High-purity medicament and preparation thereof
A high-purity, pharmaceutical technology, applied in the direction of separation methods, pharmaceutical formulations, chemical instruments and methods, etc., can solve the problems of low purity of active ingredients, low purity, and large side effects, so as to facilitate industrial continuous production and reduce production Cost, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
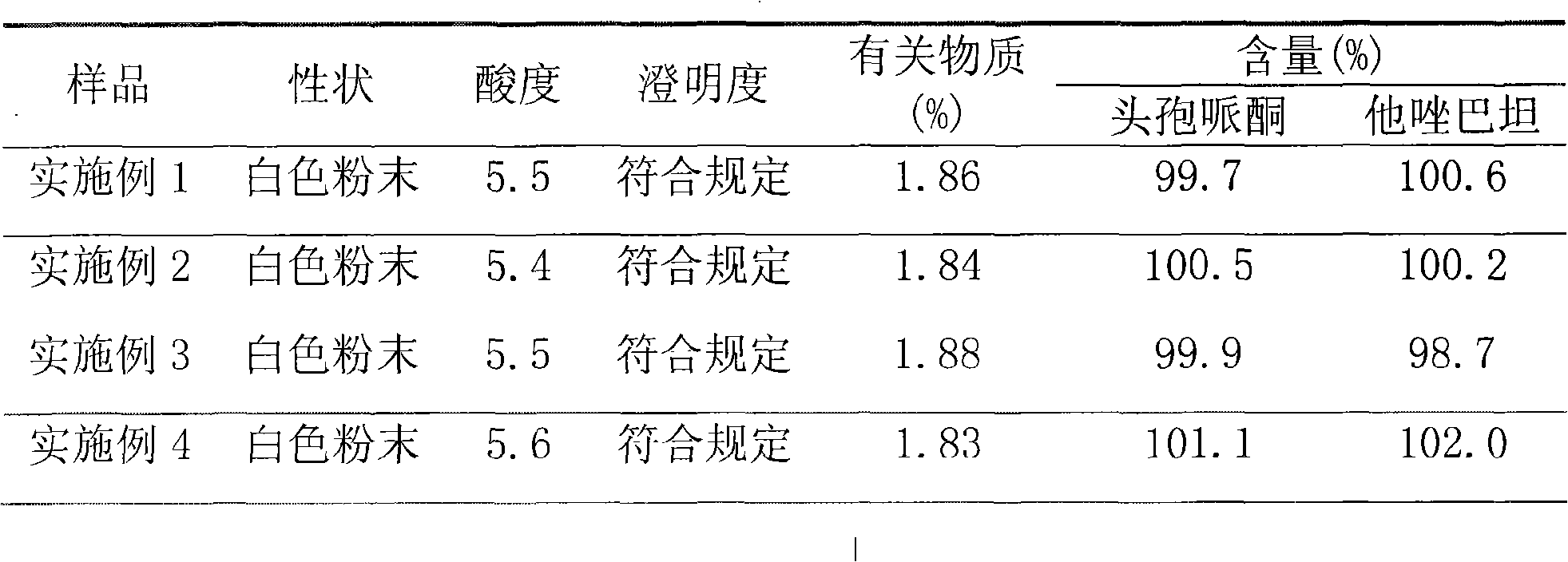
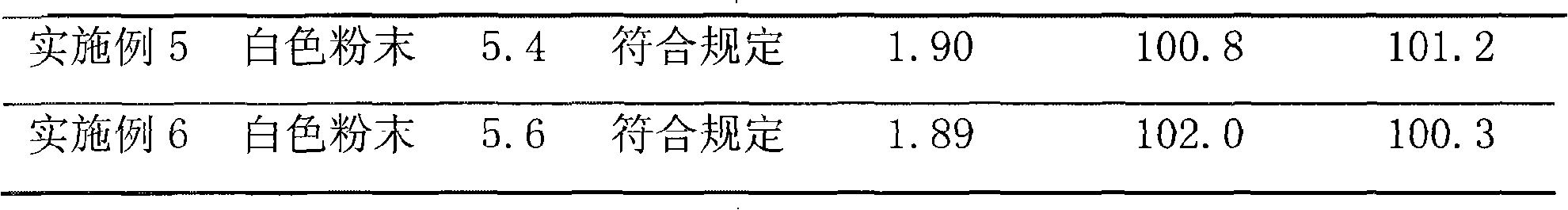
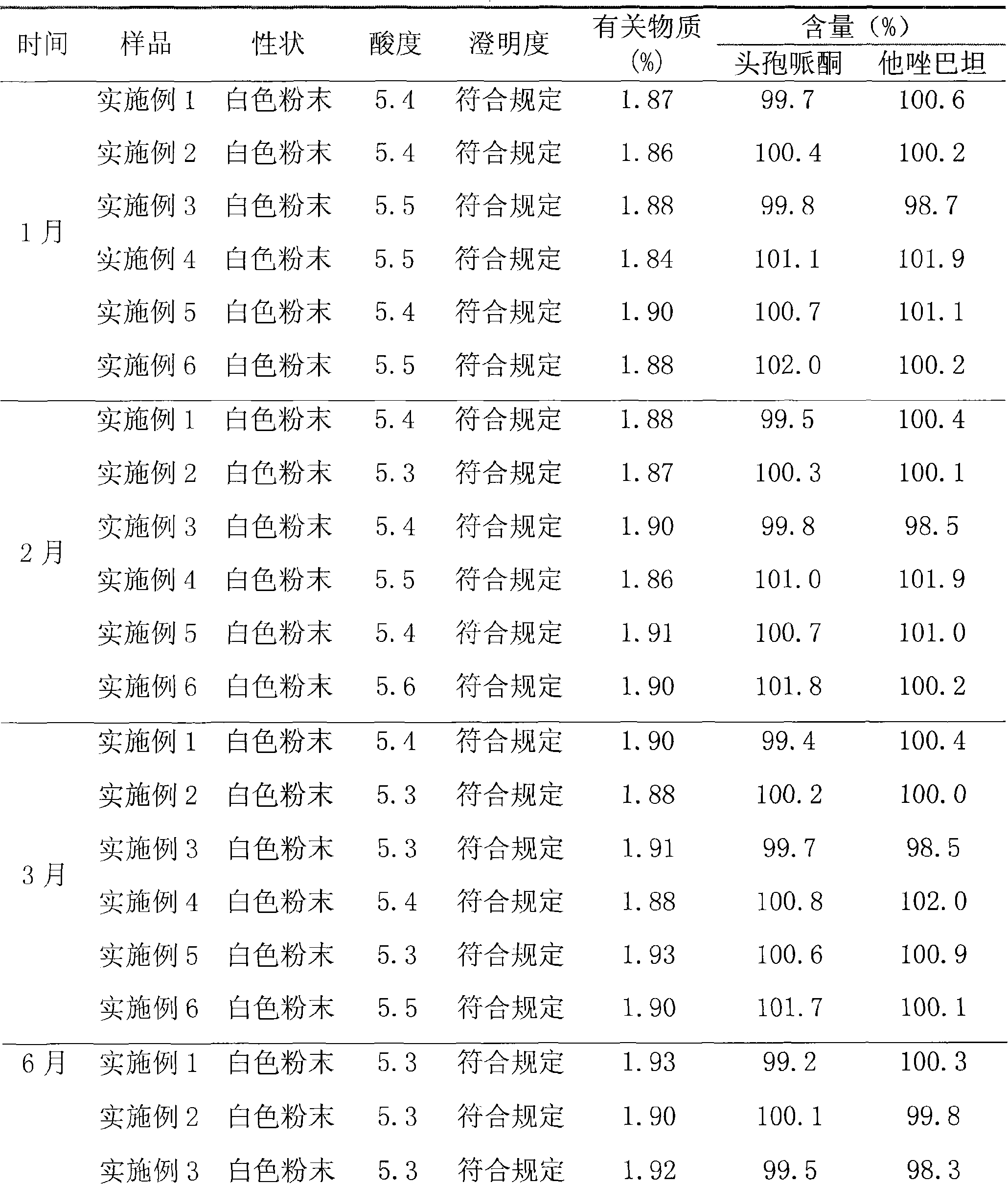
Embodiment 1
[0034] Dissolve 500 g of cefoperazone sodium raw material for injection in 2000 ml of water, and the resulting solution is subjected to gel chromatography. The filler of the chromatographic column used is Sephadex LH-20, which is eluted with 1200 ml of 95% ethanol solution, and the cefoperazone sodium content is greater than 92%. off fraction. The resulting eluted fraction was chromatographed with BUCHI medium pressure preparative chromatography, and the filler of the reverse chromatography column used was C 18 Alkyl bonded phase silica gel. Carry out elution with 1500ml of 95% ethanol solution, collect the cefoperazone sodium content not less than 99% elution fraction, reclaim solvent, obtain high-purity cefoperazone sodium raw material 424.0g after freeze-drying, and purity is 99.5%, and yield is 84.8%.
[0035] Dissolve 500 g of tazobactam sodium raw material for injection in 1800 ml of water, and perform gel chromatography on the resulting solution. The packing of the chrom...
Embodiment 2
[0038] Dissolve 1000 g of cefoperazone sodium for injection in 3000 ml of water, and carry out gel chromatography on the resulting solution. The packing of the chromatographic column used is Sephadex LH-60, and elution is carried out with 1600 ml of 80% ethanol solution, and the cefoperazone sodium content is not less than 92%. Elution fractions. The resulting eluted fraction was chromatographed with BUCHI medium pressure preparative chromatography, and the filler of the reverse chromatography column used was C 4 Alkyl bonded phase silica gel. Carry out elution with 1800ml of 70% ethanol solution, collect the elution flow fraction that cefoperazone sodium content is not less than 99%, reclaim solvent, obtain high-purity cefoperazone sodium raw material 822.1g after freeze-drying, and purity is 99.6%, and yield is 82.2%.
[0039] Dissolve 600 g of tazobactam sodium raw material for injection in 1500 ml of water, and perform gel chromatography on the resulting solution. The pac...
Embodiment 3
[0042] Dissolve 800 g of cefoperazone sodium for injection in 2400 ml of water, and carry out gel chromatography on the resulting solution. The filler of the chromatographic column used is Sephadex LH-20, which is eluted with 1200 ml of 85% ethanol solution, and the cefoperazone sodium content is not less than 92%. Elution fractions. The resulting eluted fraction was chromatographed with BUCHI medium pressure preparative chromatography, and the filler of the reverse chromatography column used was C 4 Alkyl bonded phase silica gel. Carry out elution with 1000ml of 80% ethanol solution, collect the cefoperazone sodium content not less than 99% elution fraction, reclaim solvent, obtain high-purity cefoperazone sodium raw material 667.2g after freeze-drying, and purity is 99.5%, and yield is 83.4%.
[0043] Dissolve 500 g of tazobactam sodium raw material for injection in 1500 ml of water, and perform gel chromatography on the resulting solution. The packing of the chromatography...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com