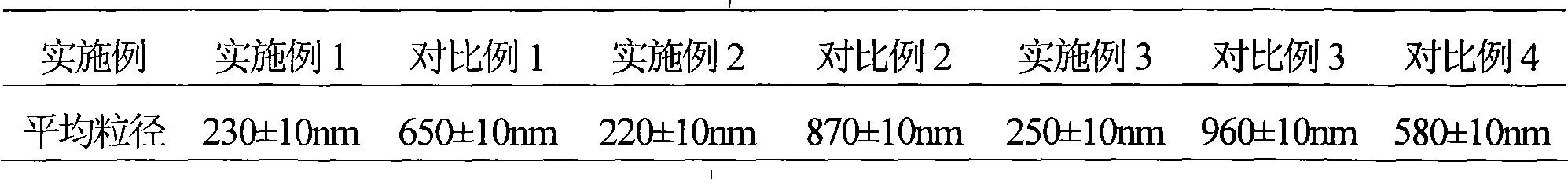
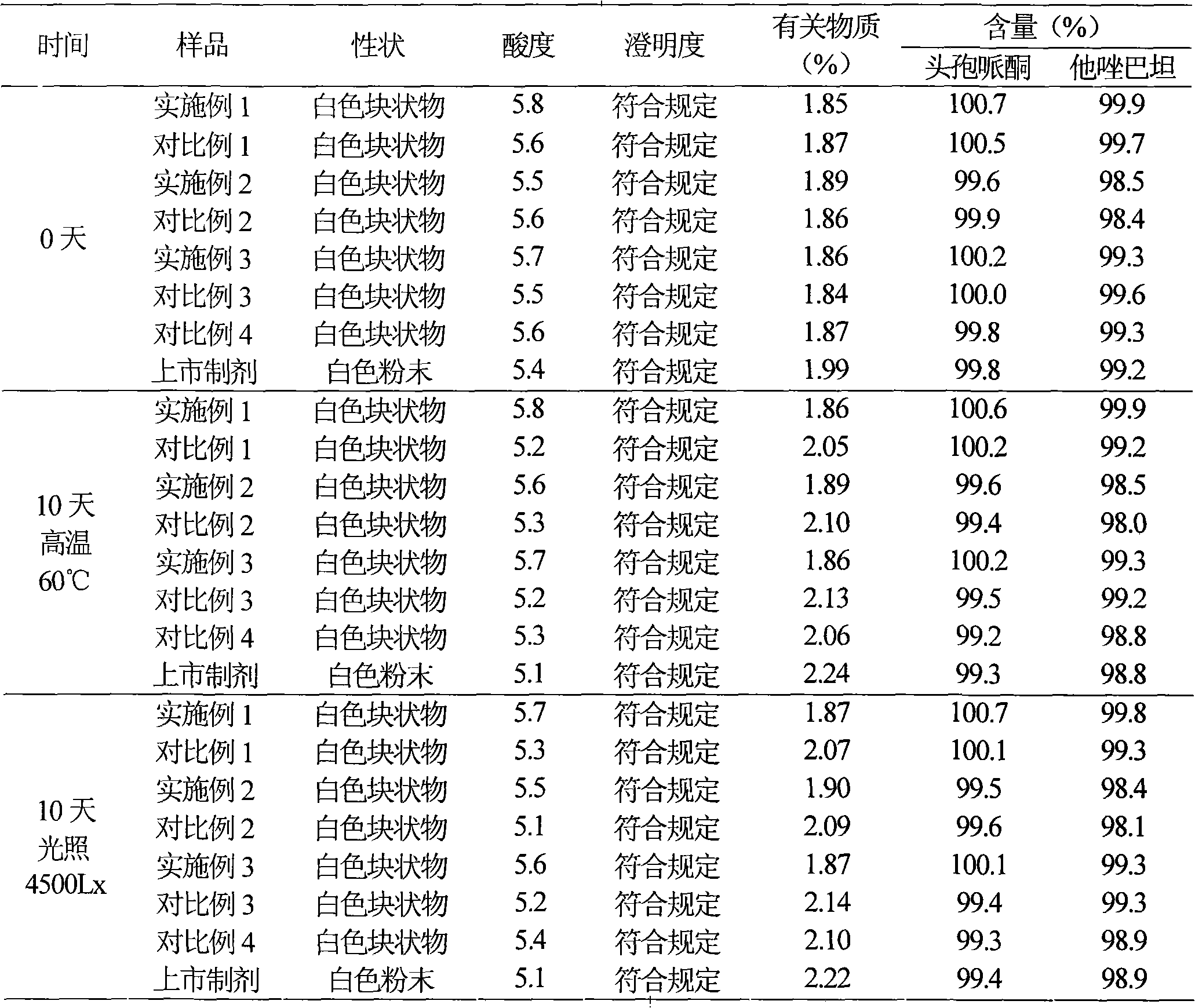
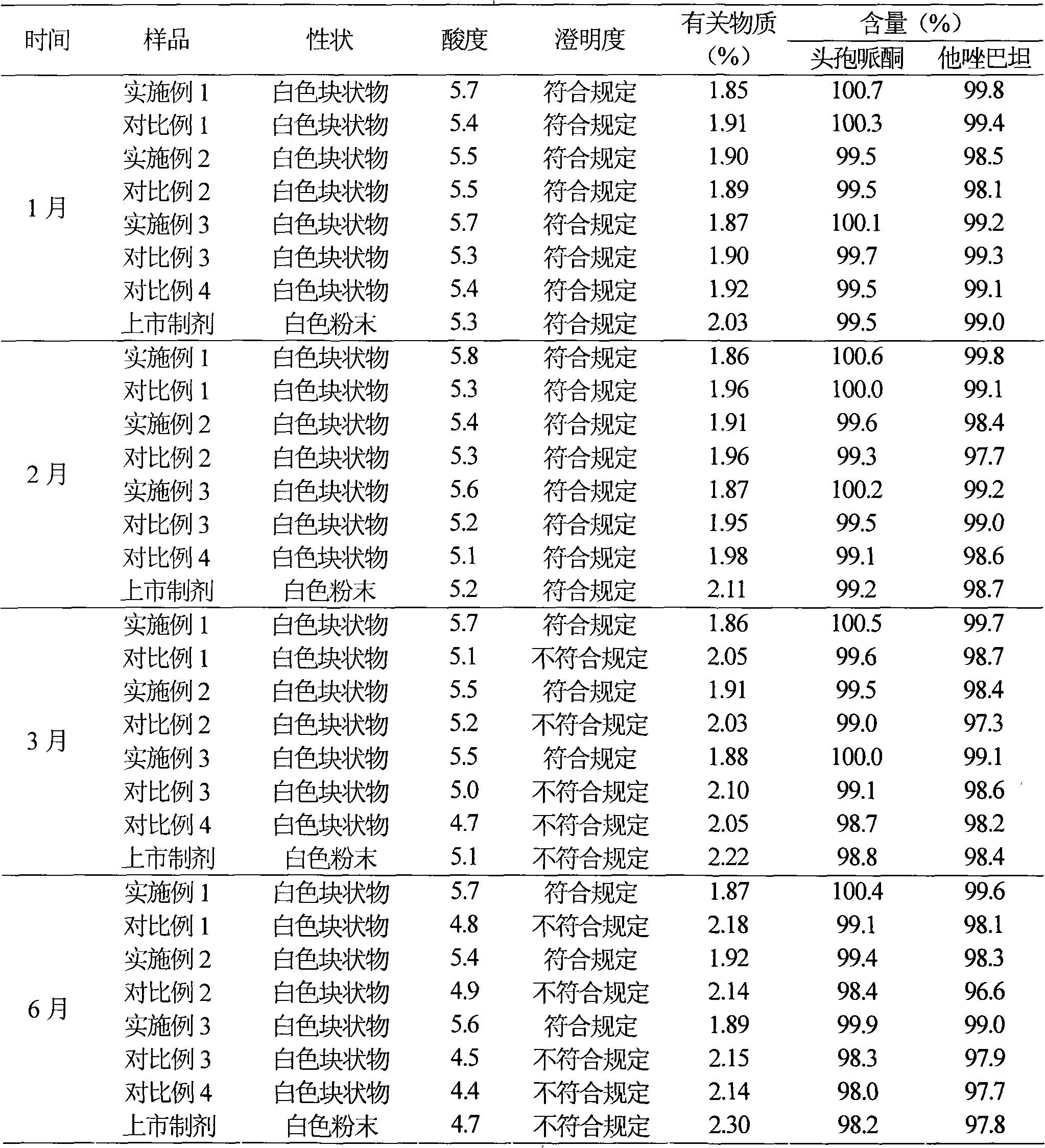
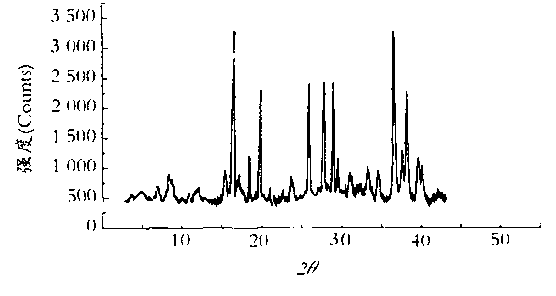
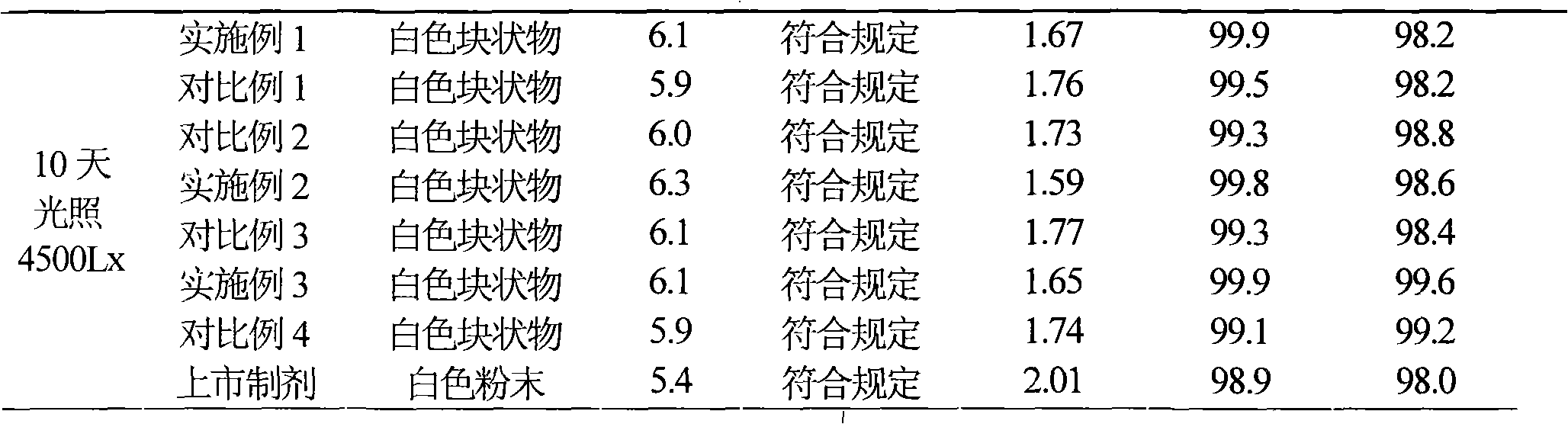
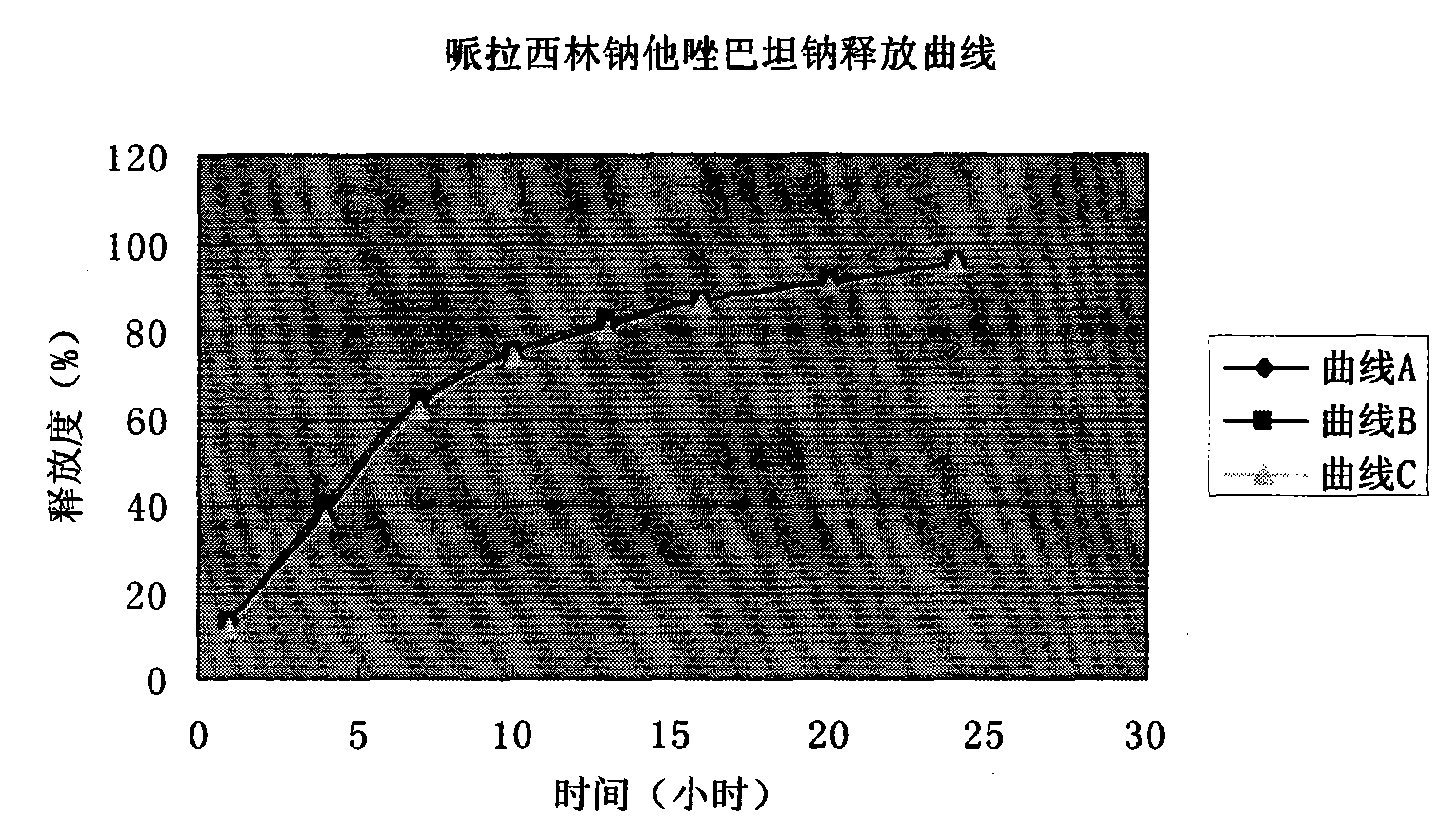
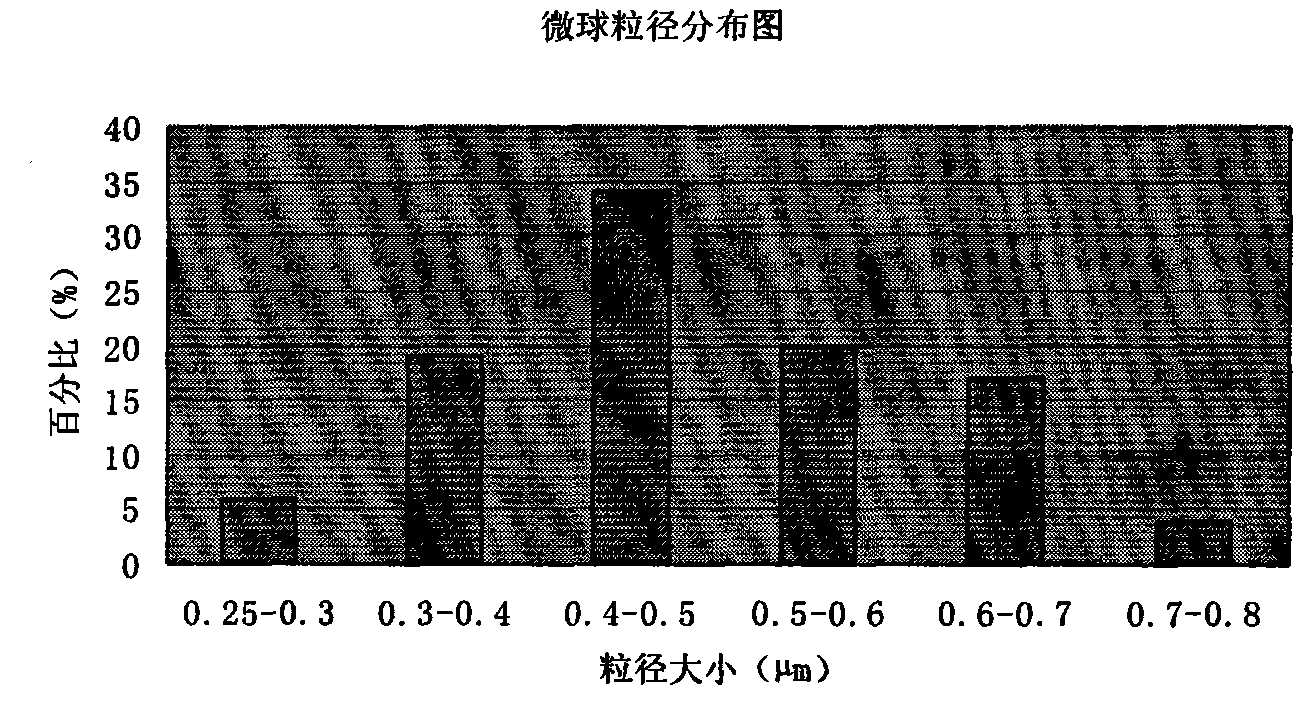
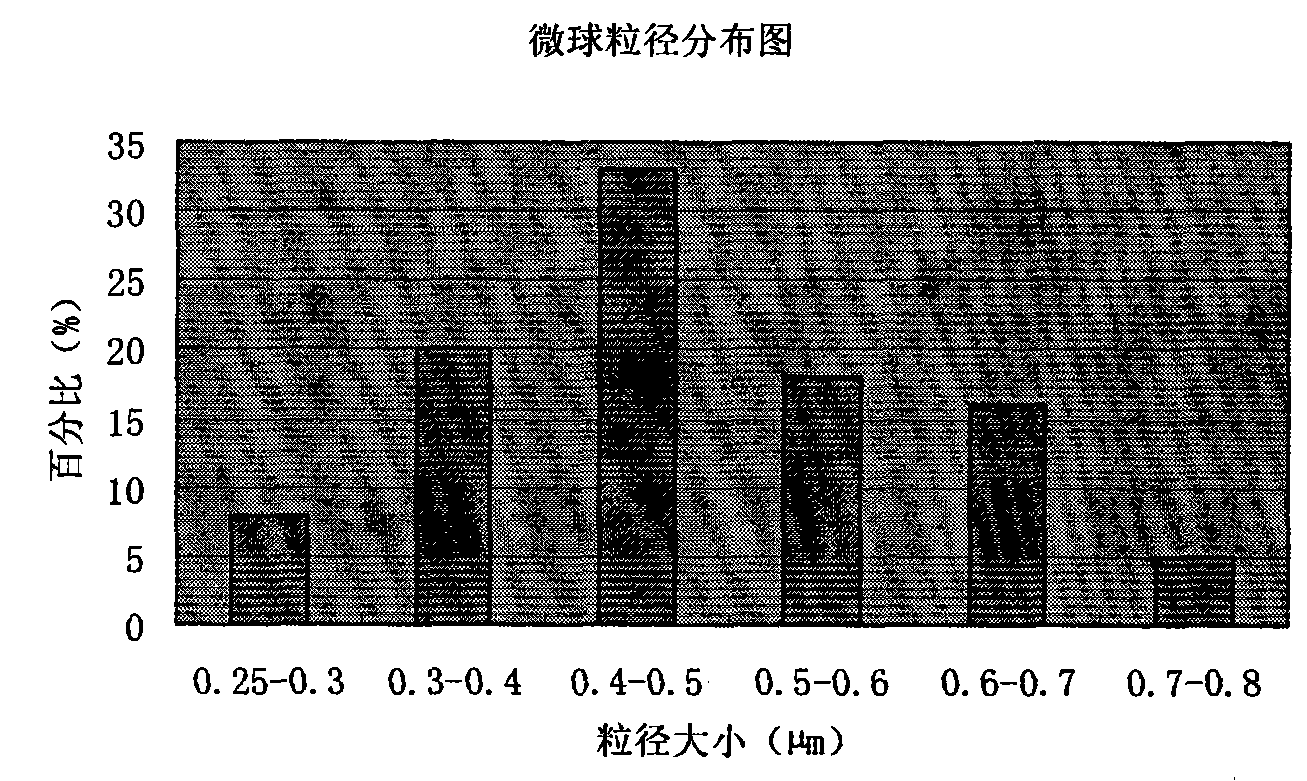
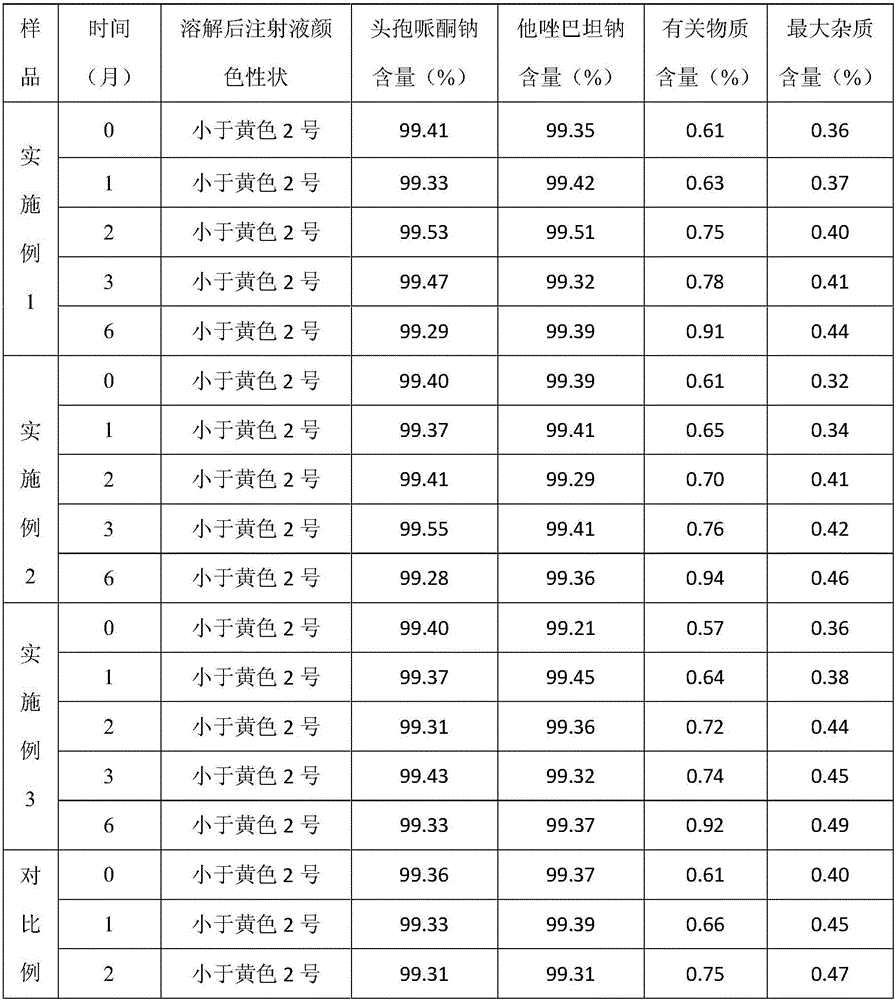
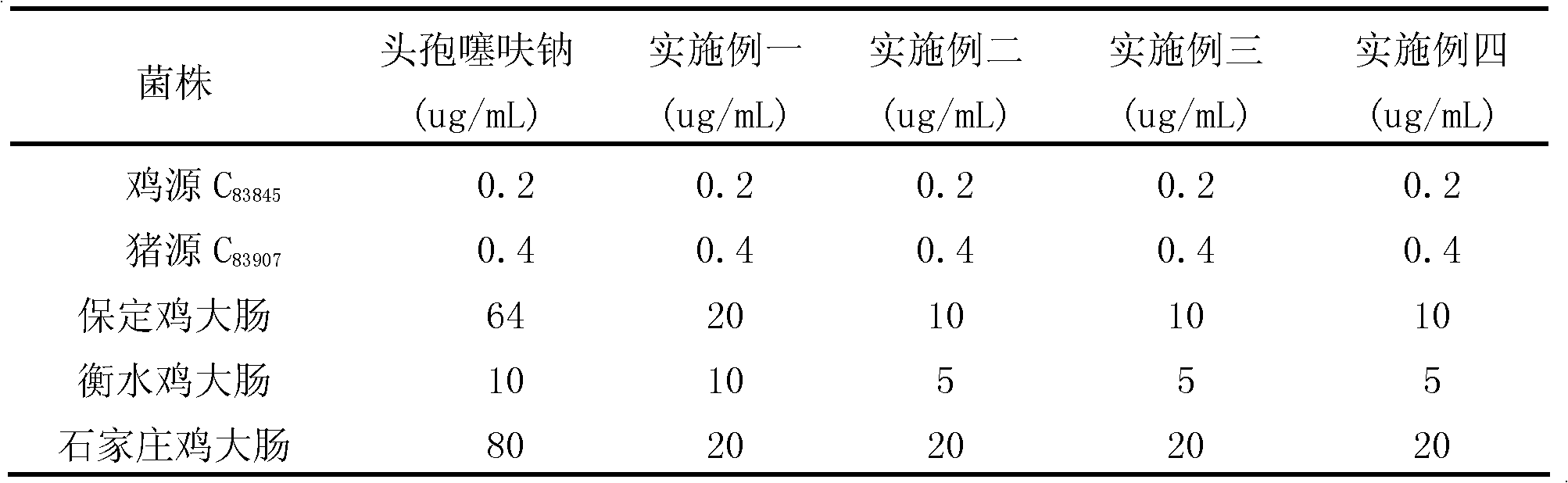
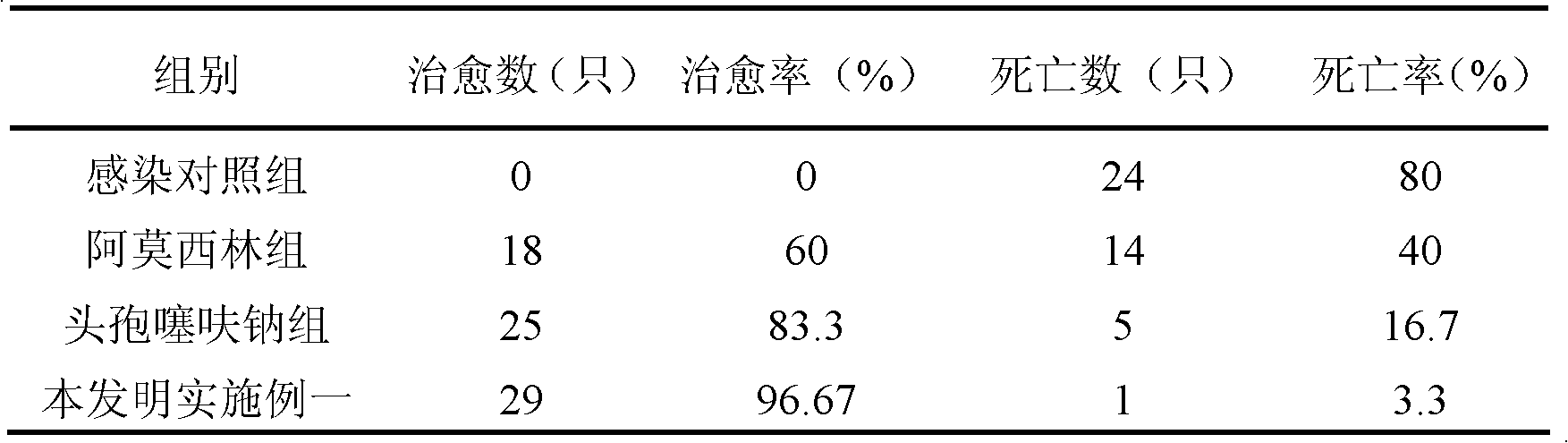
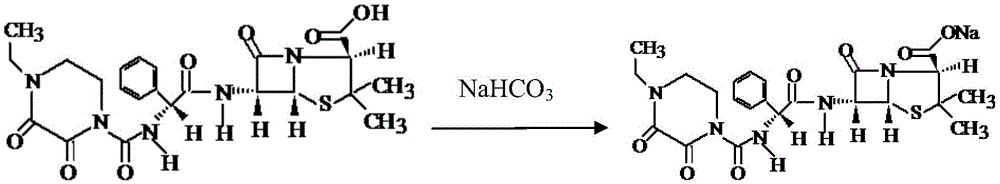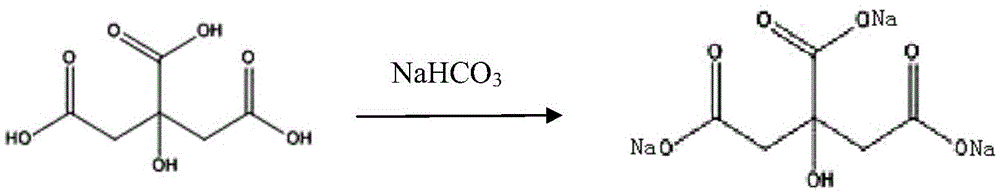Patents
Literature
114 results about "TAZOBACTAM SODIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
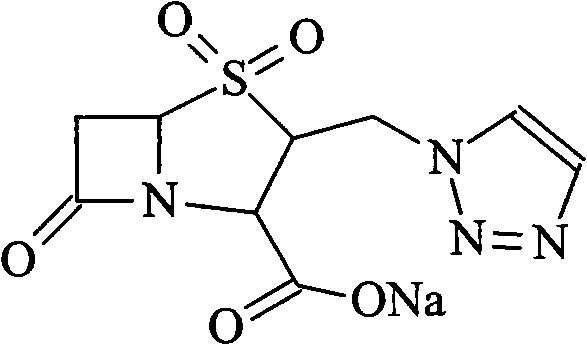
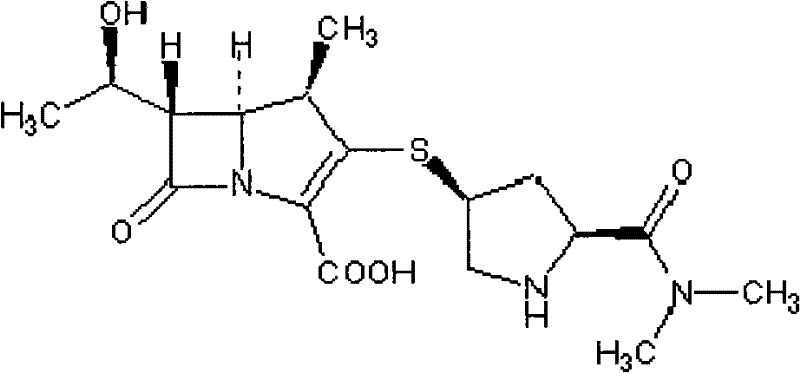
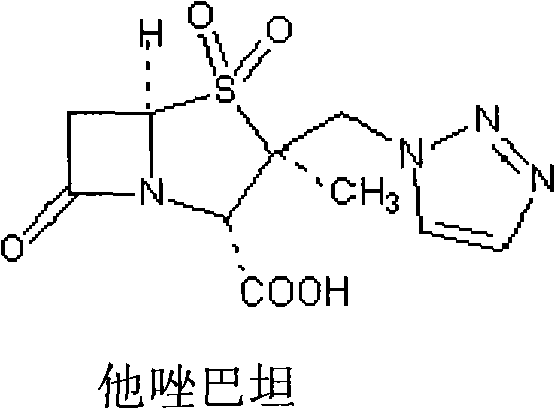
Tazobactam Sodium is the sodium salt form of tazobactam, a penicillanic acid sulfone derivative and beta-lactamase inhibitor with antibacterial activity. Tazobactam contains a beta-lactam ring and irreversibly binds to beta-lactamase at or near its active site.
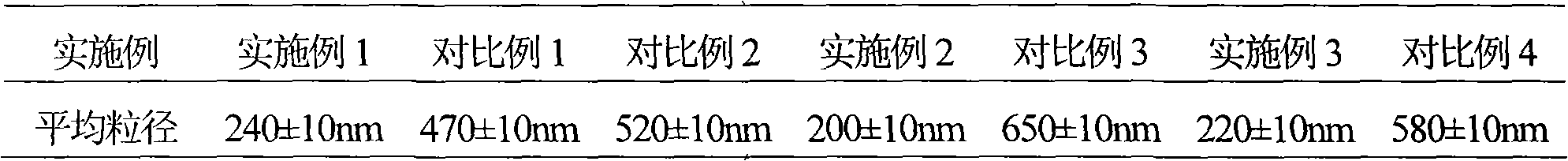
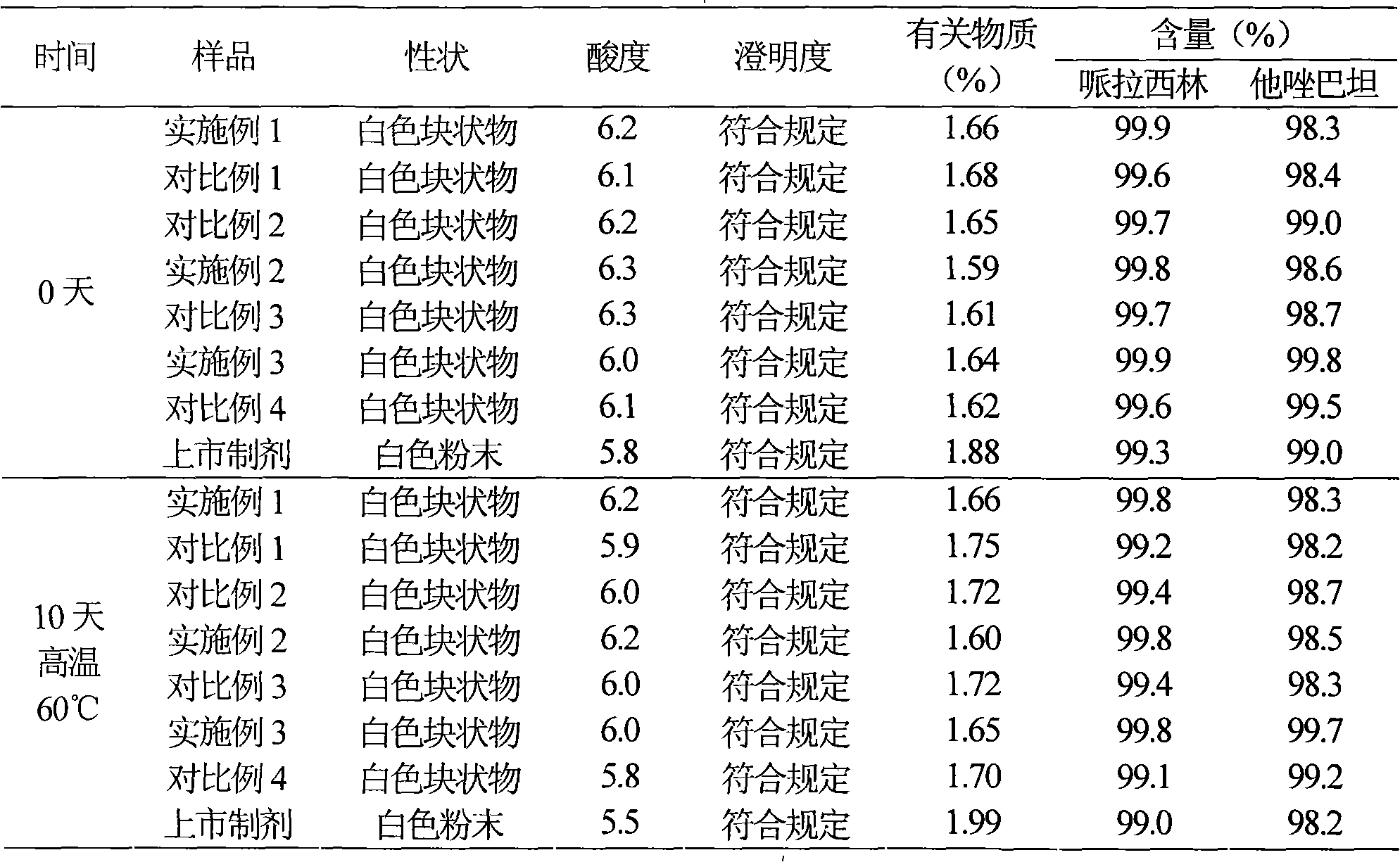
High-purity tazobactam sodium compound
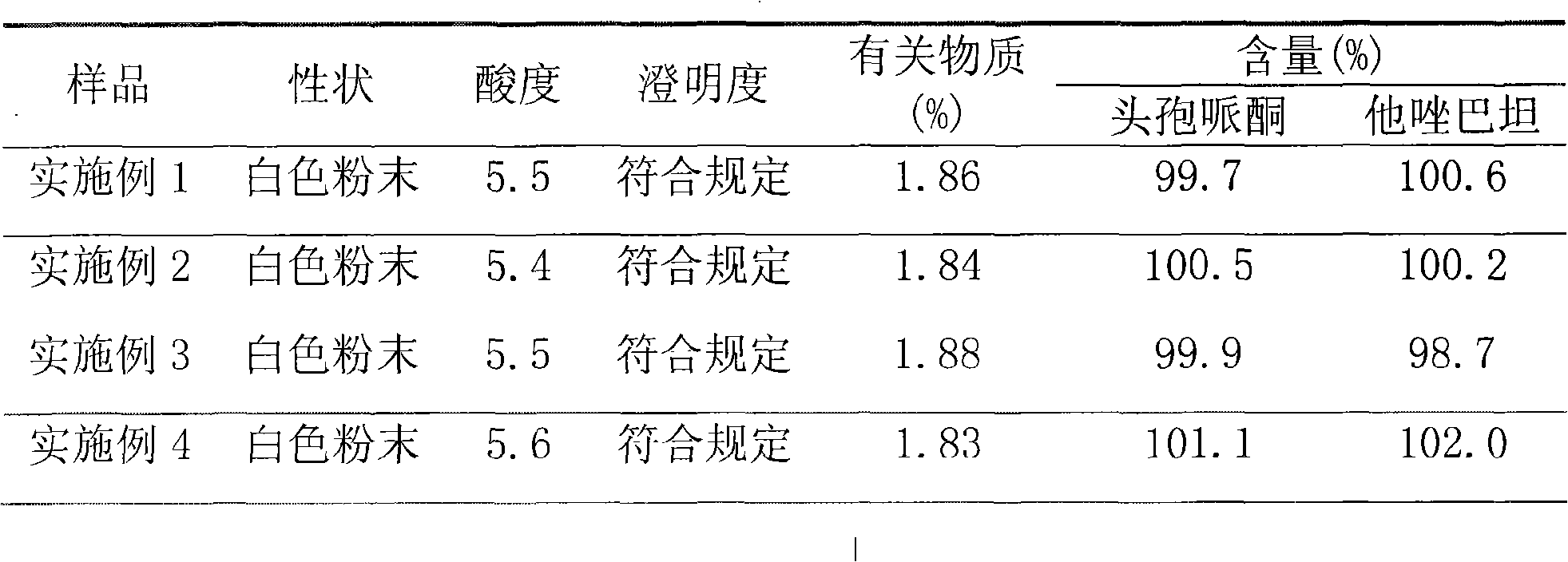
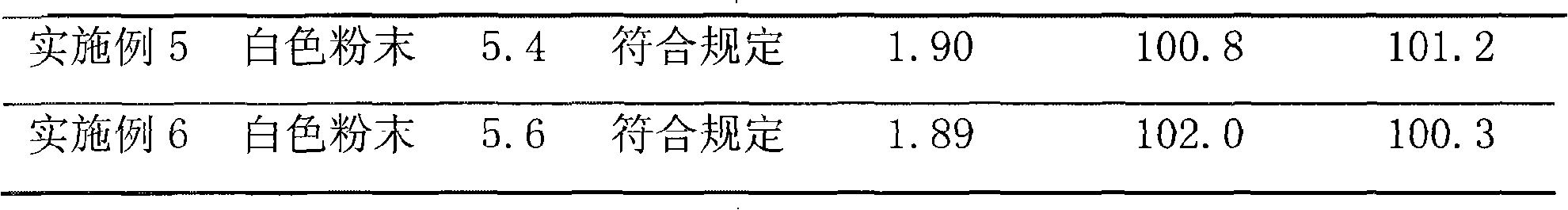
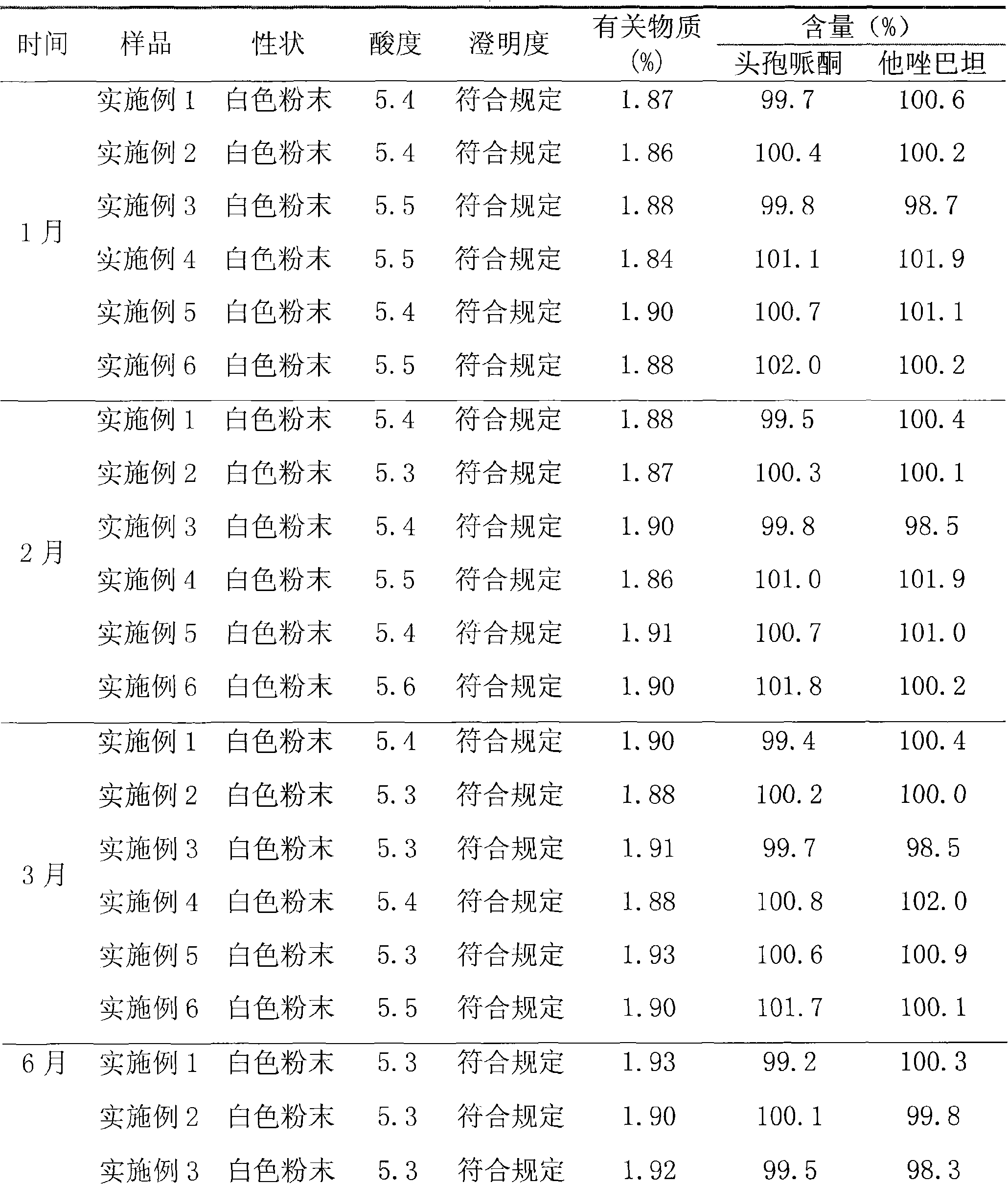
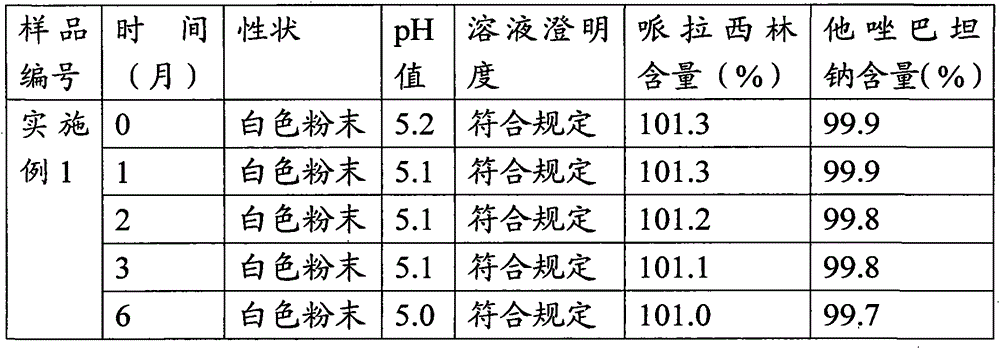
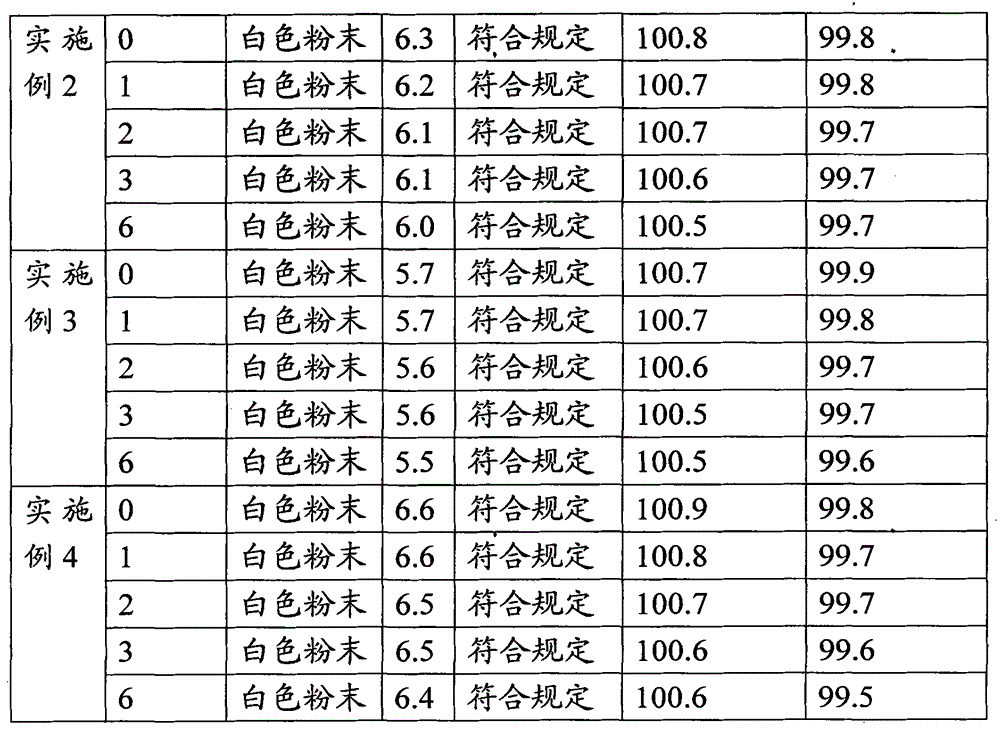
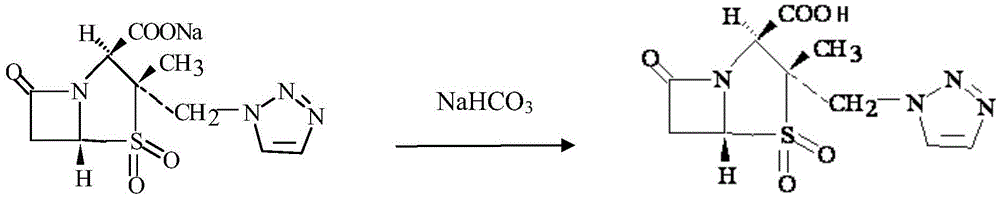
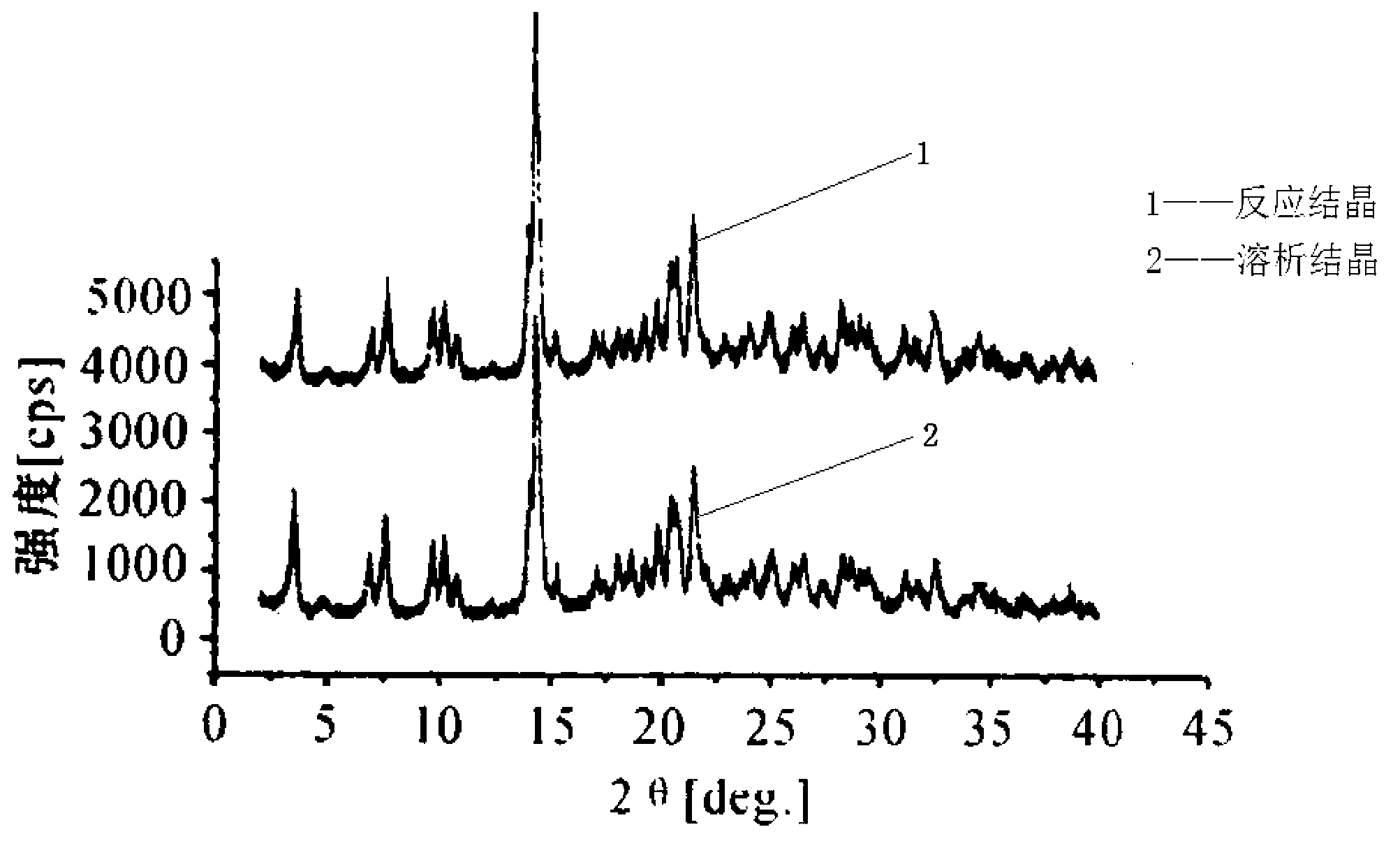
The invention relates to a high-purity tazobactam sodium compound, and particularly provides a method for refining the tazobactam sodium compound. The method comprises the following steps: a, dissolving a tazobactam sodium coarse product into water, regulating the pH value of aqueous solution to less than 7 and collecting solid precipitated from the solution; b, dissolving the solid obtained in the step a into organic solvent to obtain solution to be refined; c, putting the solution to be refined into macroporous absorption resin, performing elution and purification by using eluting agent, and collecting eluent; and d, regulating the pH value of the eluent obtained in the step c to 7 and collecting the precipitated solid to obtain refined tazobactam sodium. The refined tazobactam sodium compound prepared by the method has the purity of over 99.8 percent and the yield of over 90 percent.
Owner:HAINAN MEIDA PHARMA
High-purity medicament and preparation thereof
InactiveCN101348493AHigh purityNo pollution in the processAntibacterial agentsPowder deliveryDrug compoundTAZOBACTAM SODIUM
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of tazobactam sodium
InactiveCN102382123ASimple production processQuality improvementOrganic chemistryBeta-lactamTAZOBACTAM SODIUM
The invention relates to a preparation method of a beta-lactam inhibitor, namely tazobactam sodium. The preparation method comprises the following steps: dissolving tazobactan acid in 6-15 DEG C of cooling water, mixing with a salt forming agent solution under the condition of controlling the temperature, stirring to ensure that the solution is clear, and lyophilizing to obtain tazobactam sodium powder. The tazobactam sodium prepared by the method has high purity; and the entire technological operation is convenient, the cost is low and the production efficiency and yield are increased.
Owner:海南美好西林生物制药有限公司
Piperacillin sodium and tazobactam sodium compound preparation for injection
InactiveCN1732930AGood treatment effectStrong mixed infectionAntibacterial agentsHeterocyclic compound active ingredientsPiperacillin Sodium/ Tazobactam SodiumOtolaryngology/ENT
The invention discloses a compound preparation of piperacillin sodium and tazobactam sodium for injection, which comprises piperacillin sodium and tazobactam sodium by the weight ratio of 3-4:0.8-1.2. The preparation can be used for treating respiratory system infection, urological infection, otologia infection and skin infection.
Owner:NORTH CHINA PHARMA GROUP CORP
Suspension powder injection of cefoperazone sodium and tazobactam sodium pharmaceutical composition and new application thereof
InactiveCN101632677AUnexpected effectImprove stabilityPowder deliveryUrinary disorderFreeze-dryingBULK ACTIVE INGREDIENT
The invention discloses a suspension powder injection with a cefoperazone sodium and tazobactam sodium pharmaceutical composition as an active ingredient, and comprises the following components: 4 parts of the cefoperazone sodium, 1 part of the tazobactam sodium, 5-30 parts of an emulsifier, 1-15 parts of an auxiliary emulsifier and 1-40 parts of a freeze-drying support agent. The invention further discloses an application thereof in preparing medicines for treating cystitis.
Owner:HAINAN YONGTIAN PHARMA INST
Mezlocillin sodium compound and medicine composition thereof
The invention relates to a mezlocillin sodium compound which is determined by adopting X-ray powder diffraction and has characteristic peaks shown in 2theta of 8.9, 15.7, 16.5, 18.9, 19.8, 24.6, 26.4, 27.8, 29.0, 29.7, 31.8, 33.2, 34.7, 36.8, 37.5, 38.9 and 40.1 in a map. The invention also relates to a mezlocillin sodium compound and a medicine composition with a medicine active component of the mezlocillin sodium compound or the mezlocillin sodium compound and sulbactam sodium or tazobactam sodium. The medicine composition is a powder injection of the mezlocillin sodium compound, or a medicine mixture powder injection of the mezlocillin sodium compound and the sulbactam sodium or tazobactam sodium. The mezlocillin sodium compound has the advantages of difficulty in absorbing mixture, good flowability, high dissolving speed, kept extremely high stability, and greatly improved convenience and safety of the mezlocillin sodium.
Owner:HUNAN KELUN PHARMA
Suspension powder injection of piperacillin sodium and tazobactam sodium pharmaceutical composition and new application thereof
InactiveCN101632670AAvoid it happening againImprove stabilityPowder deliveryLyophilised deliveryPiperacillin Sodium/ Tazobactam SodiumPharmacology
The invention discloses a suspension powder injection of a piperacillin sodium and tazobactam sodium pharmaceutical composition, and further discloses an application thereof to preparing medicines for treating lung abscess.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
A kind of cefoperazone sodium tazobactam sodium pharmaceutical composition
ActiveCN102274233AReduce security risksFew kindsAntibacterial agentsHeterocyclic compound active ingredientsPowder diffractionSubstance content
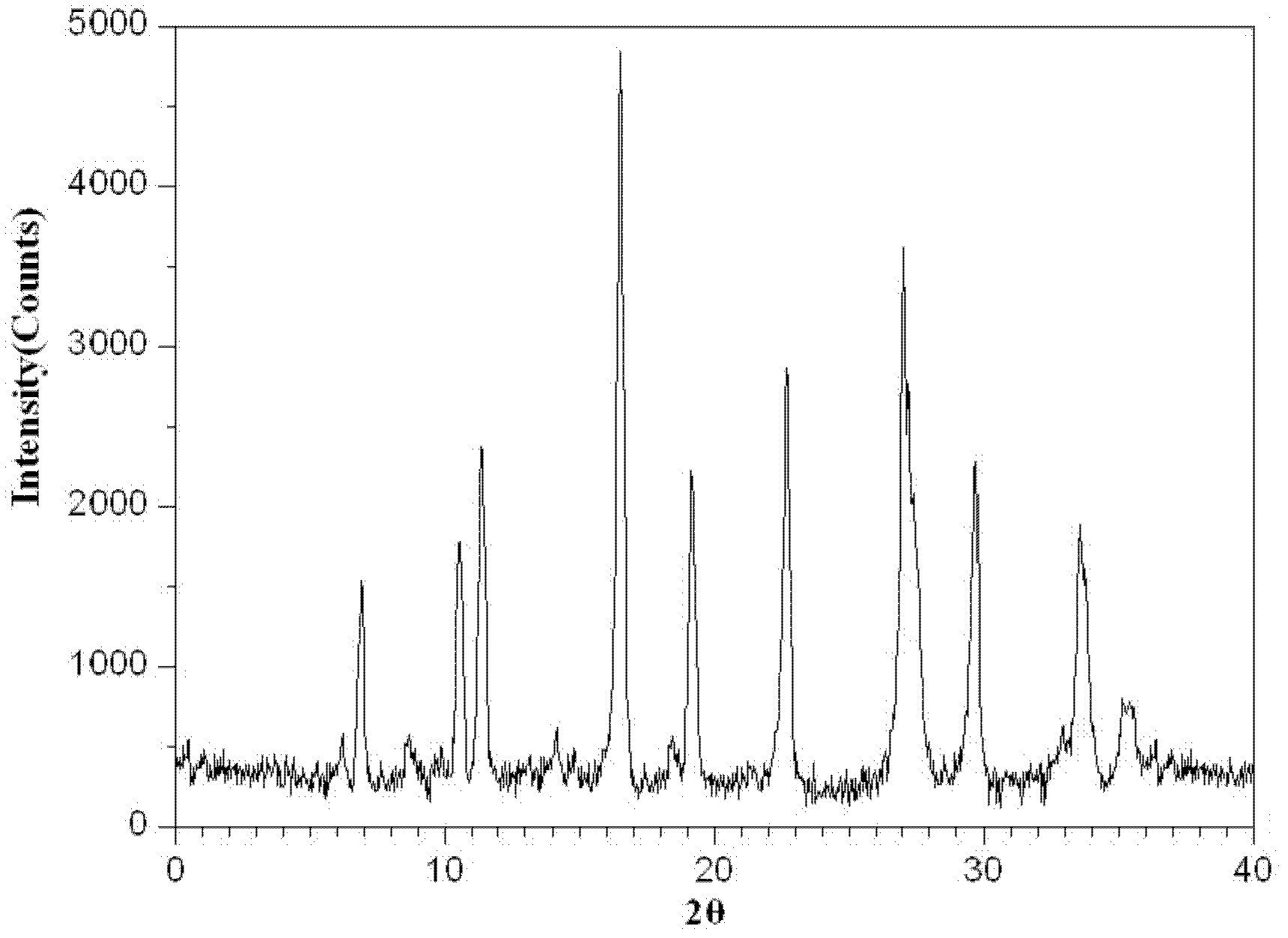
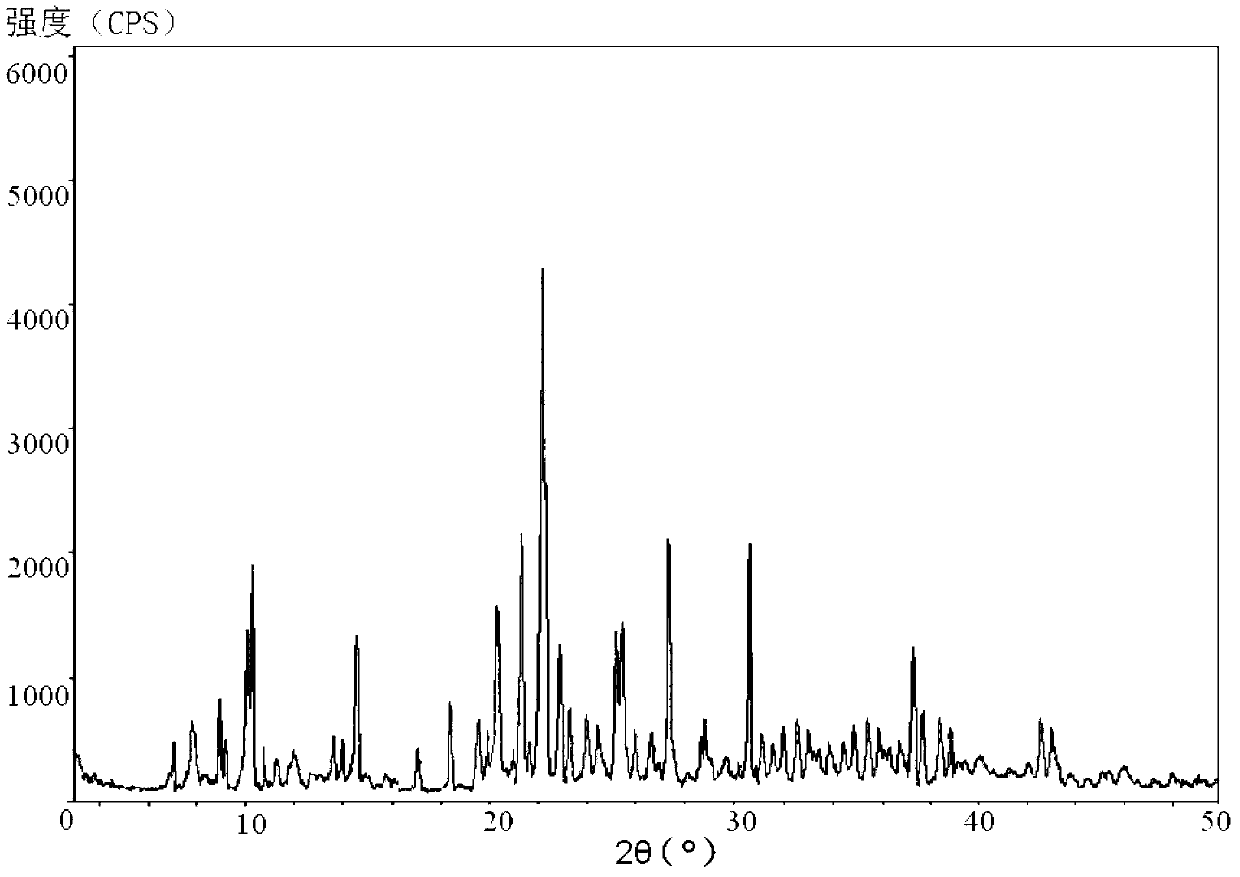
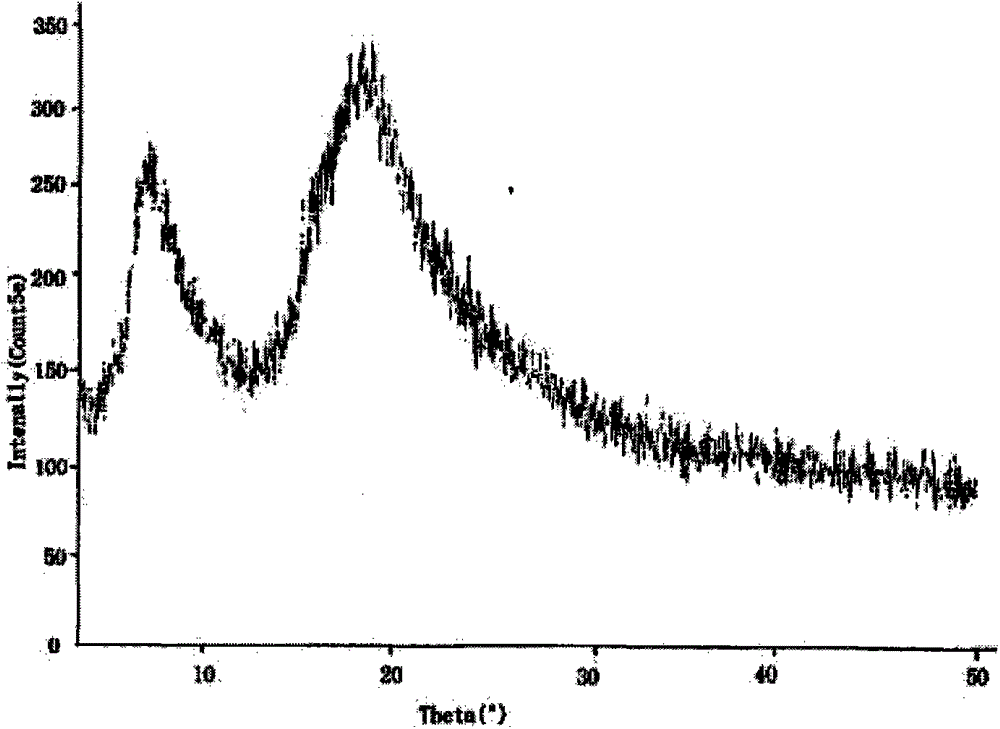
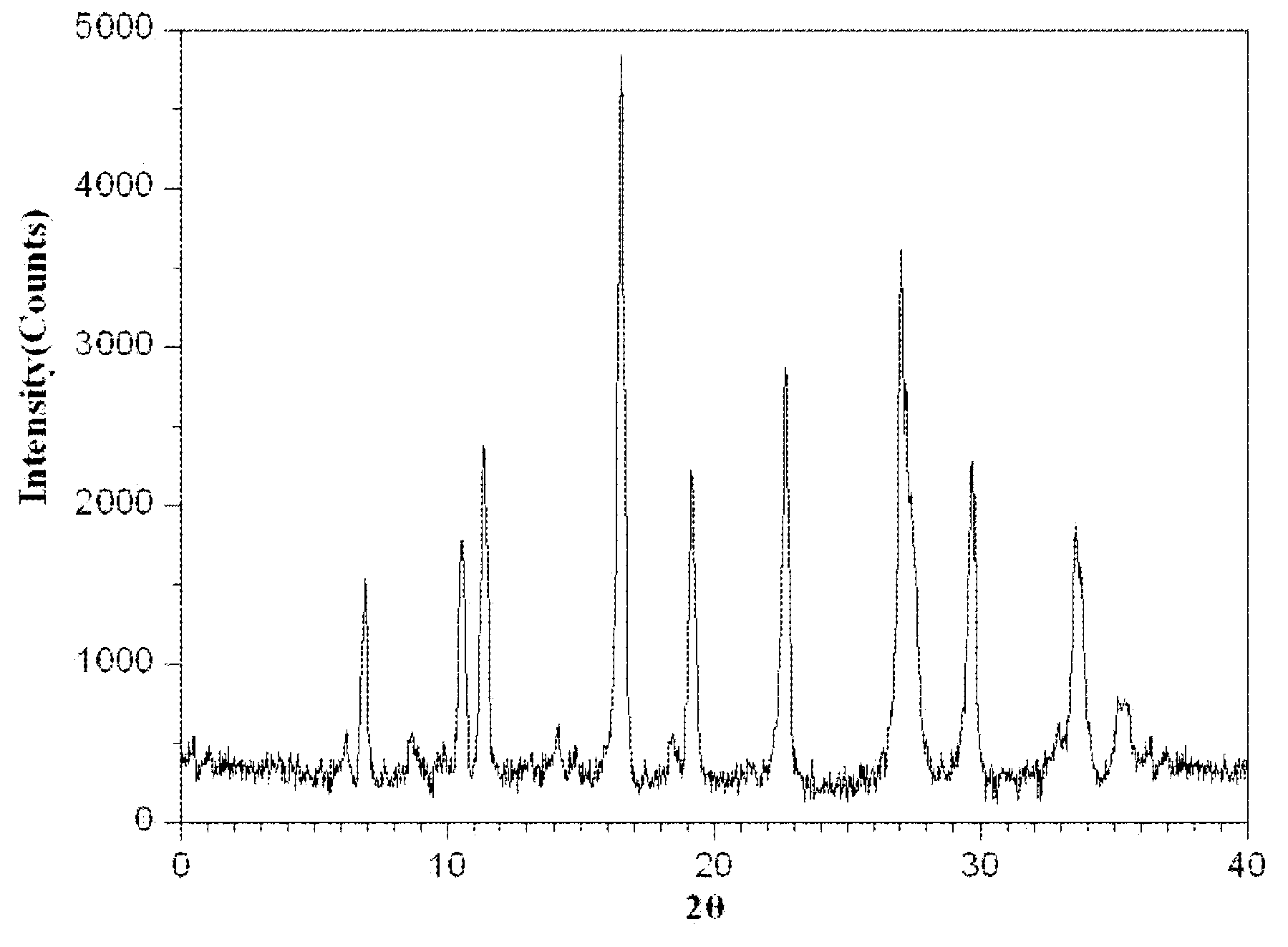
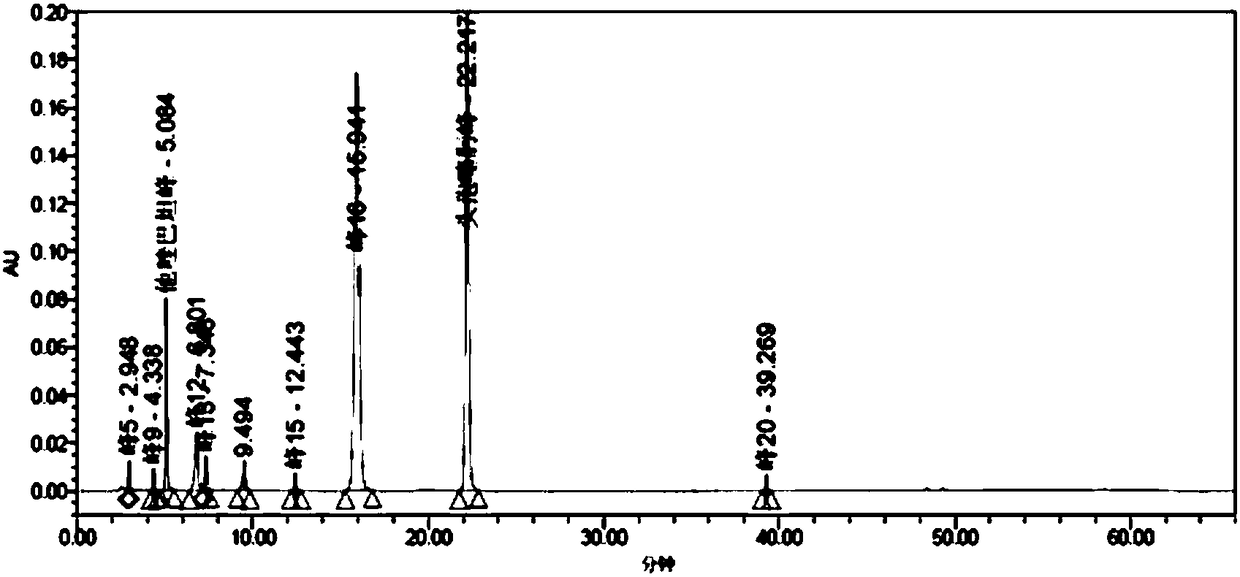
The invention relates to a medicinal composition of cefoperazone sodium and tazobactam sodium. The medicinal composition comprises the following components in part by weight: 4 to 8 parts of cefoperazone sodium and 1 part of tazobactam sodium, wherein the tazobactam sodium is measured by a powder X-ray diffraction measuring method, and characteristic diffraction peaks are shown at the positions of 6.9 degrees, 10.5 degrees, 11.4 degrees, 16.6 degrees, 19.2 degrees, 22.7 degrees, 27.0 degrees, 29.7 degrees and 33.5 degrees in an X-ray powder diffraction map expressed by a diffraction angle of between 2 theta+ / -0.2 degree. The medicinal composition has the advantages of high stability, low relevant substance content, controllable quality and the like, and the administration safety of patients is improved. The invention also relates to a method for preparing the tazobactam sodium with the technical characteristics of the characteristic diffraction peaks.
Owner:江西璟瑞药业有限公司
Injectable sterile pharmaceutical composition with piperacillin sodium and tazobactam sodium as active principles
ActiveUS20080233196A1Improve stabilityEasily soluble in waterOrganic active ingredientsBiocideSodium bicarbonateTAZOBACTAM SODIUM
A sterile pharmaceutical composition having as its active principles piperacillin sodium and tazobactam sodium of substantially the same density, mixed with sodium bicarbonate. The mixture is soluble in water to give injectable reconstituted solutions having high stability with time.
Owner:ACS DOBFAR SPA
Composite pharmaceutical composition of cefoperazone sodium and tazobactam sodium and preparation process thereof
ActiveCN104013629AImprove solubilityImprove stabilityAntibacterial agentsRespiratory disorderSolubilityAntioxidant
The invention discloses a composite pharmaceutical composition of cefoperazone sodium and tazobactam sodium. The composite pharmaceutical composition is an injection, and is prepared from the following components in parts by weight: 3.5-4.5 parts of cefoperazone sodium, 1 part of tazobactam sodium, 0.5-1 part of ambroxol hydrochloride, 0-6 parts of excipient, 0-10 parts of isotonic agent, 0-1 part of antioxidant, a proper amount of citric acid-sodium citrate and 20 parts of injection water. The composite preparation of the cefoperazone sodium and the tazobactam sodium disclosed by the invention is stable in quality and significant in curative effect, not only can three active ingredients be evenly mixed, but also the composite pharmaceutical composition is excellent in stability, good in solubleness and good in clinical use safety.
Owner:福安药业集团庆余堂制药有限公司
Piperacillin sodium-tazobactam sodium medicine composition and preparation method thereof
ActiveCN103340866ALow polymer contentImprove stabilityAntibacterial agentsHeterocyclic compound active ingredientsPiperacillin Sodium/ Tazobactam SodiumMass ratio
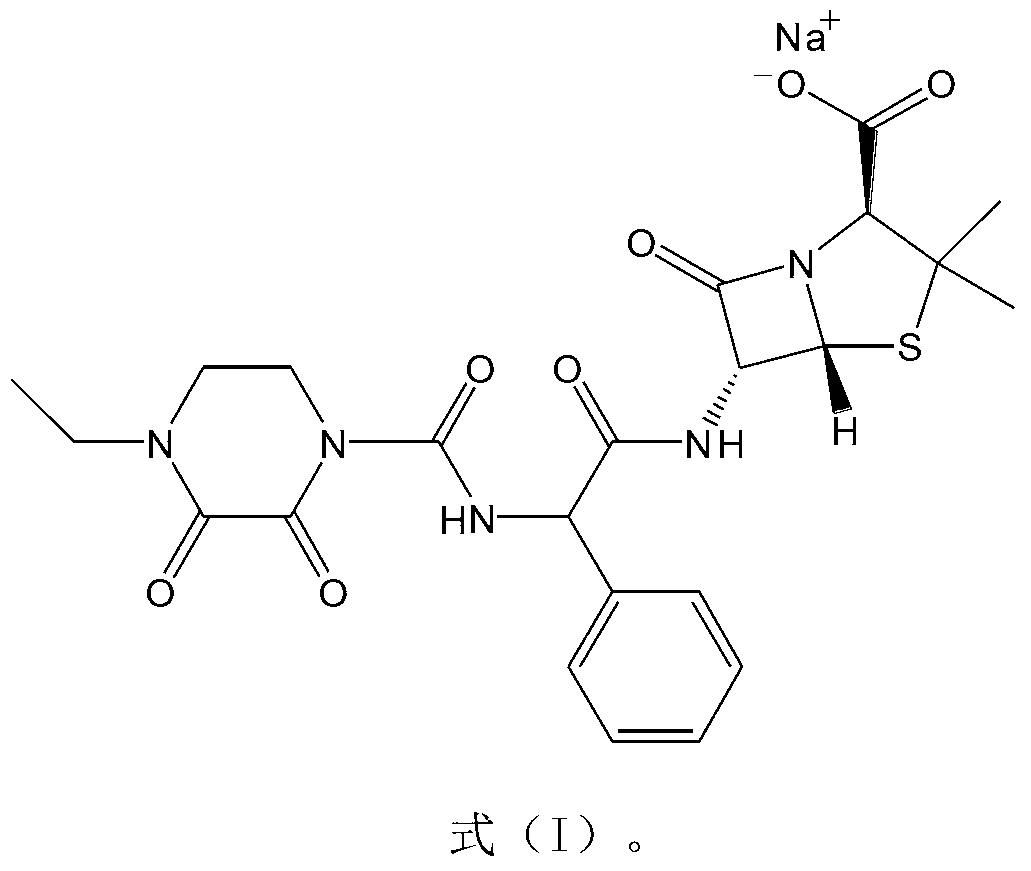
The invention belongs to the technical field of medicine, and in particular relates to a piperacillin sodium-tazobactam sodium medicine composition and a preparation method of the medicine composition. The medicine composition is a sterile powder injection; the mass ratio of the piperacillin sodium to the tazobactam sodium in the medicine composition is 4-20:1; the X-ray powder diffraction pattern of the piperacillin sodium measured by means of powder X-ray diffraction measurement method is shown in Figure 1; the chemical structural formula of the piperacillin sodium is shown in Formula (I); and the content of the piperacillin sodium polymer in the medicine composition provided by the invention is quite low and is not changed obviously in an accelerated test condition and in a long-term test condition. The medicine composition prepared from the piperacillin sodium and the tazobactam sodium provided by the invention also has the advantages of low polymer content and good stability.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Liposome injection of pharmaceutical composition comprising piperacillin sodium and tazobactam sodium
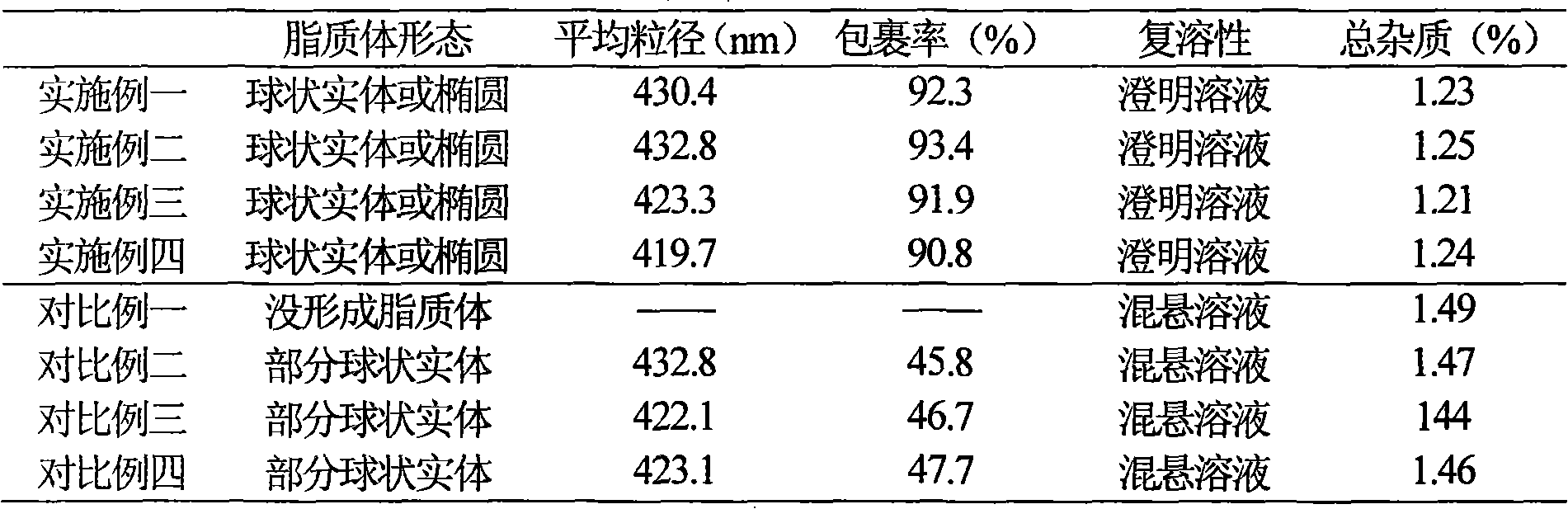
InactiveCN101890015ASimple preparation processFacilitated releaseAntibacterial agentsLiposomal deliveryPiperacillin Sodium/ Tazobactam SodiumLiposome membrane
The invention discloses a liposome injection of a pharmaceutical composition comprising piperacillin sodium and tazobactam sodium. The liposome injection mainly comprises the following components in part by weight: 4 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 4.5 to 13.5 parts of liposome membrane material and membrane material additive, 0.9 to 1.8 parts of frozen dry excipient and 0.45 to 0.9 part of antioxidant.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation method for injection cefoperazone sodium tazobactam sodium composition
InactiveCN103120692AGuaranteed uniformityAntibacterial agentsPowder deliveryCefbuperazoneTAZOBACTAM SODIUM
The invention provides a preparation method for injection cefoperazone sodium tazobactam sodium composition. The preparation method comprises the following steps of: crushing the cefoperazone sodium and the tazobactam sodium; and mixing the cefoperazone sodium and the tazobactam sodium in a weight ratio of 4:1; and separately packaging, pressing and capping the mixture. The preparation method is characterized in that the fineness of pulverization of the cefoperazone sodium and the tazobactam sodium is 50-120 meshes.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Piperacillin sodium and tazobactam sodium pharmaceutical composition and preparation method thereof
ActiveCN103550216AEasy to controlDry fastAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumPowder injection
The invention discloses a piperacillin sodium and tazobactam sodium pharmaceutical composition and a preparation method thereof. The pharmaceutical composition mainly comprises piperacillin sodium and tazobactam sodium, as well as a disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pair, and the powder injection is prepared by spraying and drying. The disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pair is adopted for playing a role in buffering the pH of a solution, thus ensuring the content stability of piperacillin sodium, and no generation of carbon dioxide; the preparation technology is simple, convenient to control, fast to dry, and applicable to continuous large-scale production. The piperacillin sodium and tazobactam sodium powder injection is fast to dissolve, stable in quality, free from crystallization and degradation products, and the solution clarity meets the specification.
Owner:REYOUNG PHARMA
Preparation process for cefoperazone sodium and tazobactam sodium for injection
A preparation process for cefoperazone sodium and tazobactam sodium for injection is disclosed, which comprises the following steps of: (1) mixing for cefoperazone sodium powder and tazobactam sodium powder; (2) cleaning for bottle: straightening a bottle, cleaning the bottle by an ultrasonic wave, and sterilizing by dry heat in a tunnel drying oven; (3) cleaning for stopper: cleaning a rubber stopper, sterilizing and drying; (4) sterile subpackaging; (5) treatment for aluminium cap; (6) lamp inspection for capping; (7) labelling; and (8) boxing.
Owner:SUZHOU ERYE PHARMA CO LTD
Preparation method of piperacillin sodium and tazobactam sodium for injection
ActiveCN105616415AAvoid decompositionImprove acidity stabilityAntibacterial agentsPowder deliverySodium bicarbonatePiperacillin Sodium/ Tazobactam Sodium
The invention discloses a preparation method of piperacillin sodium and tazobactam sodium for injection. The preparation method comprises the following steps: adding water into a blending tank, cooling to 5-10 DEG C, adding citric acid, then slowly adding sodium bicarbonate, and after reacting, reading the pH value and adjusting the pH value to be 6.3-6.6; adding piperacillin acid into the blending tank, and then dropwise adding the sodium bicarbonate solution, wherein the pH value in the dropwise adding process is controlled to be less than or equal to 7.0; after dropwise adding, adding tazobactam acid, and then dropwise adding the sodium bicarbonate solution, wherein the pH value in the dropwise adding process is controlled to be less than or equal to 7.0; after dropwise adding, carrying out vacuum removal on carbon dioxide gas by suction, and after the feed liquid in the blending tank is stabilized, reading the pH value and adjusting the pH value to be 6.0-6.5; and carrying out aseptic filtration and freeze-drying to obtain raw powder of piperacillin sodium and tazobactam sodium for injection. According to the preparation method, by adding salified citric acid, the acidity stability of the product is increased, the decomposition of piperacillin sodium and tazobactam sodium is inhibited, and the storage stability of the product is increased.
Owner:山东安信制药有限公司
Novel method for measuring compound cefotaxime sodium tazobactam sodium
The invention relates to a novel high performance liquid chromatogram (HPLC) method, which can simultaneously detect the content of two single ingredients and relevant impurities in the cefotaxime sodium tazobactam sodium compound. The two ingredients have no interferences or influences. The method has easy operation, strong specificity, high sensitivity, large linear range and good stability, and can be used for detecting a compound preparation and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Piperacillin sodium-tazobactam sodium medicinal composition microsphere injection
InactiveCN101890016AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryPiperacillin Sodium/ Tazobactam SodiumMANNITOL/SORBITOL
The invention discloses piperacillin sodium-tazobactam sodium medicinal composition microsphere injection. The injection is characterized by consisting of piperacillin sodium, tazobactam sodium, polylactic acid-polyethylene glycol (PLA-PEG), PEG600, polysorbate 80 and mannitol and particularly consisting of 4 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 4 to 9 parts of PLA-PEG, 6 to 10 parts of PEG600, 1 to 3 parts of polysorbate 80 and 2 to 4 parts of mannitol. Compared with the prior art, the piperacillin sodium-tazobactam sodium medicinal composition microsphere injection prepared by the invention has the characteristics of good stability, high entrapment rate, high preparation technology repeatability, suitability for industrial production, uniform particle distribution, few solvent residue, good injectability and excellent sustained release property.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Piperacillin sodium tazobactam sodium preparation for injection and preparation method thereof
ActiveCN104644637AImprove securityEasy to decompose and removeAntibacterial agentsPowder deliveryPiperacillin Sodium/ Tazobactam SodiumImpurity
The invention discloses a medicine composition of piperacillin sodium tazobactam sodium and a preparation method thereof. The medicine composition is sterile powder injection, wherein the weight ratio of piperacillin sodium tazobactam sodium is (2-4):1. The piperacillin sodium tazobactam sodium sterile powder injection prepared in the invention has high stability and less impurity; by utilizing the sterile powder injection, the safety and effectiveness of clinical medication are improved greatly.
Owner:NORTH CHINA PHARMA COMPANY +2
Production process of compound preparation of ceftriaxone sodium and tazobactam sodium for injection
ActiveCN101537009AHigh purityImprove the safety of useAntibacterial agentsPharmaceutical delivery mechanismFiltrationDissolution
The invention discloses a production process of compound preparation of ceftriaxone sodium and tazobactam sodium for injection, comprising the steps of weighing raw materials of ceftriaxone sodium, tazobactam sodium, sterilized water for injection, mixed liquid of ethyl acetate and isopropanol, and anhydrous ethanol based on the weight ratio of 3-5:1:2:5:9; conducing dissolution and filtration; crystallizing and washing; and lyophilizing to obtain the compound preparation of ceftriaxone sodium and tazobactam sodium for injection. The invention is applicable to the compound preparation of ceftriaxone sodium and tazobactam sodium for injection, which is produced according to the ratio of 3:1-5:1 of ceftriaxone to tazobactam; and the invention adopts evenly mixing of liquid phase, can achieve good mixing uniformity, effectively increase the purity of the preparations simultaneously, and further guarantee the advantage of high safety of clinic use.
Owner:HAIKOU QILI PHARMA
Drug composition of cefoperazone sodium and tazobactam sodium and preparation method thereof
InactiveCN103230400AReduce humidityImprove thermal stabilityAntibacterial agentsOrganic chemistryThermal stabilityStructural formula
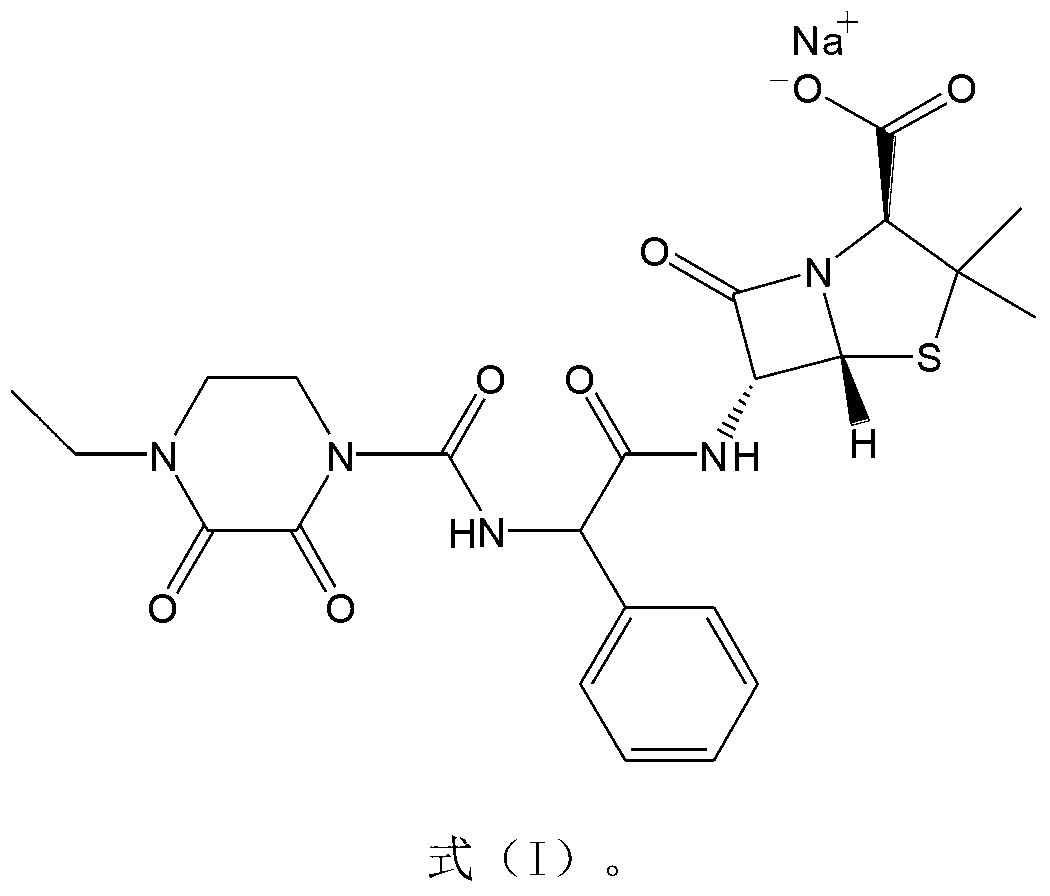
The invention belongs to the technical field of medicines and in particular relates to a drug composition of cefoperazone sodium and tazobactam sodium and a preparation method thereof. The drug composition is sterile powder injection. The mass ratio of cefoperazone sodium to tazobactam sodium in the drug composition is (4-8):1, wherein cefoperazone sodium is shown in a drawing 1, an X-ray powder diffraction pattern obtained by powder X-ray diffractometry and the chemical structural formula of cefoperazone sodium is shown in the formula (I) in the specification. Compared with the prior art, the cefoperazone sodium compound used in the drug composition has lower hygroscopicity and good thermal stability, thus improving the stability of the drug composition of cefoperazone sodium and tazobactam sodium and reducing the impurity content.
Owner:四川省惠达药业有限公司
Meropenem sodium/tazobactam sodium medicinal composition
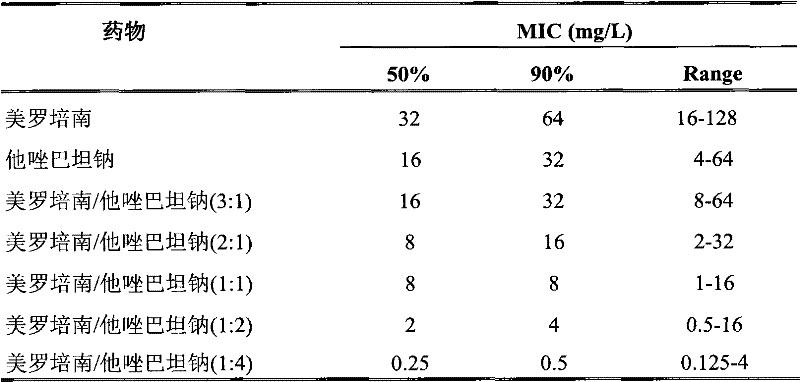
The invention provides a combined medicament, in particular a meropenem tazobactam sodium combined medicament for treating infectious diseases caused by acinetobacter baumannii. The meropenem and tazobactam sodium combined medicament has a synergistic effect and an accumulative antibacterial effect on the infectious diseases caused by the acinetobacter baumannii and particularly has a good synergistic effect and a good accumulative antibacterial effect on multiple medicine-tolerant acinetobacter baumannii strains, and can be used for curing clinical infection caused by the multiple medicine-tolerant acinetobacter baumannii strains in clinic.
Owner:深圳市新泰医药有限公司
Method for simultaneously measuring content of main components and main impurities in cefotaxime sodium tazobactam sodium for injection
InactiveCN108802206AEffective quality controlImprove targetingComponent separationFiller ExcipientPhosphate
The invention belongs to the technical field of the pharmaceutical analysis, and particularly relates to a method for simultaneously measuring content of main components and main impurities in cefotaxime sodium tazobactam sodium for injection. The method comprises the following steps: using octadecyl silane bonded silica gel as filler of a chromatographic column, using mixed solution of phosphoricacid-phosphate buffer solution and methyl alcohol in a certain proportion as a flowing phase, simultaneously detecting the content of two main components and two main impurities in the cefotaxime sodium tazobactam sodium for injection, thereby effectively controlling quality of the cefotaxime sodium tazobactam sodium for injection. In addition, the invention further develops reference solution for positioning the known impurities. The reference solution is suitable for positioning a cefotaxime impurity B and impurity F in an HPLC detecting method, and improving pertinency of impurity detection. The method is strong in specificity, high in precision, good in stability, simple and convenient in operation, and strong in pertinency, an expensive impurity reference substance is prevented frombeing used, and the method has a good application value.
Owner:JIANGSU LINGBAO PHARMA
Beta-lactam compound antibiotic composition
ActiveCN102327270AImprove liquidityImprove product quality yieldAntibacterial agentsPowder deliveryActive componentCefuroxime
The invention provides a beta-lactam compound antibiotic composition, which contains cefuroxime and a tazobactam sodium monohydrate, or cefuroxime salts and the tazobactam sodium monohydrate, wherein the weight ratio of the cefuroxime salts counted according to the cefuroxime to the tazobactam sodium monohydrate counted according to the tazobactam is (1-8):1. The beta-lactam compound antibiotic composition disclosed by the invention is used for being prepared into a clinically-acceptable medicinal preparation with the cefuroxime and salts thereof as well as the tazobactam sodium monohydrate as active components. According to the beta-lactam compound antibiotic composition, the tazobactam sodium monohydrate is mixed with the cefuroxime and salts thereof so as to improve the flowability of composition powder, enable the uniformity of the medicament to be better in the production process and improve the yield of the product; and the beta-lactam compound antibiotic composition has saved production cost and is especially suitable for popularization and application.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Medicinal composition
ActiveCN102018713BAntibacterial agentsHeterocyclic compound active ingredientsChemical compositionIon-exchange resin
Owner:BEIJING SIHUAN NEW PHARMA TECH +1
Cefmetazole-containing and beta-lactamase inhibitor-containing medicinal composition
ActiveCN101816791AAntibacterial agentsHeterocyclic compound active ingredientsCEFMETAZOLE SODIUMDrug tolerance
The invention provides a cefmetazole-containing and beta-lactanase inhibitor-containing medicinal composition, belongs to the technical field of medicaments, in particular relates to a medicinal composition consisting of 1 to 8 weight parts of cefmetazole and 1 weight part of specific beta-lactamase inhibitor. The beta-lactamase inhibitor is one of sulbactam sodium and tazobactam sodium. The medicinal composition can improve the antimicrobial spectrum of the medicament cefmetazole sodium, and reduce the drug tolerance of bacteria.
Owner:HAINAN TIANHUANG PHARMA +2
Liposome injection prepared from ceftriaxone sodium tazobactam sodium medicinal composition
InactiveCN102048740AQuality assuranceImprove stabilityAntibacterial agentsLiposomal deliverySide effectAntioxidant
The invention discloses a liposome injection prepared from ceftriaxone sodium tazobactam sodium medicinal composition, comprising the following components in parts by weight: 3 parts of ceftriaxone sodium, 1 part of azobactam sodium, 3-18 parts of liposome film material, 1.2-10 parts of addictive, 6-25 parts of frozen-dried support agent and 0.1-4 parts of antioxidant. By preparing the ceftriaxone sodium tazobactam sodium medicinal composition into liposome, the stability of a preparation can be greatly improved, the quality of medicines is improved, the toxic or side effects are reduced, the target property can be acquired and the curative effect can be improved.
Owner:HAINAN YONGTIAN PHARMA INST
Cefoperazone sodium and tazobactam sodium pharmaceutical composition for injection
ActiveCN105748482AImprove solubilityLight colorAntibacterial agentsPowder deliverySodium bicarbonateSolubility
The invention belongs to the technical field of medicines, and particularly relates to cefoperazone sodium and tazobactam sodium pharmaceutical composition for injection and a preparation method of the cefoperazone sodium and tazobactam sodium pharmaceutical composition. The pharmaceutical composition is a freeze-dried powder preparation. The mass ratio of cefoperazone sodium to tazobactam sodium in the pharmaceutical composition is 4:1. The preparation method comprises the steps as follows: firstly, tazobactam is refined; then, tazobactam sodium is prepared from refined tazobactam and sodium bicarbonate; finally, cefoperazone sodium and prepared tazobactam sodium are uniformly mixed for preparation of freeze-dried powder. Compared with like products sold in the market, the pharmaceutical composition has the advantages of good solubility, light color, few related substances, small polymer content and low adverse reaction rate; besides, effective constituents in the pharmaceutical composition are stable, and when the pharmaceutical composition is preserved for a long term, few effective constituents are degraded, the content of impurities is low, the quality performance of products is relatively good, and the medication safety of patients is guaranteed accordingly.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Pharmaceutical composition used for preventing and treating colibacillosis in livestock and poultry
InactiveCN102462686AEasy to prepareStable traitsAntibacterial agentsPeptide/protein ingredientsESCHERICHIA COLI ANTIGENBeta-Cyclodextrins
The invention relates to a pharmaceutical composition used for preventing and treating colibacillosis in livestock and poultry. Every 100 g of the pharmaceutical composition comprises 2 to 10 g of ceftiofur hydrochloride, 0.25 to 10 g of tazobactam sodium, 5 to 10 g of probenecid sodium, 0.1 to 0.5 g of dexamethasone sodium phosphate and 2 to 15 g of beta-cyclodextrin, with the balance being pharmaceutically acceptable accessories. The pharmaceutical composition has the advantages of reasonable composition, low cost, a remarkable curative effect on clinical pathogenic escherichia coli, especially on drug-resistant intractable escherichia coli, a simple preparation method, stable properties and convenience in large scale intensive production.
Owner:TIANJIN RINGPU BIO TECH
Piperacillin sodium and tazobactam sodium sterile powder injection and preparation method thereof
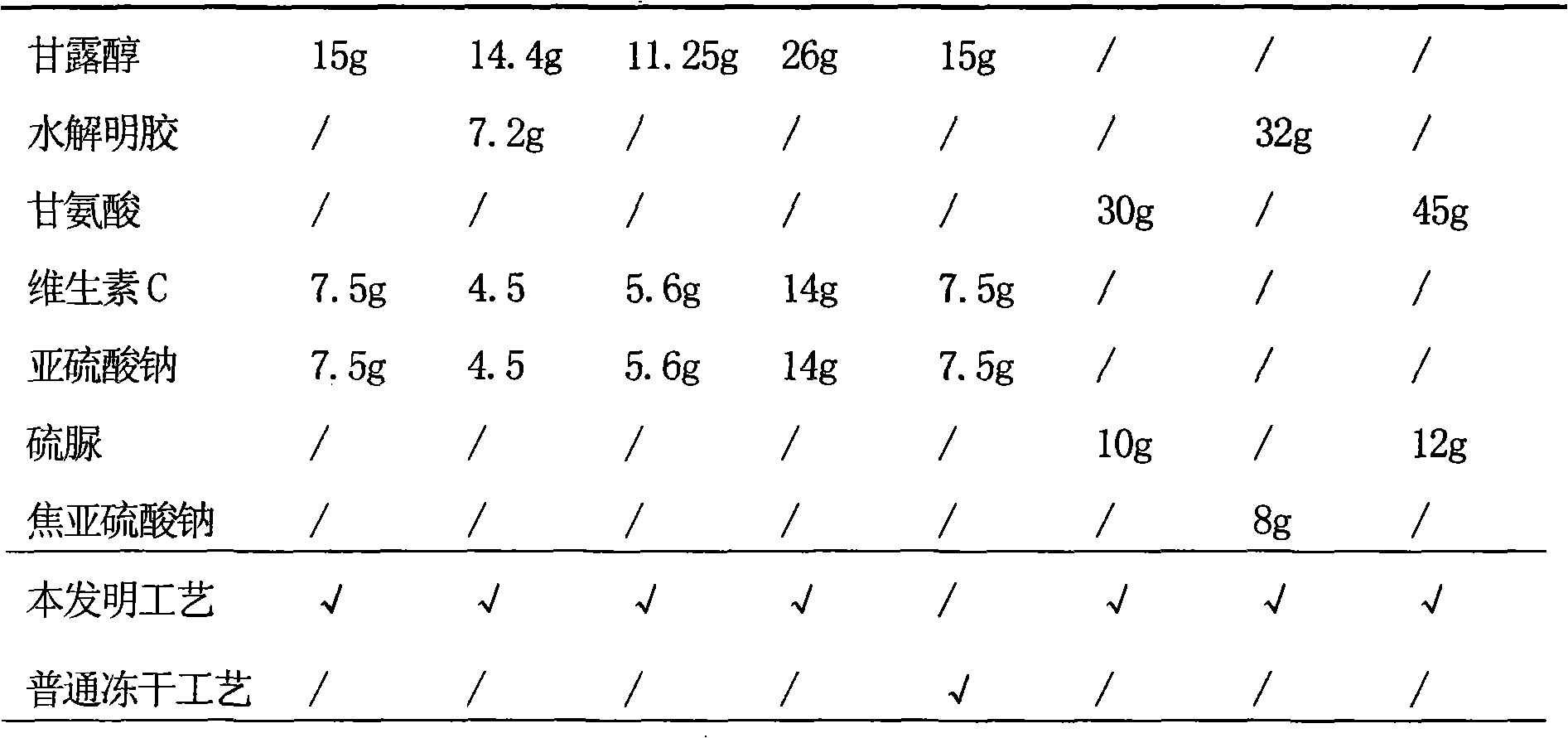
ActiveCN104922126AReduce contentGuaranteed curative effectAntibacterial agentsPowder deliveryVitamin CFiltration
The invention provides a piperacillin sodium and tazobactam sodium sterile powder injection, which comprises the following components: 6 to 8 parts of piperacillin sodium, 1 part of tazobactam sodium, 40 to 80 parts of mannitol, 10 to 30 parts of vitamin C, 8 to 15 parts of sodium pyrosulfite and 5 to 10 parts of glycine. A preparation method for the piperacillin sodium and tazobactam sodium sterile powder injection comprises the following steps of dissolving all the components in water for injection under inert gas shielding; adding activated carbon for decoloration, and then, filtering to remove the activated carbon; performing refined filtration with a microporous filtering film of 0.22mum to 0.80mum; performing freeze drying to obtain the piperacillin sodium and tazobactam sodium sterile powder injection. Compared with the prior art, the sterile powder injection has the characteristics that the stability of the sterile powder injection is effectively improved by adopting a stabilizer which consists of the mannitol, the vitamin C, the sodium pyrosulfite and the glycine, piperacillin sodium and tazobactam sodium are basically unchanged in stability during preparation and storage; the polymer content is low, the product is guaranteed to be qualified within the period of validity, and the curative effect and the safety in clinical application are guaranteed; the preparation method is simple, low in cost and suitable for industrial mass production.
Owner:HAINAN GENERAL & KANGLI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com