Suspension powder injection of cefoperazone sodium and tazobactam sodium pharmaceutical composition and new application thereof
A technology of tazobactam sodium and cefoperazone sodium, which is applied in the field of cefoperazone sodium tazobactam sodium pharmaceutical composition suspension powder injection, can solve the problems of poor stability of cefoperazone sodium and tazobactam sodium, and reduce drug side effects and drug toxicity , the effect of preventing the generation of particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
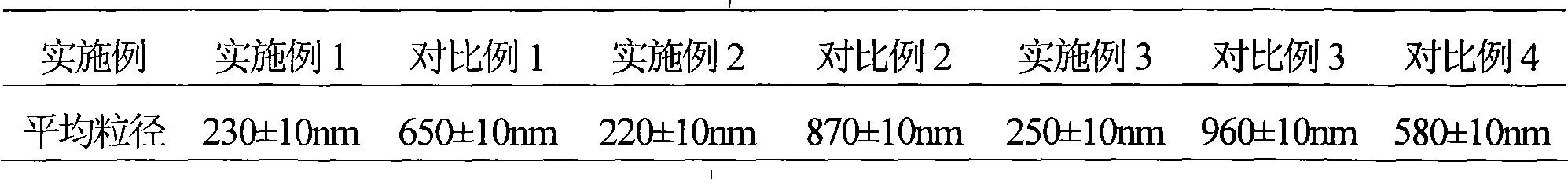
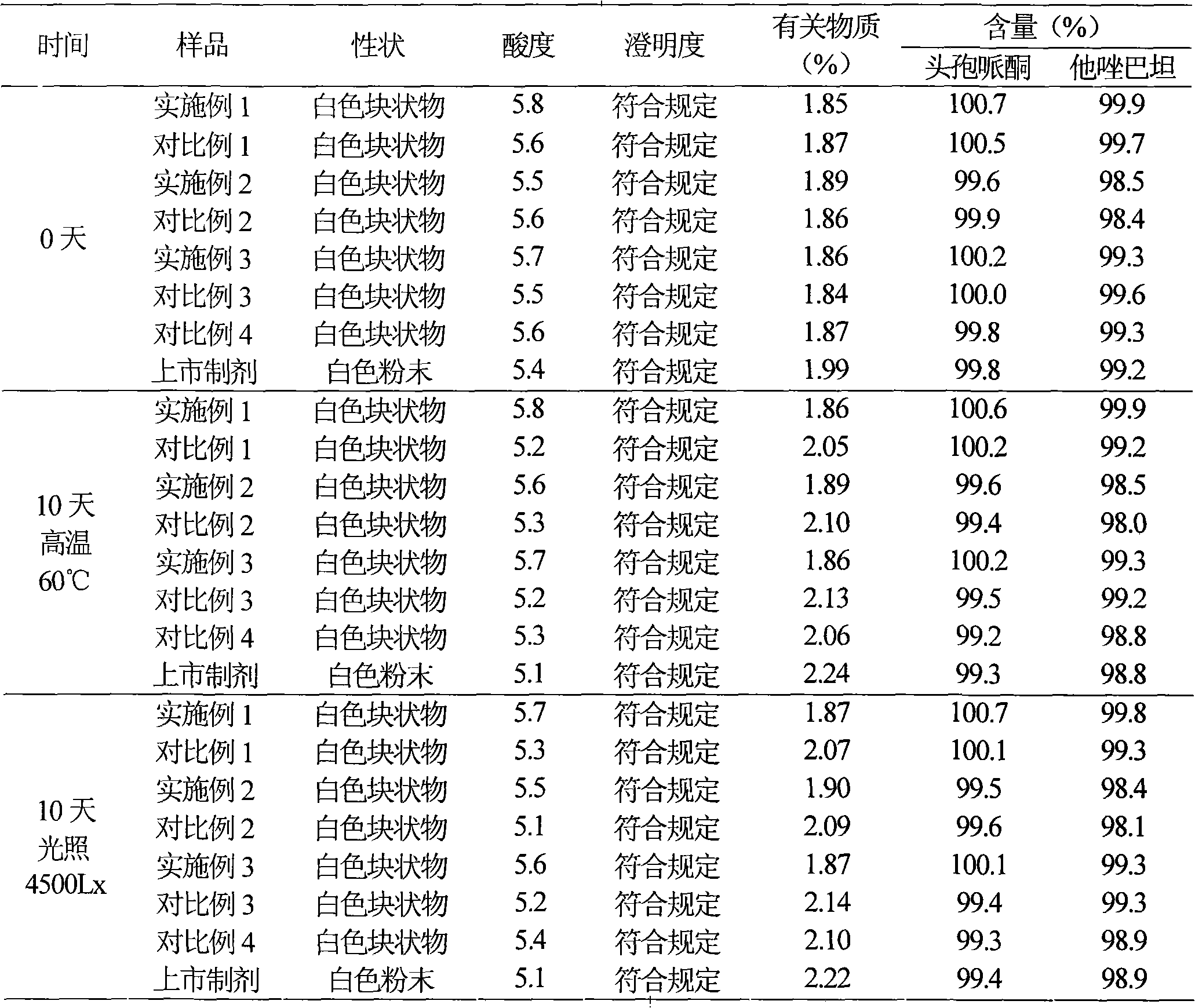
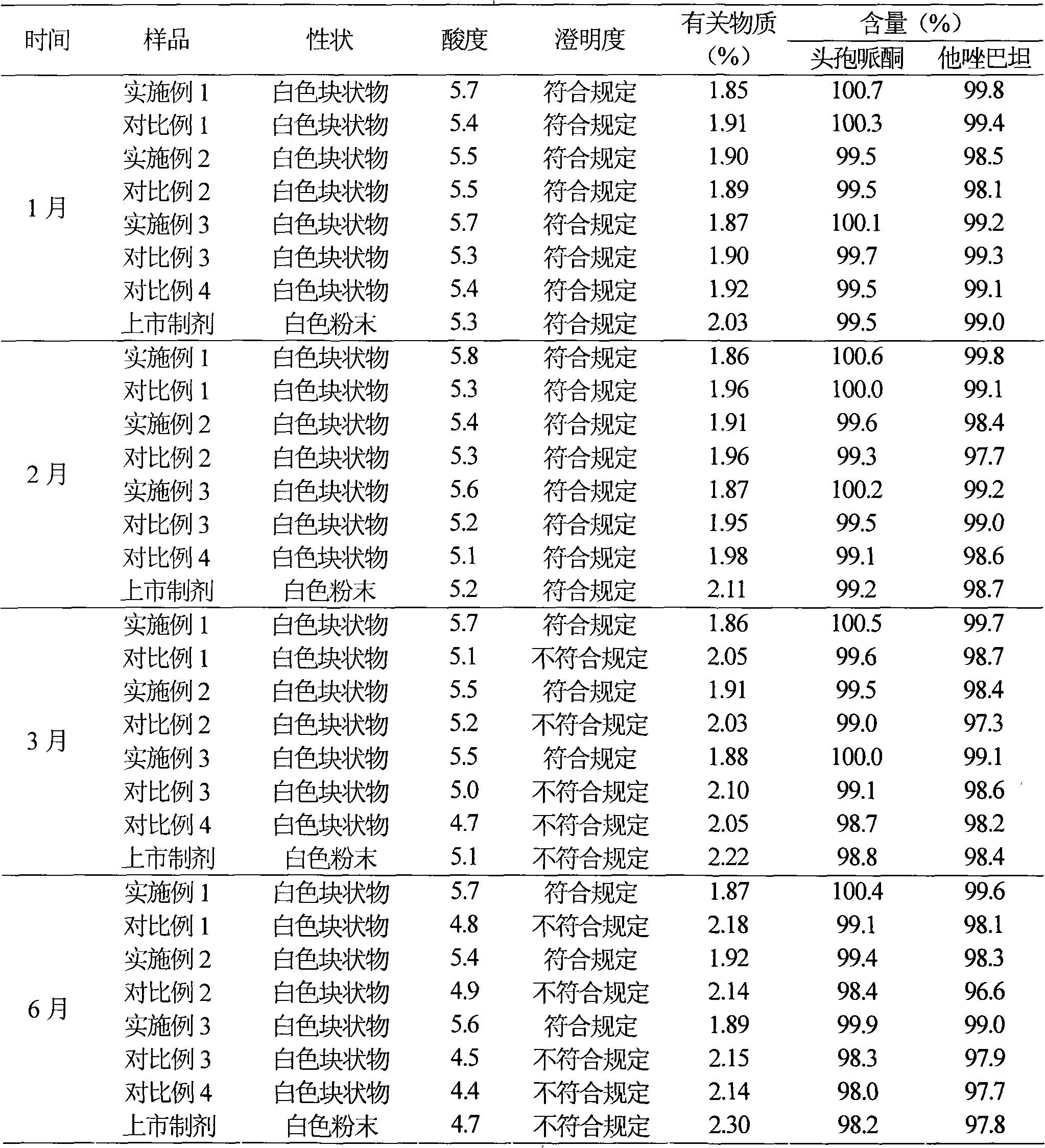
Examples
Embodiment 1
[0040] Example 1 Preparation of cefoperazone sodium tazobactam sodium suspension powder injection
[0041] Prescription (100 bottles):
[0042] Cefoperazone Sodium 160g
[0043] Tazobactam Sodium 40g
[0044] Soy Lecithin 450g
[0045] Poloxamer 188 150g
[0046] Sodium Deoxycholate 200g
[0047] Mannitol 240g
[0048] Trehalose 240g
[0049] Preparation Process
[0050] (1) Add 450g soybean lecithin, 150g poloxamer 188 and 200g sodium deoxycholate to 4500ml water for injection, then add 160g cefoperazone sodium and 40g tazobactam sodium and mix well, heat and stir in a water bath at 70°C until it melts ;
[0051] (2) adopting tissue masher to shear and stir for 20min under the condition of thermal insulation 70-90℃, rotating speed 10000r / min to obtain primary emulsion, and then circulate emulsification by high pressure homogenizer for 4 times to obtain emulsion;
[0052] (3) 240g of mannitol and 240g of trehalose were added to the emulsion, dissolved and then filt...
Embodiment 2
[0064] Example 2 Preparation of cefoperazone sodium tazobactam sodium suspension powder injection
[0065] Prescription (100 bottles):
[0066] Cefoperazone Sodium 80g
[0067] Tazobactam Sodium 20g
[0068] Soy Lecithin 150g
[0069] Poloxamer 188 50g
[0070] Sodium Deoxycholate 60g
[0071] Mannitol 50g
[0072] Trehalose 50g
[0073] Preparation Process
[0074] (1) Add 150g soybean lecithin, 50g poloxamer 188 and 60g sodium deoxycholate to 2000ml water for injection, then add 80g cefoperazone sodium and 20g tazobactam sodium and mix well, heat and stir in a water bath at 80°C until it is molten ;
[0075] (2) adopting the tissue masher to shear and stir for 10min under the condition of keeping the above-mentioned liquid at 70-90°C, the rotating speed is 15000r / min, to obtain the primary emulsion, and then circulate emulsification through a high-pressure homogenizer for 5 times to obtain an emulsion;
[0076] (3) 50g of mannitol and 50g of trehalose were added...
Embodiment 3
[0087] Example 3 Preparation of cefoperazone sodium tazobactam sodium suspension powder injection
[0088] Prescription (100 bottles):
[0089] Cefoperazone Sodium 400g
[0090] Tazobactam Sodium 50g
[0091] Soy Lecithin 750g
[0092] Poloxamer 188 250g
[0093] Sodium Deoxycholate 400g
[0094] Mannitol 500g
[0095] Trehalose 500g
[0096] Preparation Process
[0097] (1) Add 750g soybean lecithin, 250g poloxamer 188 and 400g sodium deoxycholate to 11000ml water for injection, then add 400g cefoperazone sodium and 50g tazobactam sodium and mix well, heat and stir in a 90°C water bath until it melts ;
[0098] (2) adopting the tissue masher to shear and stir for 10 minutes under the condition of keeping the above-mentioned liquid at 70-90 ℃, the rotating speed is 13000r / min, to obtain the primary emulsion, and then circulate emulsification through the high-pressure homogenizer for 5 times to obtain the emulsion;
[0099] (3) adding 500g mannitol and 500g trehalo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com