Medicinal composition of piperacillin sodium and sulbactam sodium
A technology of piperacillin sodium and sulbactam sodium, which is applied in the field of pharmaceutical preparations, can solve the problems of affecting clinical efficacy, poor storage stability of preparations, loss of calcium ions, etc., and achieves the effects of optimizing the preparation process, improving stability and using safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation method is as follows:
[0022] Preparation of piperacillin sodium: (1) at 20-25°C, add sodium bicarbonate to piperacillin acid and keep stirring, and control the pH of the reaction solution to 5.5-6.0; (2) after the reaction, add ethanol and keep stirring After 10 minutes, irradiate with infrared rays for 3-5 minutes at the same time, and continue stirring for 30-60 minutes to obtain crystals.
[0023]Preparation of sulbactam sodium: (1) Add lysine hydrochloride to sulbactam acid acetone solution at 0-5°C, and control the pH value of the reaction solution at 6.6; (2) Add lysine hydrochloride dropwise After the completion, the temperature of the reaction solution is raised to 10-25 degrees Celsius, and the sodium bicarbonate solution is added to the sulbactamic acid acetone solution, and the pH value of the reaction solution is controlled at 6.7; (3) After the reaction is completed and filtered, it is divided into three stages. In the first stage, contro...
Embodiment 2
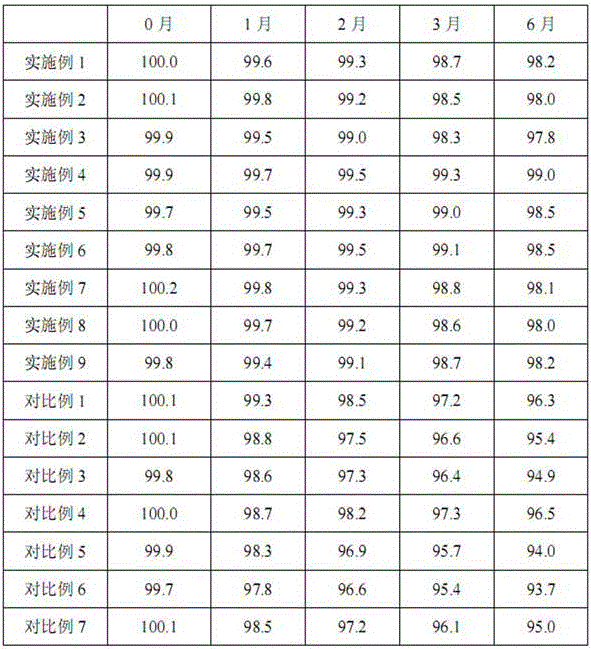
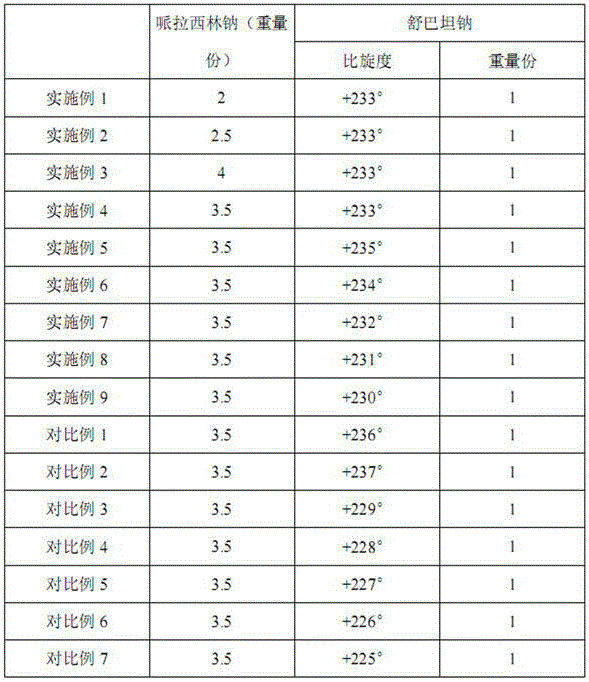
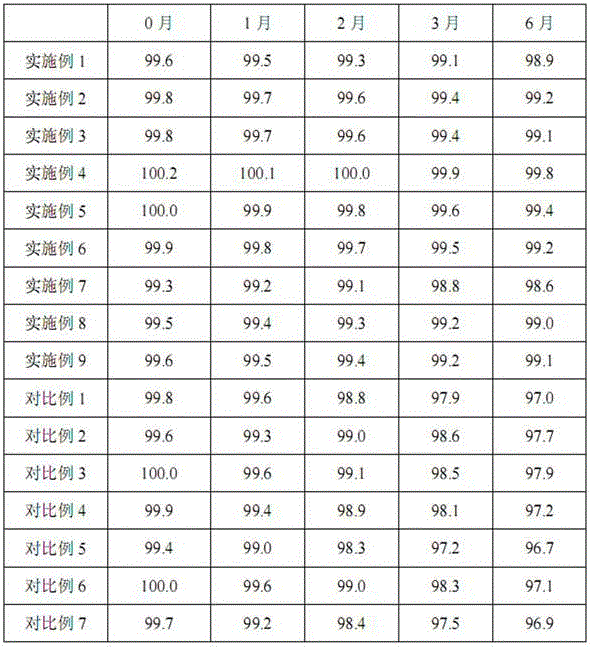
[0027] Piperacillin sodium and sulbactam sodium were prepared by the same process as in Example 1, except that the weight ratios of piperacillin sodium and sulbactam sodium were different during powder mixing, as shown in Table 1.
Embodiment 3
[0029] Piperacillin sodium and sulbactam sodium were prepared by the same process as in Example 1, except that the weight ratios of piperacillin sodium and sulbactam sodium were different during powder mixing, as shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com