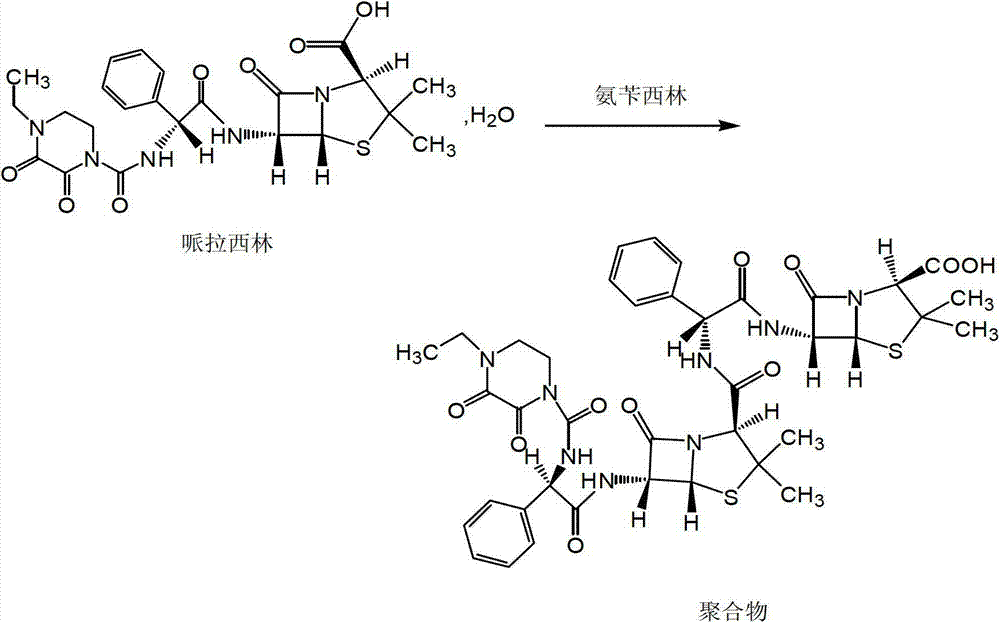
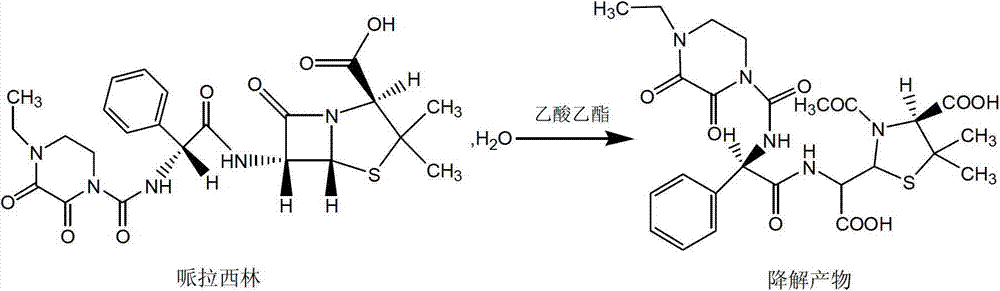
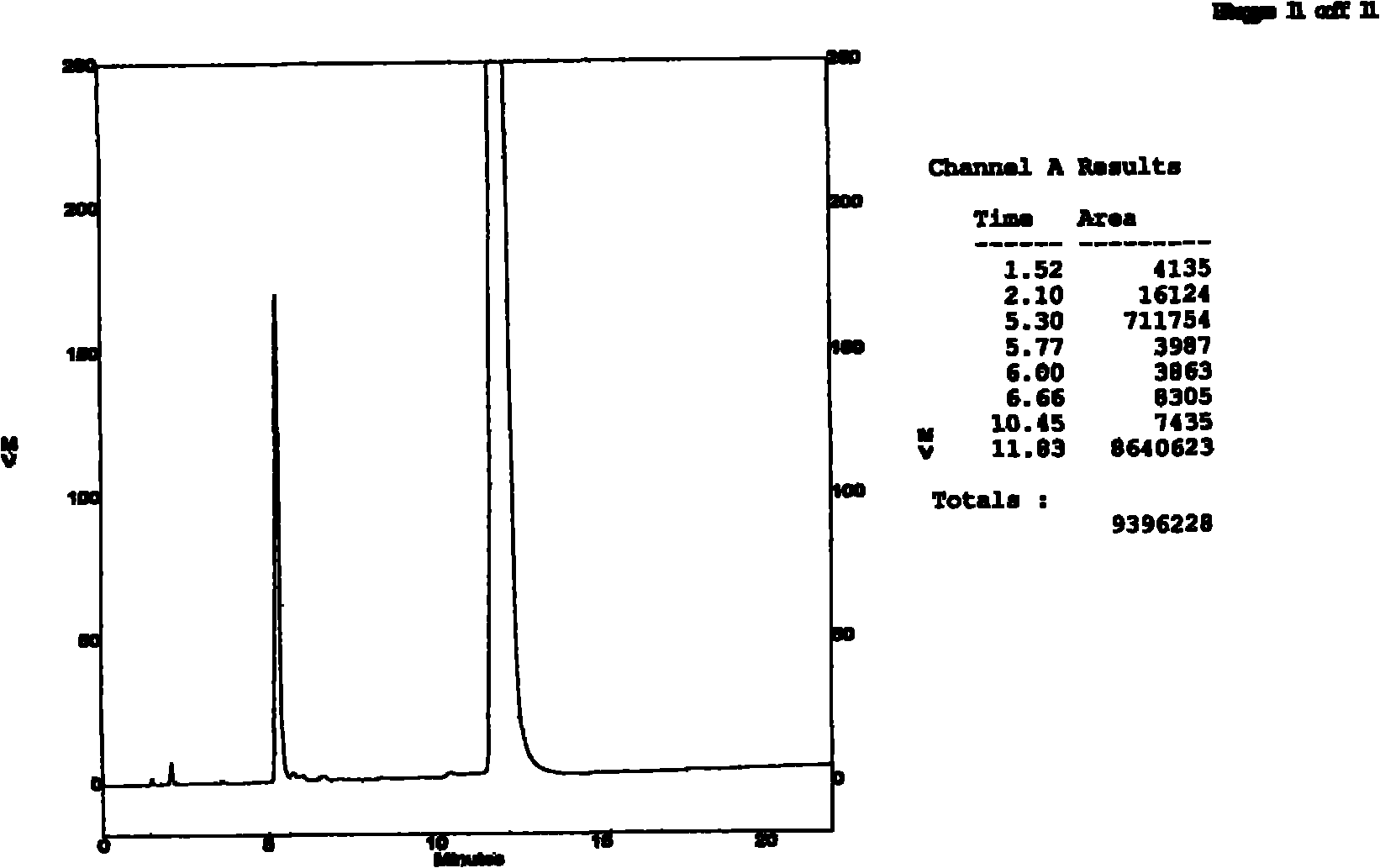
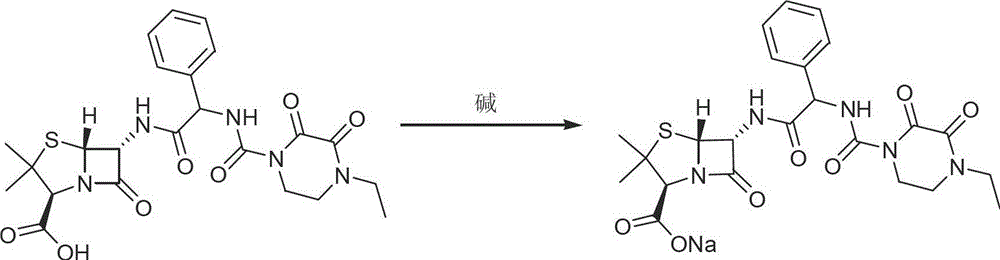
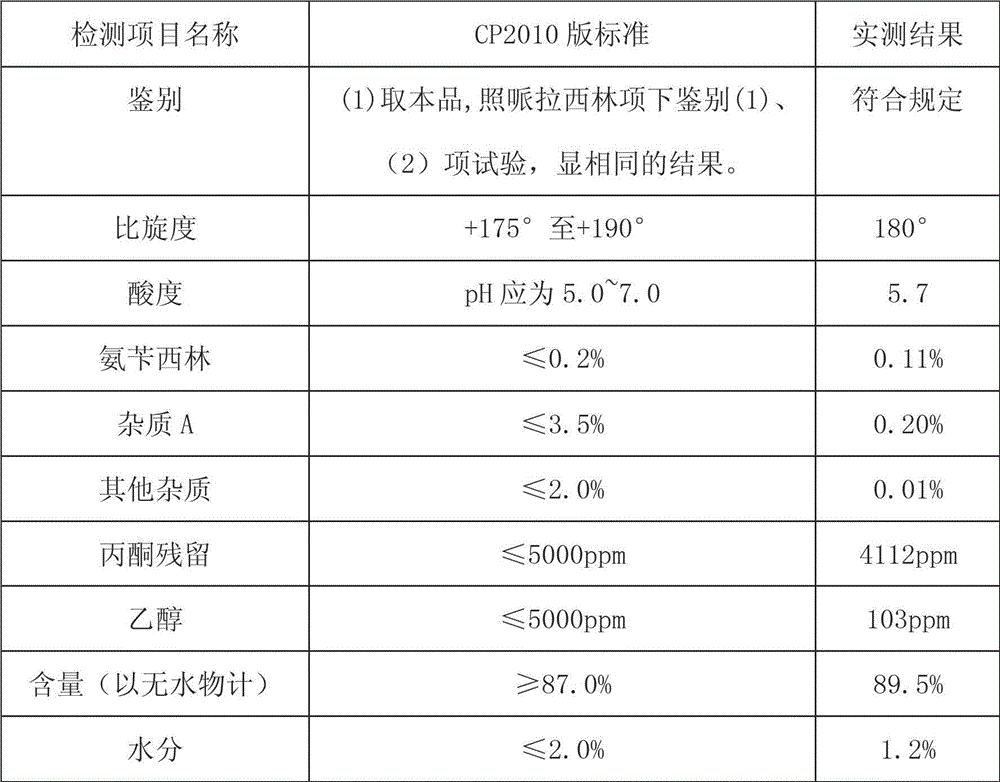
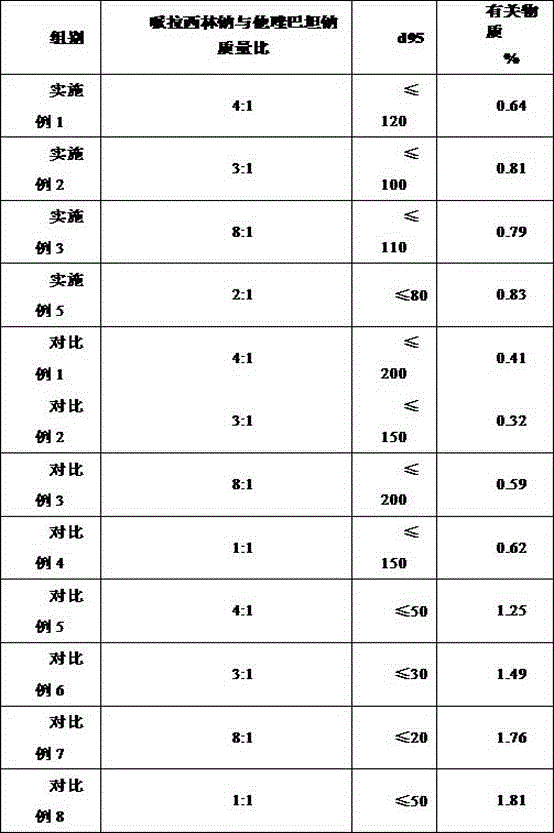
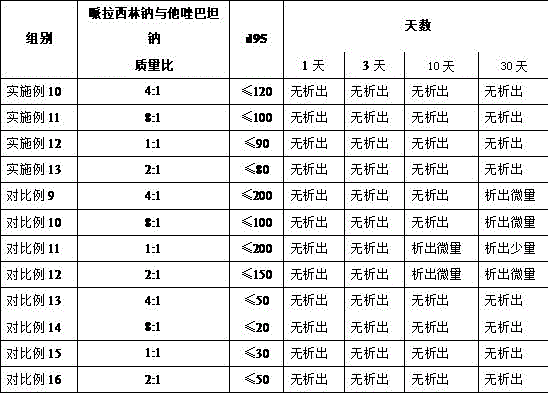
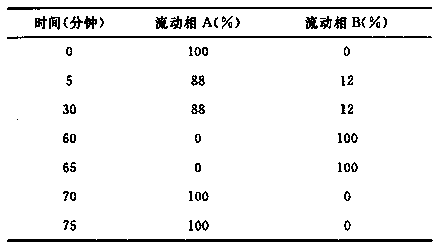
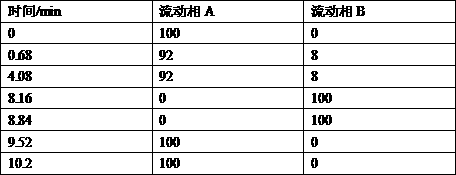
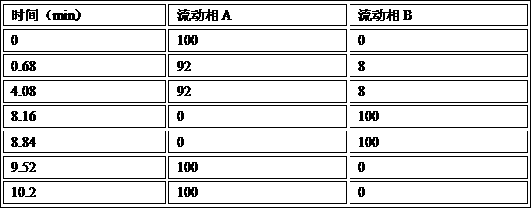
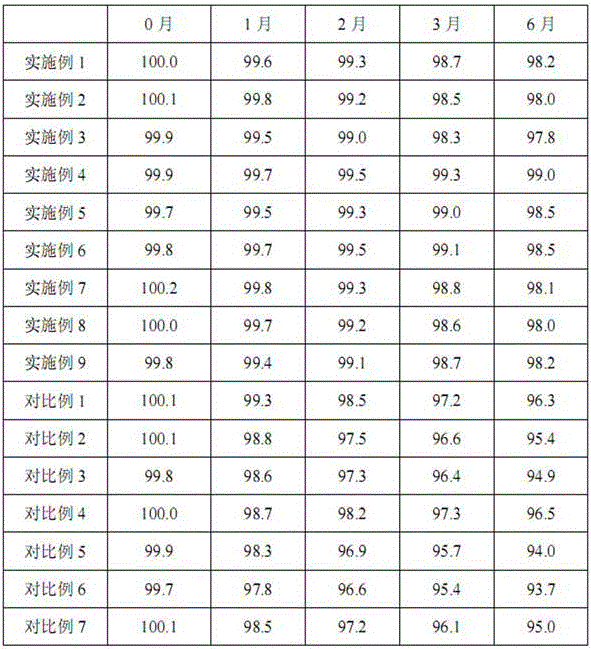
Patents
Literature
52 results about "Piperacillin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The sodium salt of piperacillin, a broad-spectrum semisynthetic, ampicillin-derived ureidopenicillin antibiotic with bactericidal activity. Piperacillin binds to and inactivates penicillin-binding proteins (PBPs), enzymes located on the inner membrane of the bacterial cell wall, resulting in the weakening of the bacterial cell wall and cell lysis. PBPs participate in the terminal stages of assembling the bacterial cell wall, and in reshaping the cell wall during cell division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity.
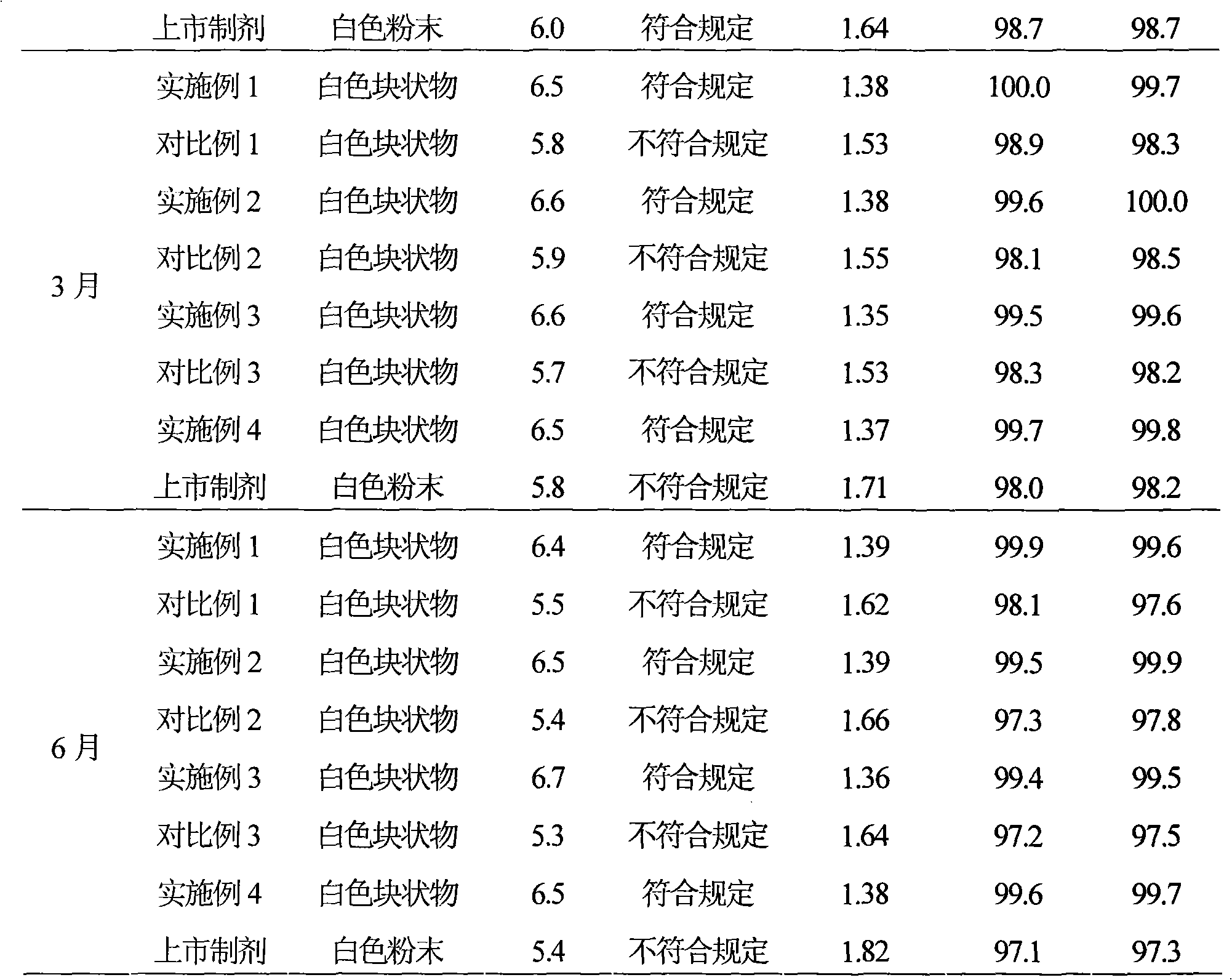
Pharmaceutical composition containing beta-lactamase restrainer and piperacillin sodium with steady content and preparation method thereof
ActiveCN101269072AContent remains constantNothing producedAntibacterial agentsPowder deliveryDrug compoundAir temperature
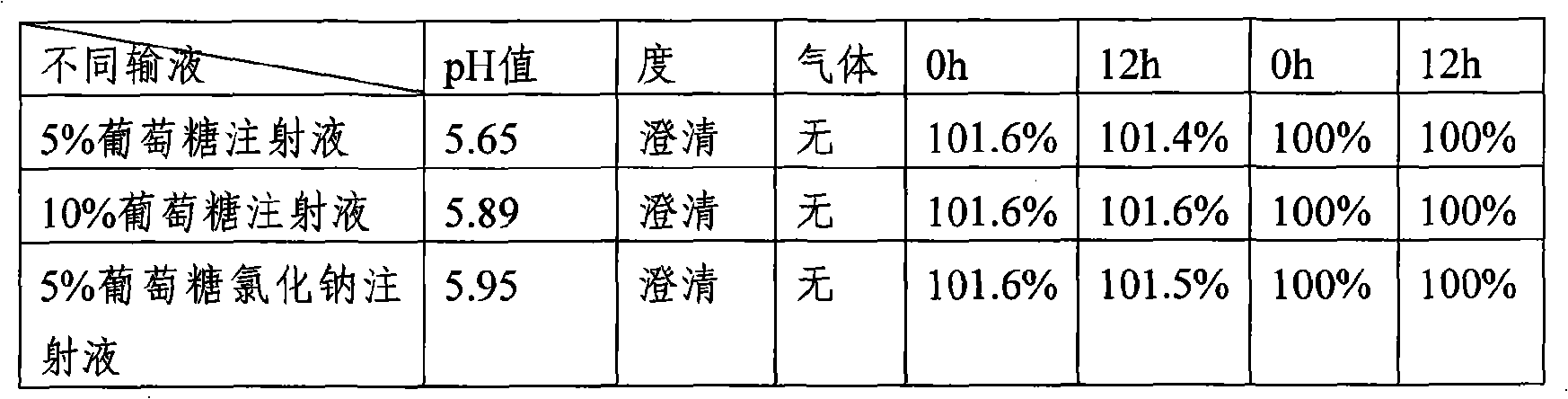
The invention provides a stable-content drug compound containing Beta-lactamases inhibitors and piperacillin sodium and the preparation method thereof. The drug compound consists of piperacillin sodium, Beta-lactamases inhibitors and pH-value regulators with the weight proportion of 1 to 100:1:0.001 to 2. Diluted and delivered in any proportion with conventional clinical transfusions, the drug compound can have a stable piperacillin sodium content, dissolve rapidly, generate no crystallization or degradation products and receive no effect from the temperature. The preparation method of the stable-content drug compound containing Beta-lactamases inhibitors and piperacillin sodium has simple preparing method and high efficiency and is fit for a large-scale industrial production.
Owner:福建丰恺思投资有限公司 +1
Suspensoid powder injection of piperacillin sodium sulbactam sodium medicine composition and novel application thereof
InactiveCN101632671AUnexpected effectImprove stabilityPowder deliveryDigestive systemFreeze-dryingPharmacology
The invention relates to suspensoid powder injection of a piperacillin sodium sulbactam medicine composition prepared by applying an emulsification suspensoid technology and freeze drying. The invention further relates to a novel application of the suspensoid powder injection in preparing a medicine treating the infection of the oral cavity.
Owner:HAINAN YONGTIAN PHARMA INST
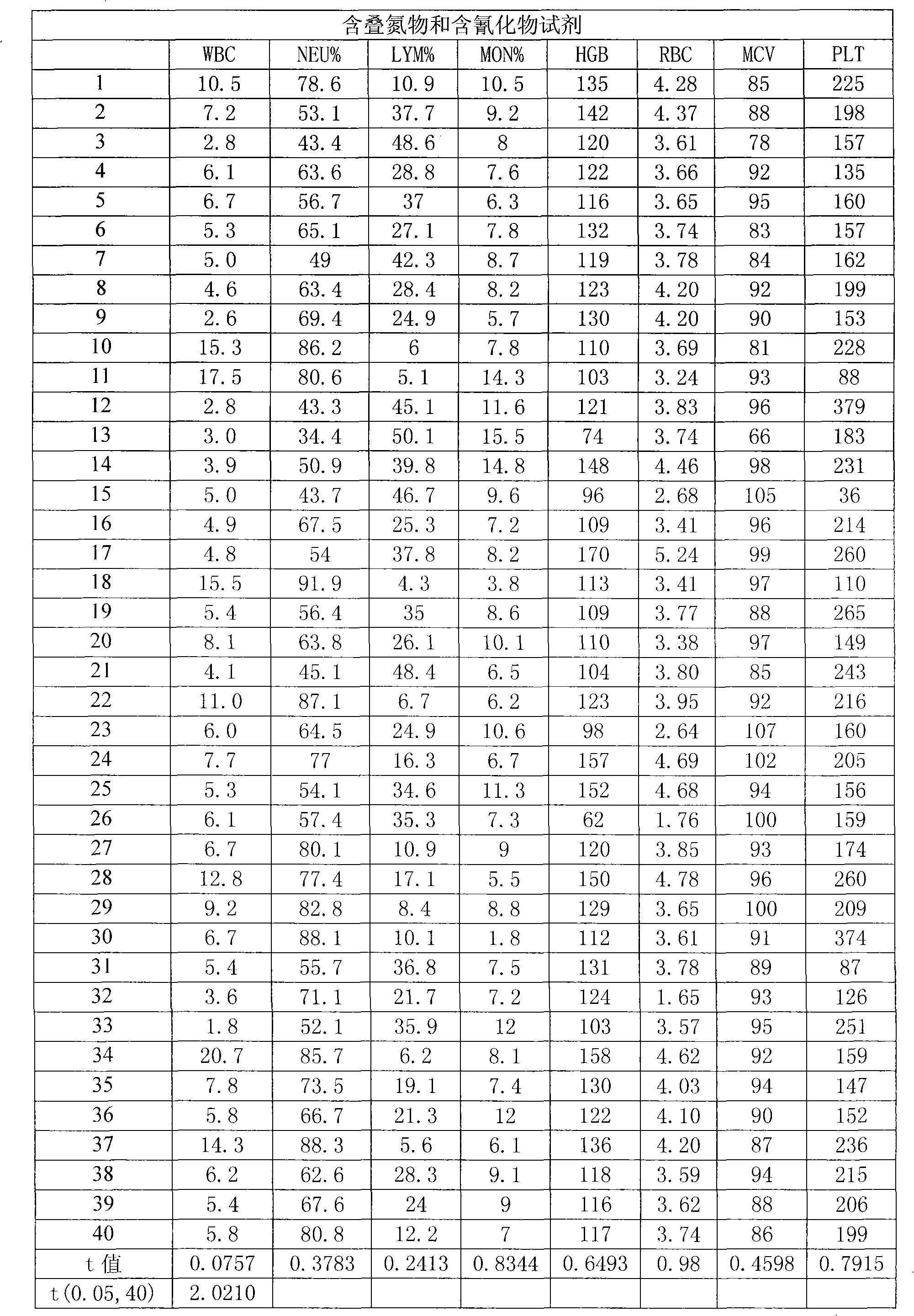
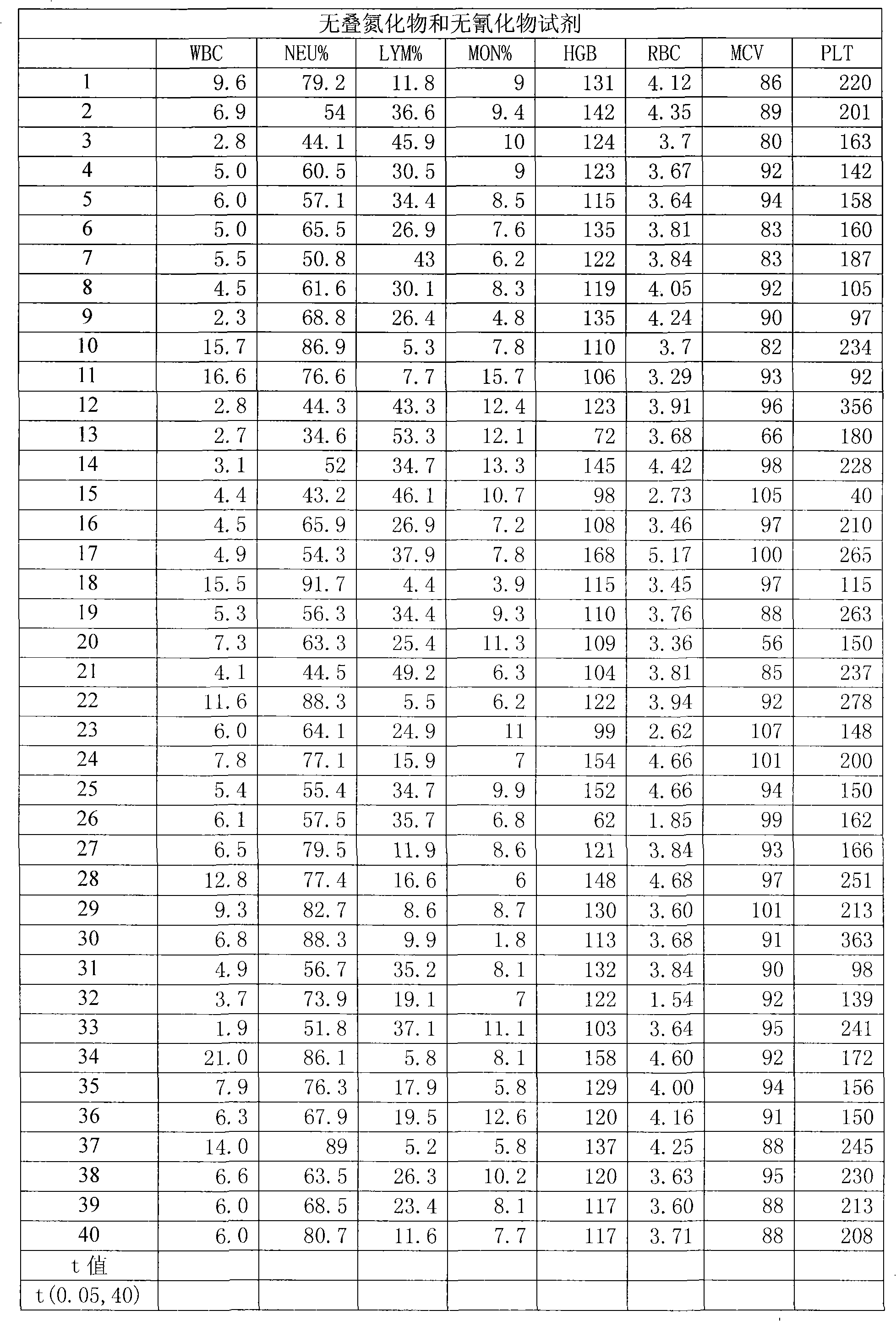
Blood cell analyzer dilution as well as hemolytic agent
InactiveCN101281193AImprove working environmentReduce harmIndividual particle analysisBiological testingNon toxicityToxicant
A blood cell analyzer diluent and a hemolytic agent, are characterized in that one litre diluent is provided with 12.0-4.0g of sodium chloride, 2.0-10.0g of sodium sulfate, 0.8-0.5g of 1,3,2-methylol urea, 0.2-0.5g of copper sulfite, 3.0-8.0g of EDTA-2Na, 0.2-0.7g of Piperacillin Sodium, a borate buffering liquid toning the ph value to 7.2-7.8, and the balance is water; one litre hemolytic agent is provided with 0.8-5.0g of potassium chloride, 0-60.0g of dodecyl trimethyl ammonium chloride, 14.0-0g of octadecyl trimethyl ammonium bromide, 6.0-10.0ml of isopropanol, carbonate or alcaine buffering liquid toning the ph value to 7.2-7.8, and the balance is water. The inventive reagent can form stable hemoglobin derivatives, and the absorption spectrum curves are similar when lambada is 540nm, lambada is 504nm, which can satisfy the clinical inspection requirement; the reagent does not contain cyanide, azide, and has non-toxicity, which can effective improve working atmosphere of operating staff, and can reduce harm of toxicant to personal health.
Owner:南昌百特生物高新技术股份有限公司
Injectable sterile pharmaceutical composition with piperacillin sodium and tazobactam sodium as active principles
ActiveUS20080233196A1Improve stabilityEasily soluble in waterOrganic active ingredientsBiocideSodium bicarbonateTAZOBACTAM SODIUM
A sterile pharmaceutical composition having as its active principles piperacillin sodium and tazobactam sodium of substantially the same density, mixed with sodium bicarbonate. The mixture is soluble in water to give injectable reconstituted solutions having high stability with time.
Owner:ACS DOBFAR SPA
Method for detecting related substances in piperacillin sodium and sulbactum sodium for injection
ActiveCN104215697ARealize determinationEfficient separationComponent separationSilanesStability indicating
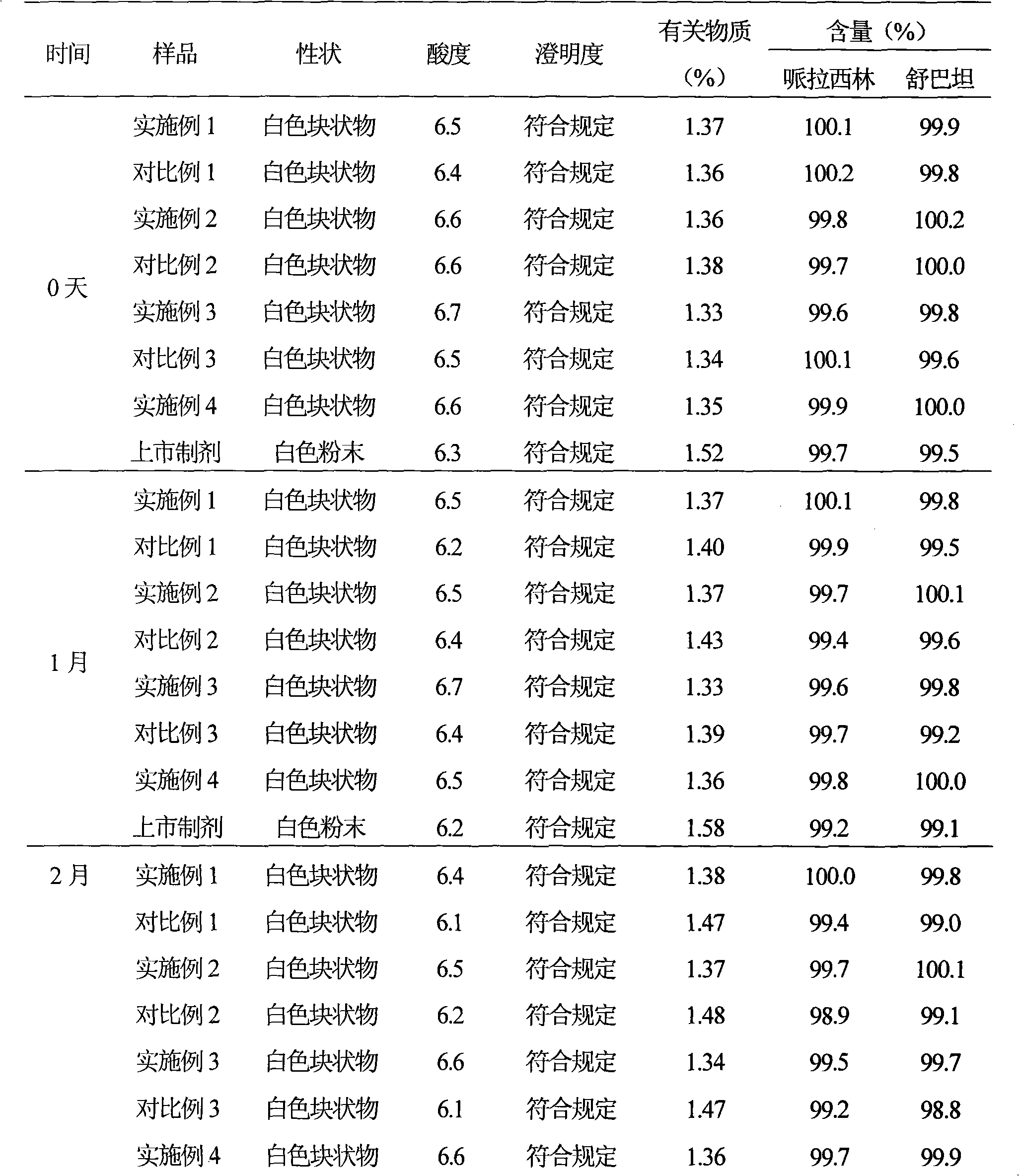
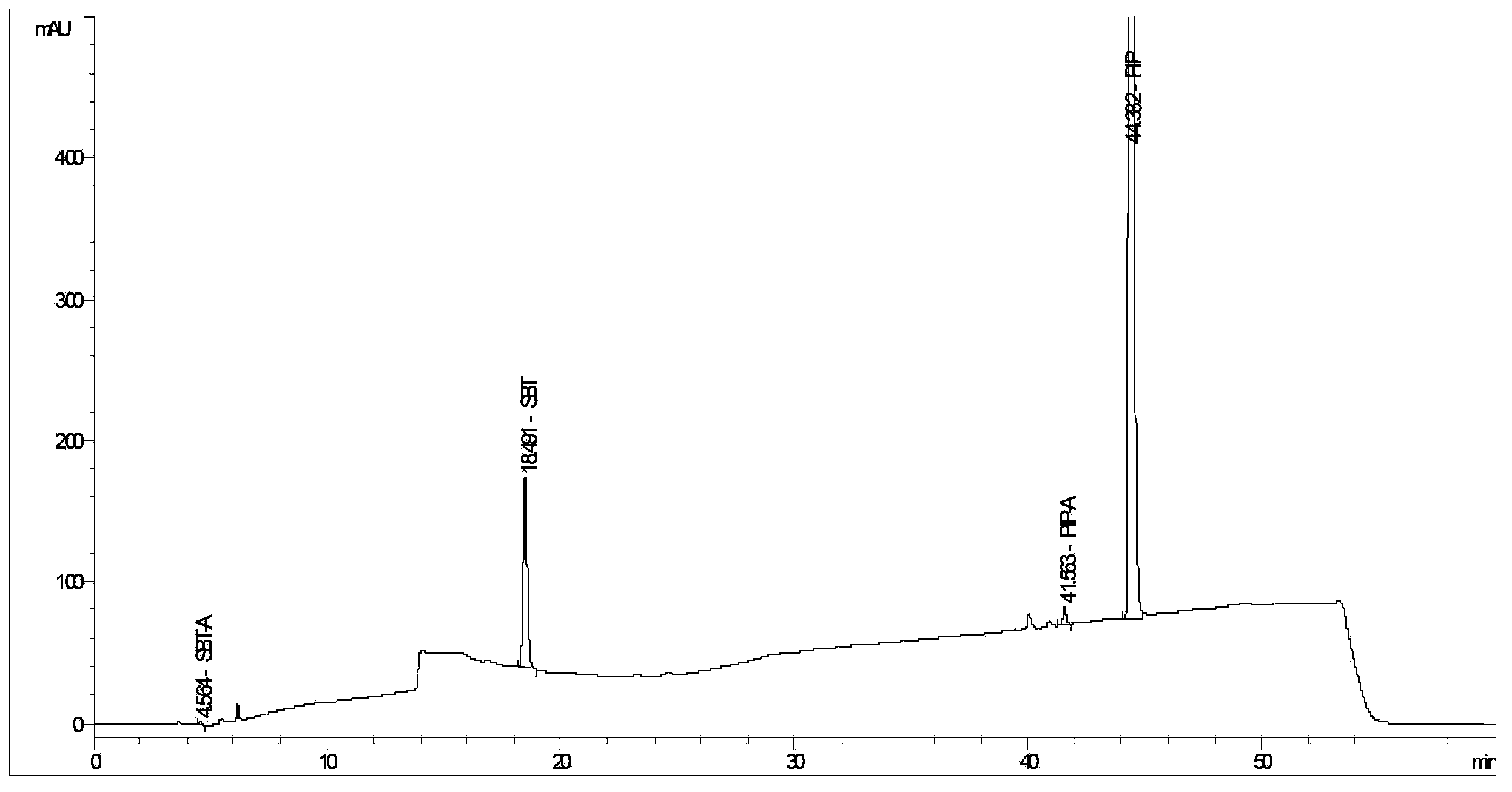
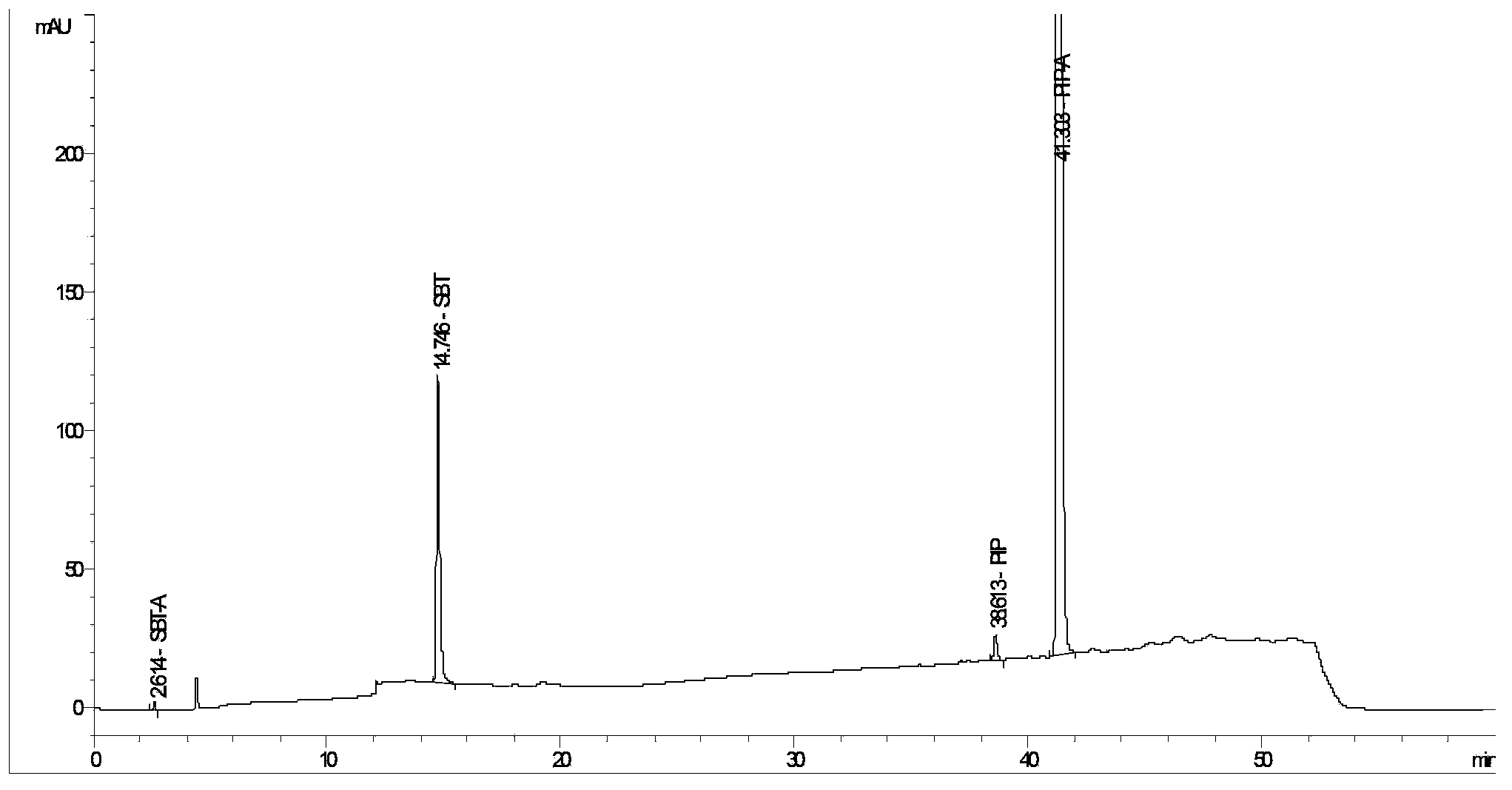
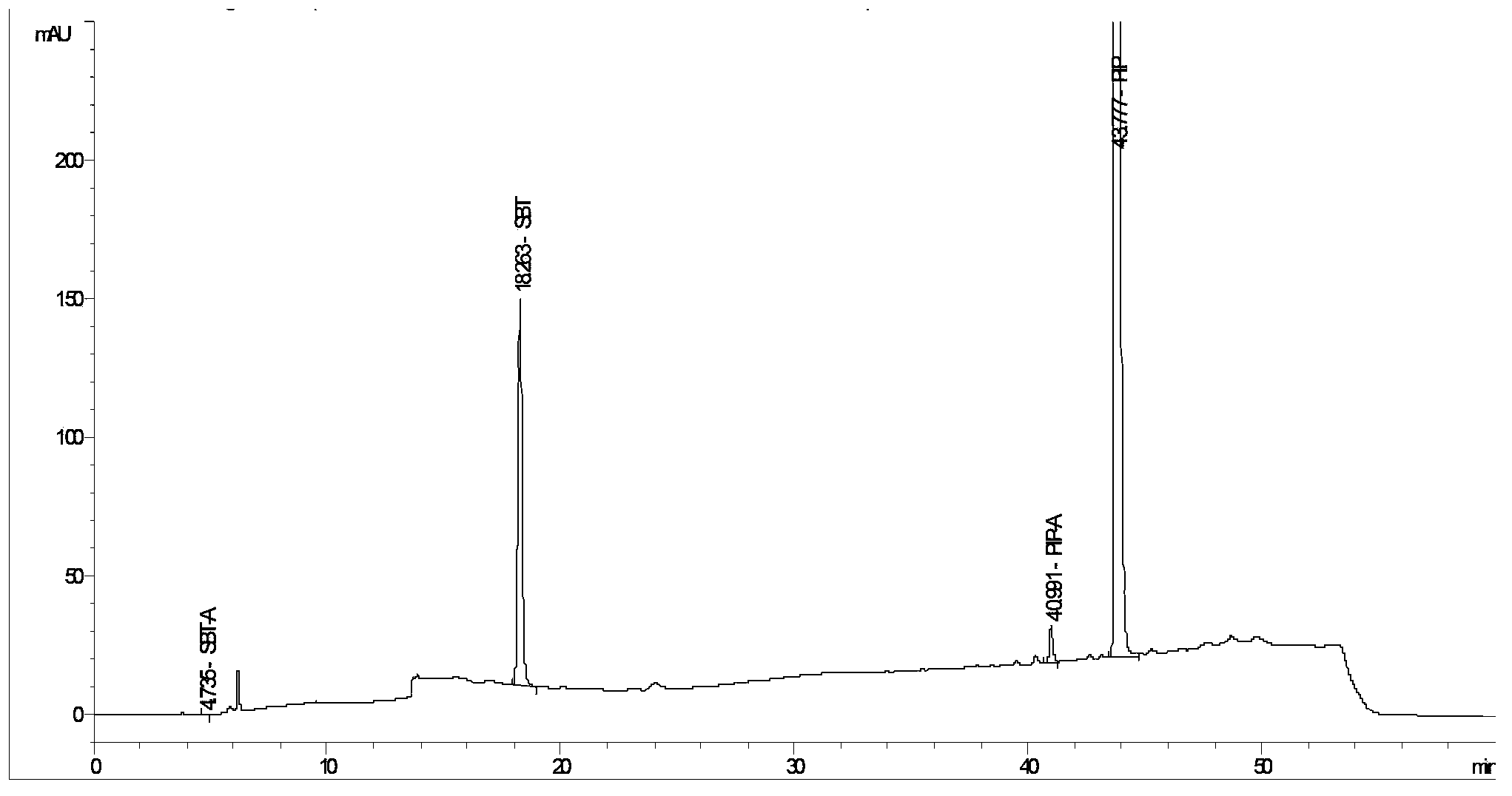
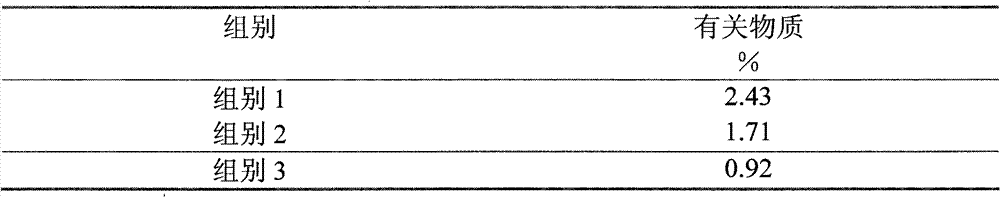
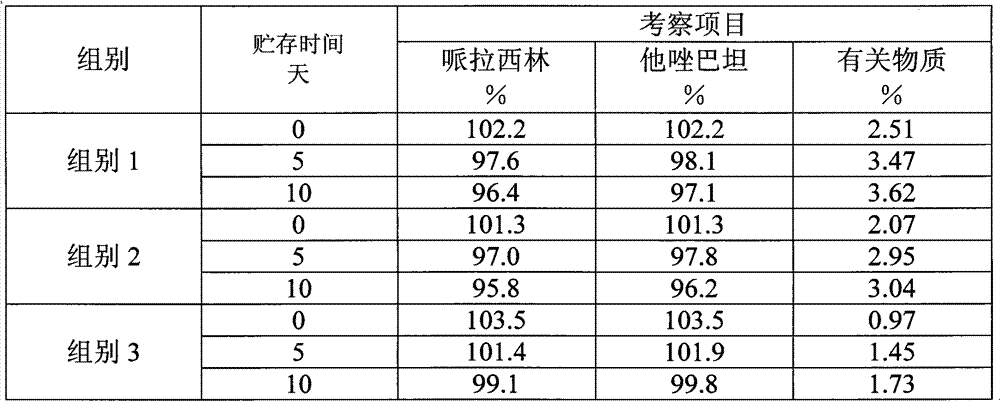
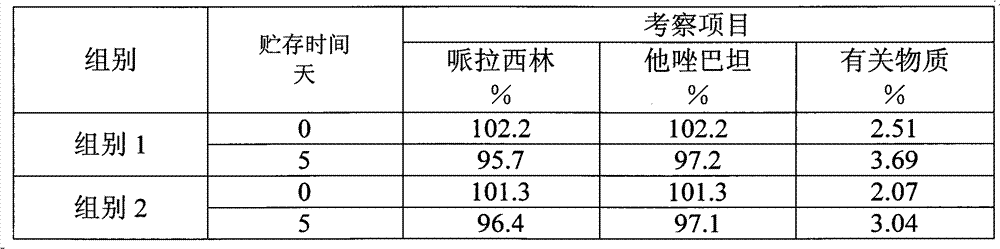
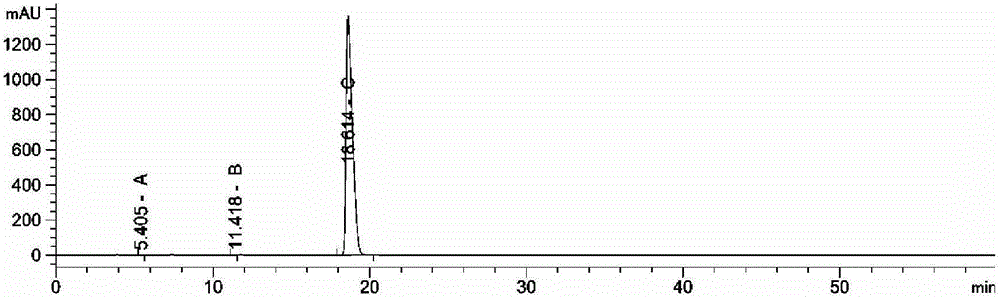
The invention provides a method for detecting related substances in piperacillin sodium and sulbactum sodium for injection. The method adopts an octadecyl silane bonded silica-gel chromatographic column to carry out gradient elution so as to rapidly and precisely complete the detection on related substances in piperacillin sodium and sulbactum sodium. In the obtained chromatogram, the main related substance (2S)-2-amino-3-methyl-3-sulfinobutyric acid and piperacillin penicilloic acid are well separated, piperacillin and sulbactum are also well separated from other related substances, and the separation degree is more than 1.5. The degradation researches and methodology on piperacillin sodium and sulbactum sodium show that the provided method has a stability indicating function, and thus the detection method can be used to control the limits of impurities in piperacillin sodium and sulbactum sodium, and can also be used to control the quality of piperacillin sodium and sulbactum sodium for injection. The method has the advantages of convenient operation and low cost, and has a good economic profit and promotion prospect.
Owner:XIANGBEI WELMAN PHARMA CO LTD +1
Compound injection composed of probenecid and penicillin antibiotic
InactiveCN1813700AReduce dosageReduce the number of dosesAntibacterial agentsPowder deliveryCurative effectAmoxicillin Sodium
The present invention relates to a compound injection preparation formed from probenecid sodium and benzylpenicillin antibiotics, belonging to the field of chemical pharmaceutical technology. The benzylpenicillin antibiotics include amoxicillin sodium and piperacillin sodium. The amoxicillin sodium and piperacillin sodium are mixed according to ratio of 1:3 and 1:5 through a conventional preparation process.
Owner:吴晓辉
Crystallization method of piperacillin
The invention discloses a crystallization method of piperacillin, belonging to the technical field of medicine. In the method, sodium bicarbonate is used as the alkali, the piperacillin is prepared into piperacillin sodium aqueous solution with concentration of 10-50%, after absorption and removal of impurities, with absence of organic solvent, the solution is treated by pure aqueous phase fractional crystallization, thus, the piperacillin acid is obtained. In the method, the frequently-used ethyl acetate, acetone or alcohol and water mixed solvent crystallization method is replaced by the pure aqueous phase crystallization method, the phenomena, for example, the product is not easy to devitrify or is tacky in devitrification, caused by the existence of organic solvent, are avoided, and the quality and the yield of products both are improved greatly. The method has the advantages of obtaining the raw material easily, being low in cost, being less harmful for environment, being safe and reliable, and being excellent in product quality.
Owner:山东安信制药有限公司 +1
Novel method for detecting piperacillin sodium and sulbactam sodium for compound injection
InactiveCN101852780AComponent separationHeterocyclic compound active ingredientsImpuritySulbactam Sodium
The invention discloses a novel high performance liquid chromatography (HPLC) method, which can detect the content and relevant impurities of two single components in the compound preparation of piperacillin sodium and sulbactam sodium simultaneously, and the two components do not interfere and influence each other. The method has the advantages of simple operation, strong specificity, high sensitivity, wide linear range, and high stability, and can be used for detecting compound preparations and materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
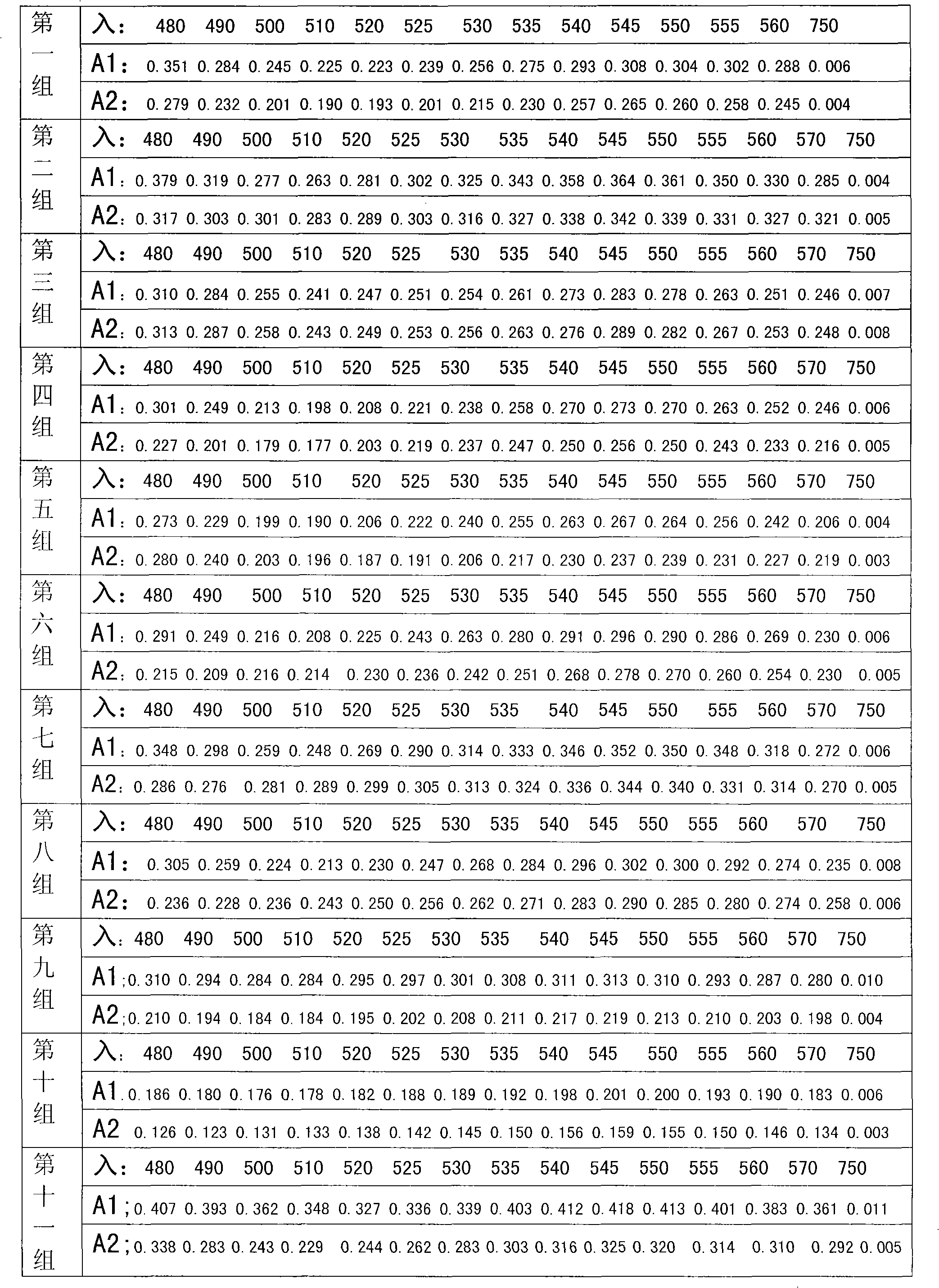
Eutectic of Piperacillin sodium and Sulbactam sodium, preparation method thereof, pharmaceutical composition containing eutectic and application of pharmaceutical composition
ActiveCN102898438ALittle difference in loadingSmall fluctuationAntibacterial agentsOrganic chemistrySolubilityX-ray
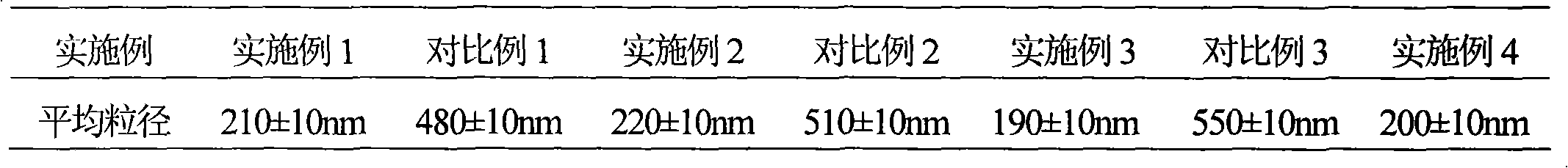
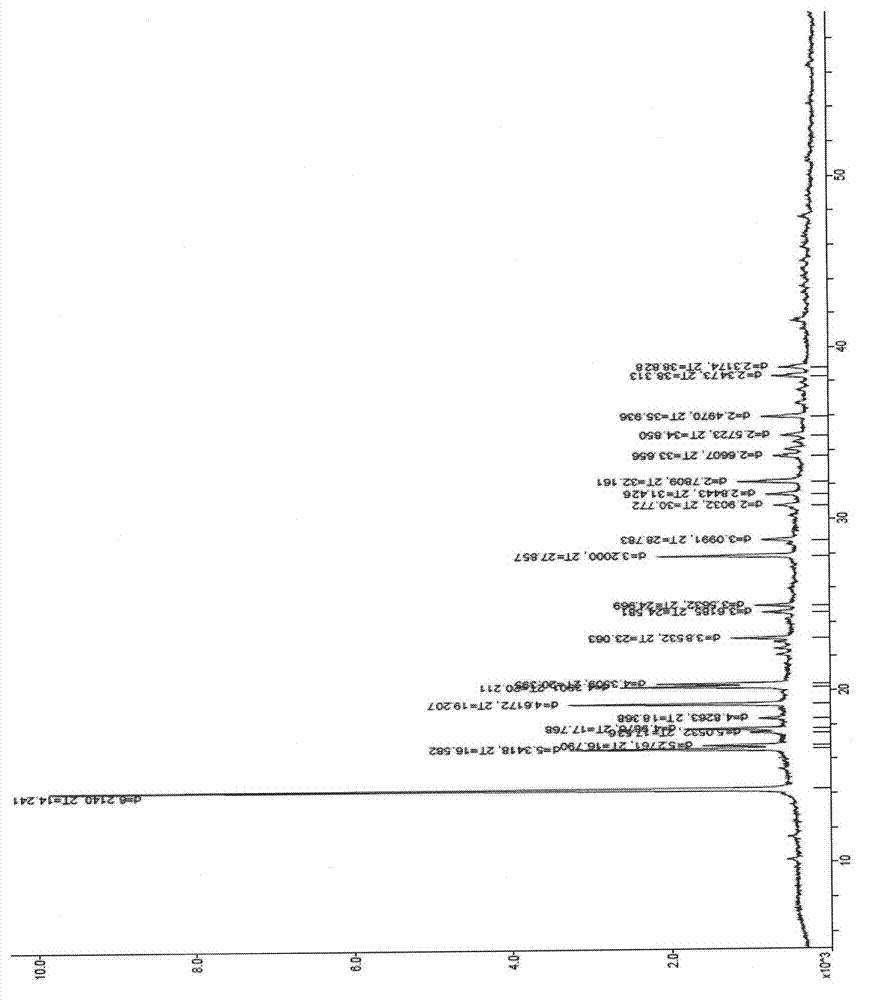
The invention relates to an eutectic of Piperacillin sodium and Sulbactam sodium, a preparation method thereof, a pharmaceutical composition containing the eutectic and an application of the pharmaceutical composition on treating infection of super bacteria producing NDM-1 and other drug-resistance bacteria. The eutectic of the Piperacillin sodium and the Sulbactam sodium comprises diffraction angles of 14.24 DEG, 16.58 DEG, 16.79 DEG, 17.77 DEG, 19.20 DEG, 20.21 DEG, 20.39 DEG, 23.06 DEG, 27.86 DEG and 32.16 DEG represented by 2[theta] in an X-ray powder diffraction analysis spectra. The eutectic provided by the invention is excellent in solubility, low in hygroscopicity, relatively small in powder volume, high in purity, low in content of related substances, and easy to filter and dry during industrial production. A compound preparation prepared by the eutectic has obviously improved stability; not only is the shelf life of the products prolonged, but also the product safety is improved, thereby reducing potential adverse risks of the drugs and further protecting the health of patients.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Refining method for piperacillin sodium
ActiveCN102807572AImprove refining effectEasy to operateOrganic chemistryActivated carbonThermal insulation
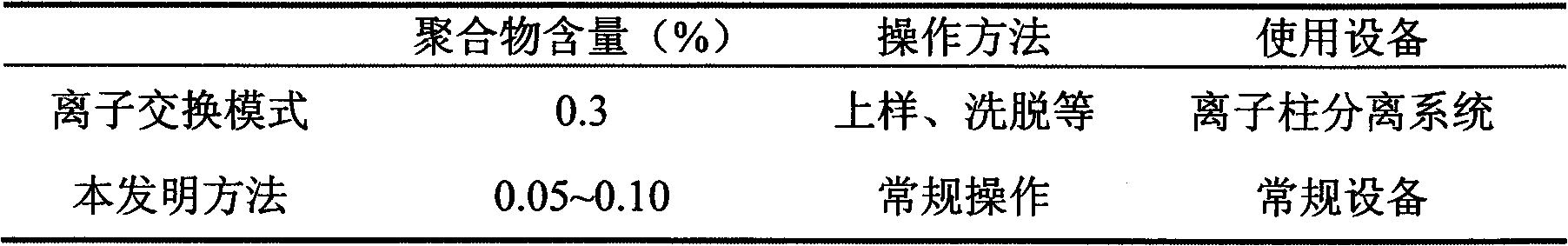
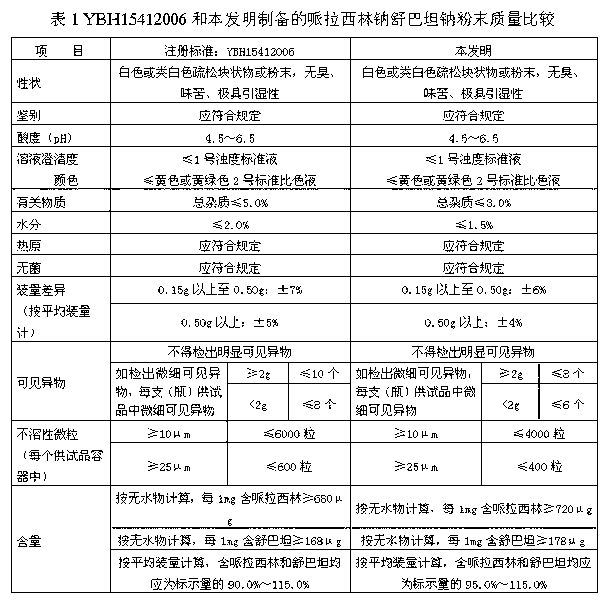
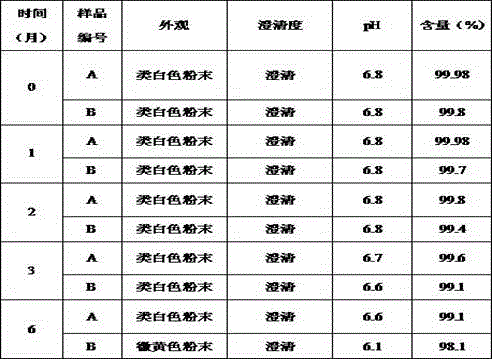
The invention discloses a refining method for a piperacillin sodium crude product, wherein the method is mainly a method for removing polymers in the crude product. The method is performed according to the following steps: heating a piperacillin sodium crude product, and dissolving in water and DMSO; carrying out a treatment with active carbon; filtering; adding acetone in a dropwise manner; cooling; carrying out thermal insulation crystallization; and filtering to obtain the piperacillin sodium refined product. Compared with the polymer removing method and other methods reported in traditional literatures, the method of the present invention has characteristics of simple operation, low production cost, and high finished product purity, and can be used for industrial purification production of piperacillin sodium crude products, wherein the method reported in the traditional literatures adopts an ion exchange manner.
Owner:JIANGSU CAREFREE PHARM CO LTD
Preparation method of piperacillin sodium sulbactam sodium for injection
InactiveCN103301131AReduce the introductionHigh content of main componentsAntibacterial agentsPowder deliveryMoistureAnaphylactic reaction
The invention relates to a preparation method of piperacillin sodium sulbactam sodium for injection. The preparation method comprises the following steps of: moving and mixing the piperacillin sodium and the sulbactam sodium in a sealed container in various directions at a weight ratio of 4:1; discharging the powder after physically mixing the powder; weighing and filling the powder into a barrel; carrying out subsequent treatment to obtain the piperacillin sodium sulbactam sodium for injection. According to the preparation method of the piperacillin sodium sulbactam sodium for injection, only the piperacillin sodium and the sulbactam sodium are selected as raw materials without introducing other novel substances, so that on one hand, the content of main ingredient of the medicament is high, on the other hand, the introduced impurities including related substances and the like are reduced. Moreover, the whole process is strictly controlled, so that the prepared piperacillin sodium sulbactam sodium for injection is low in moisture content, less in visible foreign substances and less in in-soluble particles, and therefore, the anaphylactic reaction during the injection is effectively reduced, and the medicament use is safer.
Owner:SICHUAN PHARMA
Preparation method of piperacillin sodium and sulbactum sodium composition for injection
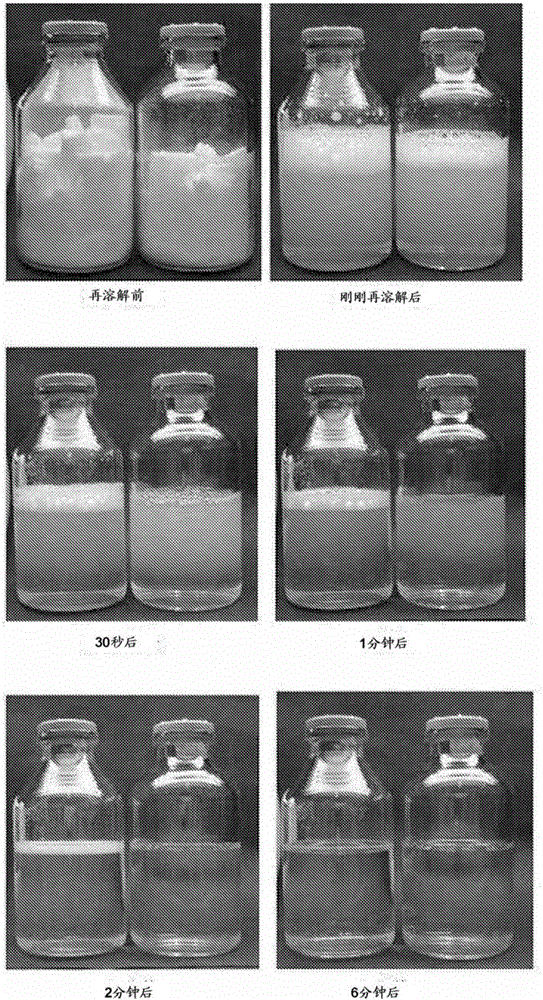
ActiveCN105560243AImprove stabilityEffective protectionAntibacterial agentsPowder deliveryFreeze-dryingDissolution
The invention relates to a preparation method of a piperacillin sodium and sulbactum sodium composition for injection. The composition comprises piperacillin sodium, ulbactum sodium, poly(alkyl cyanoacrylate), polyvinylpyrrolidone, trehalose, and glycine, and has the characteristics of good re-dissolution property and high stability. The preparation method is simple, and the composition can be obtained through freeze-drying.
Owner:SUZHOU ERYE PHARMA CO LTD
Powder mixing technology for compound antibiotic
ActiveCN103040768AReduce pollutionStable chemical qualityPowder deliveryAntiinfectivesChemical qualityOrganic solvent
The invention relates to a powder mixing technology for a compound antibiotic. The compound antibiotic is obtained by cold drying after uniform mixing of antibiotic A and antibiotic B in advance, wherein the antibiotic A is mezlocillin sodium, amoxicillin sodium, cefoperazone sodium, and piperacillin sodium, and the antibiotic B is sulbactam sodium. According to the powder mixing technology, organic solvent is omitted, the environment is less polluted, the freeze-drying technology has stable yield, and the cost is low; the freeze-drying mixed powder has good uniformity, fast dissolution rate, and stable chemical quality, so that the powder mixing technology is applicable to preparation of various of compound antibiotics, and is suitable for mass production.
Owner:山东二叶制药有限公司
Medicinal composition
ActiveCN102018713BAntibacterial agentsHeterocyclic compound active ingredientsChemical compositionIon-exchange resin
Owner:BEIJING SIHUAN NEW PHARMA TECH +1
Process for preparing piperacillin sodium by using solvent method
InactiveCN102977119AEasy to operateLow equipment requirementsOrganic chemistrySodium methoxideOrganic solvent
The invention provides a process for preparing piperacillin sodium by using a solvent method. The process comprises the following steps of: dissolving sodium methoxide into an anhydrous organic solvent; dissolving piperacillin into anhydrous acetone, cooling to the temperature of between 0 and 5 DEG C under the protection of an inert gas, dripwise adding a sodium methoxide solution, controlling the temperature to be between 0 and 5 DEG C, naturally returning the temperature of between 12 and 18 DEG C with stirring after dripwise adding, and preserving heat until the reaction is ended; and performing filter pressing on the reacted product by adopting the inert gas, drying filter cakes at the temperature of between 60 and 70 DEG C under reduced pressure, emptying with the inert gas, discharging, and thus obtaining the piperacillin sodium in accordance with Chinese pharmacopeia (CP2010). The piperacillin sodium in accordance with the Chinese pharmacopeia (CP2010) can be prepared by the process, the preparation method is easier to operate, the equipment requirement is low, and the piperacillin sodium has the characteristics of high purity, high yield, high content and low residue.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Medicine composition of piperacillin sodium and tazobactam sodium
ActiveCN105640959AHigh antibacterial activityImprove securityAntibacterial agentsPowder deliveryMedicineTraditional medicine
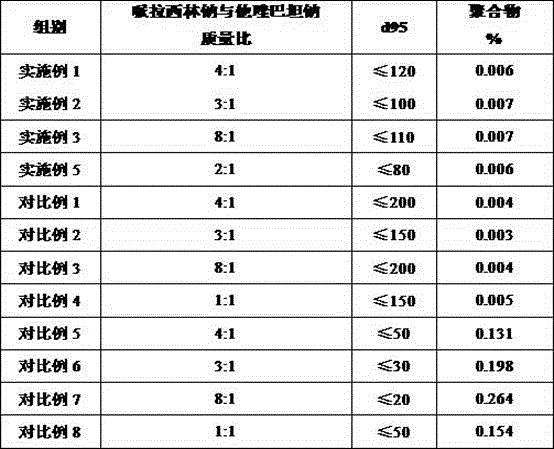
The invention discloses a medicine composition of piperacillin sodium and tazobactam sodium. Raw material medicine comprises piperacillin sodium micro powder and tazobactam sodium micro powder of d95 within the range of 120 micrometers to 180 micrometers. Experiments prove that the medicine composition is high in antimicrobial activity, good in redissolving performance and high in product safety.
Owner:闫虎林 +1
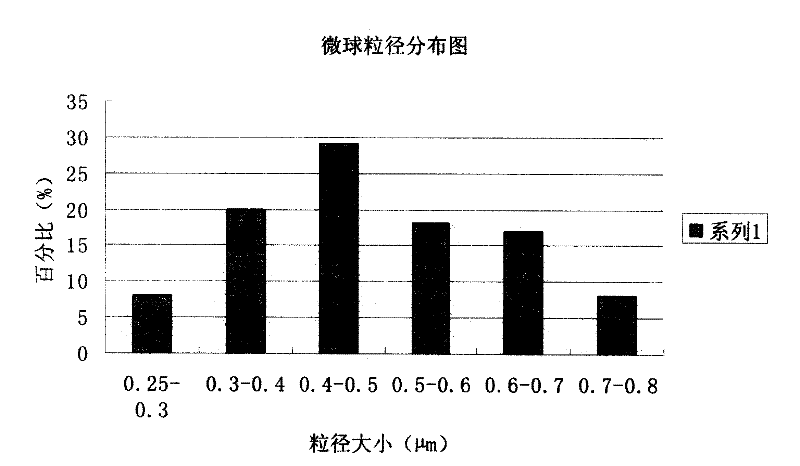
Piperacillin sodium and sulbactam sodium drug composite microsphere injection
InactiveCN101983629AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryMicrospherePolyethylene glycol
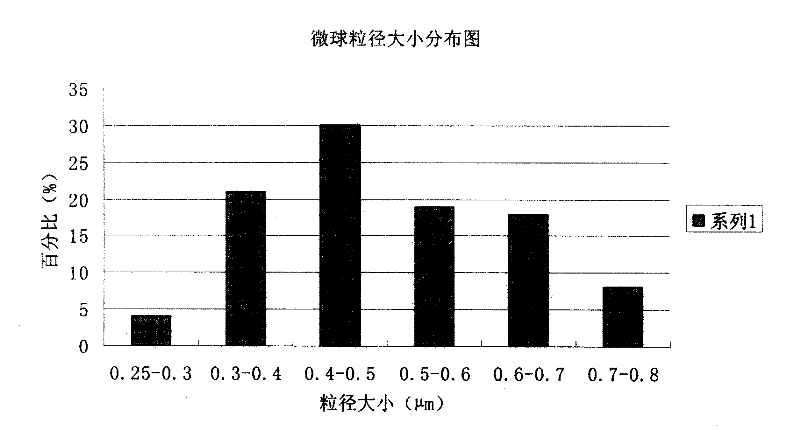
The invention discloses a piperacillin sodium and sulbactam sodium drug composite microsphere injection which is characterized by comprising the following components in parts by weight: 2-4 parts of piperacillin sodium, 1 part of sulbactam sodium, 6-10 parts of albumin, 3-5 parts of poloxamer 188, 5-7 parts of polyethylene glycol 600 (PEG600) and 2-5 parts of trehalose. Compared with the prior art, the piperacillin sodium and sulbactam sodium drug composite microsphere injection prepared by the invention has the characteristics of good stability, high entrapment rate, good reproducibility of preparation technology, even particle distribution, small dissolvent residue, good injectability and excellent slow-release property, and is suitable for industrial production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
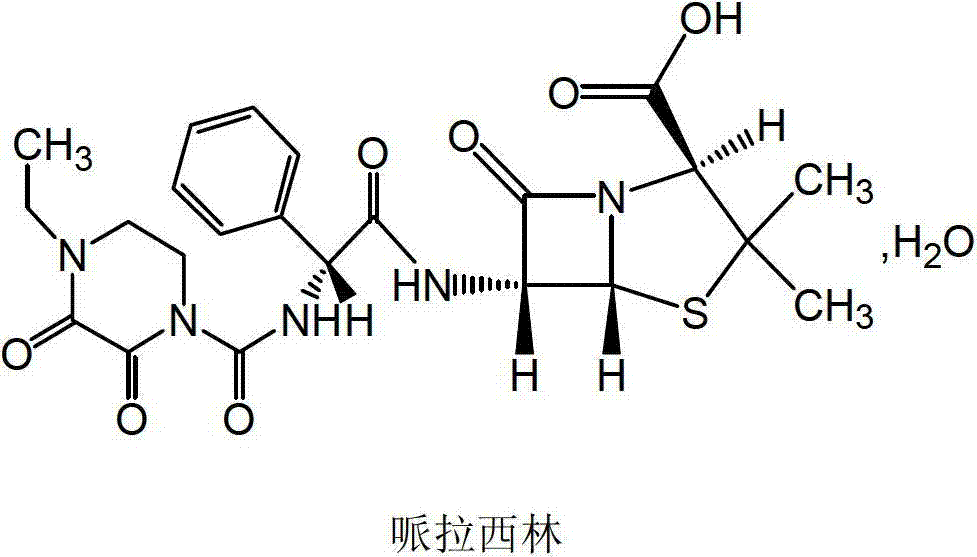
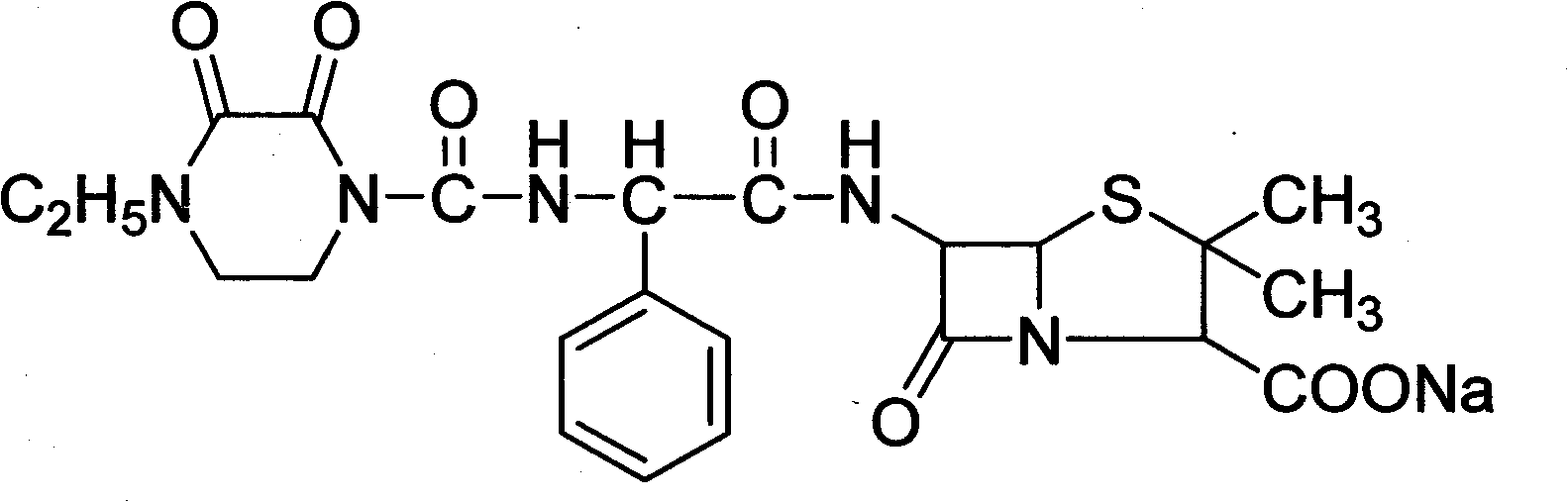
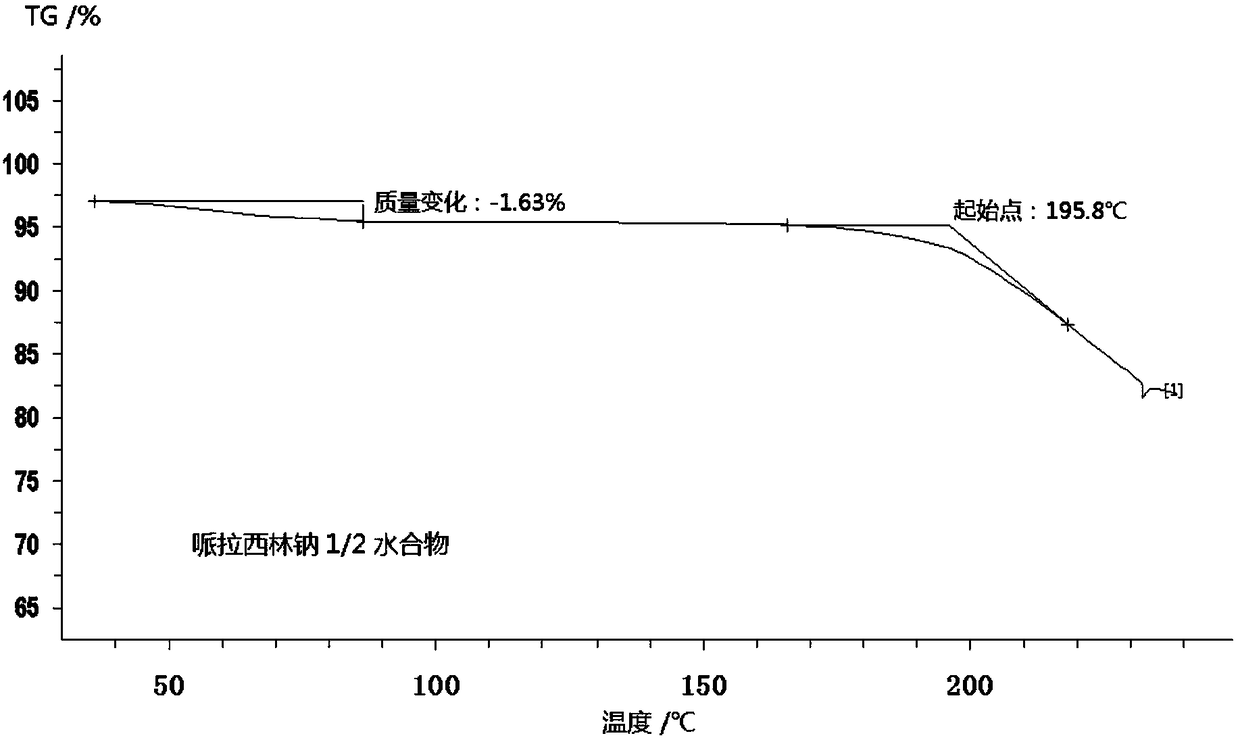
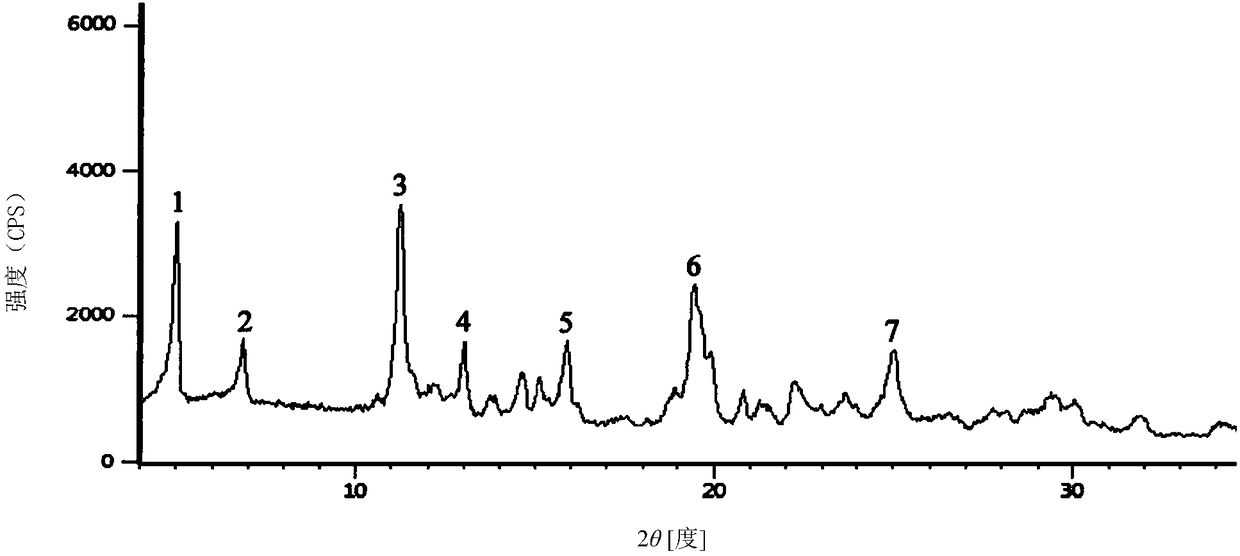
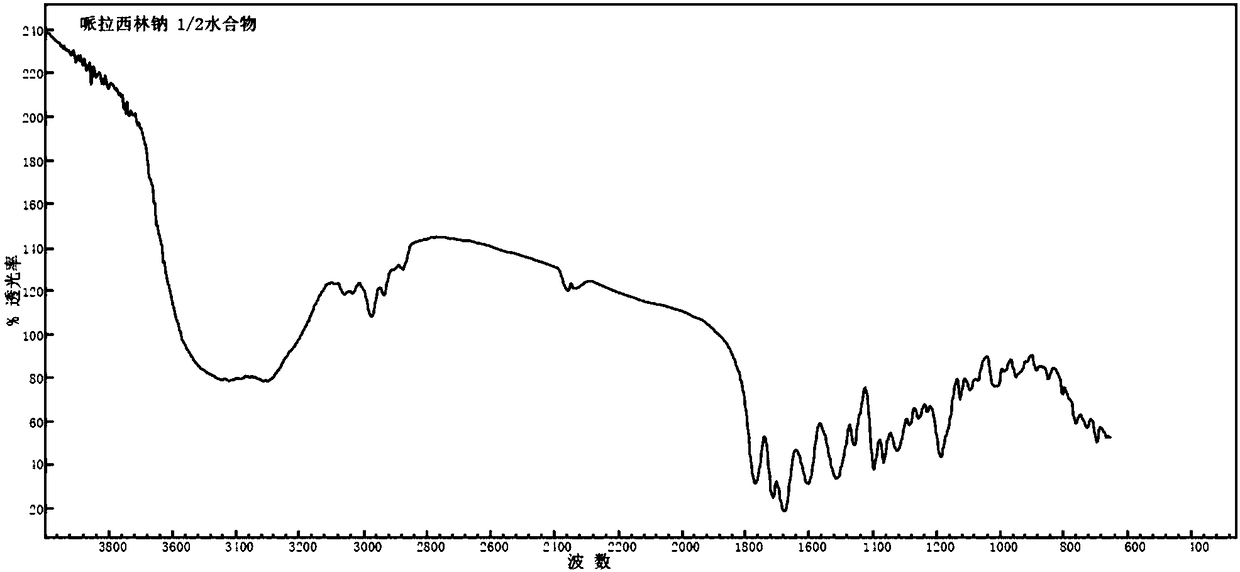
Piperacillin sodium compound containing half water
The invention discloses a piperacillin sodium compound containing half water and a preparation method thereof. Each mole of piperacillin sodium contains a half mole of water. First, ampicillin trihydrate is reacted with 4-ethyl-2,3-dioxypiperazine formyl chloride to form piperacillin acid, then piperacillin acid is reacted with sodium acetate in a mixed solution of acetone and water, isopropanol is added dropwise, and crystallization, filtration and drying are conducted to obtain the piperacillin sodium compound containing half water. The piperacillin sodium compound containing half water hashigh fluidity, low hygroscopicity and impurity content, high thermodynamic stability and a wider application prospect.
Owner:陈立平
Pharmaceutical composition containing beta-lactamase restrainer and piperacillin sodium with steady content and preparation method thereof
ActiveCN101269072BContent remains constantEliminate hidden dangersAntibacterial agentsPowder deliveryDrug compoundAir temperature
The invention provides a stable-content drug compound containing Beta-lactamases inhibitors and piperacillin sodium and the preparation method thereof. The drug compound consists of piperacillin sodium, Beta-lactamases inhibitors and pH-value regulators with the weight proportion of 1 to 100:1:0.001 to 2. Diluted and delivered in any proportion with conventional clinical transfusions, the drug compound can have a stable piperacillin sodium content, dissolve rapidly, generate no crystallization or degradation products and receive no effect from the temperature. The preparation method of the stable-content drug compound containing Beta-lactamases inhibitors and piperacillin sodium has simple preparing method and high efficiency and is fit for a large-scale industrial production.
Owner:福建丰恺思投资有限公司 +1
Eutectic of Piperacillin sodium and Sulbactam sodium, preparation method thereof, pharmaceutical composition containing eutectic and application of pharmaceutical composition
ActiveCN102898438BImprove stabilityExtend your lifeAntibacterial agentsOrganic chemistrySolubilityX-ray
The invention relates to an eutectic of Piperacillin sodium and Sulbactam sodium, a preparation method thereof, a pharmaceutical composition containing the eutectic and an application of the pharmaceutical composition on treating infection of super bacteria producing NDM-1 and other drug-resistance bacteria. The eutectic of the Piperacillin sodium and the Sulbactam sodium comprises diffraction angles of 14.24 DEG, 16.58 DEG, 16.79 DEG, 17.77 DEG, 19.20 DEG, 20.21 DEG, 20.39 DEG, 23.06 DEG, 27.86 DEG and 32.16 DEG represented by 2[theta] in an X-ray powder diffraction analysis spectra. The eutectic provided by the invention is excellent in solubility, low in hygroscopicity, relatively small in powder volume, high in purity, low in content of related substances, and easy to filter and dry during industrial production. A compound preparation prepared by the eutectic has obviously improved stability; not only is the shelf life of the products prolonged, but also the product safety is improved, thereby reducing potential adverse risks of the drugs and further protecting the health of patients.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Medicinal composition
ActiveCN102018713AAntibacterial agentsHeterocyclic compound active ingredientsChemical compositionActive ingredient
The invention discloses a medicinal composition. The medicinal composition comprises piperacillin sodium and tazobactam sodium serving as raw material medicaments, and is characterized in that: the piperacillin sodium serving as the raw material medicament is prepared from piperacillin acid through ion exchange resin; and the tazobactam sodium serving as the raw material medicament is prepared from tazobactam acid through the ion exchange resin. Experiments prove that a preparation prepared from the medical composition has higher quality.
Owner:BEIJING SIHUAN NEW PHARMA TECH +1
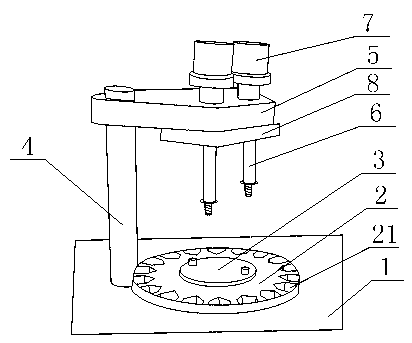
Automatic filling machine for piperacillin sodium and sulbactam sodium medicine powder used for injection
InactiveCN103287594ASimple structureIncrease the areaLiquid fillingSolid materialSmall footprintEngineering
The invention discloses an automatic filling machine for piperacillin sodium and sulbactam sodium medicine powder used for injection. The automatic filling machine comprises an operating platform and a rotating device fixed on the operating platform. A round medicine bottle fixing tray is fixed on the rotating device. Grooves are formed in the periphery of the medicine bottle fixing tray, a supporting rod is fixed on the operating platform, and a fixing plate is fixed at the top of the supporting rod. A seal plate is movably connected with the bottom of the fixing plate. A stop block is fixed at the bottom of the fixed plate. Medicine placing boxes are fixed on the fixing plate, conduits are perpendicularly fixed on the seal plate, and a driving device is connected to the seal plate. The automatic filling machine for the piperacillin sodium and sulbactam sodium medicine powder used for the injection has the advantages of being simple in structure, small in occupied area, economical and practical.
Owner:SICHUAN PHARMA
Preparation method of penicillin antibiotic impurity
PendingCN111197064AEasy to operateShort reaction timeOrganic chemistryFermentationFlucloxacillinMycinamicins
The invention relates to a preparation method of a penicillin antibiotic impurity. The penicillin antibiotic impurity has a structure represented by formula (I), and in the formula (I), R represents side chains of penicillin, mezlocillin, piperacillin sodium and flucloxacillin. The method comprises the following steps: (1) mixing penicillin antibiotic, beta-lactamase and water; (2) controlling thetemperature to be 37 DEG C or below; (3) purifying by preparative chromatography, and concentrating; and (3) mixing and reacting with an alcohol solvent. The method is simple to operate and short inreaction time, the purity of the product is 90% or above, and the product can be directly used as a reference substance to qualitatively and quantitatively research penicillin antibiotics, so that theproduct quality is effectively controlled.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
Suspensoid powder injection of piperacillin sodium sulbactam sodium medicine composition and novel application thereof
InactiveCN101632671BImprove stabilityEnsure product quality is qualifiedPowder deliveryDigestive systemFreeze-dryingPharmacology
Owner:HAINAN YONGTIAN PHARMA INST
Piperacillin sodium and sulbactam sodium drug composite microsphere injection
InactiveCN101983629BImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryMicrosphereMedicine
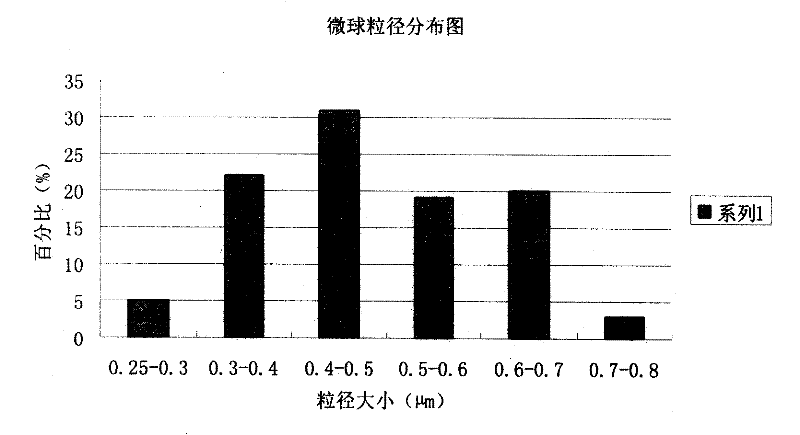
The invention discloses a piperacillin sodium and sulbactam sodium drug composite microsphere injection which is characterized by comprising the following components in parts by weight: 2-4 parts of piperacillin sodium, 1 part of sulbactam sodium, 6-10 parts of albumin, 3-5 parts of poloxamer 188, 5-7 parts of polyethylene glycol 600 (PEG600) and 2-5 parts of trehalose. Compared with the prior art, the piperacillin sodium and sulbactam sodium drug composite microsphere injection prepared by the invention has the characteristics of good stability, high entrapment rate, good reproducibility of preparation technology, even particle distribution, small dissolvent residue, good injectability and excellent slow-release property, and is suitable for industrial production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Method for producing freeze-dried preparation
InactiveCN106714799AHigh pHAvoid decompositionAntibacterial agentsPowder deliveryAqueous sodium hydroxideFreeze-drying
The present invention addresses the main problem of providing a novel method for producing a freeze-dried preparation (combination drug) for injection that comprises tazobactam sodium and piperacillin sodium. An example of the production method according to the present invention is as follows. A method for producing a freeze-dried preparation for injection, said method being characterized by comprising: (a) a step for blowing carbon dioxide gas into an aqueous sodium hydroxide solution; and (b) a step for dissolving tazobactam and piperacillin in the solution obtained in step (a). According to the present invention, a freeze-dried preparation showing good defoaming performance after redissolution can be obtained.
Owner:SAWAI PHARMA
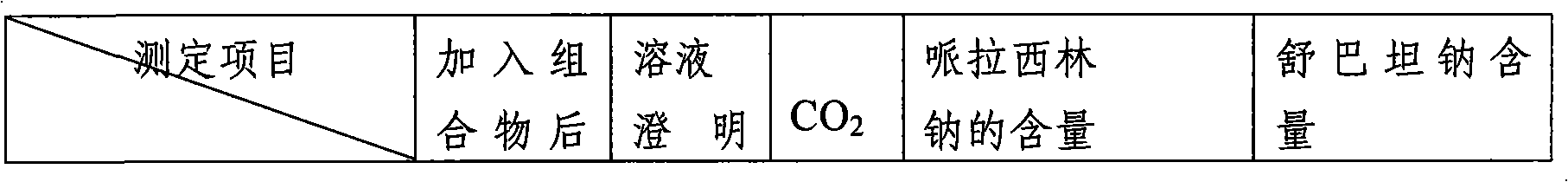
A method for detecting related substances of piperacillin sodium and sulbactam sodium for injection
The invention provides a method for detecting relevant substances in piperacillin sodium and sulbactam sodium for injection. According to the method, octadecylsilane bonded silica gel is adopted as filler and tetrabutyl ammonium hydroxide solution-acetonitrile is adopted as a moving phase for gradient elution, and measurement of the relevant substances in piperacillin sodium and sulbactam sodium is realized quickly and accurately.
Owner:SUZHOU ERYE PHARMA CO LTD
Medicinal composition of piperacillin sodium and sulbactam sodium
ActiveCN105640958ASimple preparation processImprove stabilityAntibacterial agentsOrganic chemistry methodsSpecific rotationSulbactam Sodium
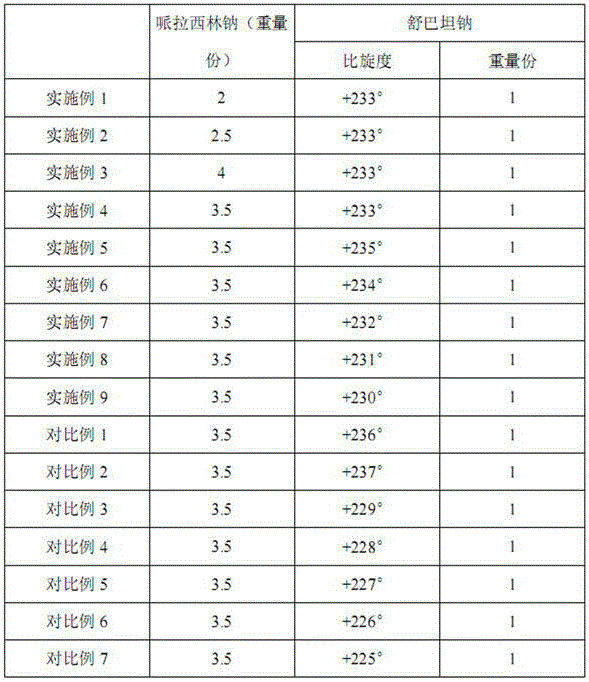
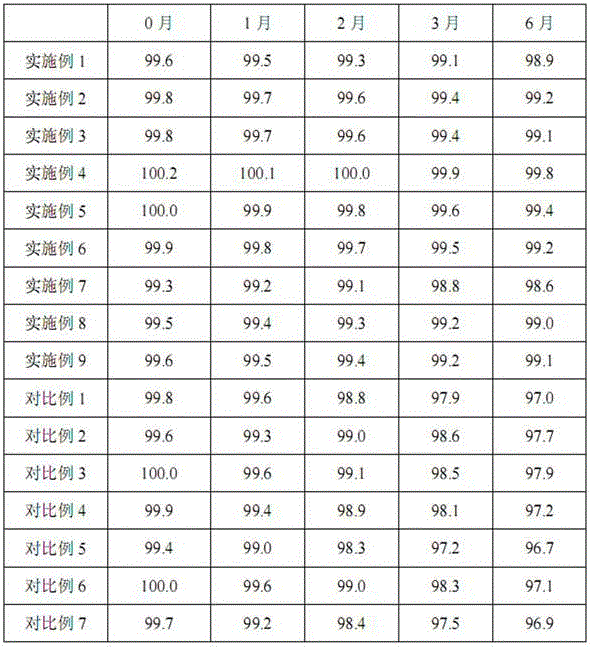
The invention discloses a medicinal composition of piperacillin sodium and sulbactam sodium. The medicinal composition comprises the following components in parts by weight: 2-4 parts of piperacillin sodium and 1 part of sulbactam sodium with specific rotation being from +230 degrees to +235 degrees. The medicinal composition disclosed by the invention has the advantages that the sulbactam sodium with the specific rotation being from +230 degrees to +235 degrees is prepared by optimizing a preparation process of the sulbactam sodium; by optimization of the preparation process of the piperacillin sodium, the stability of the medicinal composition is favorably improved and the use is safer.
Owner:SICHUAN PHARMA
Groove sterilizing cart for preparing piperacillin sodium used for injection
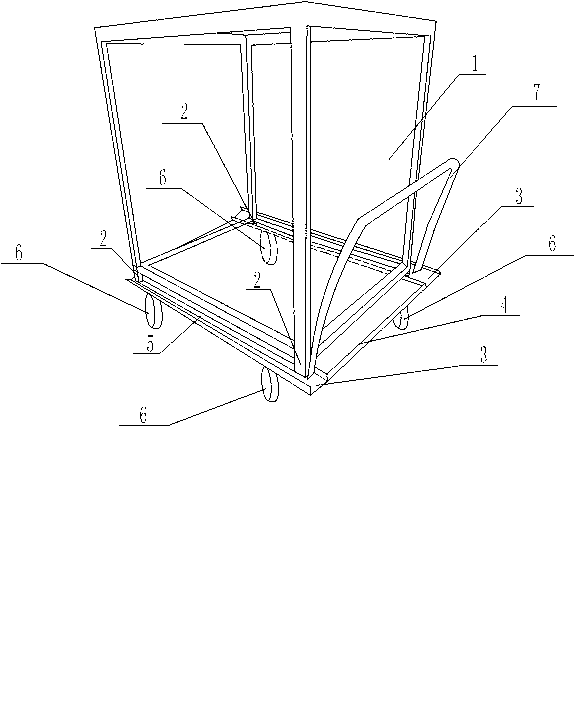
InactiveCN103287468ASmooth shippingPlay limitHand carts with multiple axesEngineeringPiperacillin Sodium
The invention discloses a groove sterilizing cart for preparing piperacillin sodium used for injection. The groove sterilizing cart comprises a tray rack (1) and a transport cart. Supporting legs (2) are arranged at the four corners of the lower surface of the tray rack (1). The transport cart is composed of a cart plate (4) and cart wheels (6). The cart wheels (6) are installed below the cart plate (4), grooves (3) are formed in the two sides of the cart plate (4), and each two supporting legs (2) are arranged in one same groove (3). Due to the arrangement of the grooves and the supporting legs on the groove sterilizing cart, the tray rack is convenient to place on the transport cart, the grooves play a role in limiting the supporting legs and preventing the displacement of the supporting legs, the tray rack is prevented from generating displacement or disengaging from the cart plate, so that the tray rack can be firmly located on the cart plate, and the situation that a whole transportation process of sterilizing is smoothly completed is guaranteed.
Owner:SICHUAN PHARMA
A kind of piperacillin sodium sulbactam sodium sterile powder injection and preparation method thereof
ActiveCN104887679BGuaranteed curative effectEnsure safetyAntibacterial agentsPowder deliveryGlycineCurative effect
The invention provides a piperacillin sodium and sulbactam sodium sterile powder-injection. The piperacillin sodium piperacillin sodium sterile powder-injection is prepared from piperacillin sodium, sulbactam sodium and a stabilizer. The stabilizer is composed of mycose, sodium pyrosulfite and glycine. The piperacillin sodium and sulbactam sodium sterile powder-injection comprises, by weight, 2-4 parts of the piperacillin sodium, 1 part of the sulbactam sodium, 20-40 parts of the mycose, 8-15 parts of the sodium pyrosulfite and 10-20 parts of the glycine. Compared with the prior art, the stabilizer is composed of the mycose, the sodium pyrosulfite and the glycine. Therefore, the stability of the piperacillin sodium and the sulbactam sodium in the preparation and storage process is effectively improved. It is guaranteed that products are qualified within the period of validity. The powder-injection is dissolved fast. No crystallization or turbidity exists. The curative effect and safety of clinical usage are guaranteed. A preparation method is simple. The cost is low. The powder-injection is suitable for industrial mass production.
Owner:HAINAN GENERAL & KANGLI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com