Synthesis method of meropenem
A synthesis method and technology of meropenem, applied in the field of preparation of meropenem, can solve problems such as short route, achieve the effects of short route, mild reaction conditions, and avoid the use of precious metal rhodium catalysts
Inactive Publication Date: 2011-02-02
SHANGHAI BUDDY BIO PHARM INTERMEDIATES
View PDF3 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This method has a short route, simple operation, and simple and easy-to-get raw materials, but there are also some areas that need improvement.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
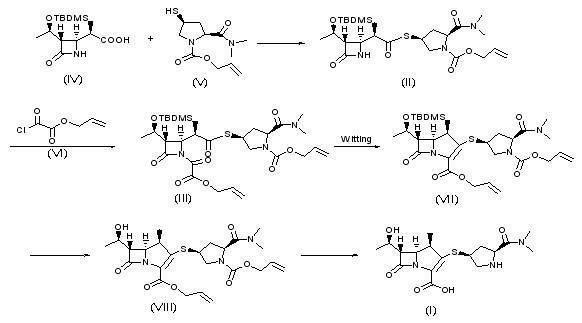
The invention relates to a synthesis method of meropenem, which particularly comprises the following reaction routes: dissolving a compound of the formula (IV) and a compound of the formula (V) into an organic solvent, and then performing a condensation reaction under the action of condensing agent to obtain a compound of the formula (II); dissolving the compound of the formula (II) and a compound of the formula (VI) into methyl benzene, acetic ether or tetrahydrofuran, and then performing a reaction under the action of alkali to generate a compound of the formula (III); dissolving the compound of the formula (III) into cyclohexane, n-octane, the methyl benzene or dimethyl benzene, and then performing a Wittig cyclization reaction under the action of an organic phosphor agent to obtain a compound of the formula (VII); dissolving the compound of the formula (VIII) into a solvent consisting of one of or a plurality of methanol, ethanol, tert-butyl alcohol, isobutanol, isopropanol, the tetrahydrofuran, dioxane, acetone, dichloromethane, chloroform and water, and performing hydrogenation under the action of a palladium catalyst to remove allyl and obtain a target product (I). The synthesis method of the meropenem has the advantages of high yield, mild reaction condition, little environmental pollution, and brief routes.
Description
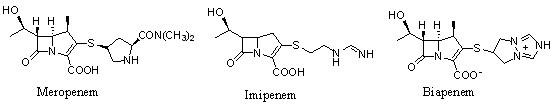
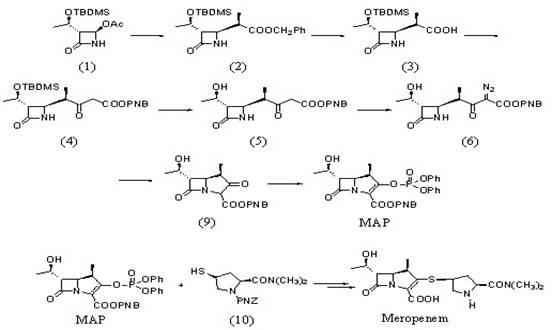
technical field The invention relates to a β-methyl carbapenem antibiotic, in particular to a preparation method of meropenem. Background technique Carbapenem (Carbapenem) is a new type of β-lactam antibiotic, known for its broad antibacterial spectrum and strong antibacterial effect, such as Meropenem (Meropenem), imipenem (Imipenem) And Biapenem (Biapenem), etc., play an important role in curing severe infections. As for the synthesis method of penems, most of the previous studies were to synthesize the corresponding penem side chain compound and the mother core MAP respectively, and then condense the two to remove the protecting group to obtain the penem product. For example, USP4933333 uses 4-acetoxyazetidinone (4AA) as the starting material to obtain the mother core MAP after several steps of reaction. Meropenem is obtained by condensation and deprotection of the mother nucleus and the side chain. However, this method is cumbersome, the synthesis steps are long, a...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D477/20C07D477/04C07D477/08
CPCC07D477/08C07D477/20C07D477/04
Inventor 张工
Owner SHANGHAI BUDDY BIO PHARM INTERMEDIATES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com