Preparation method for injection cefoperazone sodium tazobactam sodium composition
A technology of cefoperazone sodium and tazobactam sodium, which is applied in the direction of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, and pharmaceutical formulations, and can solve the problem of losing antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
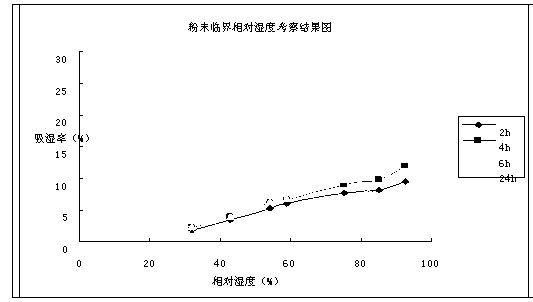
Image
Examples
Embodiment 1
[0014] Specification: 1.0g (Example 1a)
[0015] prescription:
[0016] Cefoperazone sodium 800g (calculated as cefoperazone)
[0017] Tazobactam sodium 200g (calculated as tazobactam)
[0018]
[0019] Makes 1000 bottles
[0020] Specification: 2.0g (Example 1b)
[0021] prescription:
[0022] Cefoperazone sodium 1600g (calculated as cefoperazone)
[0023] Tazobactam sodium 400g (calculated as tazobactam)
[0024]
[0025] Makes 1000 bottles
[0026] Preparation:
[0027] 1. Grind cefoperazone sodium and tazobactam sodium to 80 meshes respectively, weigh the two raw materials according to the prescription amount, and set aside;
[0028] 2. Add the above two raw materials into the mixer and mix them evenly, and detect the content of the two components;
[0029] 3. Dispensing, control the filling volume between 97% and 103% of the marked filling volume, divide 1.0g into 10ml low borosilicate glass control injection bottle (Example 1a), and divide 2.0g into 25ml l...
Embodiment 2
[0036] Specification: 1.0g
[0037] prescription:
[0038] Cefoperazone sodium 800g (calculated as cefoperazone)
[0039] Tazobactam sodium 200g (calculated as tazobactam)
[0040]
[0041] Makes 1000 bottles
[0042] Preparation:
[0043] 1. Grind cefoperazone sodium and tazobactam sodium to 60 meshes respectively, weigh the two raw materials according to the prescription amount, and set aside;
[0044] 2. Add the above two raw materials into the mixer and mix them evenly, and detect the content of the two components;
[0045] 3. Divide into 10ml low-borosilicate glass control injection bottles, control the filling volume between 97% and 103% of the marked filling volume, press the stopper and tie the cap;
[0046] 4. Paste the inner label and pack according to 1 bottle / box;
[0047] 5. After passing the inspection, the finished product will be obtained.
[0048] Note: The above steps 1~3 require the ambient humidity to be controlled within 45%.
Embodiment 3
[0050] Specification: 1.0g
[0051] prescription:
[0052] Cefoperazone sodium 800g (calculated as cefoperazone)
[0053] Tazobactam sodium 200g (calculated as tazobactam)
[0054]
[0055] Makes 1000 bottles
[0056] Preparation:
[0057] 1. Grind cefoperazone sodium and tazobactam sodium to 100 mesh respectively, weigh the two raw materials according to the prescription amount, and set aside;
[0058] 2. Add the above two raw materials into the mixer and mix them evenly, and detect the content of the two components;
[0059] 3. Divide into 10ml low-borosilicate glass control injection bottles, control the filling volume between 97% and 103% of the marked filling volume, press the stopper and tie the cap;
[0060] 4. Paste the inner label and pack according to 1 bottle / box;
[0061] 5. After passing the inspection, the finished product will be obtained.
[0062] Note: The above steps 1~3 require the ambient humidity to be controlled within 45%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com