Patents
Literature
162 results about "Meropenem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
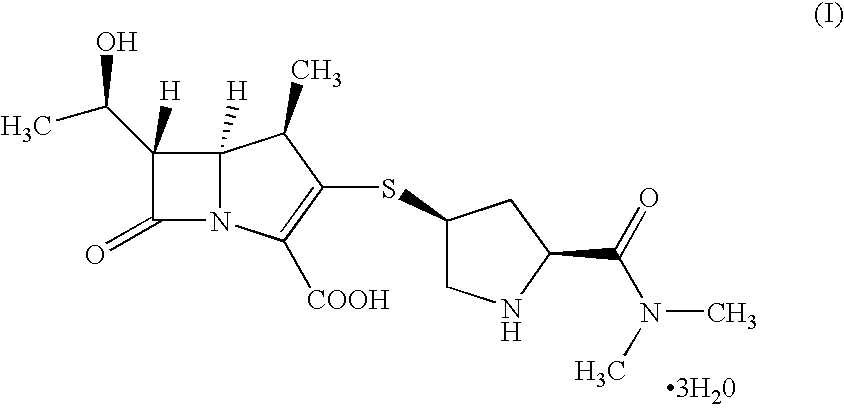
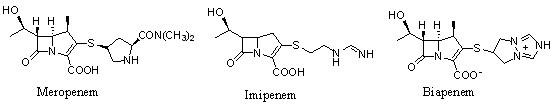
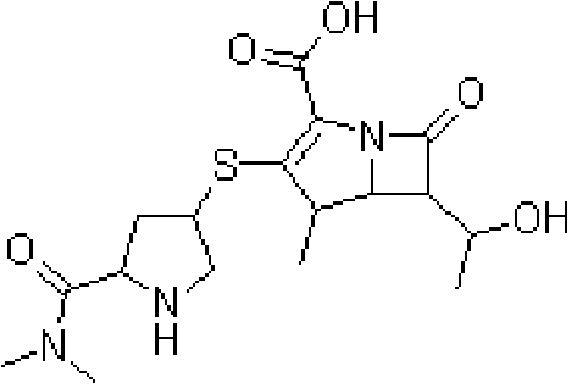
Meropenem is used to treat a wide variety of bacterial infections.
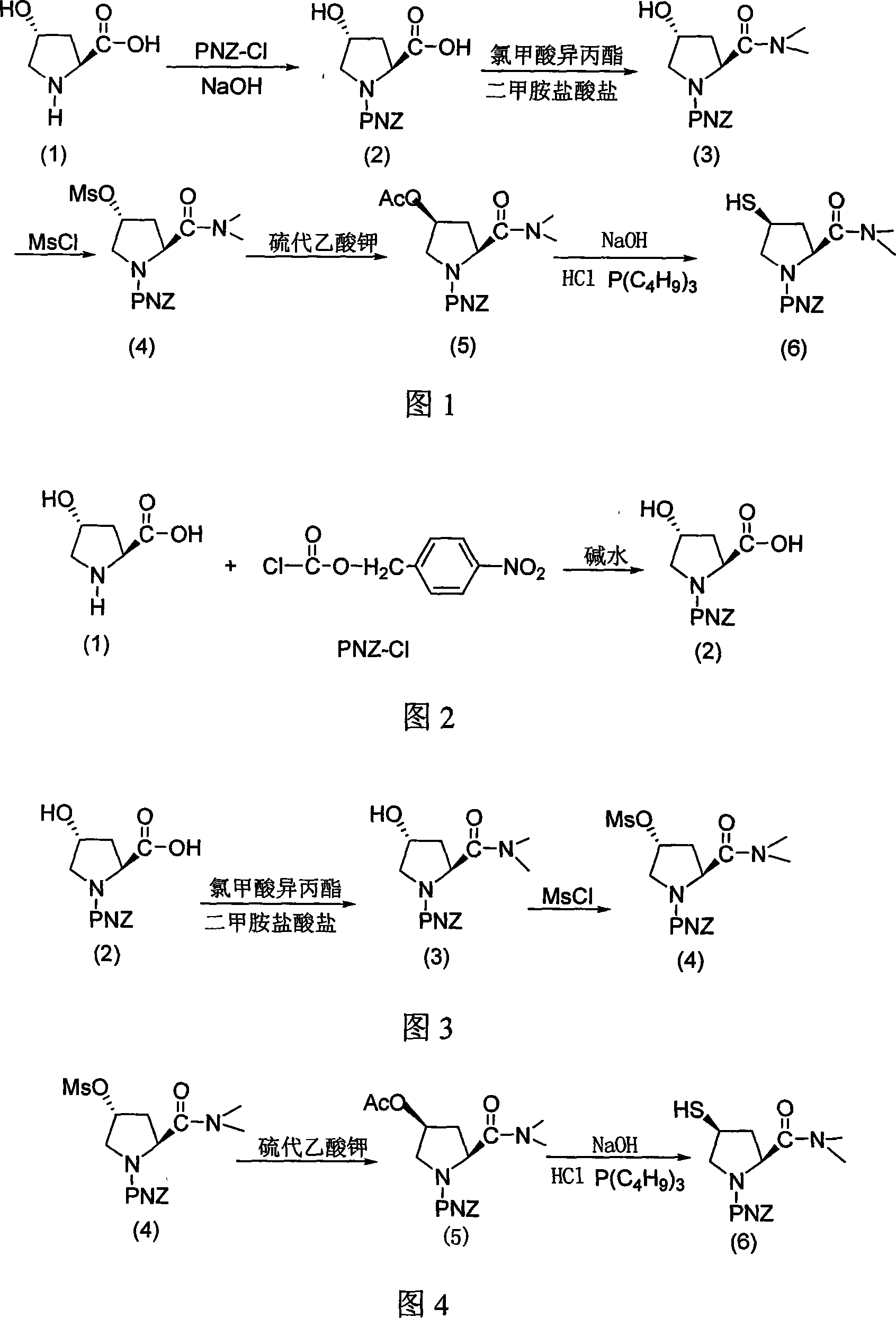
Preparation method of meluopeinan
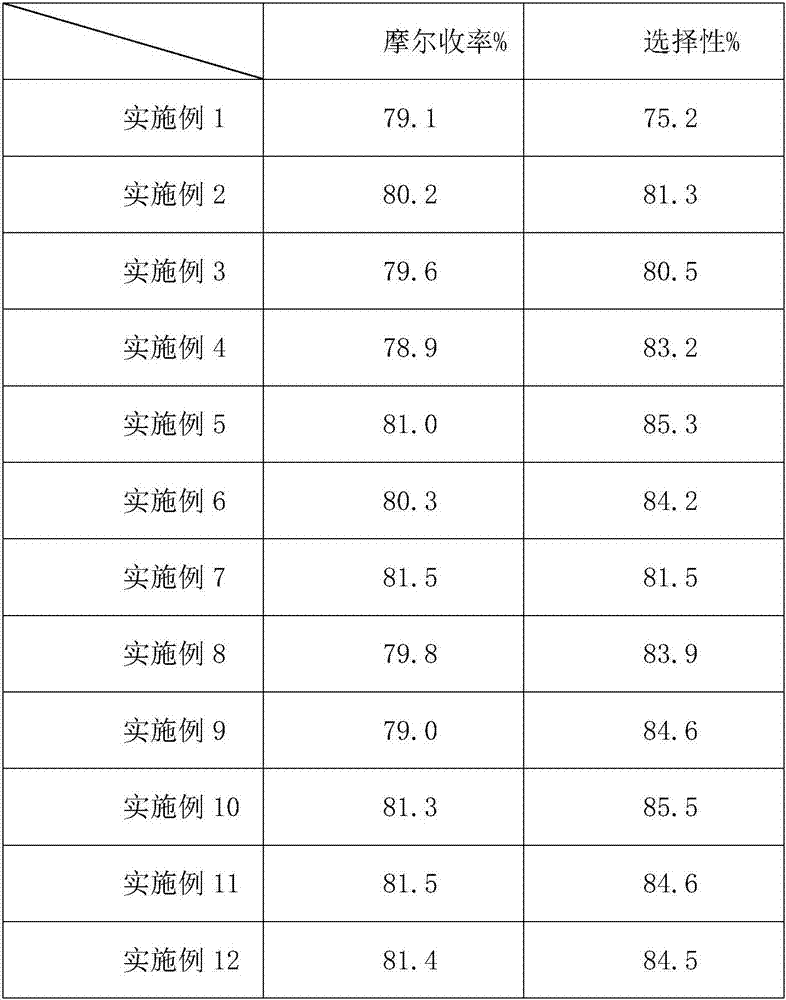
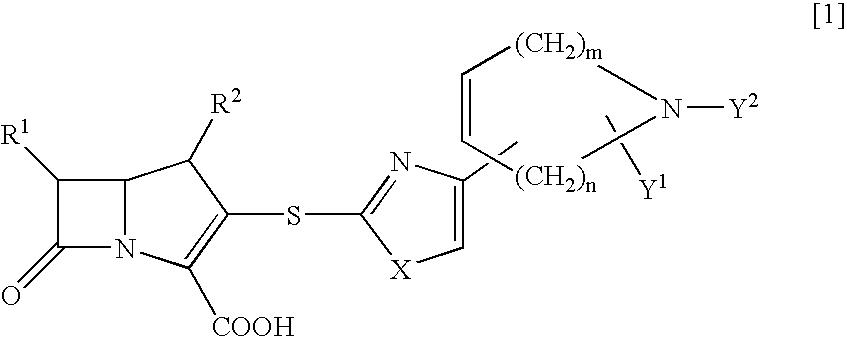
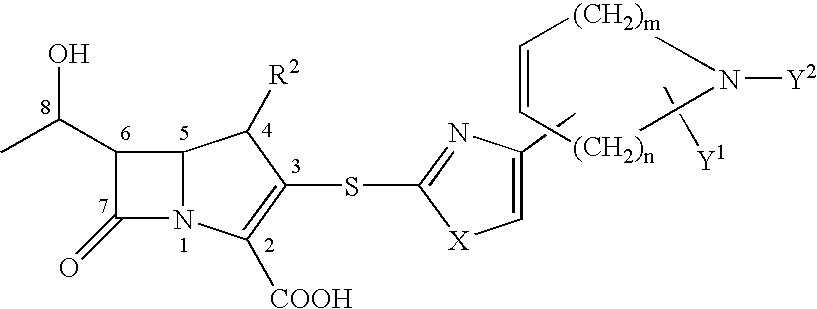
InactiveCN1948312AReduce typesReduce the use effectAntibacterial agentsOrganic chemistryState of artChemical structure
The present invention relates to a preparation method of beta-methylcarbapenem antibiotic-meropenem. Said invention provides its chemical structure formula, and concrete steps of its preparation method.
Owner:SHENZHEN HAIBIN PHARMA +1
Process for The Preparation of Beta-Lactam Antibiotic
InactiveUS20090264643A1Simple and viable processSimple processAntibacterial agentsOrganic chemistryMeropenemAntibiotics beta lactam
Owner:ORCHID CHEM & PHARM LTD
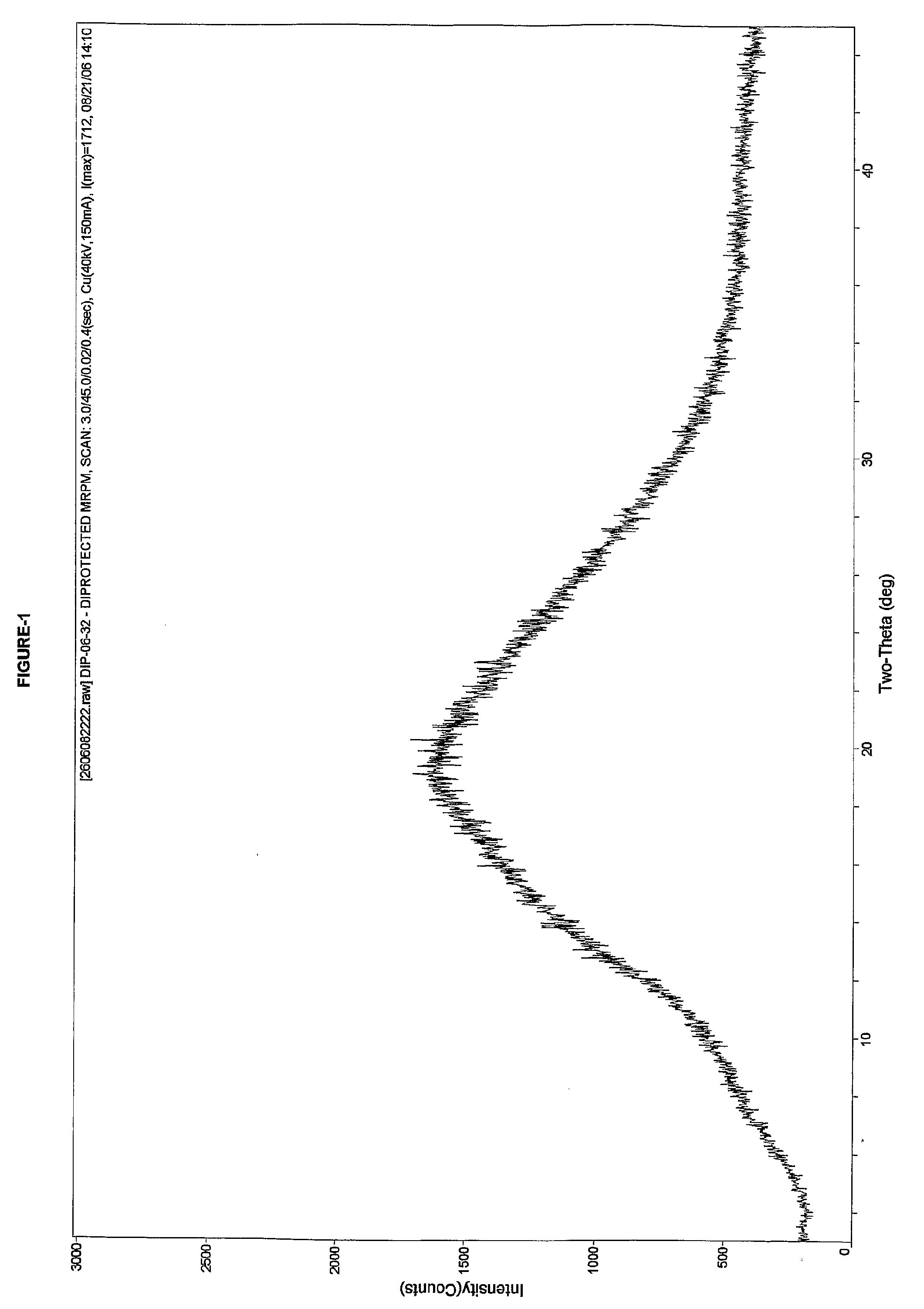
Crystallization refining method of Meropenem
ActiveCN101921276AIncrease the amount of feedIncrease production capacityOrganic chemistryOrganic solventAlcohol
The invention relates to a crystallization refining method of Meropenem, which has low impurity content. The crystallization refining method of Meropenem comprises the following steps: a) dissolving Meropenem crude into methyl alcohol; b) decoloring, decarbonizing, degerming and filtering; c) adding the mixture of water and precipitator into filtrate, and precipitating, wherein the precipitator is the one or the mixture of two-four types of C2-C6 alcohol, C3-C8 ketone, C2-C8 ether or tetrahydrofuran at any ratio, and the volume ratio of water to precipitator is 1:3-1:5; d) filtering to obtain crystallization; e) washing the crystallization by mixed solvent of water and organic solvent of alcohol and esters; and f) drying to obtain the finished product. The invention utilizes the characteristic that Meropenem is easy to dissolve in methyl alcohol, and a small quantity of methyl alcohol can cause Meropenem to dissolve at normal temperature; same equipment can increase inventory for multiple times if being compared with the prior art, and production capability is obviously improved. Because a small quantity of methyl alcohol is used, a great quantity of crystallization solvent can be added, crystallization is complete, yield is high, and impurities can be thoroughly removed.
Owner:JIANGSU DESANO PHARMA
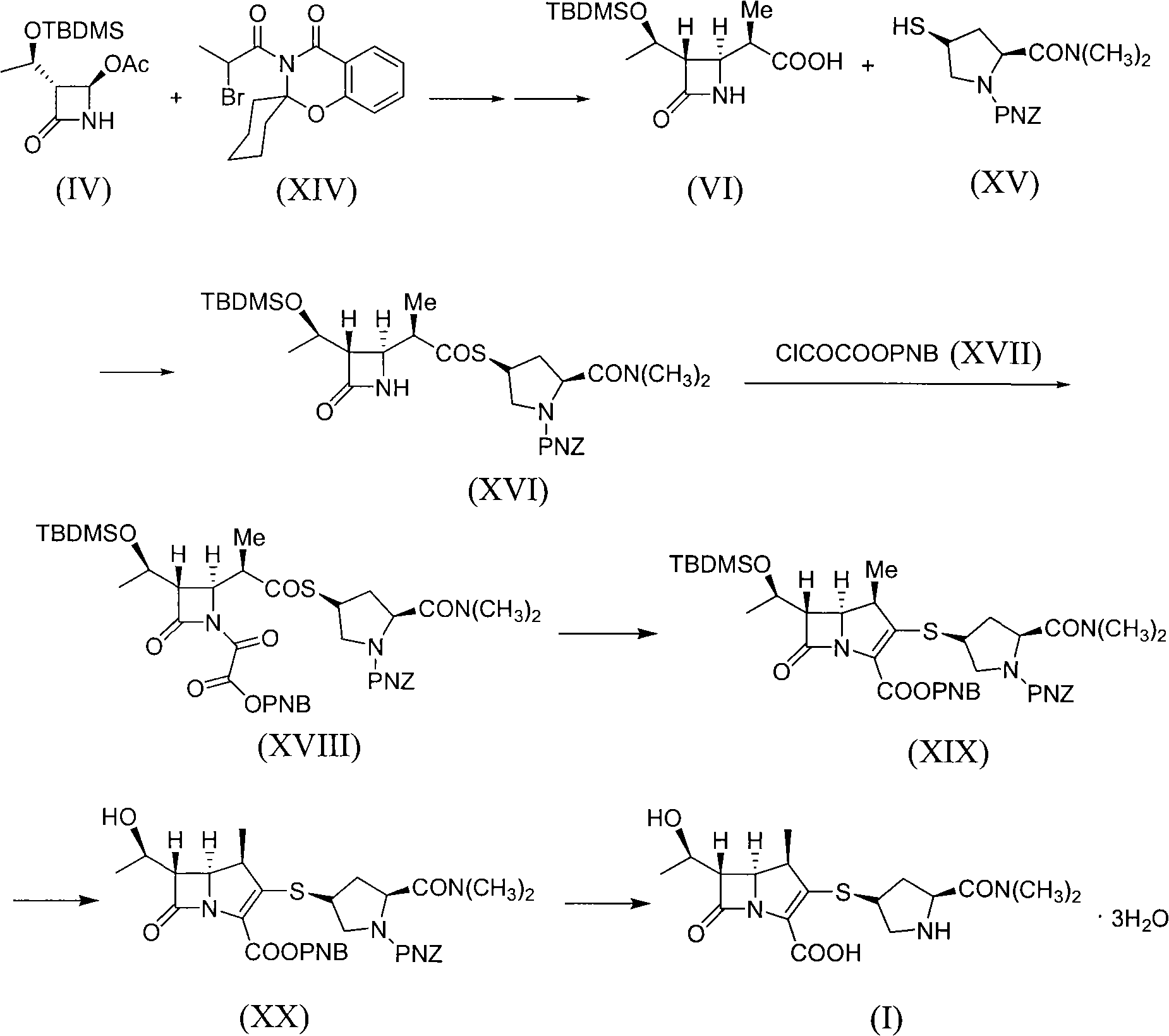
Preparation of meropenem
ActiveCN101348486AReduce usageReduce pollutionAntibacterial agentsOrganic chemistryHydrogenMeropenem
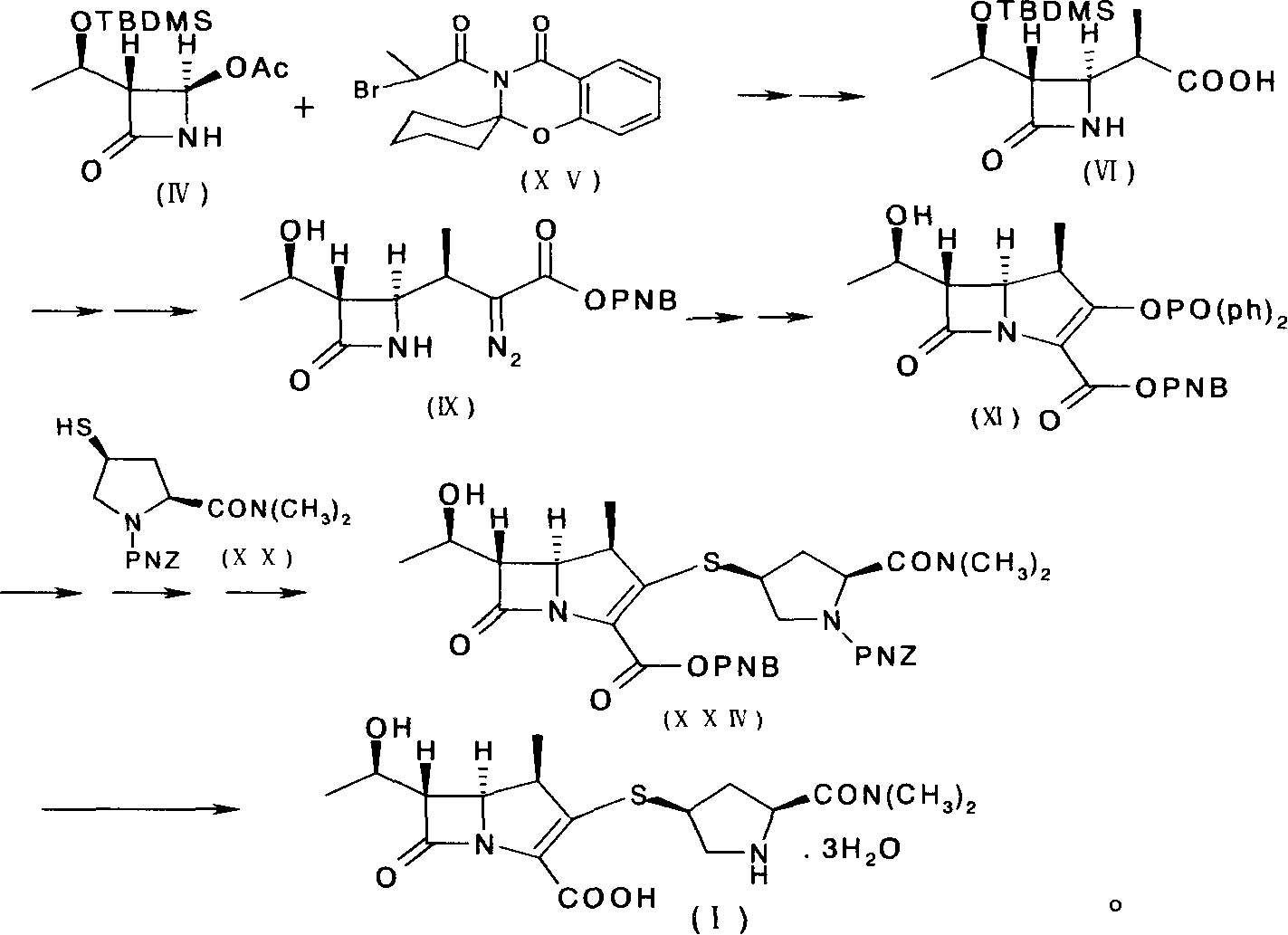
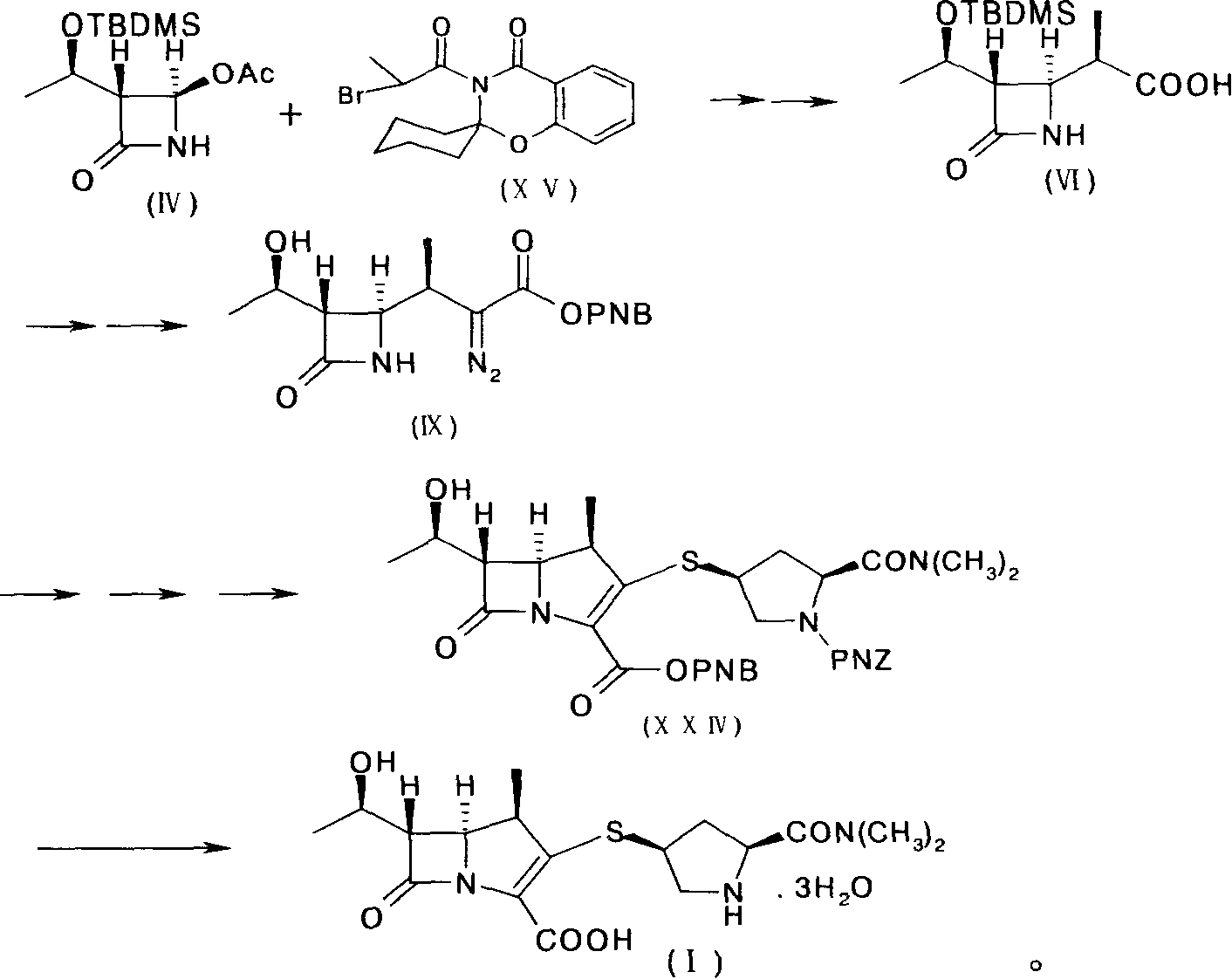
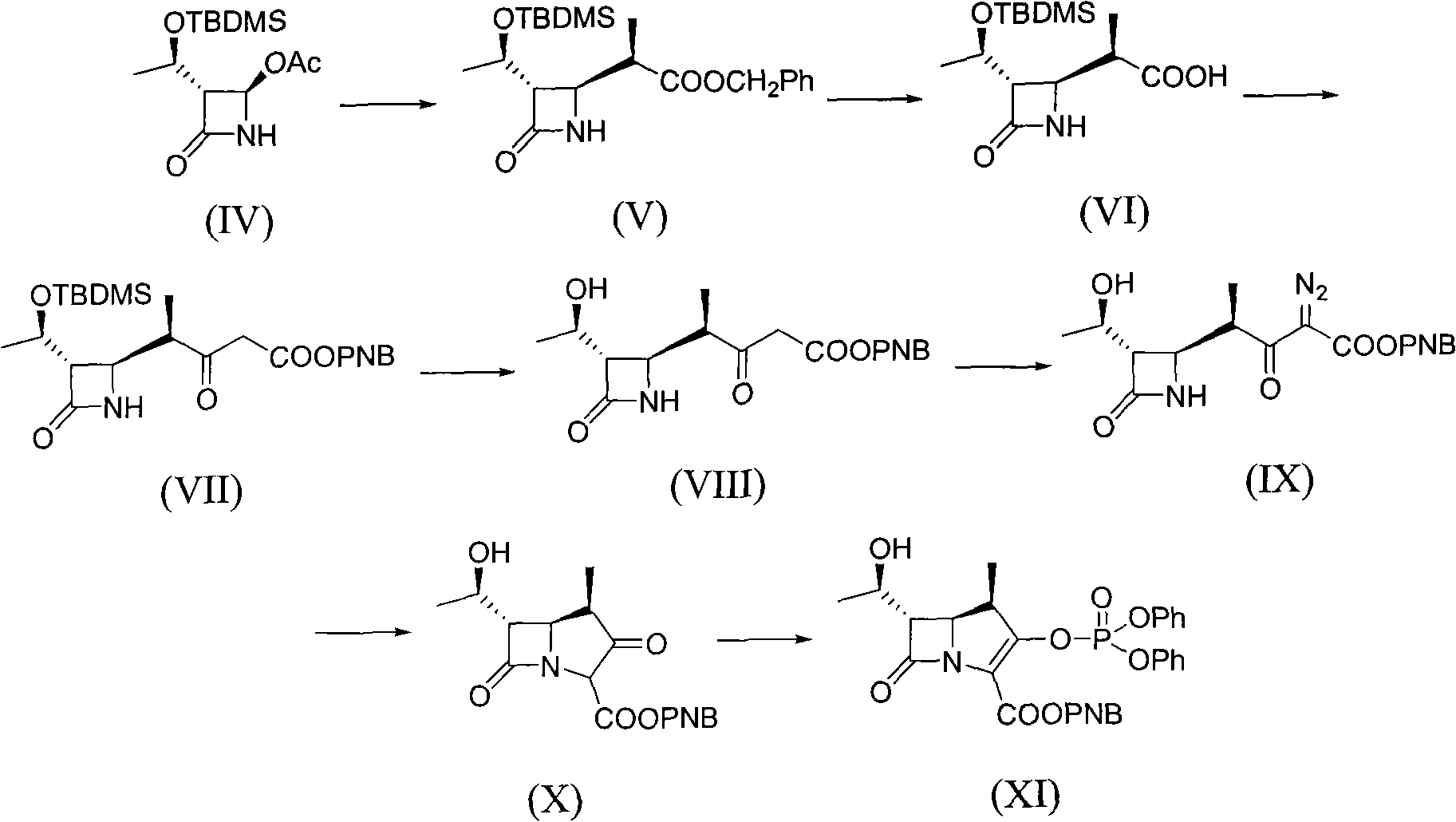
The invention provides a method for preparing Meropenem. The method comprises the following steps that: 1) a compound shown in formula (IV) reacts with a compound shown in formula (XIV) to produce a compound with the formula (VI); 2) the compound shown in formula (VI) reacts with a compound shown in formula (XV) to produce a compound shown in formula (XVI), 3) the compound XVI reacts with a compound shown in formula XVII to obtain a compound shown in formula XVIII, 4) the compound shown in formula XVIII is used to prepare a compound shown in the formula XIX; 5) the compound shown in the formula XIX is hydrolyzed to obtain a compound and; and 6) the compound shown in formula XX is deoxidized under the action of catalysts and hydrogen to produce Meropenem shown in formula (I). The method adopted by the invention has shorter synthetic procedures, simplified operation, simple raw material with easy access, and no requirement on the catalyst of precious metal of rhodium.
Owner:SHENZHEN HAIBIN PHARMA
Palladium tin carbon catalyst for meropenem synthesis and preparation method
InactiveCN102133527AHigh activityIncrease profitOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsMeropenemBULK ACTIVE INGREDIENT
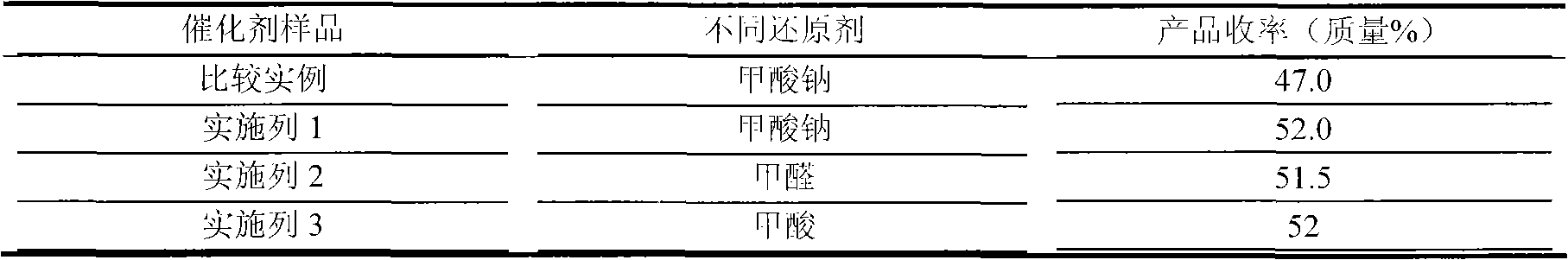
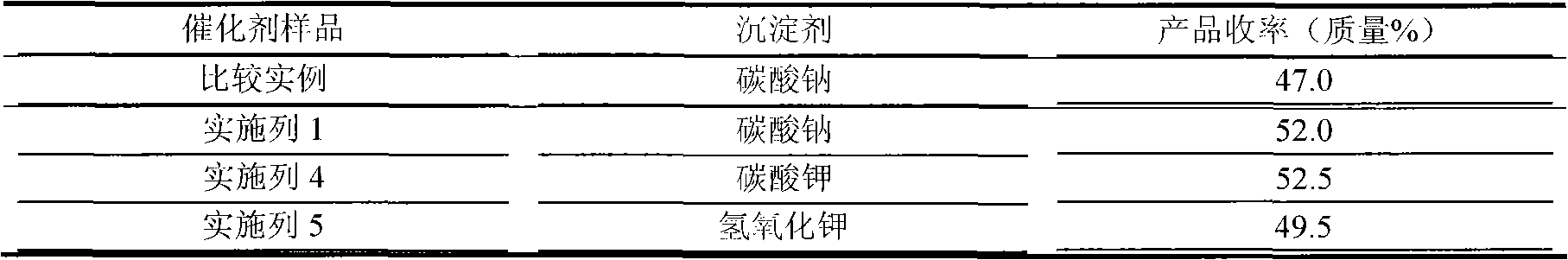
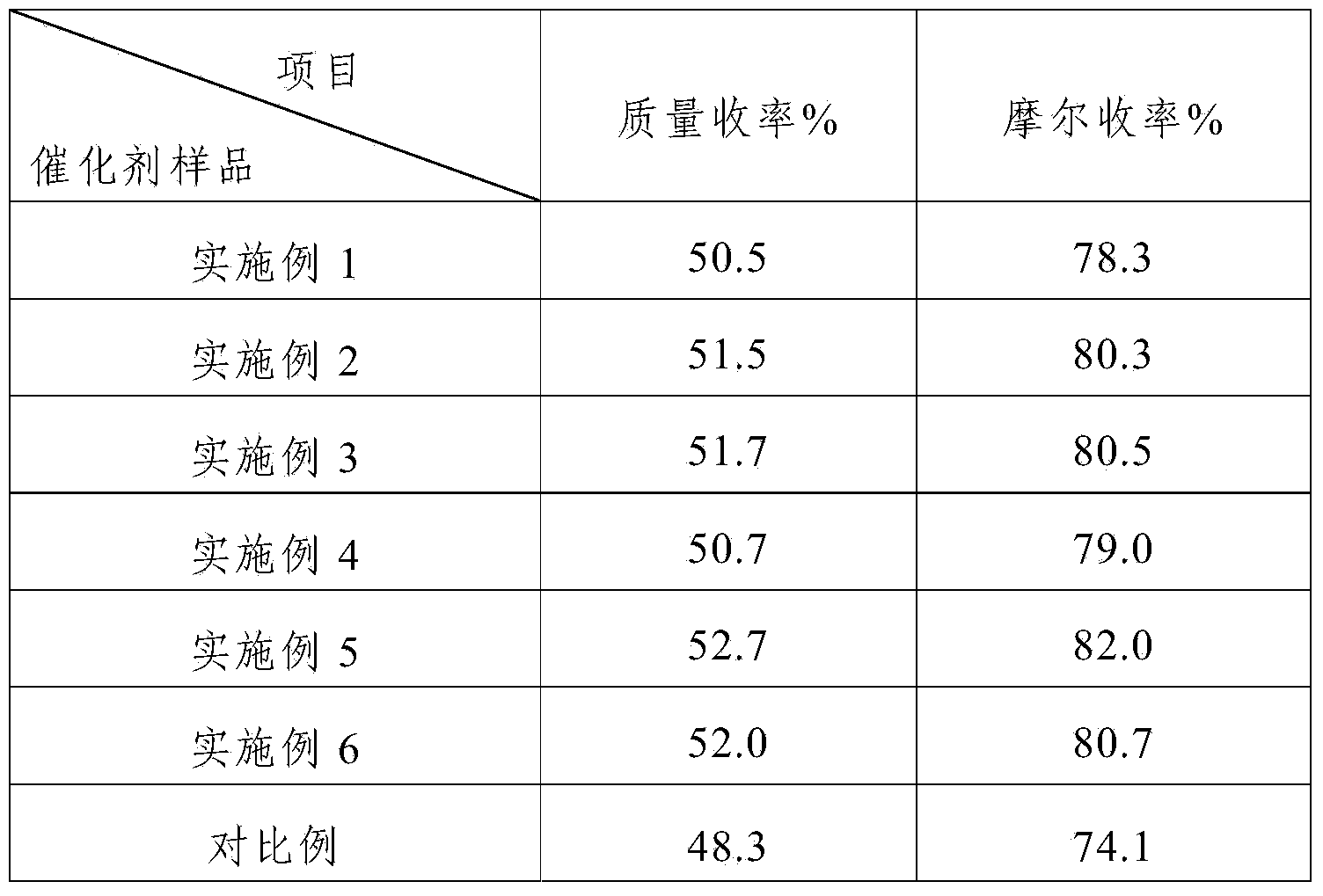
The invention discloses a palladium carbon catalyst for meropenem synthesis and a preparation method. In the catalyst, powdery active carbon is used as a carrier and loads active ingredient Pd metal and active ingredient Sn. The preparation method comprises the following steps of: after the active carbon carrier is sequentially treated by using inorganic acid and sodium hypochlorite, washing the active carbon carrier to be neutral for later use by using pure water; preparing active ingredient solution by using water-soluble Pd containing compound and diluted hydrochloric acid solution of stannous chloride in a ratio; pulping the active carbon of metered weight by using aqueous solution of alkali compound with certain concentration, heating the pulp to the temperature of between 40 and 65 DEG C with stirring and stabilizing the pulp for 1 hour; and guiding the mixed solution of Pd and Sn in a metering ratio into the active carbon pulp, continually stirring the solution for 3 to 6 hours to load the Pd and the Sn on the active carbon to obtain a catalyst precursor, ageing the catalyst precursor, and reducing the catalyst precursor by using a reducing agent to obtain a catalyst product. The palladium carbon noble metal content can be reduced to below 5 percent from 10 percent, the yield of the product is improved by 3 to 4 percent, and the using cost is effectively reduced when the yield of the product is improved.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Preparation method of palladium-carbon catalyst for synthesizing meropenem
ActiveCN103894190AReduce consumptionLow operating environment requirementsMetal/metal-oxides/metal-hydroxide catalystsMeropenemSlurry
The invention discloses a preparation method of a palladium-carbon catalyst for synthesizing meropenem. The preparation method comprises the following steps: (1) putting wood charcoal into a boiling alkali compound water solution, carrying out backflow treatment, washing by virtue of pure water, and drying to obtain an active carbon carrier; (2) preparing an active ingredient solution, and regulating the pH value of the active ingredient solution; (3) pulping the active carbon carrier, and stabilizing the active carbon carrier at a stirring condition, so as to obtain active carbon slurry; (4) adding the active ingredient solution into the active carbon slurry after the pH value of the active ingredient solution is regulated, and stirring to obtain a catalyst precursor; and (5) aging the catalyst precursor, and reducing the catalyst precursor by virtue of a reducing agent, so as to obtain a catalyst finished product. According the preparation method, a low-content alkali compound is utilized for processing the carrier so as to obtain a proper carrier with a reasonable surface chemical structure, so that the consumption of a reagent is reduced; chelated palladium ions of a certain size are prepared by regulating the pH value of the active component solution, so that purposes of controlling the activity and selectivity of the catalyst are realized; the preparation method has no special control point, is simple in process and is beneficial to the industrial large scale production.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Preparation method of meropenem
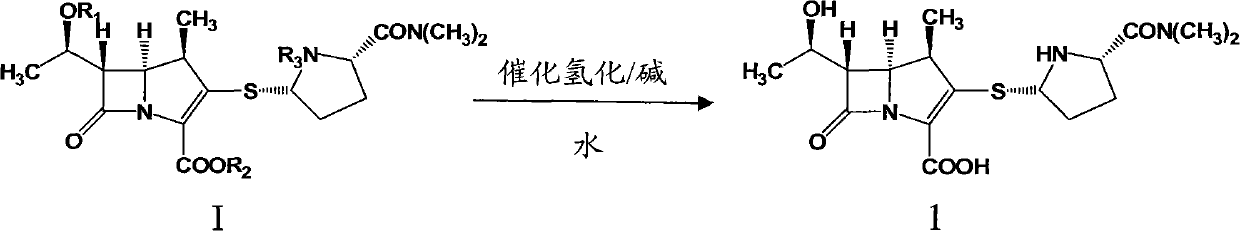
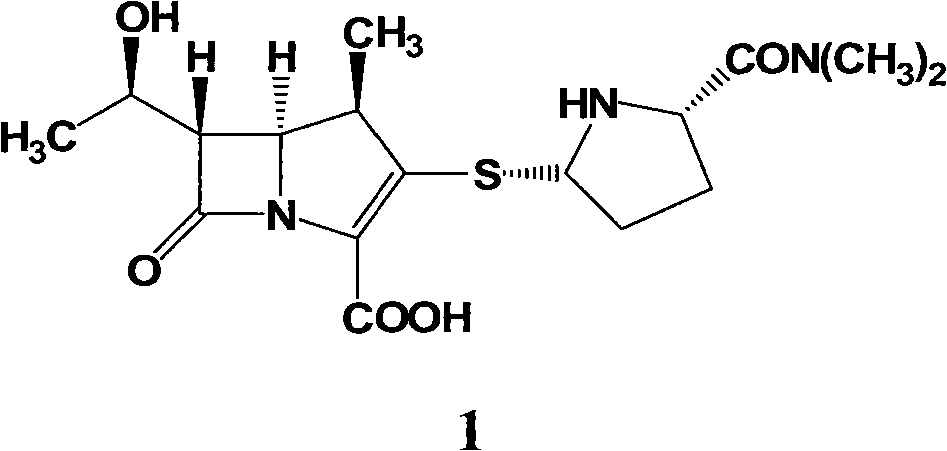
InactiveCN102731504ASolve dissolveReduce degradationOrganic chemistryBulk chemical productionMeropenemSolvent
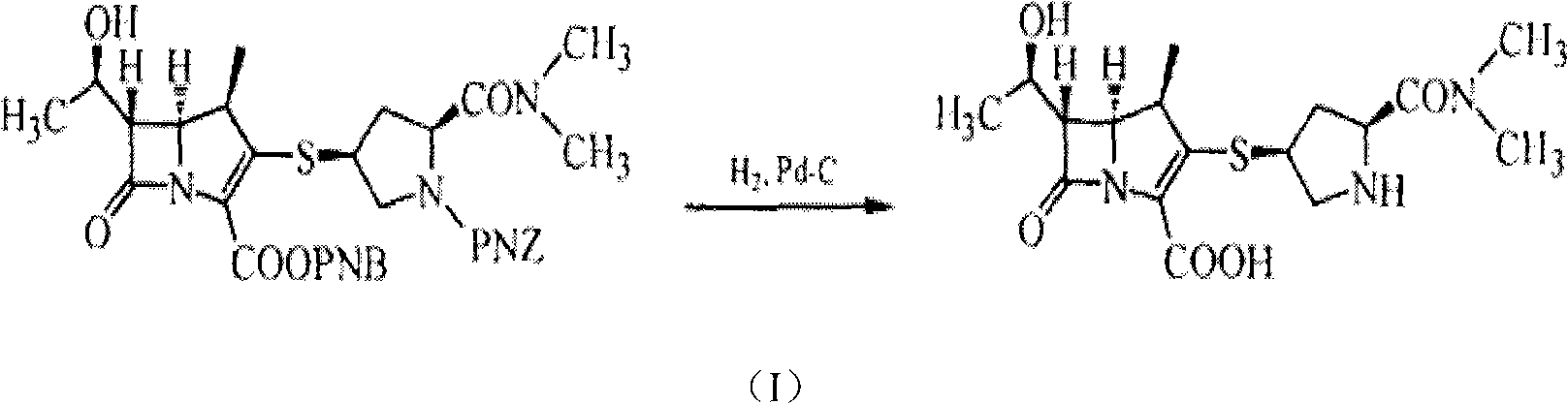
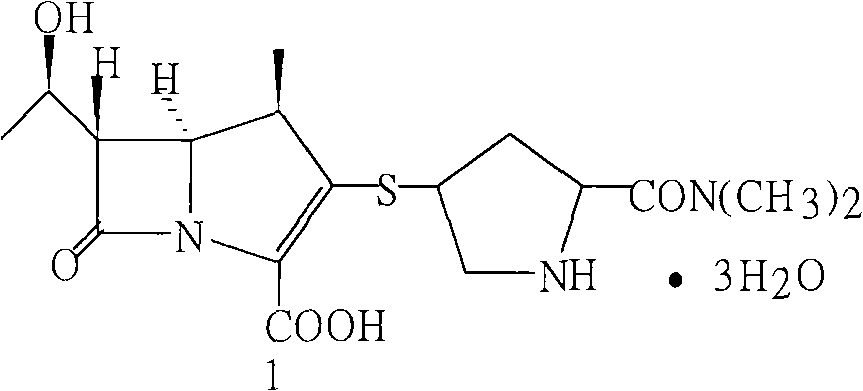
The invention relates to a preparation method of meropenem as shown in the formula 1 and its hydrate. According to the method, a meropenem intermediate I is used as a raw material and a single solvent water is used as a reaction solvent to perform a hydrogenated deprotection reaction in the presence of alkali and a catalyst. The single solvent water is used as a reaction solvent in the method provided by the invention, thus solving the problem of dissolving the catalyst by the reaction solvent, reducing product degradation and raising product purity. In addition, the preparation method is economical, safe and environmentally friendly, and is more suitable for industrial operation at large scale.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
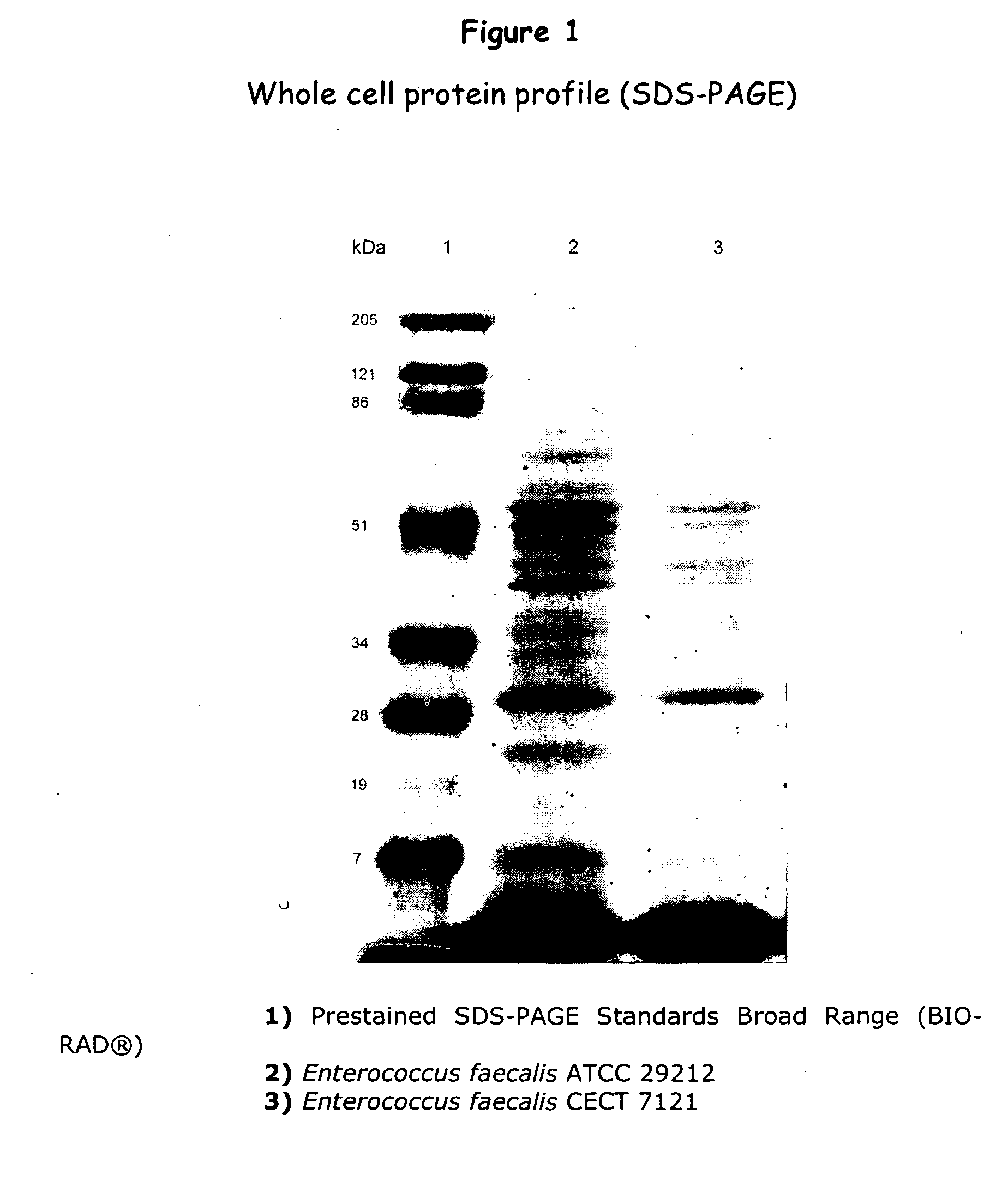
New lactic bacteria useful as probiotics
InactiveUS20080063666A1Pleasant flavorHealthy digestion and intestineBiocideBacterial antigen ingredientsDiseaseAmpicillin
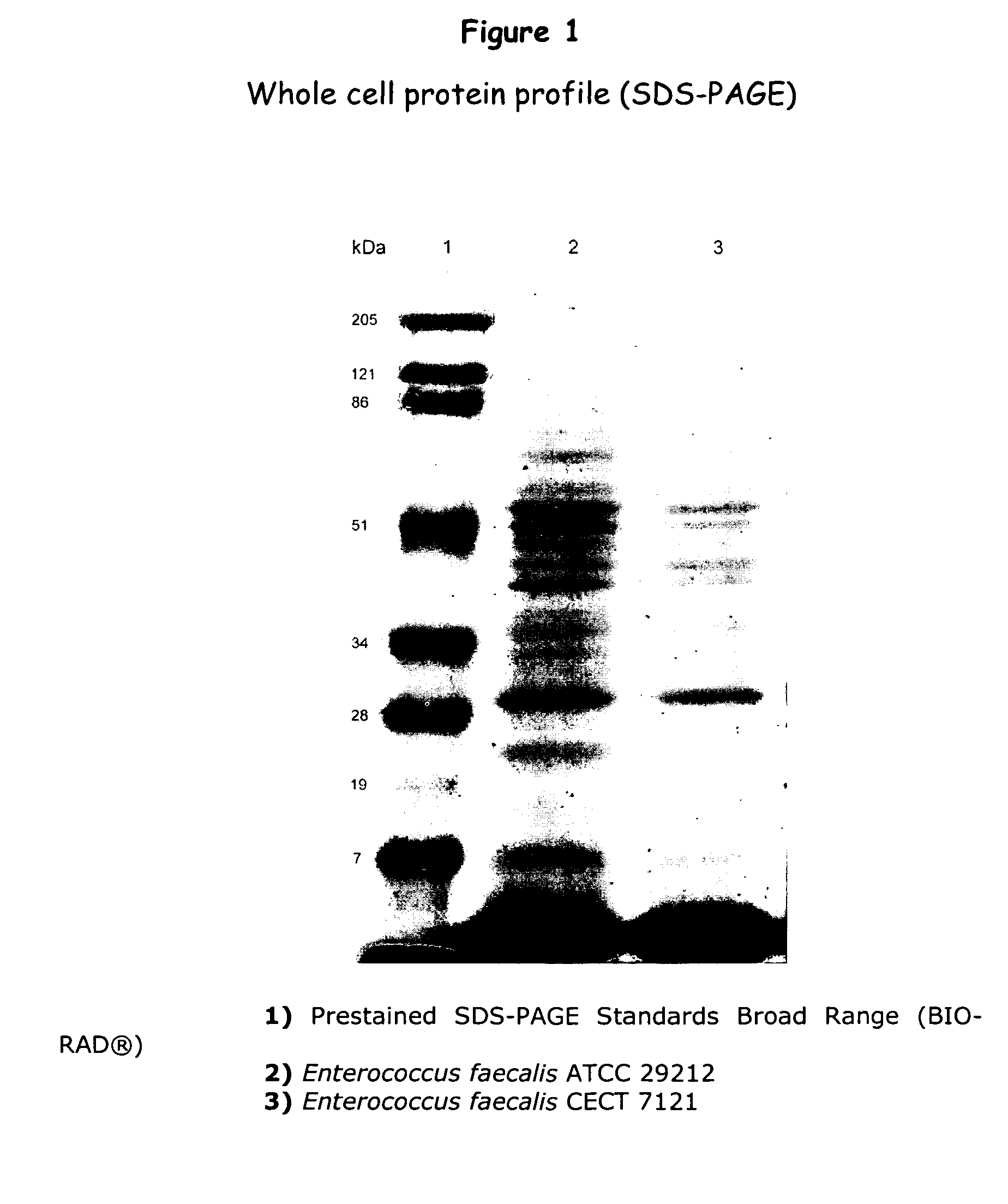
An isolated strain of Enterococcus faecalis GALT deposited under number C E C T 7121 of the group of lactic bacteria is disclosed, which is capable of surviving and colonizing the gastrointestinal tract of humans and / or animals and showing beneficial probiotic activity for the health of humans and animals. The strain E. faecalis GALT and / or a culture supernatant and / or metabolites thereof shows no in vitro multiresistance to antibiotics of common use in human clinics as glycopeptides, such as vancomycin, teicoplanine; carbapenemes, such as impipenem, meropenem; and ampicillin. The strain E. faecalis GALT contains no red blood cell-destroying hemolysins of human, ovine and equine origin; and it does not produce any gelatinase, DNase and decarboxylases. The strain E. faecalis GALT is useful for the preparation of a composition intended for the treatment and / or prophylaxis of disorders associated with colonization by pathogenic microorganisms of the gastrointestinal tract; for use as a regulator of the immune response in human and animals, as well as for the preparation of a composition. The invention is also directed to methods and uses of the strain E. faecalis GALT.
Owner:ALLENDE MIGUEL ANGEL GARCIA
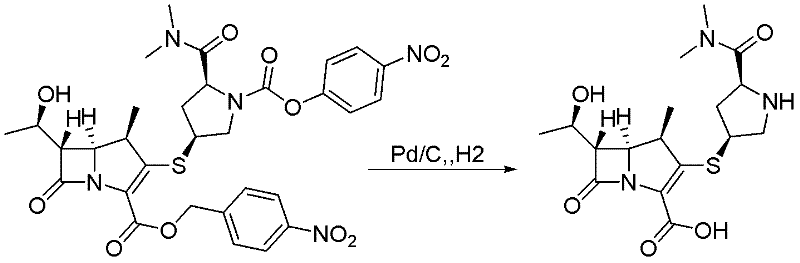
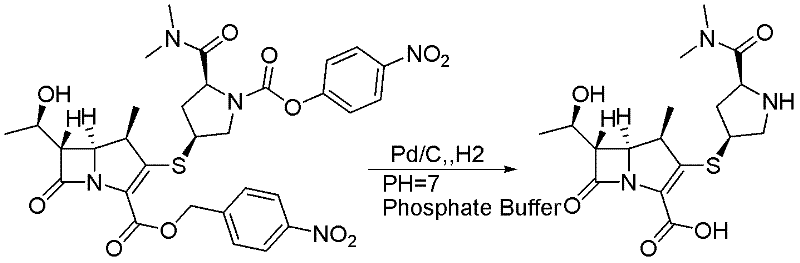
Method for preparing meropenem
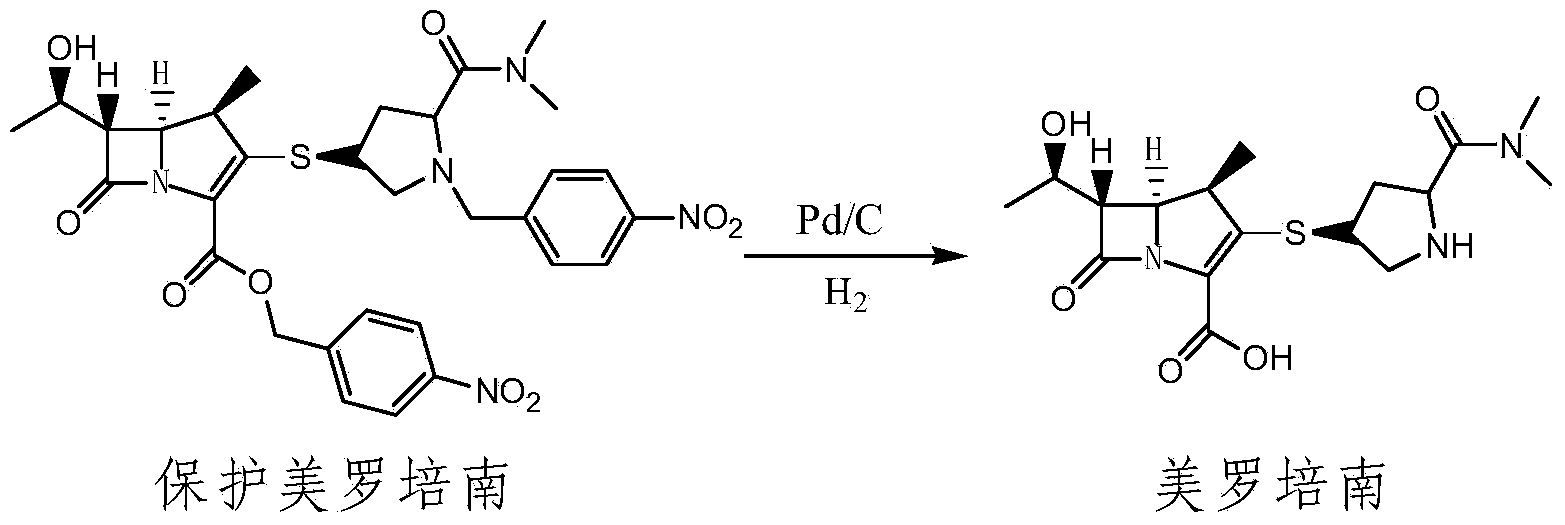
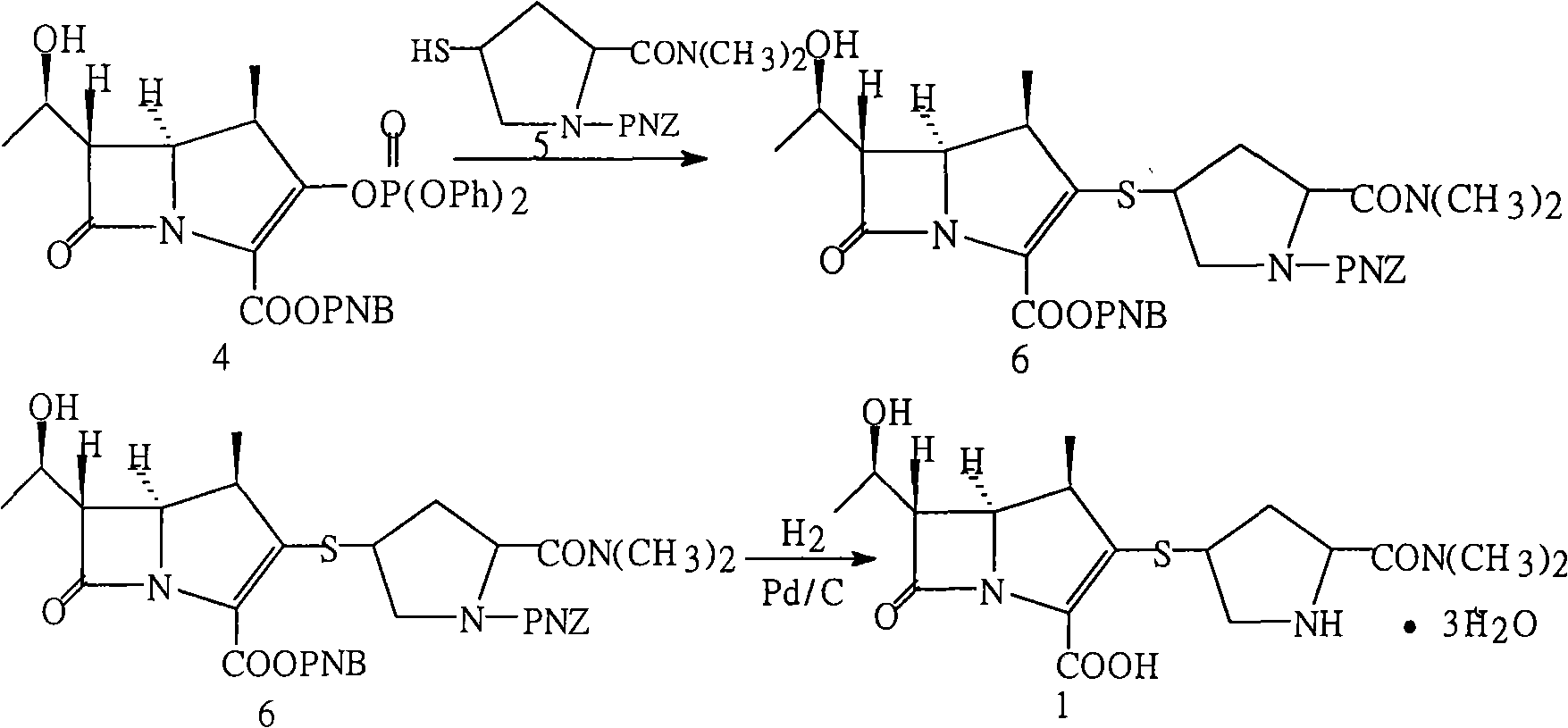
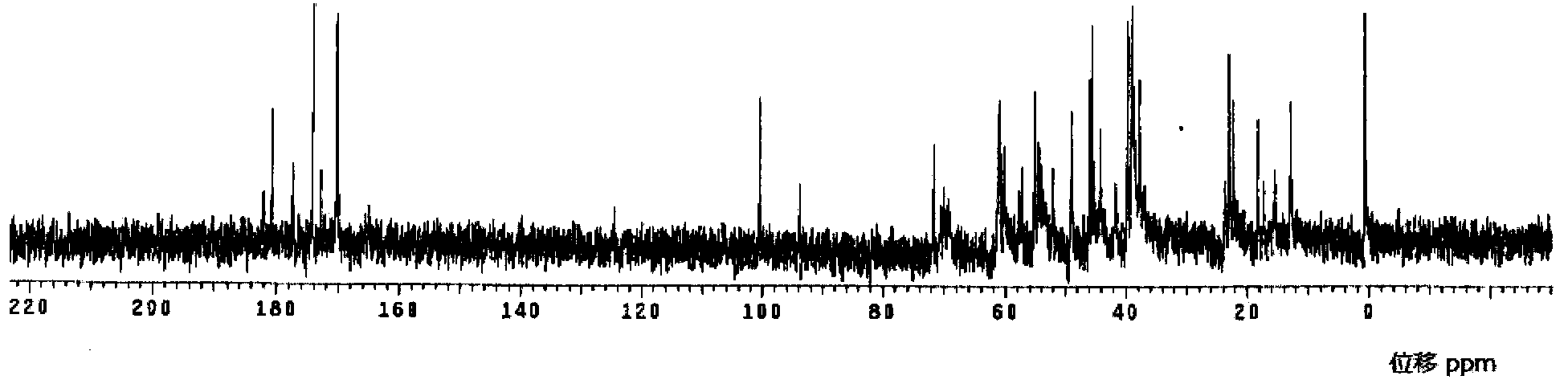
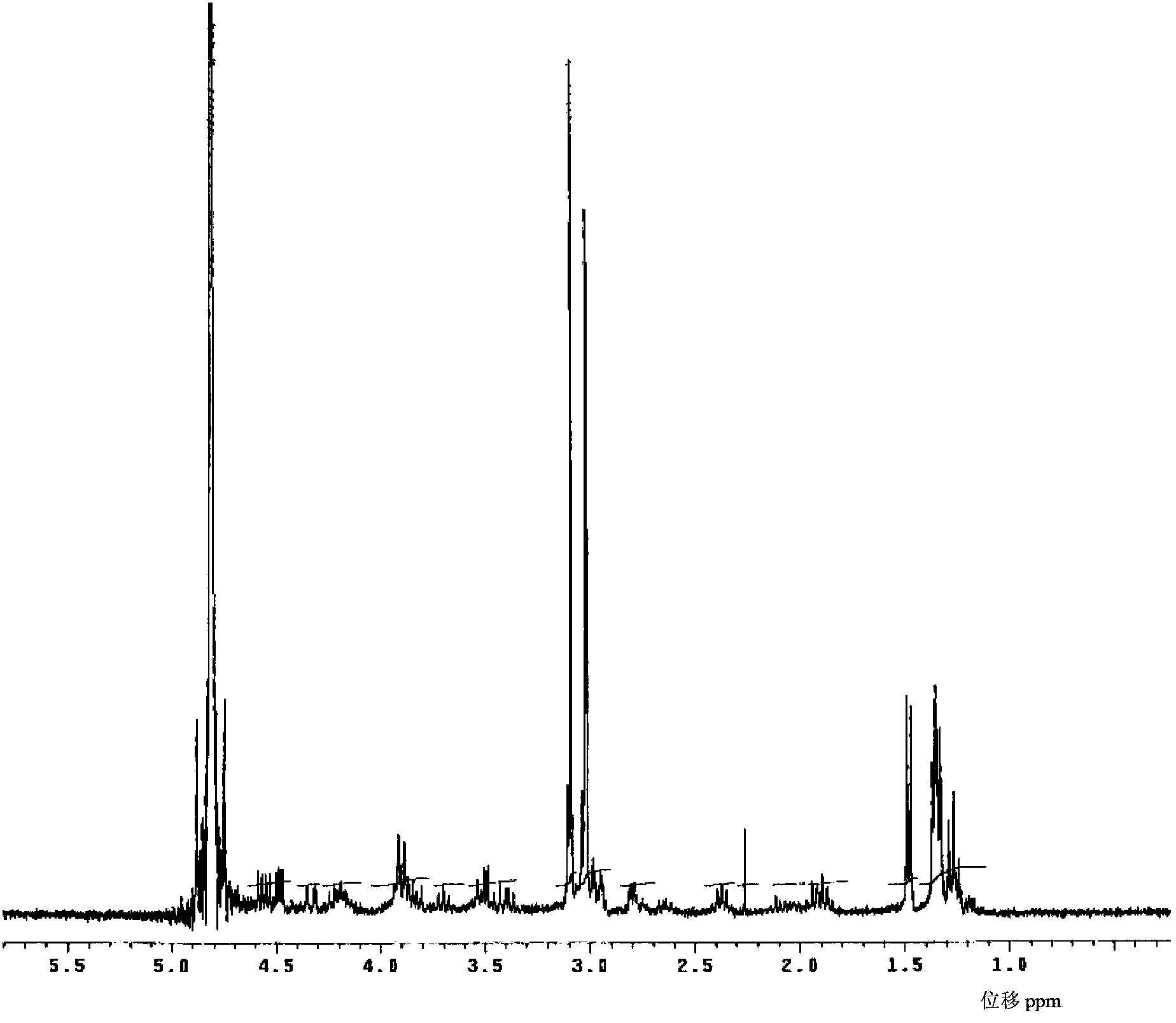
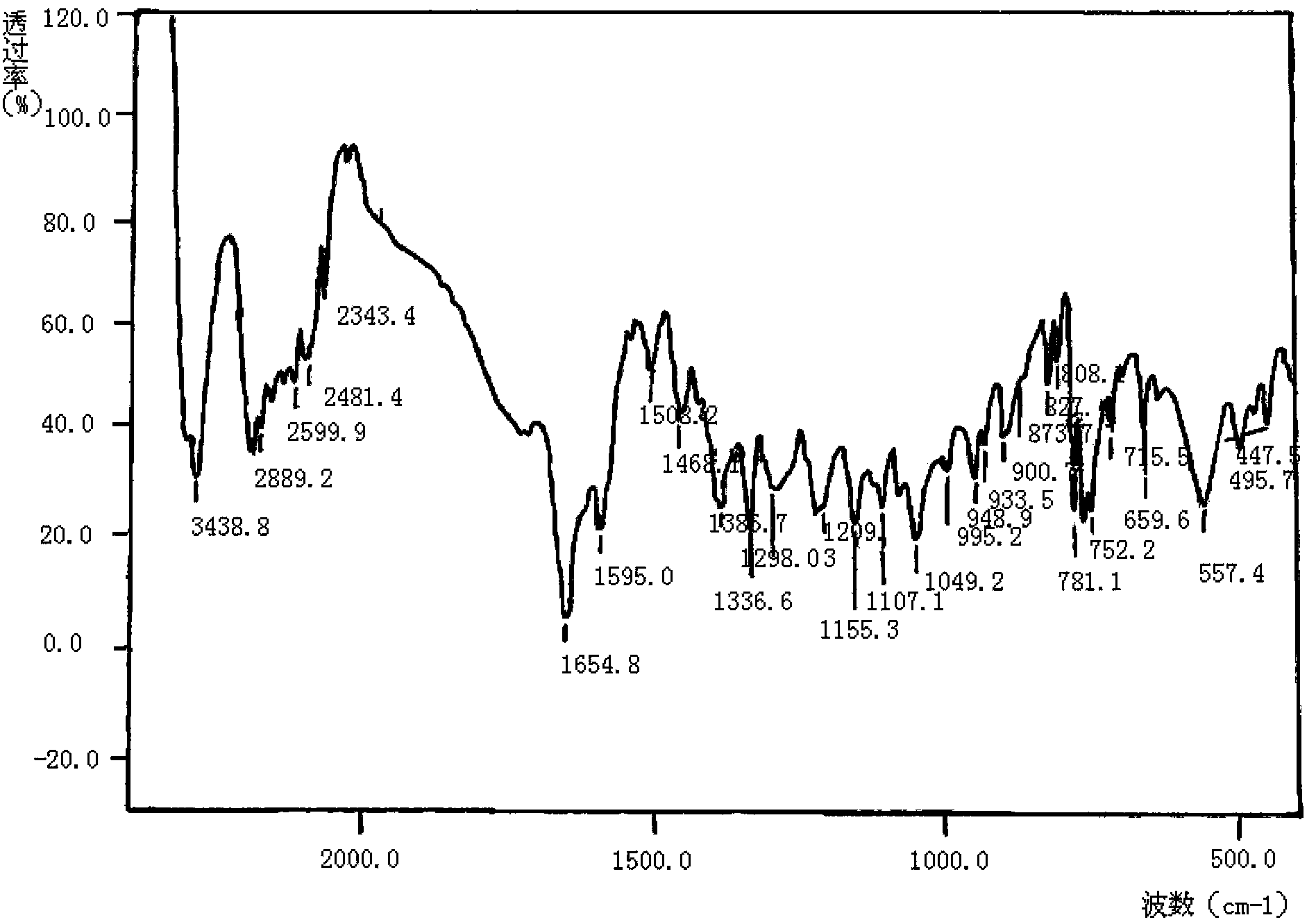
The invention relates to a method for preparing meropenem. The method comprises the following step of: reducing beta-methyl carbapenem protected by a meropenem precursor into meropenem under the transferring and catalyzing actions of hydrogen. The method has the advantages of mild chemical reaction conditions, stable process conditions, easiness for operating, high transformation rate, high yield, product purity stabilized over 99 percent, recycling of solvents and catalysts in the entire process, and great saving in the production cost, and is a practicably synthesizing process suitable for large-scale production; and a novel thought and a novel method for a penem compound are provided.
Owner:ASYMCHEM LAB TIANJIN +4
Lactic bacteria useful as probiotics
InactiveUS7927584B2Pleasant flavorHealthy digestion and intestineBiocideBacterial antigen ingredientsDiseaseMetabolite
Owner:ALLENDE MIGUEL ANGEL GARCIA
Method for preparing meropenem
InactiveCN102153554ASimple production processEasy to produceOrganic chemistryCondensation processMeropenem
The invention relates to a method for preparing meropenem, and belongs to the field of chemical Chinese herbal medicines. In the method, the meropenem is prepared by performing catalytic hydrogenolysis on meropenem from a key intermediate 9H of the meropenem directly by the conventional catalytic cyclization esterification protective condensation process without separation. The method simplifies a production process, is high in yield and low in cost, production links are reduced obviously, the efficiency is improved, and the cost is saved.
Owner:重庆天地药业有限责任公司
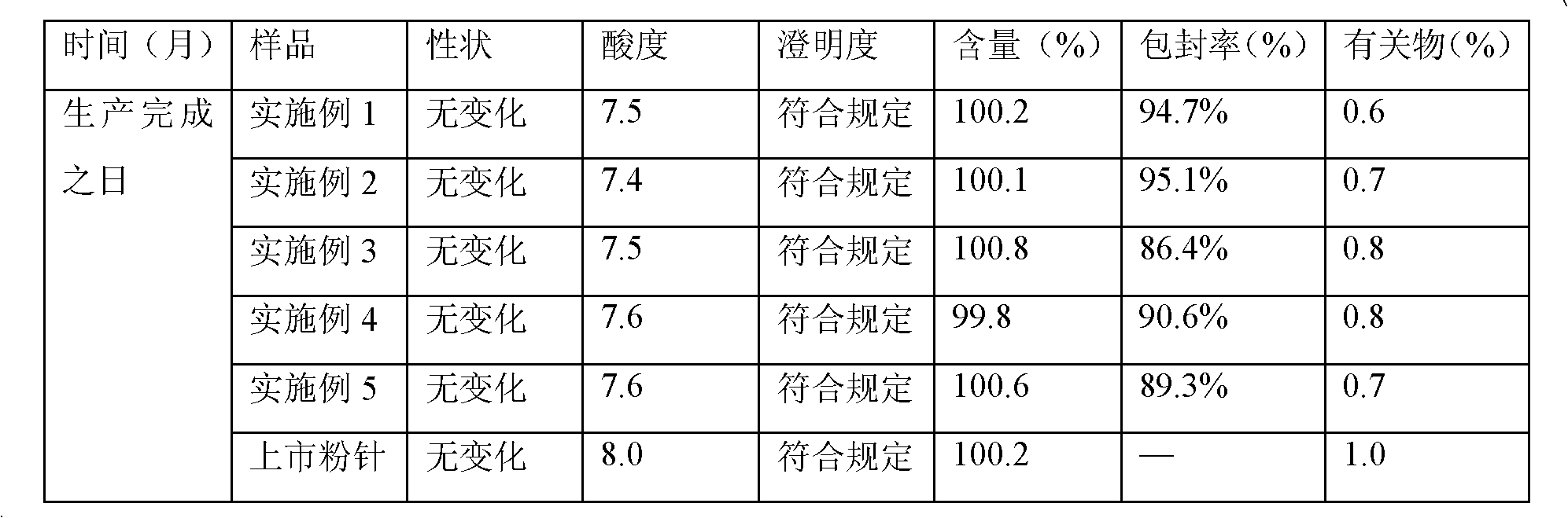
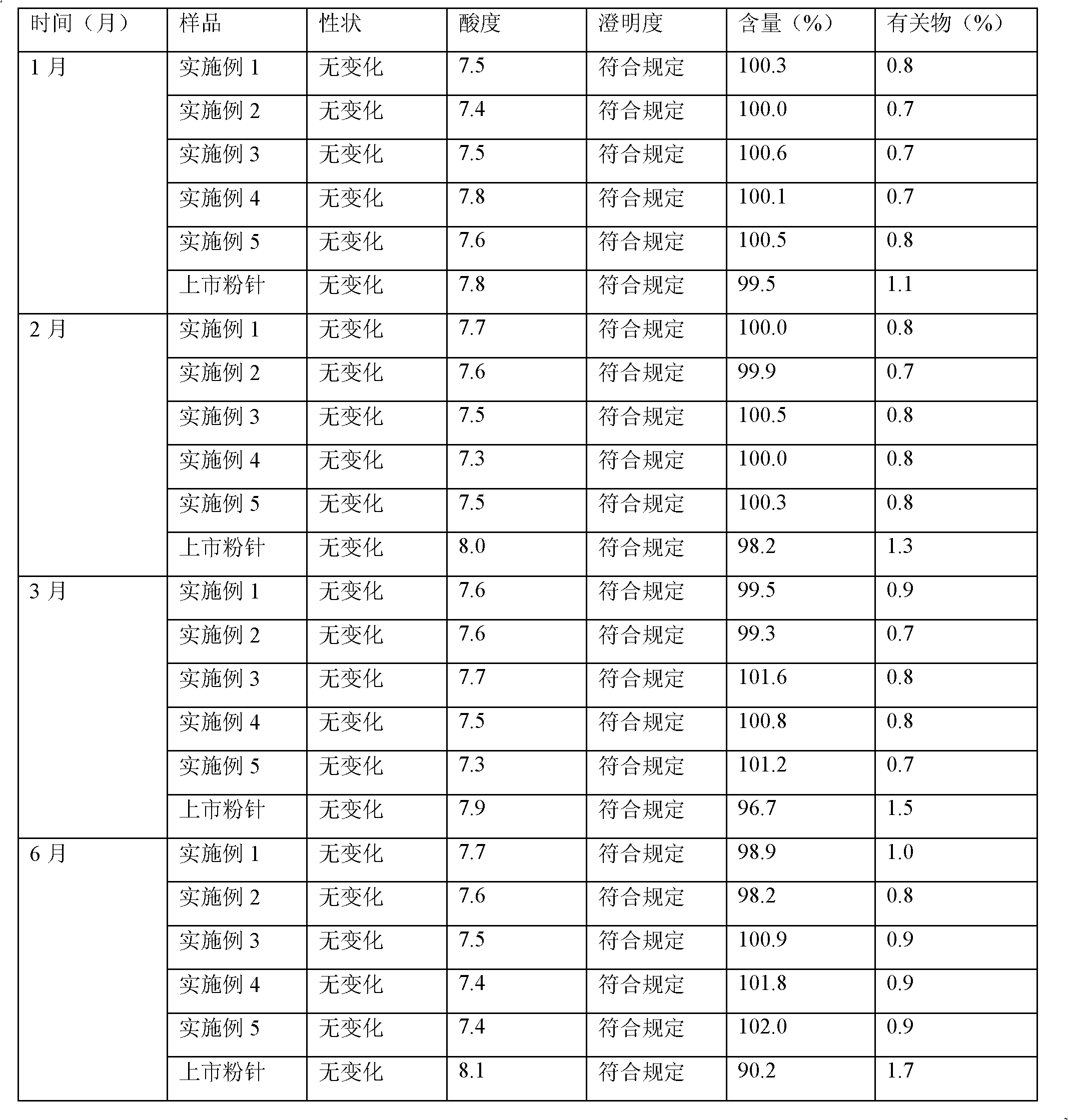
Meropenem raw medicine, preparation method thereof and pharmaceutical composition containing same
ActiveCN103570720AHigh purityThe status of impurities is clearAntibacterial agentsOrganic active ingredientsSolubilityMeropenem
The invention discloses a meropenem raw medicine which is characterized in that the content of meropenem in the raw medicine is 98.0-101.0% by weight based on anhydride; the content of the impurity A and impurity B in the related substances of the raw medicine is not greater than 0.25% respectively; the content of any unknown single impurity is not greater than 0.05%; the total content of other impurities except the A and B is not greater than 0.25%; the acetone residue is not greater than 300ppm. The invention also discloses a meropenem pharmaceutical composition for injection, and the pharmaceutical composition takes the meropenem raw medicine provided by the invention as an active ingredient and has excellent stability. The meropenem raw medicine provided by the invention has the advantages of high purity, clear impurity condition, low solvent residue, good solubility and good long-term storage stability, and can guarantee the effectiveness and safety of the medicine.
Owner:XINXIANG HAIBIN PHARMA
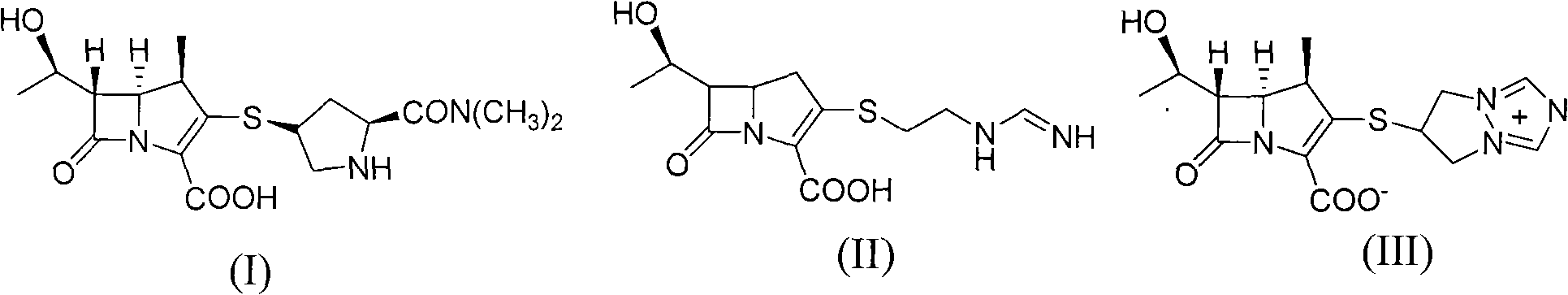
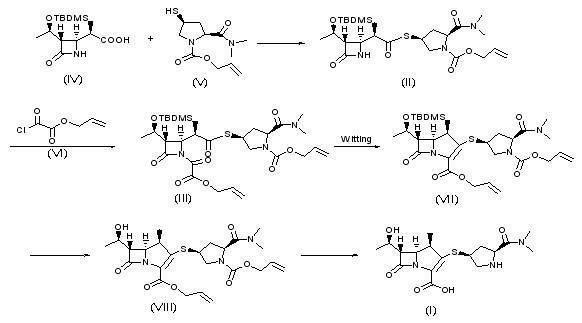
Synthesis method of meropenem
The invention relates to a synthesis method of meropenem, which particularly comprises the following reaction routes: dissolving a compound of the formula (IV) and a compound of the formula (V) into an organic solvent, and then performing a condensation reaction under the action of condensing agent to obtain a compound of the formula (II); dissolving the compound of the formula (II) and a compound of the formula (VI) into methyl benzene, acetic ether or tetrahydrofuran, and then performing a reaction under the action of alkali to generate a compound of the formula (III); dissolving the compound of the formula (III) into cyclohexane, n-octane, the methyl benzene or dimethyl benzene, and then performing a Wittig cyclization reaction under the action of an organic phosphor agent to obtain a compound of the formula (VII); dissolving the compound of the formula (VIII) into a solvent consisting of one of or a plurality of methanol, ethanol, tert-butyl alcohol, isobutanol, isopropanol, the tetrahydrofuran, dioxane, acetone, dichloromethane, chloroform and water, and performing hydrogenation under the action of a palladium catalyst to remove allyl and obtain a target product (I). The synthesis method of the meropenem has the advantages of high yield, mild reaction condition, little environmental pollution, and brief routes.
Owner:SHANGHAI BUDDY BIO PHARM INTERMEDIATES
Meropenem freeze-dried preparation for injection and preparation method thereof
ActiveCN102058545AReduce hydrolysisReduce oxidationAntibacterial agentsOrganic active ingredientsLipid formationAdditive ingredient
The invention discloses a meropenem freeze-dried preparation for injection and a preparation method thereof. 1,000 milliliters of water for injection comprises the following ingredients: 2 to 15 grams of meropenem, 20 to 100 grams of oil for injection, 0 to 20 grams of stabilizing agent, 10 to 80 grams of emulsifying agent, 10 to 30 grams of glycerol and 50 to 200 grams of freeze-dried protective agent. The preparation method comprises the following steps of: dispersing the emulsifying agent into the oil for injection and adding the meropenem; adding the glycerol into the water for injection; stirring and mixing uniformly to form an aqueous phase; emulsifying and homogenizing an oil phase and the aqueous phase to obtain emulsion; and preparing freeze-dried emulsion. A meropenem lipid microsphere preparation provided by the invention can obviously reduce hydrolysis and oxidation of active ingredients and can maintain the integrity of the lipid microsphere structure so as to obviously improve the stability of medicament storage.
Owner:石药集团中诺药业(石家庄)有限公司 +1
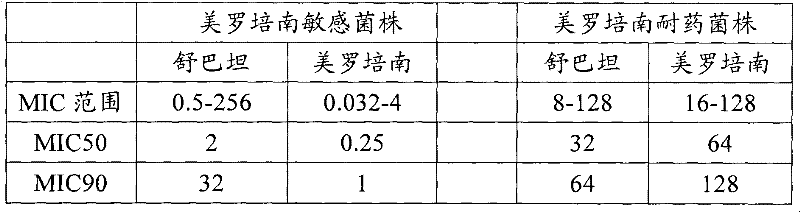
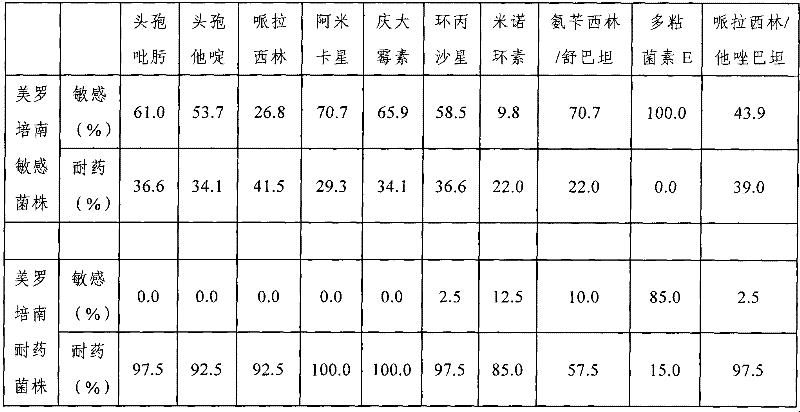
Anti-Acinetobacter baumannii drug combination and application thereof
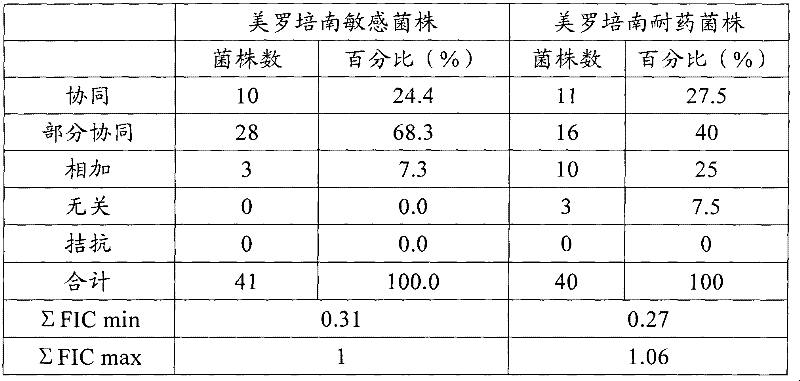
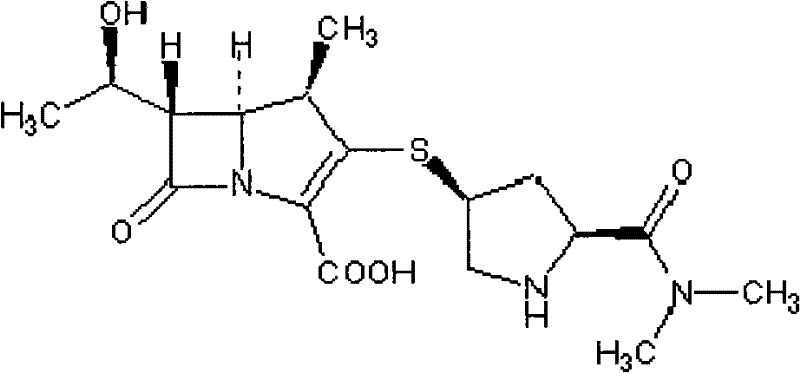
The invention provides an anti-Acinetobacter baumannii drug combination and an application thereof. The drug combination contains meropenem and sulbactam with the weight ratio of meropenem to sulbactam being 1:2-4. The invention is designed by a checkerboard method. The synergetic antibacterial effect of the combined use of meropenem and sulbactam to carbapenems sensitive and drug resistant Acinetobacter baumannii is found by broth microdilution detection. As shown in the test result, no matter meropenem drug resistant strain or meropenem sensitive strains, most of them show a synergetic and partly synergetic effect to the combined use of meropenem and sulbactam and the strains which show a synergetic effect account for 25%.
Owner:LIVZON PHARM GRP INC
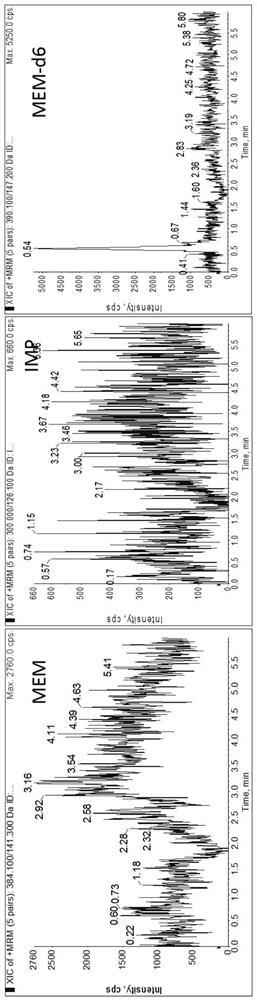
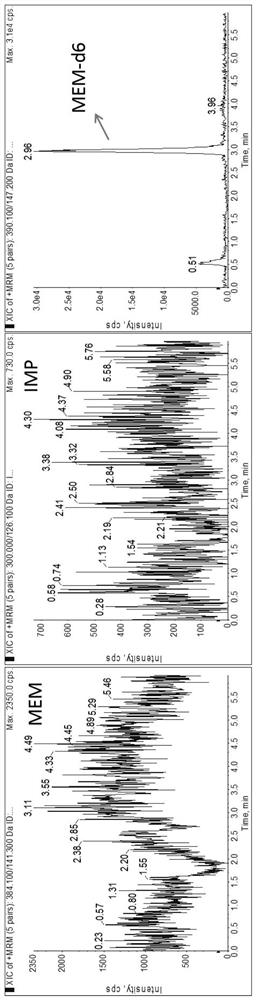
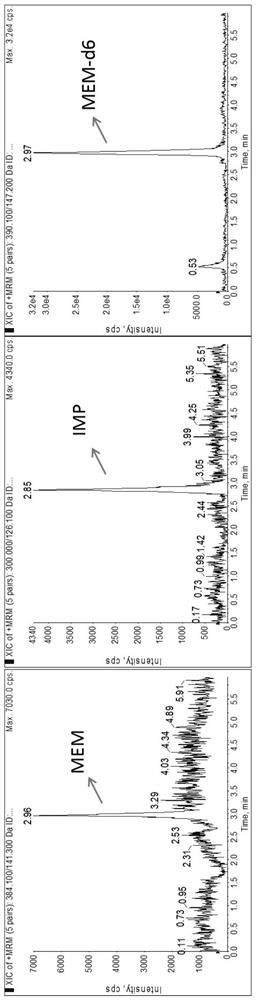
Method for detecting plasma protein binding rate of meropenem or imipenem by combining liquid chromatography-mass spectrometry technology with ultrafiltration technology
According to a method for detecting the plasma protein binding rate of meropenem or imipenem by combining a liquid chromatography-mass spectrometry technology with an ultrafiltration technology, the liquid chromatography-tandem mass spectrometry detection technology is combined with the ultrafiltration technology, and the total concentration of meropenem or imipenem in plasma and the free concentration of drugs in ultrafiltrate are respectively detected. And an MOPs stabilizer is added during sample treatment, so that the problem of poor drug stability is solved, and the determination result is more accurate. The method can be used for rapidly detecting the protein binding rate of the drug at high throughput, and is particularly suitable for unstable drug molecules.
Owner:PEKING UNIV THIRD HOSPITAL +1
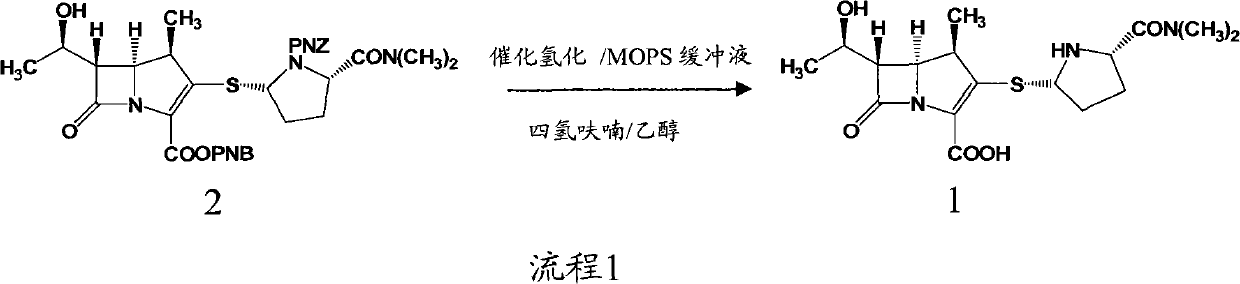
Deprotection method in meropenem synthesis
InactiveCN102336756ASimple processReaction conditions are easy to controlOrganic chemistryAlcoholOrganic solvent
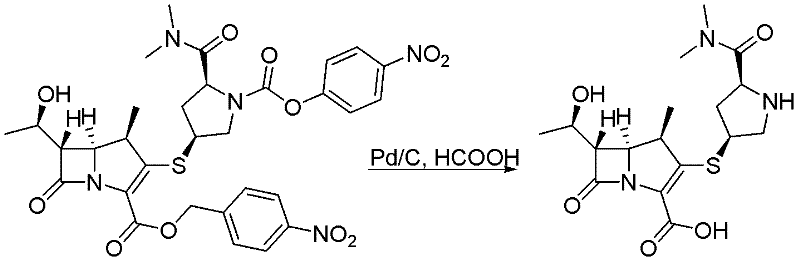
A deprotection method in meropenem synthesis comprises the following steps of: removing p-nitrobenzyl and p-nitrobenzyl oxygen formyl from meropenem in an organic solvent 1 or a hydrous organic solvent 1 (such as an alcohol or ether or ester compound under the action of a catalyst and a reducing agent, filtering, and adding an organic solvent 2 (such as acetonitrile or acetone) to precipitate meropenem. The method provided by the invention can be carried out at normal pressure, has advantages of low equipment investment, simple operation, mild reaction, good selectivity, high yield and the like, and is suitable for industrial production.
Owner:HUBEI YITAI PHARMA
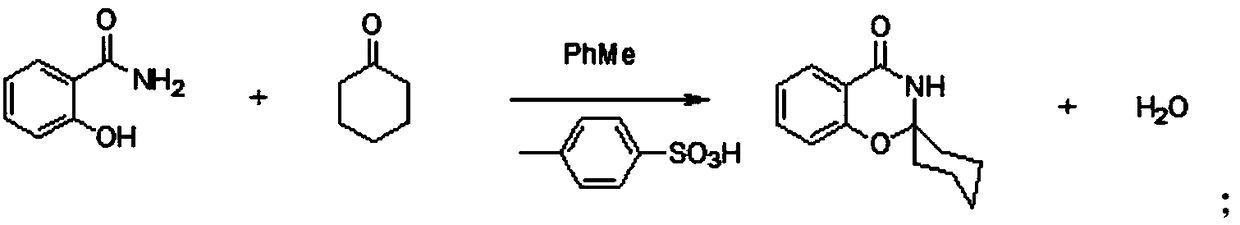
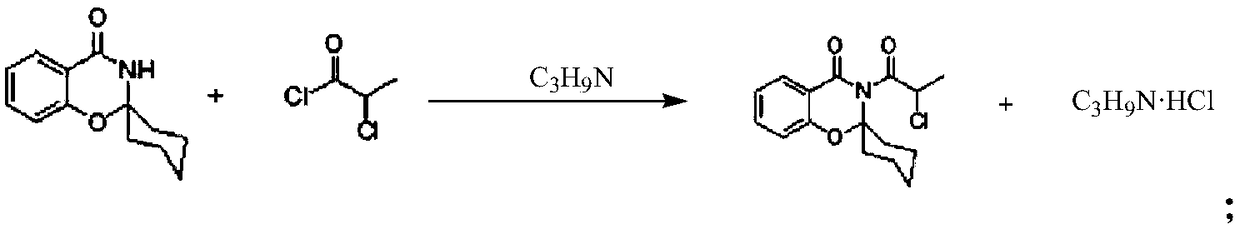
3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof
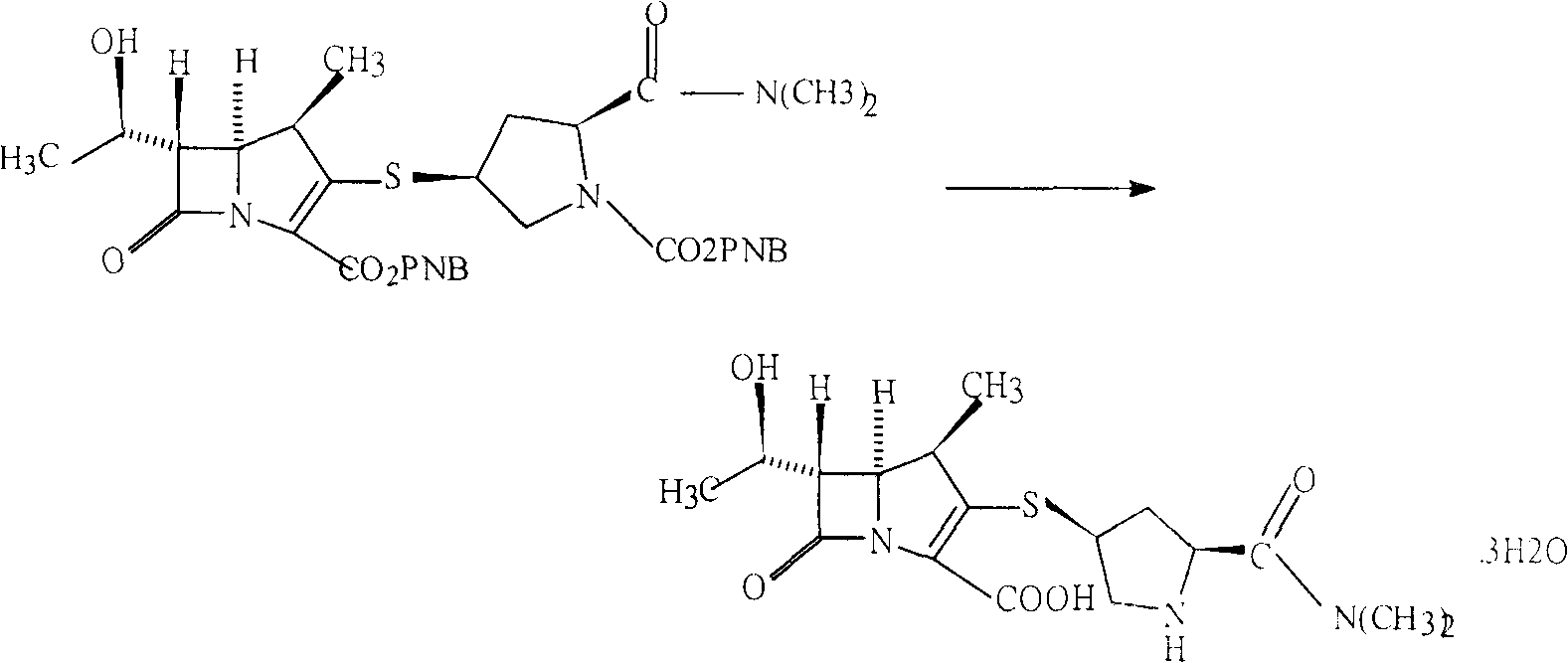
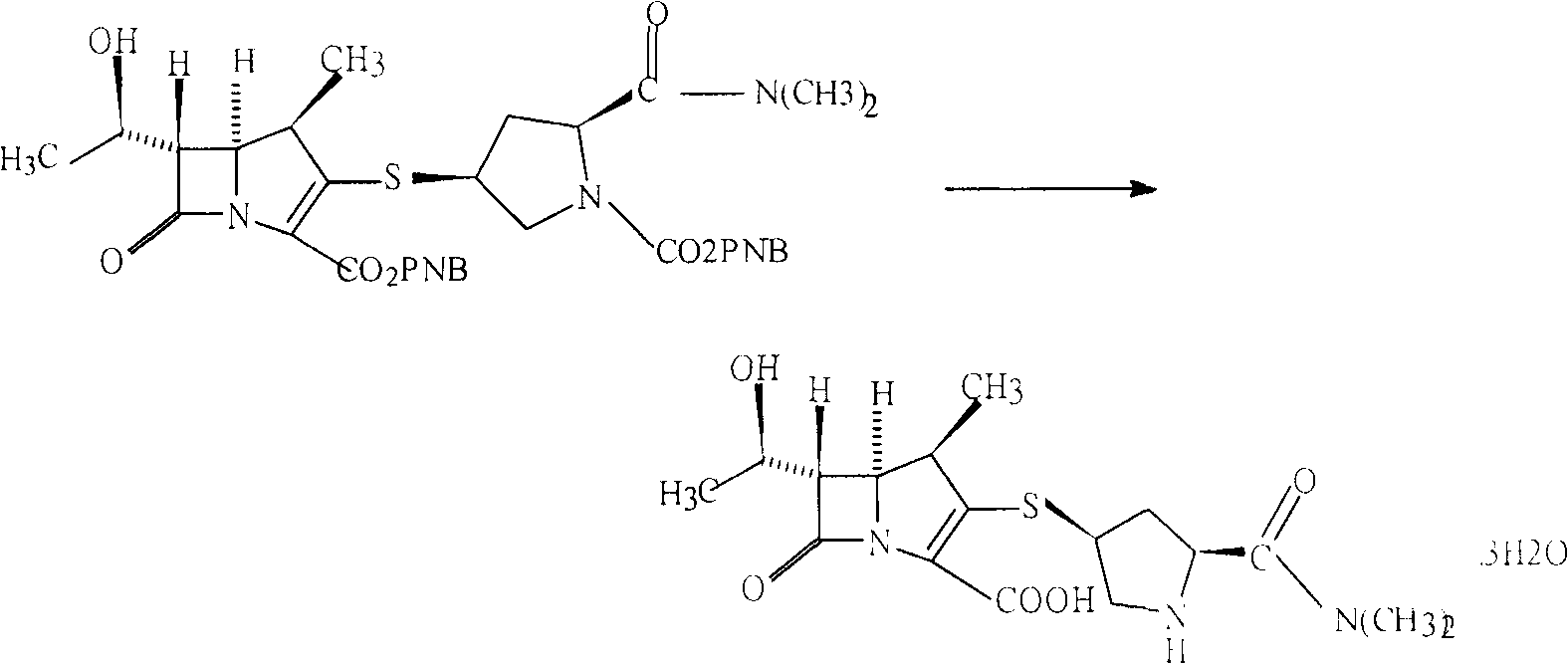
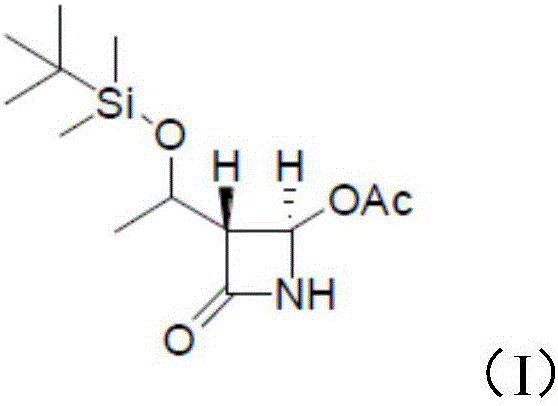
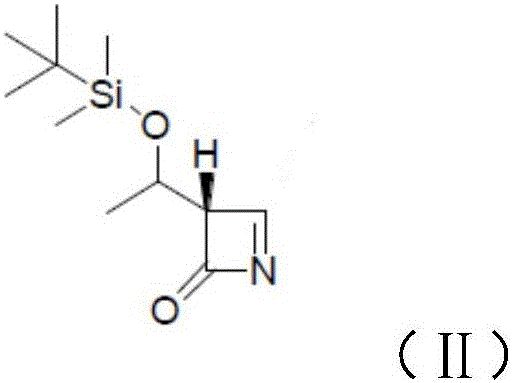
InactiveCN106478538ASynthetic raw materials are readily availableFew reaction stepsOrganic chemistryFiltrationMeropenem
The invention relates to the field of material synthesis, in particular to 3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof, aiming to solve the problem of restricted development of meropenem due to complicated existing methods for synthesizing 3-(2-Bromo-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one, strict reaction conditions and low yield. A method for preparing the 3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one includes steps of 1, placing intermediates I, pyridine, chloropropionyl chloride and reactive solvents into a reactor according to a molar ratio of 1:(1-3):(1-3):(5-10), carrying out reaction at the temperature of 30-50 DEG C under the control for 3-5 h, concentrating the solvents and cooling and crystallizing reaction products to obtain F-6; 2, adding mixed liquid of organic solvents and salt solution into the F-6 obtained in the step 1, completely dissolving the F-6, stirring the F-6 and the mixed liquid, allowing the F-6 and the mixed liquid to stand still and then layer, cooling separated organic phases obtained by means of layering, precipitating out solid, carrying out suction filtration on the solid and drying the solid to obtain refined products. Raw materials for synthesizing the F-6 are easily available, the method includes few reaction steps, reaction conditions are mild, only few byproducts are generated, the method is easy and convenient to implement, and the used solvents can be recycled.
Owner:曹子领
Application of dithiocarbamate derivative to antibacterial field
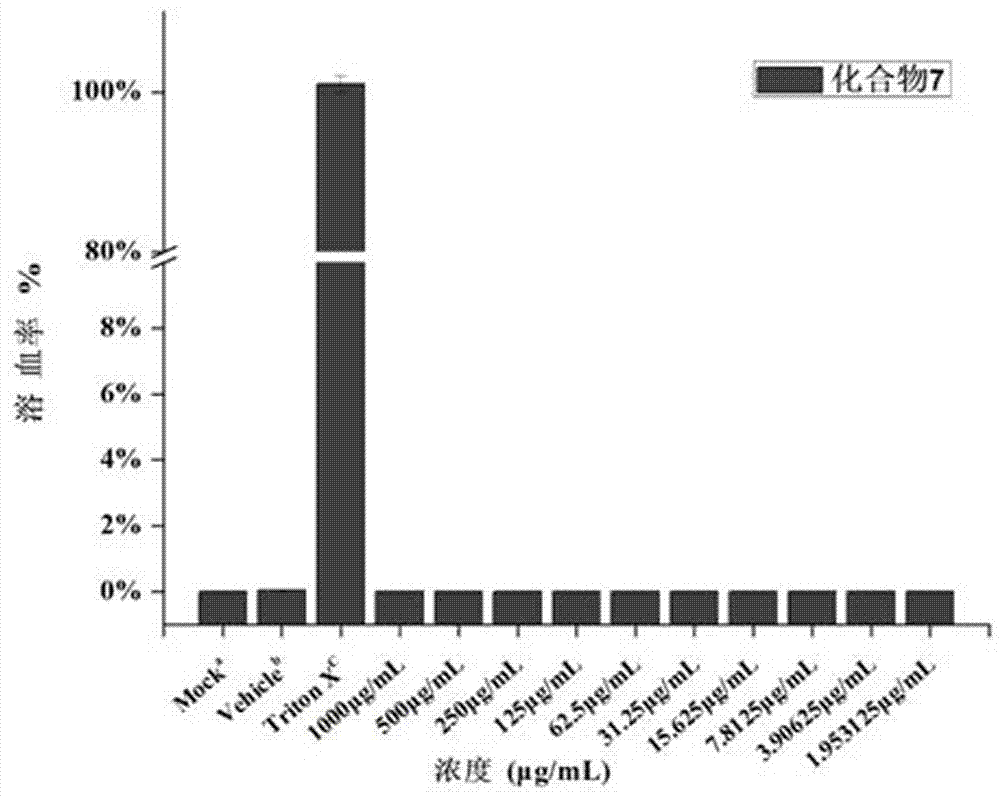
ActiveCN107184581AImprove the bactericidal effectAntibacterial agentsEster active ingredientsMinimum inhibitory concentrationRed blood cell
Owner:ZHENGZHOU UNIV
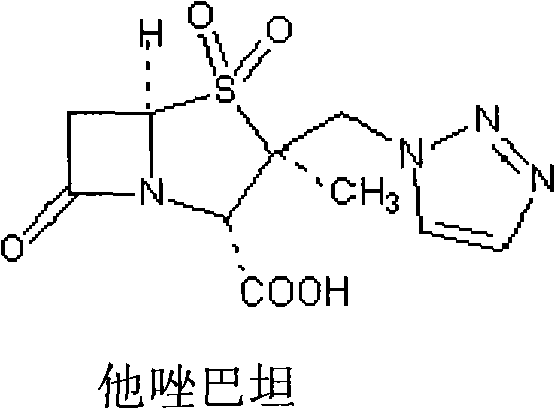
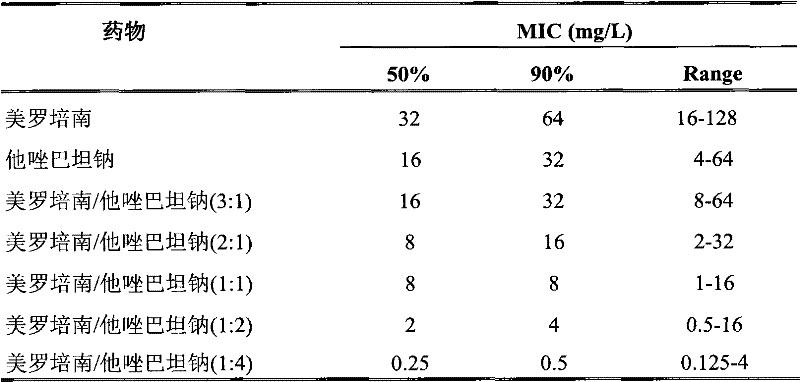
Meropenem sodium/tazobactam sodium medicinal composition
The invention provides a combined medicament, in particular a meropenem tazobactam sodium combined medicament for treating infectious diseases caused by acinetobacter baumannii. The meropenem and tazobactam sodium combined medicament has a synergistic effect and an accumulative antibacterial effect on the infectious diseases caused by the acinetobacter baumannii and particularly has a good synergistic effect and a good accumulative antibacterial effect on multiple medicine-tolerant acinetobacter baumannii strains, and can be used for curing clinical infection caused by the multiple medicine-tolerant acinetobacter baumannii strains in clinic.
Owner:深圳市新泰医药有限公司
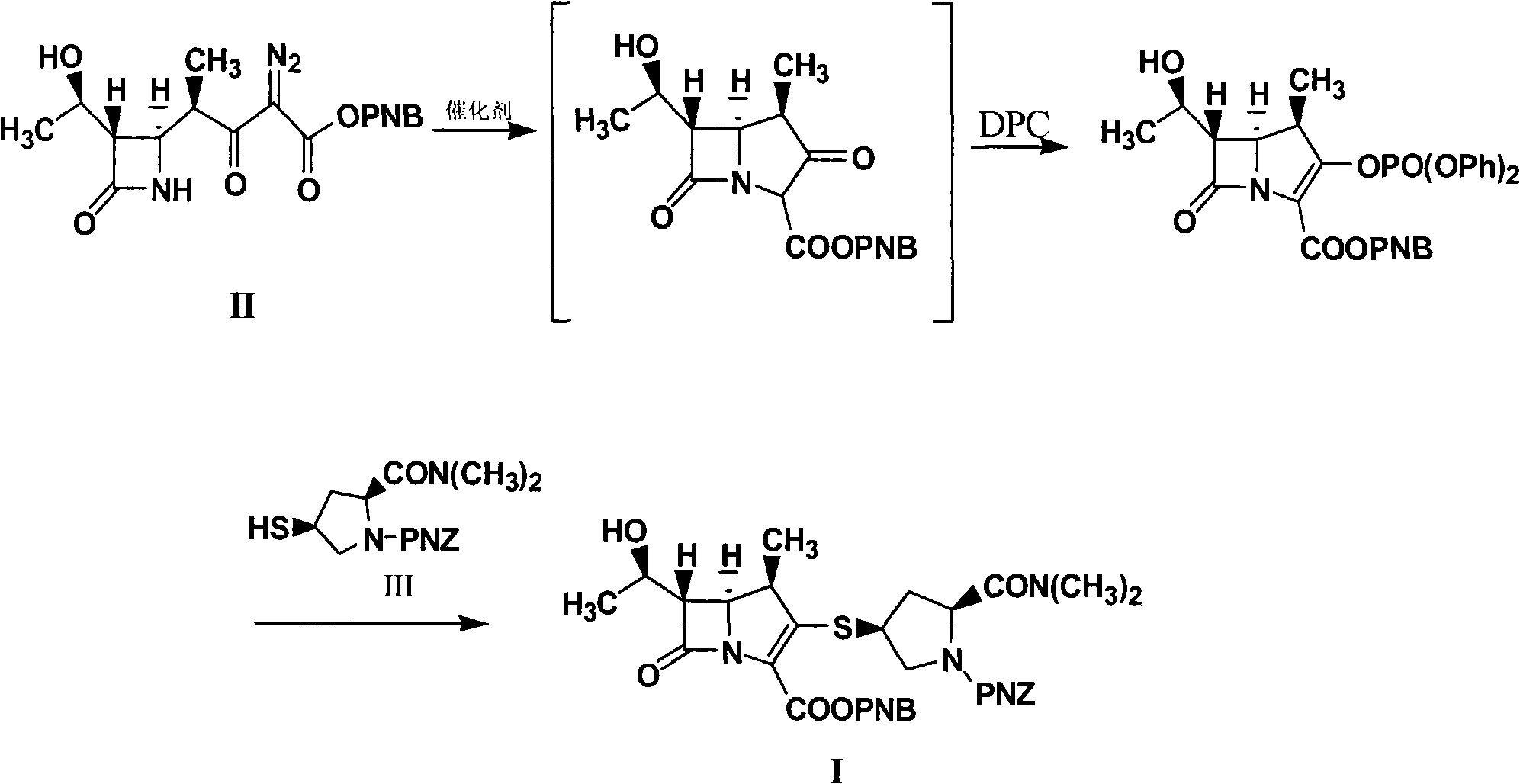
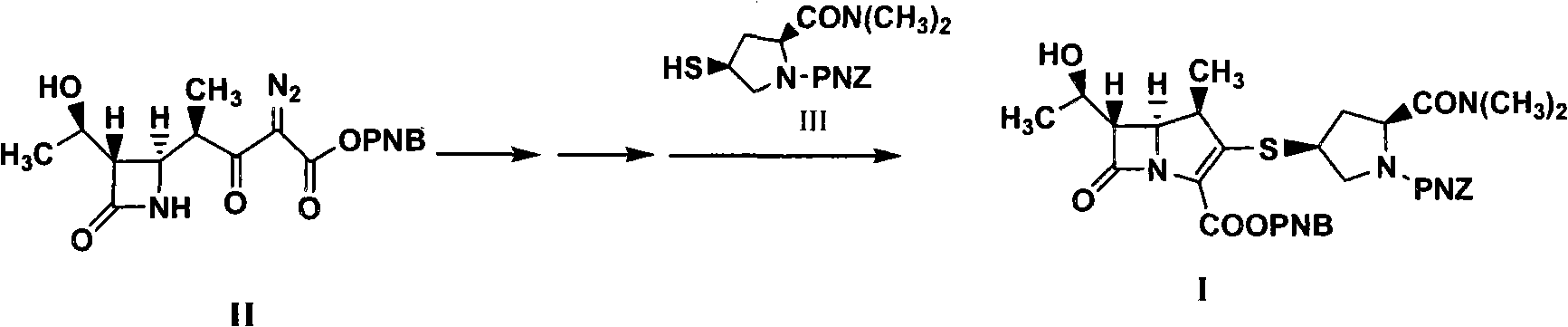
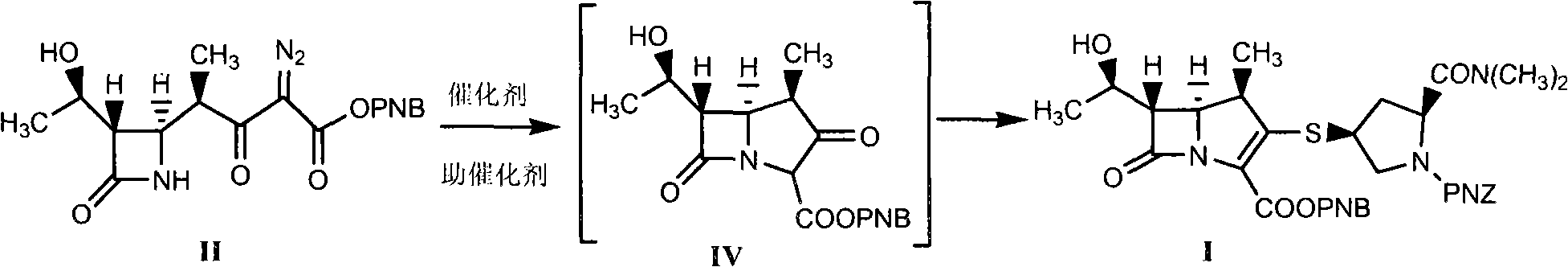
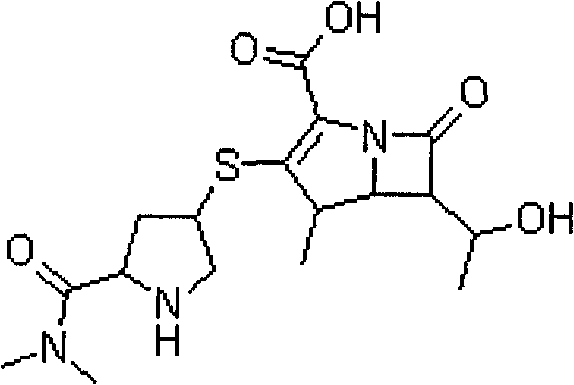
Method for synthesizing meropenem intermediate
The invention relates to a method for synthesizing a meropenem intermediate, which comprises the following steps: (1) in an inert gas atmosphere, adding an organic solvent, a compound II, a noble metal catalyst and auxiliary catalysts into a reactor, heating the materials to perform a reaction, and monitoring the reaction by TLC or HPLC; (2) at the end of the reaction, adding the organic solvent, cooling the reaction solution, adding an organic alkali or DPC, and monitoring a reaction by the TLC or HPLC; (3) at the end of the reaction, cooling the reaction solution, adding the organic alkali and a compound III, performing a reaction at a controlled temperature, and monitoring the reaction by the TLC or HPLC; and (4) washing, removing a water phase, dehydrating an organic phase, filtering to remove solid, concentrating the organic phase, adding the organic solvent for crystallization, filtering the solution, washing the crystals, and drying the crystals to obtain the target compound I. Compared with the techniques of the same kind, the method, which is simple and convenient in operation, of the invention has the advantages of adding two auxiliary catalysts at the same time, greatly reducing the consumption of noble metal catalyst, making raw materials react more completely, producing less impurities and improving product yield.
Owner:XINXIANG HAIBIN PHARMA
Stable meropenem injection and preparation method thereof
ActiveCN102188395AImprove protectionImprove stabilityAntibacterial agentsPowder deliveryFreeze-dryingCholesterol
The invention provides a stable meropenem injection and a preparation method thereof. The injection is liposome freeze-dried preparation, which comprises the following components by weight: 5 to 15 parts of meropenem, 80 to 160 parts of phosphatide, 50 to 110 parts of cholesterol, 0.15 to 0.50 parts of vitamin E, 300 to 550 parts of sodium deoxycholate, and 30 to 100 parts of sugar. The liposome freeze-dried preparation provided in the invention has advantages of small particle size, high entrapment rate, low percolation rate, and good stability. Therefore, a problem of instability of ordinary powder injection under a room temperature during long time infusion can be solved, and thus the stable meropenem injection is suitable for clinical practice.
Owner:石药集团中诺药业(石家庄)有限公司 +1
Method for synthesizing meropenem intermediate
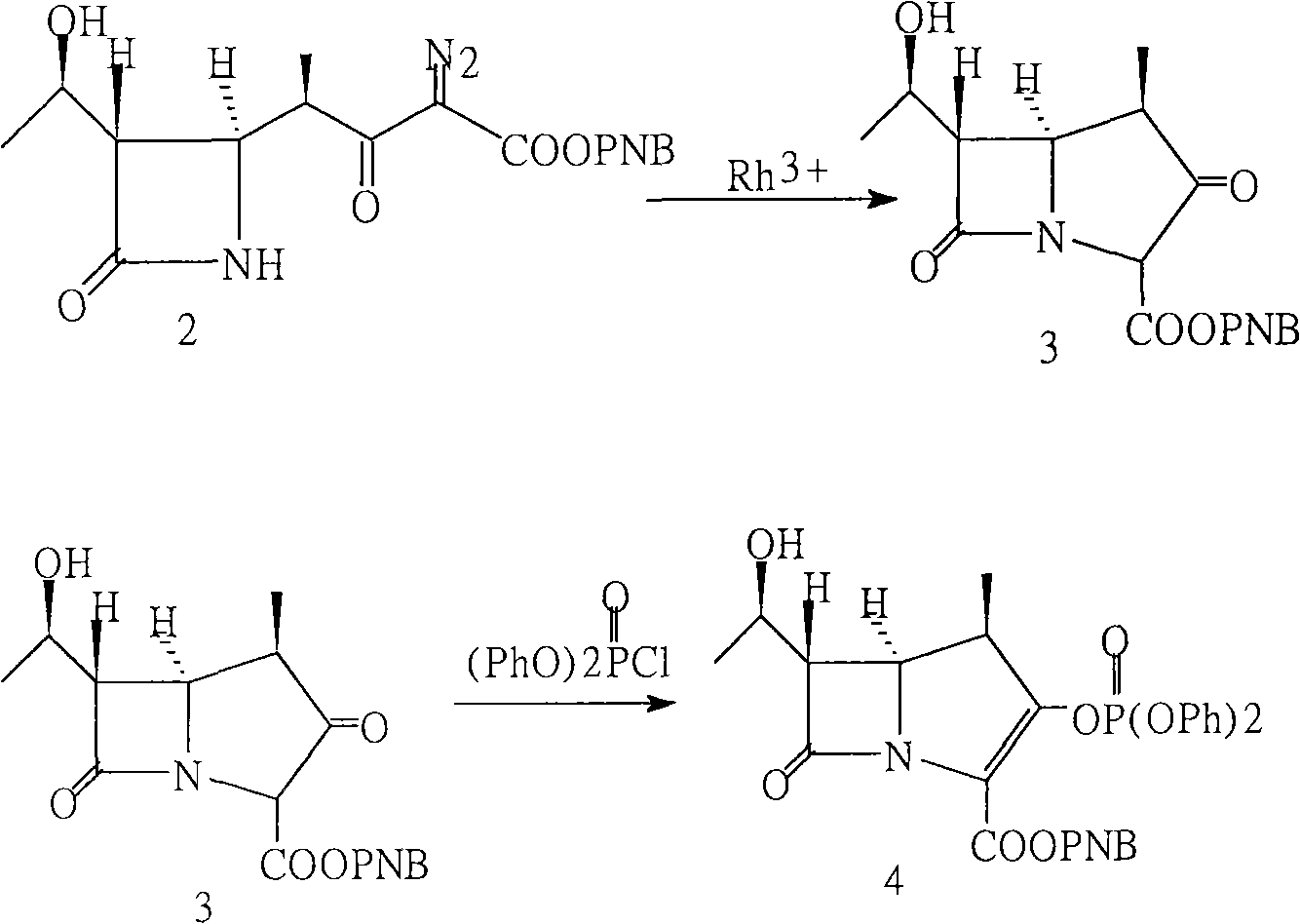
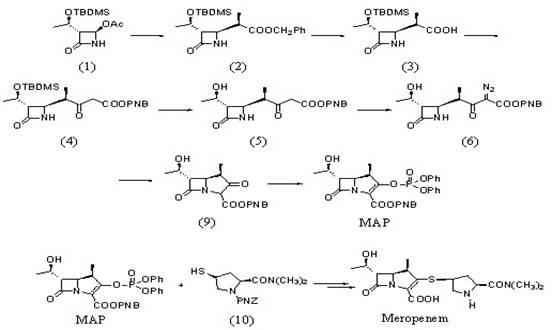
InactiveCN101225069ASave man hoursReduce energy consumptionOrganic chemistrySide chainSynthesis methods
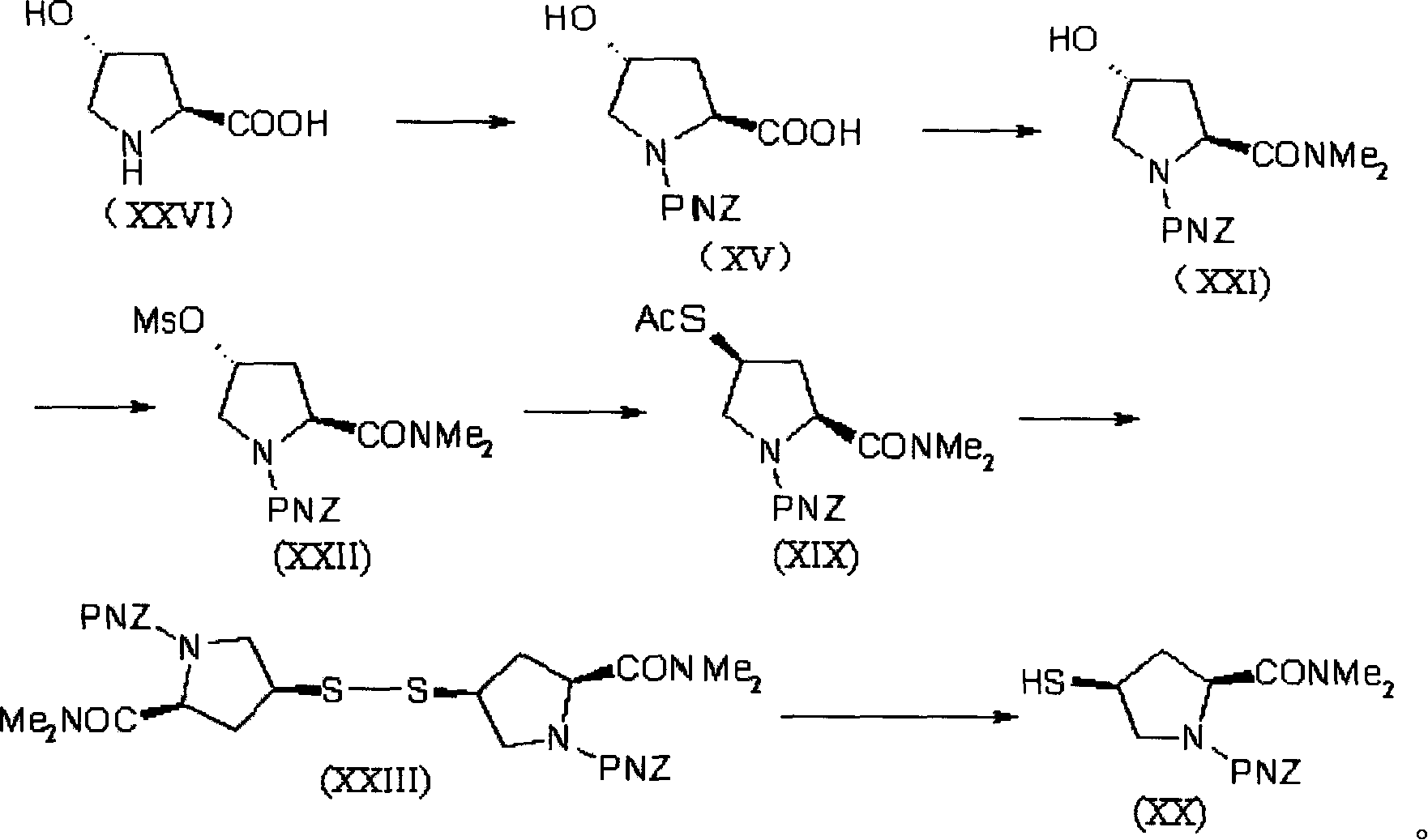
The invention provides a synthesis method of a side chain H of a meropenem, which is characterized in that a new radical is introduced respectively for three functional groups of 4R hydroxy L hydroxyproline and a certain spatial configuration is kept. The synthesis method for the side chain H of a meropenem has the advantages of moderate process conditions, stable product quality, and high yield, which is applicable to the industrial production on a large scale.
Owner:TIANJIN JINGYE FINE CHEM
Meropenem raw medicine, preparation method thereof and pharmaceutical composition containing same
ActiveCN103570718AHigh purityThe status of impurities is clearAntibacterial agentsOrganic active ingredientsSolubilityMeropenem
The invention discloses a meropenem raw medicine which is characterized in that the content of meropenem in the raw medicine is 98.5-101.0% by weight based on anhydride; the content of the impurity A and impurity B in the related substances of the raw medicine is not greater than 0.25% respectively; the content of any unknown single impurity is not greater than 0.05%; the total content of other impurities except the A and B is not greater than 0.2%; the acetone residue is not greater than 400ppm and preferably not greater than 100ppm. The invention also discloses a meropenem pharmaceutical composition for injection, and the pharmaceutical composition takes the meropenem raw medicine provided by the invention as an active ingredient and has excellent stability. The meropenem raw medicine provided by the invention has the advantages of high purity, clear impurity condition, low solvent residue, good solubility and good long-term storage stability, and can guarantee the effectiveness and safety of the medicine. In the invention, the process is simple and compact, the cost is remarkably low, and the control is simple. The invention is suitable for industrial large-scale aseptic production of the meropenem raw medicine and pharmaceutical preparation.
Owner:SHENZHEN HAIBIN PHARMA +1
Metal beta-lactamase inhibitor open chain pyridine carboxylic acid derivative and preparation method thereof
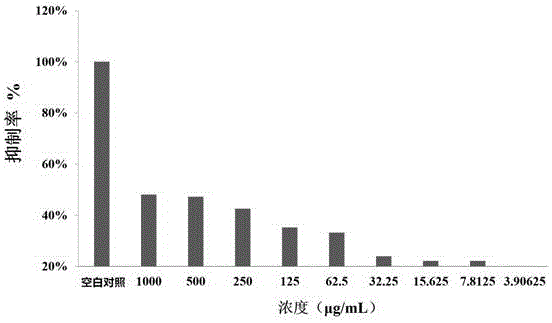
ActiveCN106496110AMIC (minimum bactericidal concentration) value decreasedPromote research and developmentAntibacterial agentsOrganic active ingredientsCytotoxicityMeropenem
Belonging to the field of medicinal chemistry, the invention discloses a metal beta-lactamase inhibitor open chain pyridine carboxylic acid derivative and a preparation method thereof. The compound has the following structure shown as the specification. The derivative can restore the sensitivity of metal beta-lactamase enterobacteriaceae bacteria to carbapenem antibiotics. In vitro antibacterial experiment results prove that the derivative can reduce the MIC value of carbapenem resistant Escherichia coli (producing NDM-1 type metal beta-lactamase) to meropenem by about 40960 times maximumly. In vitro red blood cell toxicity experiments also prove that the compound has very small red blood cell toxicity. Therefore, the compound is expected to be used as a candidate drug of novel metal beta-lactamase inhibitor.
Owner:ZHENGZHOU UNIV
Preparation method of meropenem intermediate 4-BMA
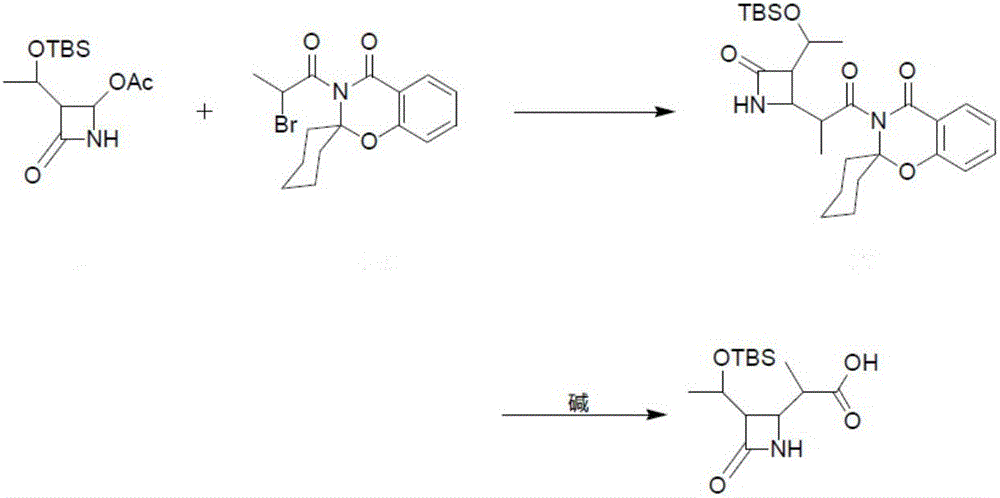
ActiveCN106397473ALess impuritiesReduced activityGroup 4/14 element organic compoundsMeropenemHydrolysis
The invention provides a preparation method of a meropenem intermediate 4-BMA. Low-reaction-activity propionyl spirobenzoxazine cyclohexane is adopted, the meropenem intermediate 4-BMA is prepared through Reformatsky reaction and hydrolysis reaction in sequence, and in the preparation process, side reactions participating in reaction are few, further obtained product impurities are less, and the prepared 4-BMA is high in purity. In addition, the propionyl spirobenzoxazine cyclohexane is adopted as a raw material, the reaction yield is not affected, on the contrary, the reaction yield is greatly improved, and high reaction yield is obtained. Further, the adopted synthetic process is simple, post-treatment is convenient and easy to operate, the raw material and a catalyst are cheap and easy to obtain, safe and environmentally friendly, and the preparation cost is low. An experimental result shows that the mole yield of the prepared meropenem intermediate 4-BMA is 90.0% or above, and the purity is 98.5% or above.
Owner:XINXIANG HAIBIN PHARMA
Meropenem palladium-carbon catalyst preparation method and catalyst prepared through same
ActiveCN106861682ALarge particle sizeHigh catalytic activityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsPalladium on carbonActivated carbon
The invention belongs to the field of chemical catalyst preparation and particularly discloses a meropenem palladium-carbon catalyst preparation method. The palladium-carbon catalyst is obtained by dipping palladium hydroxide solution on an activated carbon carrier and restoring through a reducing agent. The preparation method comprises the steps: 1) utilizing a palladium-contained compound to prepare palladium hydroxide; 2) preparing palladium hydroxide solution; 3) throwing activated carbon into the palladium hydroxide solution to prepare dipping slurry; 4) performing chemical reduction reaction on the dipping slurry. The invention further discloses meropenem palladium-carbon catalyst prepared through the method. The palladium-carbon catalyst disclosed by the invention has a simple preparation technological process and strong selective pertinence; furthermore, preparation raw materials are simple and easy to obtain, and a reaction product is green and environment-friendly; the palladium-carbon catalyst is mainly applied to preparation of meropenem in carbapenems antibiotics and also has other wide application in the field of medical treatment.
Owner:JIANGXI HANS PRECIOUS METALS CO LTD +1
Novel Antimicrobial Medicament
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
A preparation method of a meropenem intermediate
InactiveCN108774188AThe process is simple and reliableShorten the production cycleOrganic chemistryCyclohexanoneReaction temperature
A preparation method of a meropenem intermediate is disclosed. The method includes adding salicylamide and cyclohexanone into an organic solvent that is toluene; reacting the mixture under the function of a catalyst that is p-toluenesulfonic acid; then performing material centrifugation, rinsing and centrifugation until a product is dry to obtain white crystals; raising the temperature until the obtained material is dissolved; then adding an organic solvent that is toluene and a catalyst that is n-propylamine; then adding 2-chloropropionyl chloride dropwise and reacting the mixture until the reaction is finished; performing vacuum distillation to remove the toluene; performing centrifugation after crystallization; and after a centrifugation product is dry, discharging and drying. The moleratio of the salicylamide, the cyclohexanone, the p-toluenesulfonic acid, the n-propylamine and the 2-chloropropionyl chloride is 1:1.3-1.7:0.01-0.04:0.2-0.5:0.2-0.5. The salicylamide, the cyclohexanone and the 2-chloropropionyl chloride are adopted as raw materials, the p-toluenesulfonic acid and the n-propylamine are adopted as catalysts, the one-time product yield is 92.4% by reaction temperature control and material ratio control, and the mother liquor recovery rate is 5%.
Owner:湖北宇阳药业有限公司
Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same
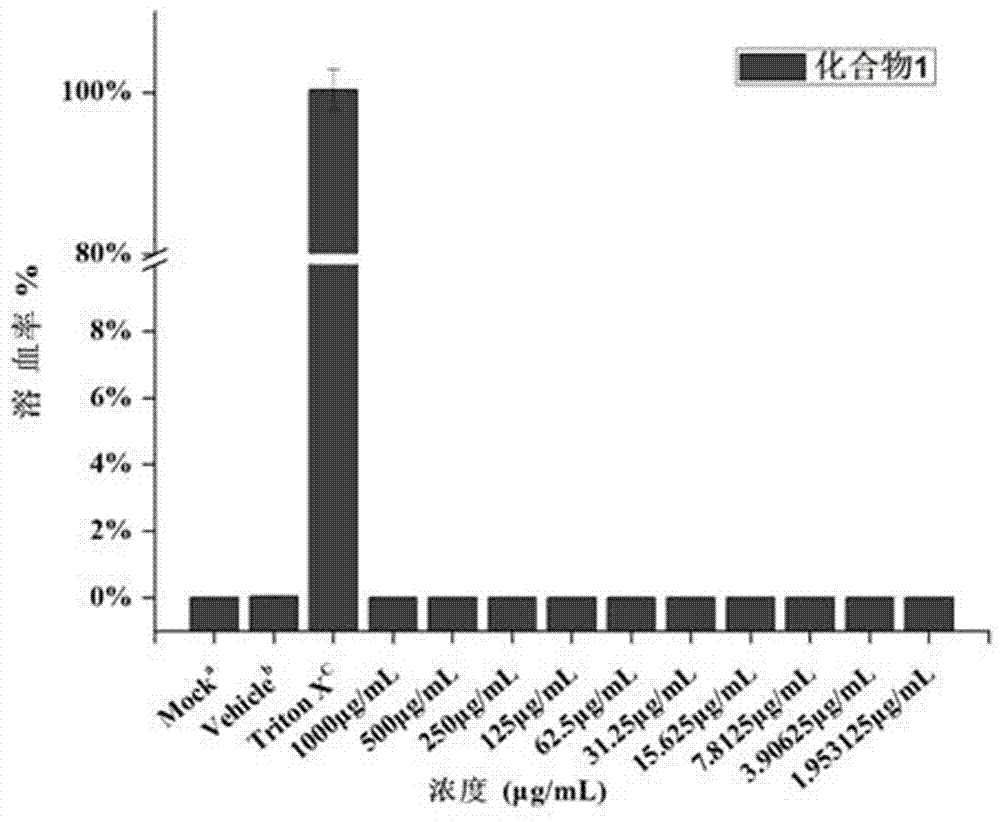
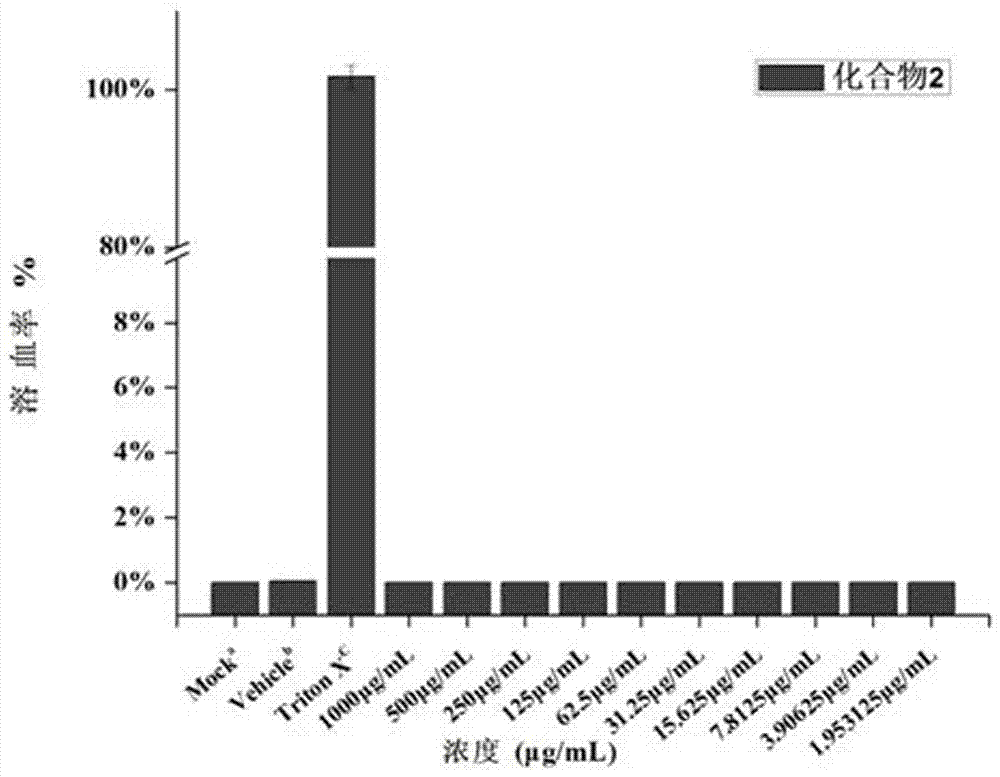
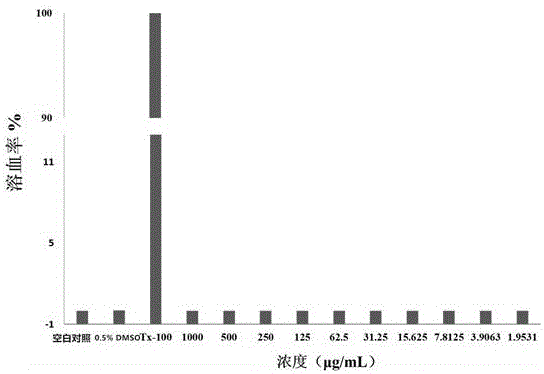
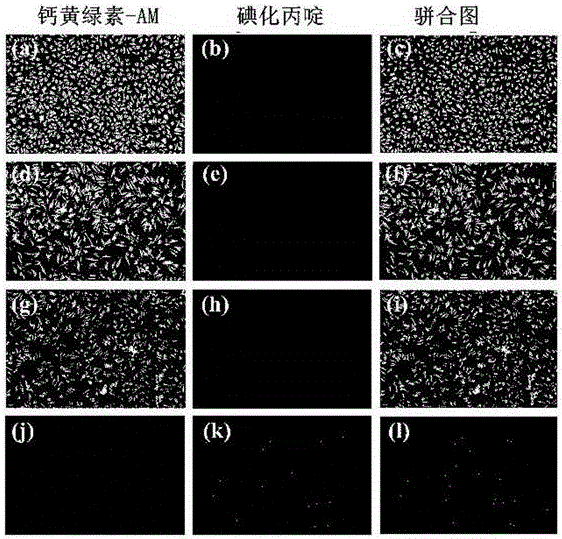
ActiveCN106220588ALow toxicityHigh antibacterial activityAntibacterial agentsOrganic chemistryRed blood cellMeropenem
The invention belongs to the field of medical chemistry and discloses a metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and a preparation method of the same. The compound is represented as the structural formulas 1, 2 or 3 as follows, wherein R is sodium, a methyl group, an n-hexyl group, an n-decyl group or an n-dodecyl group. The inhibitor is combined with a carbapenem antibiotic (e.g. meropenem) to achieve the inhibition activity of metal [beta]-lactamase, so that the sensitivity to the carbapenem antibiotic of a bacterial strain having drug resistance against the carbapenem is recovered. The inhibitor has significant effects on a bacterial strain producing NDM-1 enzyme and having drug resistance against the carbapenem. A red blood cell haemolytic test and a cytotoxicity test prove that the compound is low in toxicity. The series of the compounds is hopeful to serve as a potential candidate medicine of the metal [beta]-lactamase inhibitor.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof 3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof](https://images-eureka.patsnap.com/patent_img/c5aa933e-2687-4a26-bc01-85edd5bbc6ff/519711DEST_PATH_IMAGE002.png)
![3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof 3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof](https://images-eureka.patsnap.com/patent_img/c5aa933e-2687-4a26-bc01-85edd5bbc6ff/DEST_PATH_IMAGE001.png)
![3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof 3-(2-chloro-1-oxopropyl)-spiro[2H-1,3-benzoxazine-2,1'-cyclohexan]-4(3H)-one and synthesis and application thereof](https://images-eureka.patsnap.com/patent_img/c5aa933e-2687-4a26-bc01-85edd5bbc6ff/DEST_PATH_IMAGE003.png)
![Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same](https://images-eureka.patsnap.com/patent_img/8e3380f8-46f7-4f90-9f36-80b500392661/160722101833.PNG)
![Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same](https://images-eureka.patsnap.com/patent_img/8e3380f8-46f7-4f90-9f36-80b500392661/160722101844.PNG)
![Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same Metal [beta]-lactamase inhibitor cyclo-amidodithioformate derivative and preparation method of same](https://images-eureka.patsnap.com/patent_img/8e3380f8-46f7-4f90-9f36-80b500392661/160722101849.PNG)