactam sodium composition, comprises the following components of: cefoperazone sodium, sulbactam sodium, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride. The invention also provides a preparation method of the cefoperazone sodium / sulbactam sodium composition. The method comprises the following steps of: weighing components as prescription; dissolving the components in water, and adjusting pH to 5.0-6.5 with phosphoric acid or sodium hydroxide solution; and adsorbing with activated carbon, filtering with microporous filtering film, filling, freeze drying, subpackaging under aseptic condition, or freeze drying, pulverizing, sieving and then subpackaging under aseptic condition. The composition adds sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride in the prescription, so as to improve the stability of the compound preparation. The composition can be stored in a dark, cool and dry place, so as to ensure product quality within validity period.
Owner:管小明
Preparation of cefoperazone and sulbactam sodium mixed powder
The invention relates to a preparation method of cefoperazone sodium / sulbactam sodium mixed powder. Cefoperazone acid and sulbactam acid are prepared into water solution with 20 to 40 percent by weight according to the weight ratio of 0.9 to 2.1: 1, a salt forming agent is used for regulating the pH of material liquid to 6.0 to 6.5, a filter membrane with 0.22Mum is used for filtration, then the material liquid is arranged in a freeze-drying box; and the cefoperazone sodium / sulbactam sodium mixed powder product is obtained after freeze-drying. The product of the mixed powder obtained after freeze-drying has good uniformity, rapid dissolution speed and stable main quality indicators of product content, water content, pH value, clarification of the solution and so on.
Owner:QILU ANTIBIOTICS PHARMA
Sulbactam compound for treating infectious diseases, and preparation method therefor
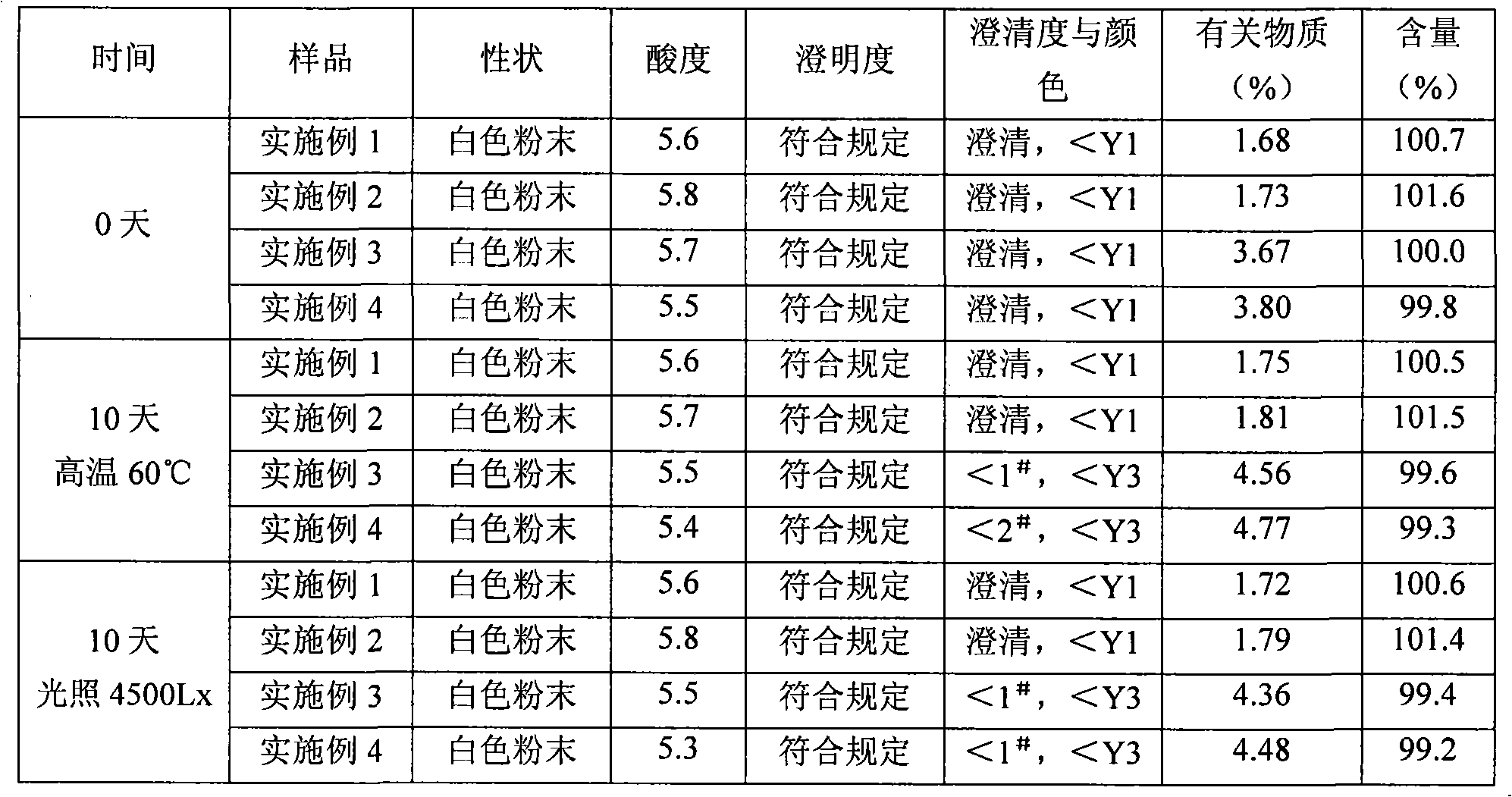
InactiveCN104876946ANot easy to absorb moistureLow impurity contentAntibacterial agentsPowder deliveryChemical compoundMoisture absorption
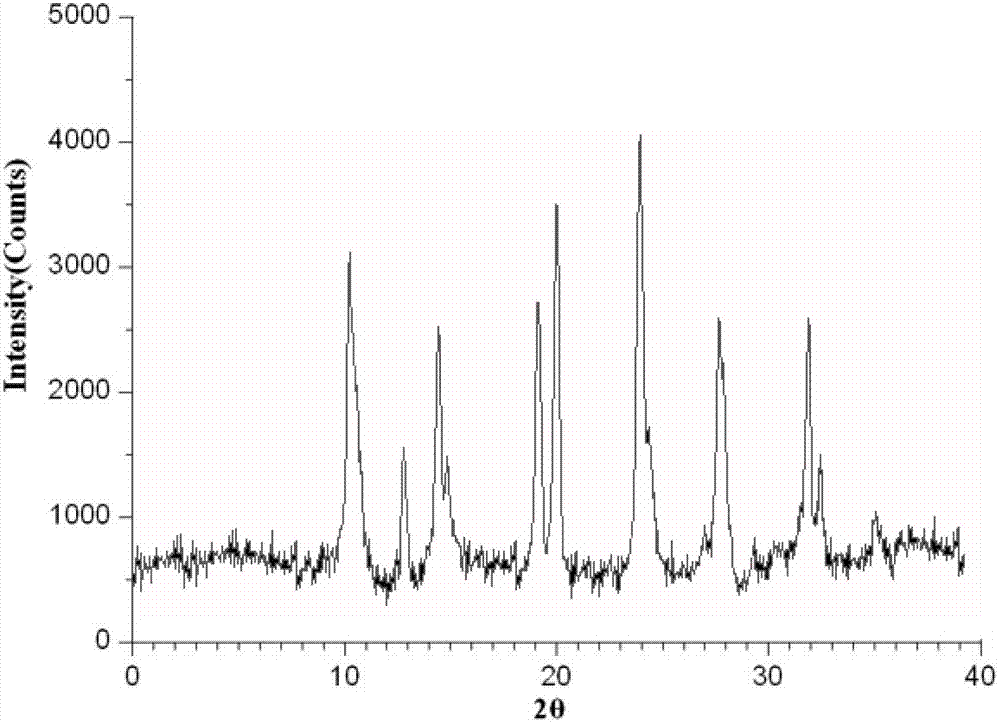
The invention discloses a sulbactam compound for treating infectious diseases, and belongs to the field of medical technology. The sulbactam compound is in the form of crystals and is measured through the X-ray diffraction (XRD) measurement process. Characteristic diffraction peaks appear at 8.8, 11.6, 12.4, 14.6, 16.5, 17.1, 17.7, 18.8, 19.1, 19.6, 21.5, 21.9, 23.4, 24.4, 25.3, 26.8, 27.1, 27.6, 28.0, 29.6, 30.6, 30.9, 31.7, 32.7, 33.2, 34.6, 35.6, 36.1, 37.0, 38.2, 40.2, 41.2, 41.9, 42.4, 43.6 and 44.2 parts of an X-ray powder diffraction spectrum, wherein the diffraction angle of the X-ray powder diffraction spectrum is within the range of 20+ / -0.2 degrees. The sulbactam compound and the preparation thereof are difficult in moisture absorption and stable in long-term storage, and greatly improves the medication safety.
Owner:王雪雁
Novel method for measuring compound cefotaxime sodium sulbactam sodium
The invention relates to a novel high performance liquid chromatogram (HPLC) method, which can simultaneously detect the content of two single ingredients and relevant impurities in the cefotaxime sodium sulbactam sodium compound. The two ingredients have no interferences or influences. The method has easy operation, strong specificity, high sensitivity, large linear range and good stability, and can be used for detecting a compound preparation and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Sulbactam sodium compound and medical composition of sulbactam sodium compound and mezlocillin sodium
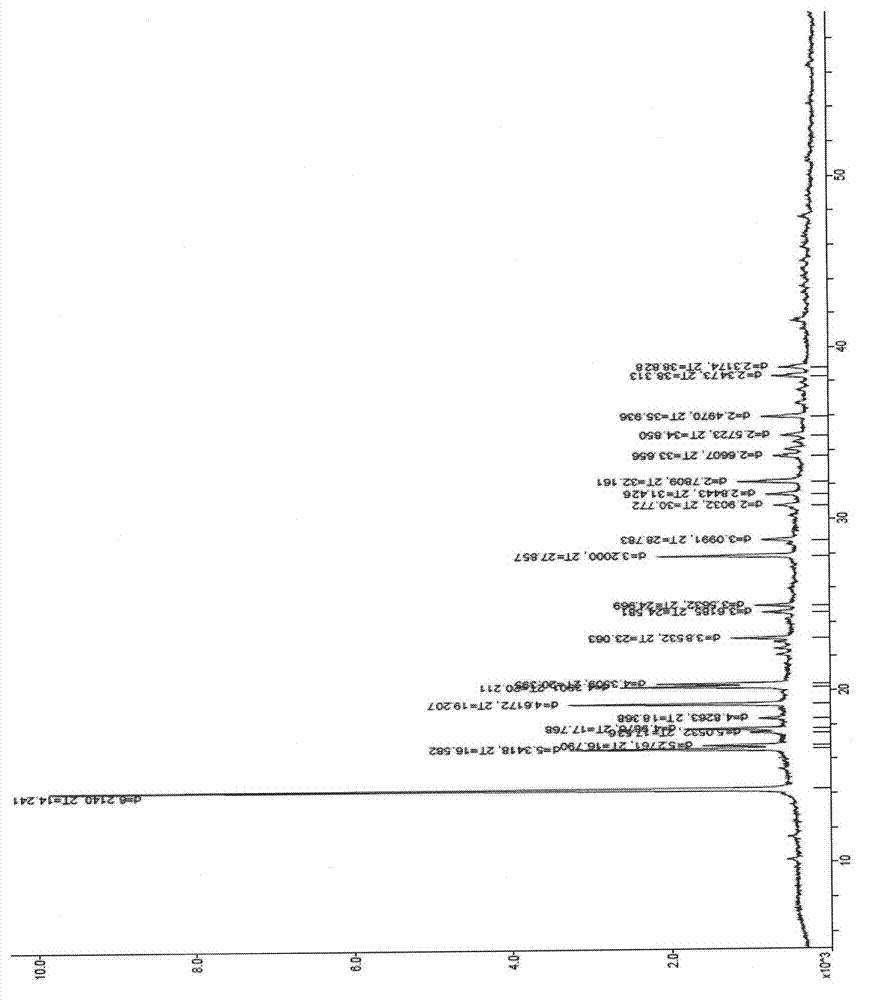
ActiveCN103113390ALess impurity content in storageGood storage stabilityAntibacterial agentsOrganic chemistryChemical compositionPowder diffraction
The invention belongs to the technical field of medicines, and in particular relates to a sulbactam sodium compound and a medical composition of the sulbactam sodium compound and mezlocillin sodium. The sulbactam sodium compound is shown as by an X-ray powder diffraction spectrogram I measured by a Cu-K alpha ray. The sulbactam sodium compound provided by the invention has better storage stability. The invention further provides a preparation method of the sulbactam sodium compound as well as a medical composition of the sulbactam sodium compound and mezlocillin sodium. The medical composition has better storage stability and higher using safety performance.
Owner:SHANXI C&Y PHARMACEUTICAL GROUP CO LTD
Liposome injection based on drug combination of mezlocillin sodium and sulbactam sodium
InactiveCN101804052AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsAntioxidantDissolution
The invention provides a liposome injection based on a drug combination of mezlocillin sodium and sulbactam sodium. The liposome injection comprises the following components: mezlocillin sodium, sulbactam sodium, liposome carriers, frozen and dried supporting agent and optional existing antioxidant, wherein the liposome carriers are particularly hydrogenated soybean phosphatidylcholine and octadecylamine. The liposome injection of the invention has good preparation stability, prevents the liposome from being cracked under the action of dehydration, fusion and ice crystal generation in the freezing-drying process, and keeps good entrapment rate of the liposome after re-dissolution through hydration.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Amoxicillin sodium/sulbactam sodium composition microsphere injection
InactiveCN101890008AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryMicrosphereAmoxicillin Sodium
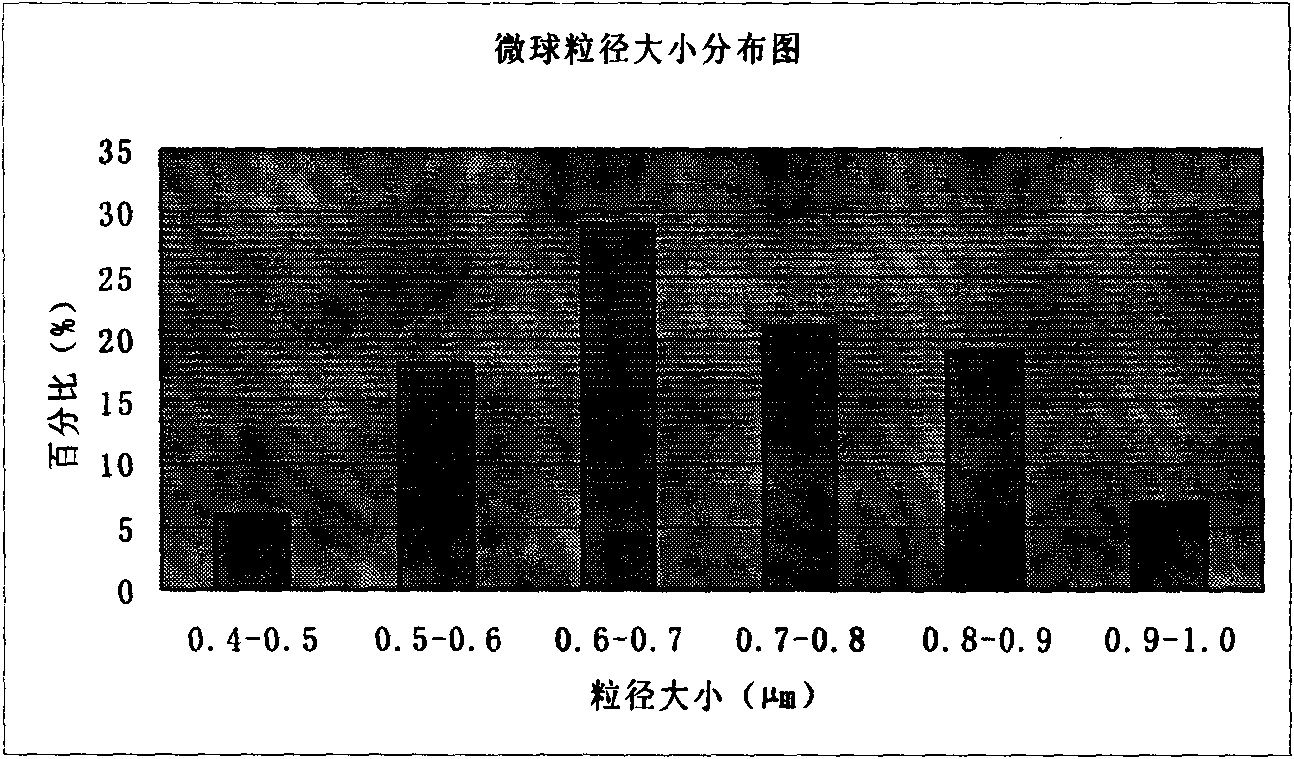
The invention discloses an amoxicillin sodium / sulbactam sodium composition microsphere injection, which is characterized by comprising 2 parts of amoxicillin sodium, 1 part of sulbactam sodium, 1.5-6 parts of PLGA, 1.5-3 parts of tween 80, 1.5-3 parts of propylene glycol and 0.5-1.0 part of trehalose by weight. Compared with the prior art, the microsphere injection has good stability, high encapsulation efficiency, good preparation process repeatability, uniform particle distribution, less solvent residues, good injectability and excellent slow-release characteristic and is suitable for industrial production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method of piperacillin sodium sulbactam sodium for injection
InactiveCN103301131AReduce the introductionHigh content of main componentsAntibacterial agentsPowder deliveryMoistureAnaphylactic reaction
The invention relates to a preparation method of piperacillin sodium sulbactam sodium for injection. The preparation method comprises the following steps of: moving and mixing the piperacillin sodium and the sulbactam sodium in a sealed container in various directions at a weight ratio of 4:1; discharging the powder after physically mixing the powder; weighing and filling the powder into a barrel; carrying out subsequent treatment to obtain the piperacillin sodium sulbactam sodium for injection. According to the preparation method of the piperacillin sodium sulbactam sodium for injection, only the piperacillin sodium and the sulbactam sodium are selected as raw materials without introducing other novel substances, so that on one hand, the content of main ingredient of the medicament is high, on the other hand, the introduced impurities including related substances and the like are reduced. Moreover, the whole process is strictly controlled, so that the prepared piperacillin sodium sulbactam sodium for injection is low in moisture content, less in visible foreign substances and less in in-soluble particles, and therefore, the anaphylactic reaction during the injection is effectively reduced, and the medicament use is safer.
Owner:SICHUAN PHARMA
Preparation method of lyophilization sulbactam sodium material medicine
InactiveCN101914104ANo useNo pollution in the processOrganic chemistryForeign matterSodium bicarbonate
The invention belongs to the field of medicine and chemical industry. The invention relates to a preparation method of a lyophilization sulbactam sodium material medicine, which comprises the following steps of: (1) adding sodium bicarbonate into sulbactam acid suspension to obtain a lyophilization sulbactam sodium material liquid medicine; (2) filtering the liquid medicine by utilizing a filter; (3) uniformly pouring the sterile liquid medicine into a material disk of a freeze drier, setting the temperature of a baffle plate to be -40 DEG C to -10 DEG C for freezing for 1-6h, then setting the temperature of the baffle plate to be -10 DEG C to 10 DEG for keeping for 1-10h, then setting the temperature of the baffle plate to be 10 DEG C to 20 DEG C for drying 1-20h, then setting the temperature of the baffle plate to be 20-50 DEG C for drying for 1-30h, finally setting the temperature of the baffle plate to be 50-65 DEG C for drying 1-20h; and (4) grinding and uniformly mixing the lyophilization powder prepared from the step (3) to obtain the lyophilization sulbactam sodium material medicine. The products prepared by the preparation method of a lyophilization sulbactam sodium material medicine has the advantages of good clarity index and high content; and bad factors of solvent residues, clarity foreign matters, turbidity and the like prepared by utilizing a solvent method can be avoided.
Owner:SHIJAZHUANG ZHONGSHUO PHARMA CO LTD +1
New method for detecting compound ceftazidime and sulbactam sodium
InactiveCN101650356AComponent separationTesting medicinal preparationsAdditive ingredientCeftazidime
The invention provides a new method for detecting compound ceftazidime and sulbactam sodium, which can detect the content of two single ingredients and relative impurities in the ceftazidime and sulbactam sodium compound at the same time. The method prevents mutual interference and influence of two single ingredients, is characterized by simple and easy operation, strong specificity, high sensitivity, large linear range and excellent stability, and can be used for detecting compound preparation and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Novel cefoperazone sodium and sulbactam sodium pharmaceutical composition for injection
InactiveCN106309448ASimple stepsEasy to operateAntibacterial agentsPowder deliverySulbactam SodiumChloride sodium
The invention discloses a novel cefoperazone sodium and sulbactam sodium pharmaceutical composition for injection, including the following components: 23o-24o of sulbactam sodium, sodium cefoperazone of specific rotation, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride, of where the weight weight-ratio of sulbactam sodium, cefoperazone sodium, sodium dihydrogen phosphate, disodium hydrogen phosphate and sodium chloride is 1:1-4:0.25-0.30:0.16-0.20:0.11-0.15. The invention also discloses a preparing method of the described new cefoperazone sodium and sulbactam sodium pharmaceutical composition for injection. The novel cefoperazone sodium and sulbactam sodium pharmaceutical composition has good stability, which can ensure qualified products within date expiration leads to clinically safe medication use.
Owner:南昌立健药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com