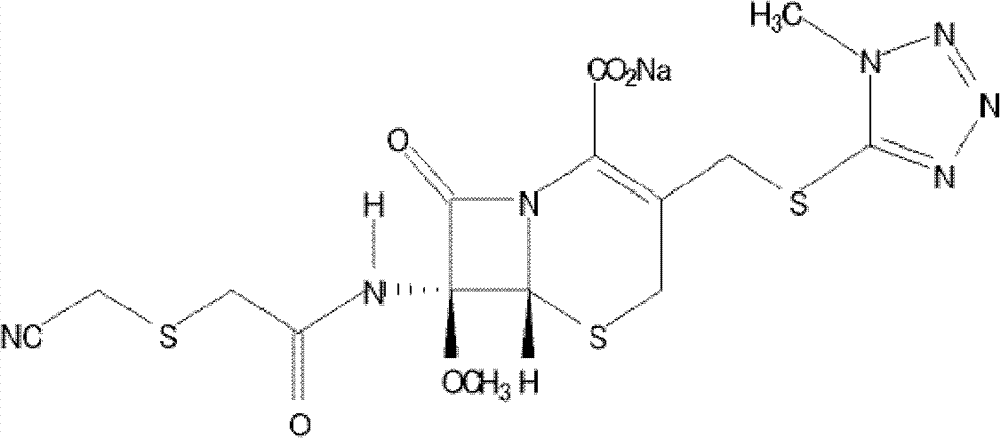
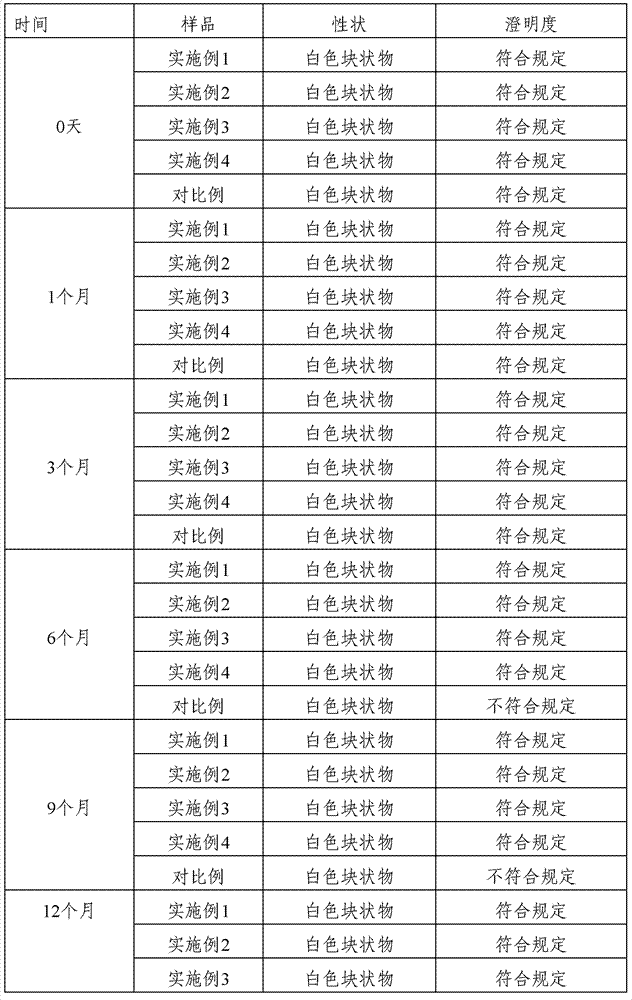
Pharmaceutical composition containing cefmetazole sodium compound, and preparation method thereof
A technology of cefmetazole sodium and cefmetazole acid, which is applied in the field of medicine and can solve problems such as high side effects and allergy rates, and instability of cefmetazole sodium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Prescription: (100 bottles)
[0019] Cefmetazole Acid 25g
[0021] Mannitol 0.1g
[0022] Preparation Process
[0023] 1) Dissolve the prescribed amount of cefmetazole acid, sodium carbonate and mannitol in water for injection, add water to 1000ml, and stir at 150r / min to make the reaction complete;
[0024] 2) Add activated carbon to the above-mentioned fully reacted solution and stir, adjust the pH to 6 with sodium hydroxide, filter to remove carbon, then filter through a filter membrane, and fill;
[0025] 3) Cool down the above-mentioned filled solution rapidly to freezing in a freeze dryer, maintain freezing at -40°C for 3 hours, vacuumize, slowly heat up to below the eutectic point, and vacuum-dry until the vacuum degree is maintained at 5-10pa Continue to heat up while heating up to 25° C. within 2 hours, and maintain until the degree of vacuum no longer changes to obtain a freeze-dried powder. Example 2
Embodiment 2
[0026] Prescription: (100 bottles)
[0027] Cefmetazole Acid 25g
[0028] Sodium carbonate 2.53g
[0029] Mannitol 0.2g
[0030] 1) Dissolve the prescribed amount of cefmetazole acid, sodium carbonate and mannitol in water for injection, add water to 1000ml, and stir at 190r / min to make the reaction complete;
[0031] 2) Add activated carbon to the above-mentioned fully reacted solution and stir, adjust the pH to 6 with sodium hydroxide, filter to remove carbon, then filter through a filter membrane, and fill;
[0032] 3) Rapidly cool down the above-mentioned filled solution to freezing in a freeze dryer, maintain freezing at -30°C for 5 hours, vacuumize, slowly raise the temperature to below the eutectic point, and vacuum-dry until the vacuum degree is maintained at 5-10pa Continue to heat up while heating up to 30° C. within 4 hours, and maintain until the degree of vacuum no longer changes to obtain a freeze-dried powder.
Embodiment 3
[0034] Prescription: (100 bottles)
[0035] Cefmetazole Acid 25g
[0036] Sodium carbonate 2.53g
[0037] Mannitol 0.3g
[0038] Preparation Process
[0039] 1) Dissolve the prescribed amount of cefmetazole acid, sodium carbonate and mannitol in water for injection, add water to 1000ml, and stir at 160r / min to make a complete reaction;
[0040] 2) Add activated carbon to the above-mentioned fully reacted solution and stir, adjust the pH to 6 with sodium hydroxide, filter to remove carbon, then filter through a filter membrane, and fill;
[0041] 3) Cool down the above-mentioned filled solution rapidly to freezing in a freeze dryer, maintain freezing at -35°C for 4 hours, vacuumize, slowly raise the temperature to below the eutectic point, and vacuum-dry until the vacuum degree is maintained at 5-10pa Continue to heat up while heating up to 30° C. within 3 hours, and maintain until the degree of vacuum no longer changes to obtain a freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com