A method for detecting related substances of piperacillin sodium and sulbactam sodium for injection
A technology of cillin sodium sulbactam sodium and related substances, which is applied in the field of detection of related substances of piperacillin sodium sulbactam sodium for injection, and can solve problems such as long detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Detection of related substances of piperacillin sodium and sulbactam sodium for injection
[0027] 1. Solution configuration
[0028] Test solution: piperacillin sodium sulbactam sodium powder for injection (manufacturer: Suzhou Erye Pharmaceutical Co., Ltd., specification 2:1) was dissolved in mobile phase A and diluted to a solution with a concentration of 2.5 mg / ml. Shake well, as the test solution;
[0029] Reference substance solution: Accurately measure 1ml of the test solution, put it in a 100ml measuring bottle, dilute to the mark with mobile phase A, shake well, and use it as a reference solution;
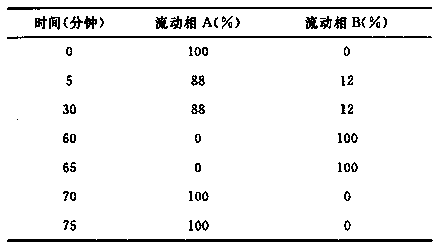
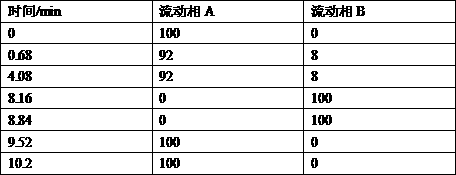
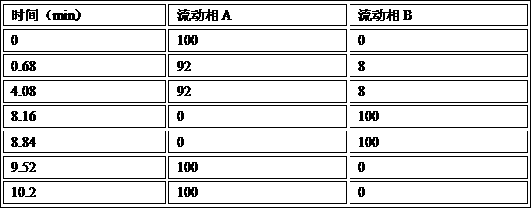
[0030] Mobile phase A: tetrabutylammonium hydroxide solution [water: 3.12% sodium dihydrogen phosphate solution: 20% tetrabutylammonium hydroxide solution (576:200:9.6)]-acetonitrile (786:200), adjust the pH to 6.0 with phosphoric acid mobile phase A;
[0031] Mobile phase B: tetrabutylammonium hydroxide solution [water: 3.12% sodium dihydrogen phos...
Embodiment 2
[0042] Example 2: System suitability test of piperacillin sodium and sulbactam sodium for injection
[0043] Using the same detection conditions as in Example 1, the column temperature, flow rate and pH of the mobile phase were investigated respectively, and the results are shown in Table 1.
[0044] Table 1. System suitability test of piperacillin sodium and sulbactam sodium for injection
[0045]
[0046] Note: Piperacillin impurity E is the first peak, and its resolution is not shown on the spectrum. The separation of piperacillin impurity E and sulbactam impurity A is the resolution under sulbactam impurity A, followed by By analogy, the degree of separation under the item of piperacillin impurity E is represented by “——”.
[0047] The above test results show that when the column temperature and flow rate have slight changes, the resolution of the system applicability test of this method is better. However, when the pH value of the mobile phase changes, the number of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com