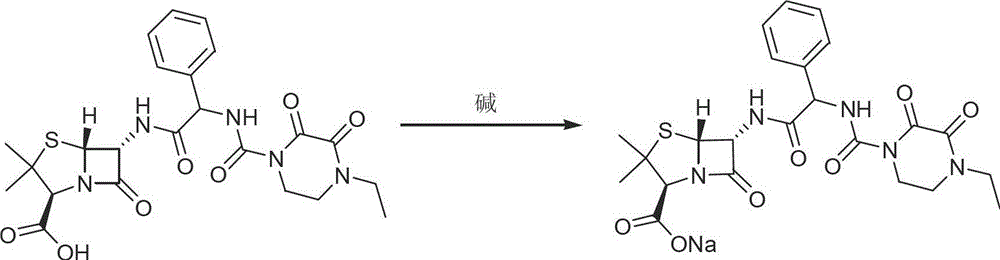
Process for preparing piperacillin sodium by using solvent method
A piperacillin sodium, solvent method technology, applied in the direction of organic chemistry, etc., can solve the problems of complex operation, the moisture can not reach moisture ≤ 2.0%, expensive equipment, etc., to achieve the effect of simple operation, low equipment requirements, and low residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
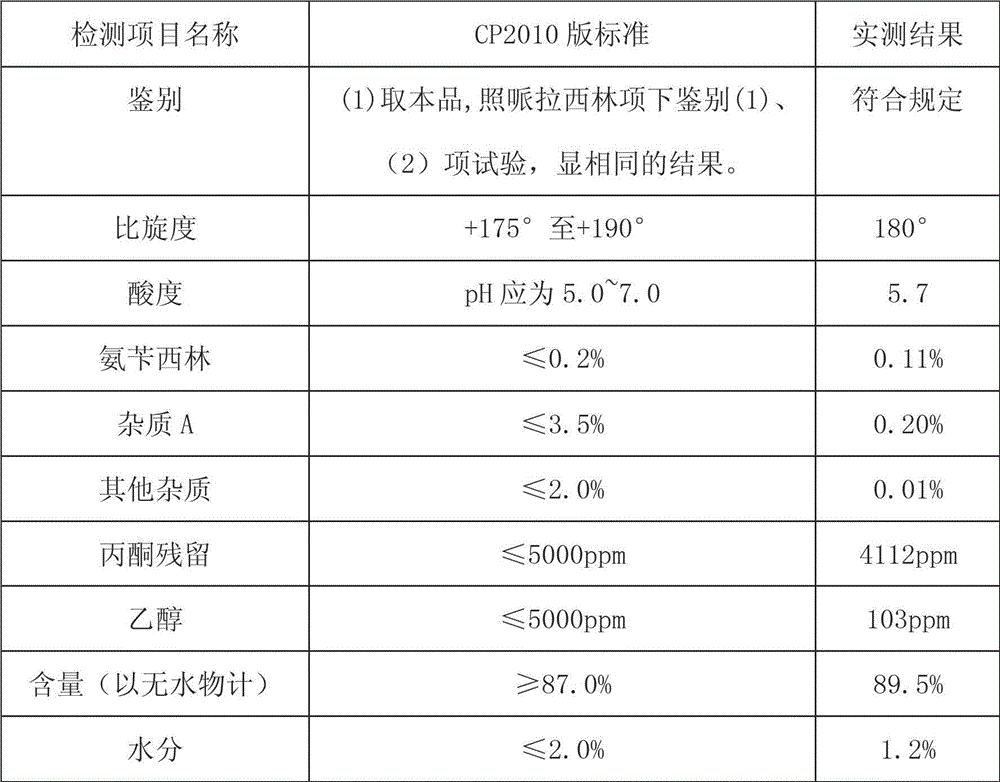
[0017] Dissolve 3.2g of sodium methoxide in 80ml of absolute ethanol; dissolve 30g of piperacillin in 1080ml of anhydrous acetone, lower the temperature to 0~5°C under the protection of helium, add the above sodium methoxide solution dropwise, control the temperature at 0~5°C, drop The addition time is about 2 hours, the dropwise addition is completed, the temperature is kept for 10 hours, the temperature is naturally returned to 15°C after stirring, the filter cake is pressure-filtered with helium, the filter cake is dried under reduced pressure at 65°C for 12 hours, and the material is discharged after being evacuated with helium gas. CP2010) piperacillin sodium 22.0g. Following is the detection result to the product that makes:
[0018]
Embodiment 2
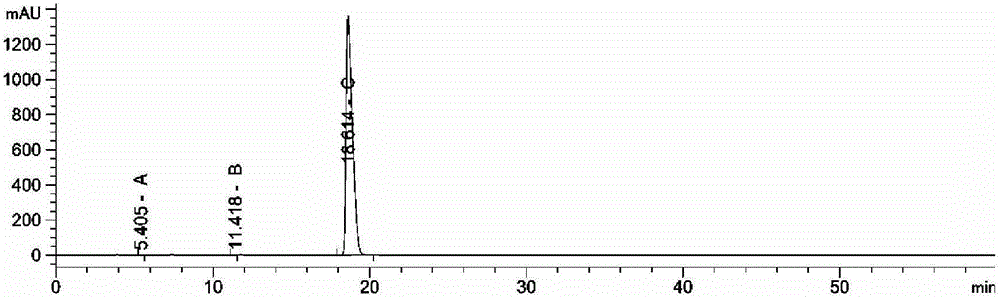
[0020] Dissolve 3.2g of sodium methoxide in 80ml of absolute ethanol; dissolve 30g of piperacillin in 1080ml of anhydrous acetone, lower the temperature to 0~5°C under nitrogen protection, add the above sodium methoxide solution dropwise, control the temperature at 0~5°C, and add dropwise The time is about 2 hours, the dropwise addition is completed, keep warm for 10 hours, stir and naturally return to 15°C, nitrogen pressure filtration, filter cake is dried under reduced pressure at 65°C for 12 hours, and the material is discharged after being evacuated with nitrogen, and the obtained product conforms to the Chinese Pharmacopoeia (CP2010) Piperacillin Sodium 22.5g, purity spectrum see figure 1 , the content was 90.2% after comparison with the standard product of China National Institute for the Control of Pharmaceutical and Biological Products. Wherein A peak is ampicillin; B peak is impurity A; C peak is piperacillin sodium.
[0021]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com