Cefmetazole-containing and beta-lactamase inhibitor-containing medicinal composition
A technology of lactamase and cefmetazole, which is applied in the field of medicine, can solve the problems of poor stability, insensitivity or drug resistance of AmpC enzyme, achieve good hydrolytic stability, good synergistic effect, and improve the effect of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
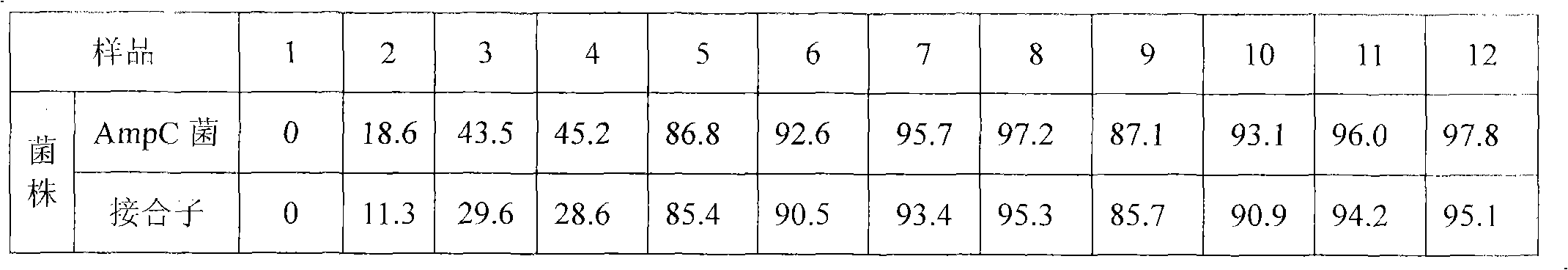
Image
Examples
Embodiment 1
[0037] Embodiment 1 freeze-dried powder injection
[0038] Composition: 100g of cefmetazole sodium, 50g of sulbactam sodium, 100g of mannitol, an appropriate amount of pH regulator, and water for injection to 2000ml.
[0039] Preparation:
[0040] 1), measure cefmetazole sodium and sulbactam sodium according to the formula;
[0041] 2), dissolving medicine and mannitol in water for injection with 80% prescription water consumption, after the solution is aseptically filtered through a 0.22 μm filter membrane, supplementing the prescription amount of water for injection, adjusting pH, filtering, subpackaging, freeze-drying, to obtain .
Embodiment 2
[0042] Embodiment 2 freeze-dried powder injection
[0043] Composition: 100g of cefmetazole sodium, 25g of sulbactam sodium, 100g of mannitol, an appropriate amount of pH regulator, and water for injection to 2000ml.
[0044] Preparation:
[0045] 1), measure cefmetazole sodium and sulbactam sodium according to the formula;
[0046] 2) Dissolving medicine and mannitol in water for injection with 80% of the prescribed water consumption, after the solution is aseptically filtered through a 0.22 μm filter membrane, supplementing the prescribed amount of water for injection, adjusting pH, filtering, subpackaging, and freeze-drying to obtain .
Embodiment 3
[0047] Embodiment 3 freeze-dried powder injection
[0048] Composition: 100g of cefmetazole sodium, 12.5g of sulbactam sodium, 120g of glucose, appropriate amount of pH regulator, and water for injection to 2000ml.
[0049] Preparation
[0050] 1), measure cefmetazole sodium and sulbactam sodium according to the formula;
[0051] 2) Dissolving medicine and glucose in water for injection with 80% of the prescribed water consumption, after the solution is aseptically filtered through a 0.22 μm filter membrane, supplementing the prescribed amount of water for injection, adjusting pH, filtering, subpackaging, and freeze-drying to obtain final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com