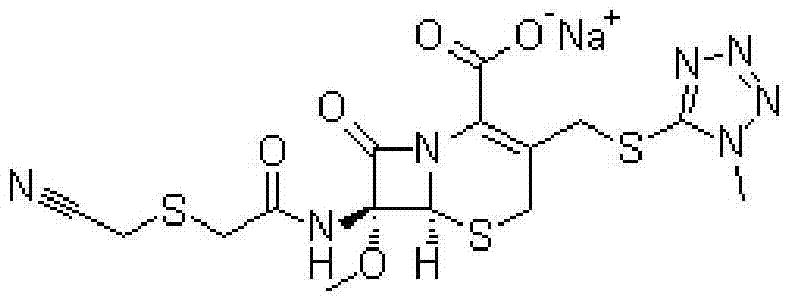
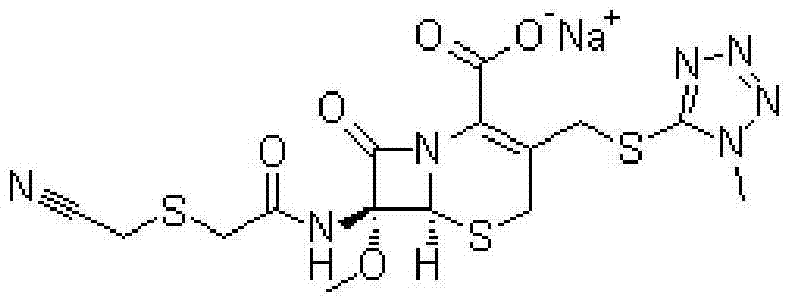
A kind of cefmetazole sodium compound and pharmaceutical composition thereof
A technology of cefmetazole sodium and cefmetazole, which is applied in the field of second-generation cephalosporin compounds and their pharmaceutical compositions, can solve the problem of cefmetazole sodium being unstable to light and heat, and the content of cefmetazole sodium decreases and increases. Adverse reaction opportunities and other issues, to achieve the effect of good distribution in the body, good antibacterial efficacy, and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A pharmaceutical composition of cefmetazole sodium, the pharmaceutical composition is an injection, and the preparation method of the injection consists of the following steps:
[0026] (1) Dissolve 6 g of cefmetazole sodium, 0.05 g of mannitol, 0.2 g of citrulline and 0.1 g of sodium citrate in water for injection, add water to 1000 ml, and stir well;
[0027] (2) Add activated carbon to the solution stirred uniformly in step (1) and stir, adjust pH=6 with sodium hydroxide, filter through membrane fine filter after decarburization, and fill;
[0028] (3) Cool the solution filled in step (2) to freezing rapidly in a freeze dryer, maintain freezing at -40°C to -30°C for 3-5 hours, vacuumize, freeze and vacuum dry for 24 hours, vacuum press Plastic cap, rolled cap, obtains cefmetazole sodium injection.
Embodiment 2
[0030] A pharmaceutical composition of cefmetazole sodium, the pharmaceutical composition is an injection, and the preparation method of the injection consists of the following steps:
[0031] (1) Dissolve 8 g of cefmetazole sodium, 0.2 g of mannitol, 0.7 g of citrulline and 0.5 g of sodium citrate in water for injection, add water to 1000 ml, and stir well;
[0032] (2) Add activated carbon to the solution stirred uniformly in step (1) and stir, adjust pH=6 with sodium hydroxide, filter through membrane fine filter after decarburization, and fill;
[0033] (3) Cool the solution filled in step (2) to freezing rapidly in a freeze dryer, maintain freezing at -40°C to -30°C for 3-5 hours, vacuumize, freeze and vacuum dry for 24 hours, vacuum press Plastic cap, rolled cap, obtains cefmetazole sodium injection.
Embodiment 3
[0035] A pharmaceutical composition of cefmetazole sodium, the pharmaceutical composition is an injection, and the preparation method of the injection consists of the following steps:
[0036] (1) Dissolve 9 g of cefmetazole sodium, 0.08 g of mannitol, 0.5 g of citrulline and 0.25 g of sodium citrate in water for injection, add water to 1000 ml, and stir well;
[0037] (2) Add activated carbon to the solution stirred uniformly in step (1) and stir, adjust pH=6 with sodium hydroxide, filter through membrane fine filter after decarburization, and fill;
[0038] (3) Cool the solution filled in step (2) to freezing rapidly in a freeze dryer, maintain freezing at -40°C to -30°C for 3-5 hours, vacuumize, freeze and vacuum dry for 24 hours, vacuum press Plastic cap, rolled cap, obtains cefmetazole sodium injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com