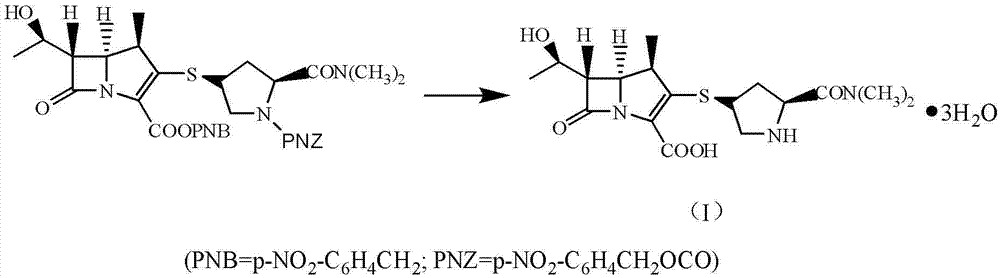
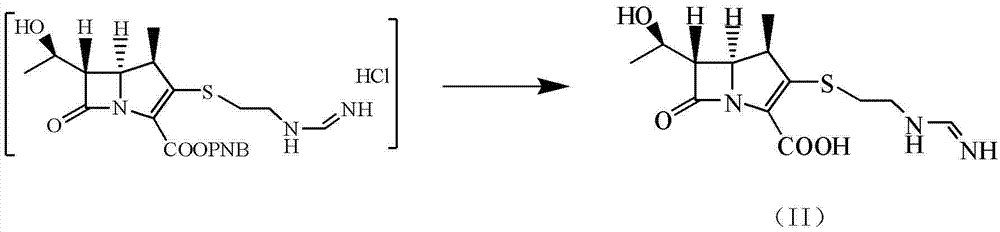
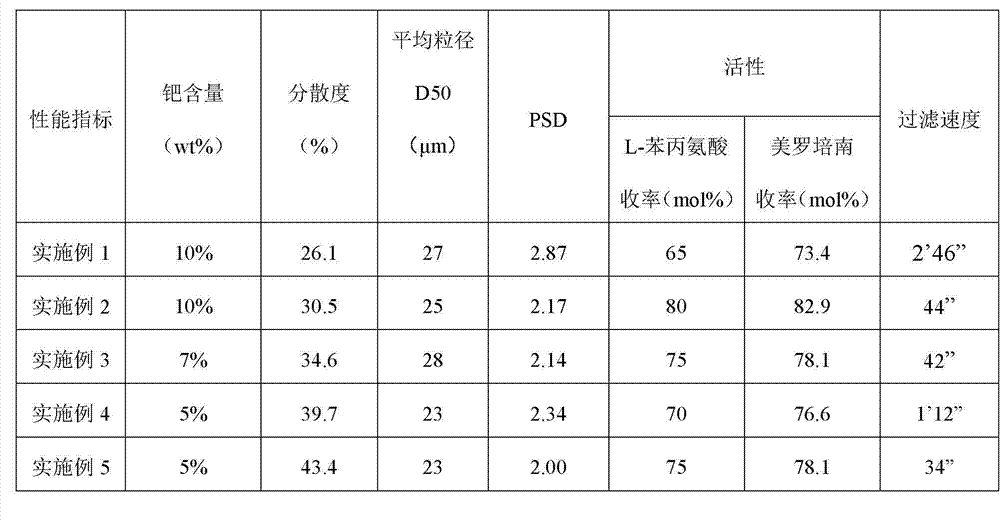
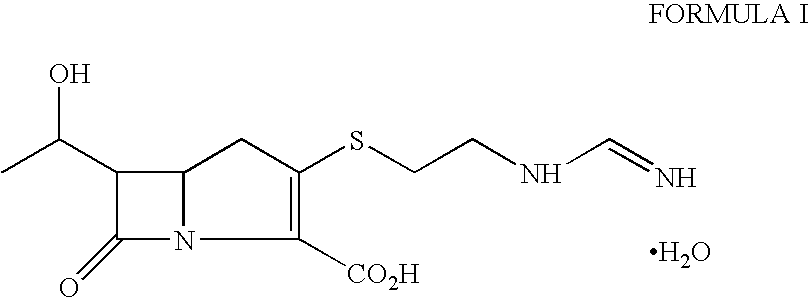
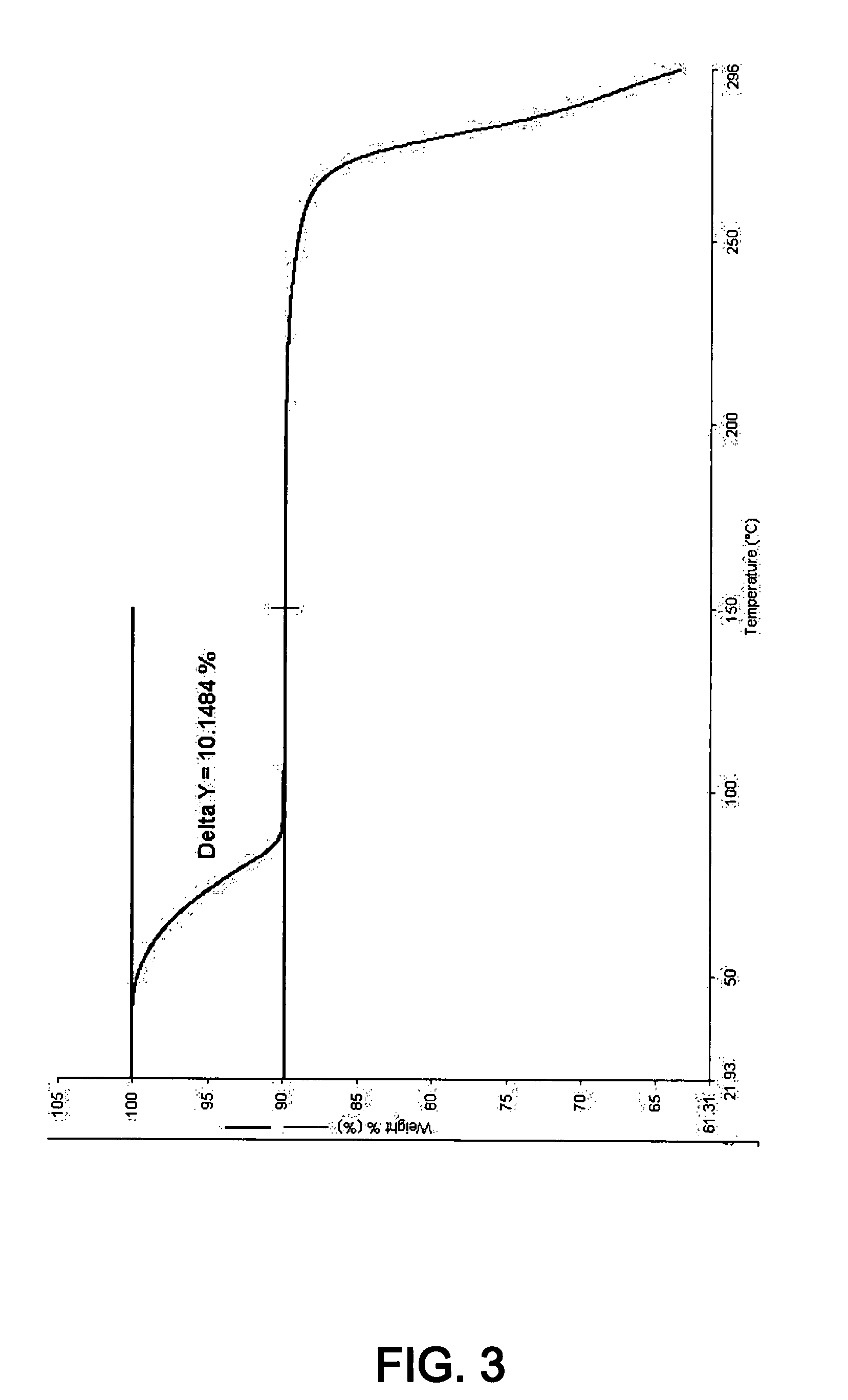
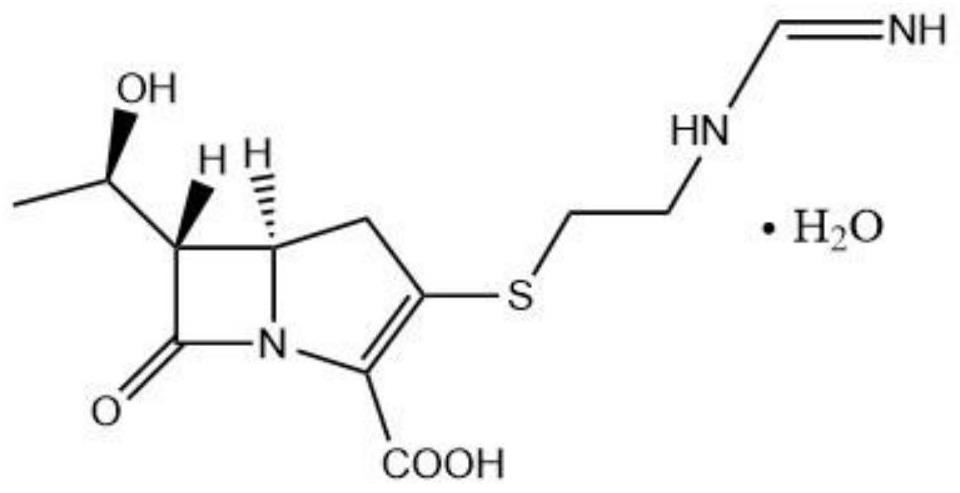
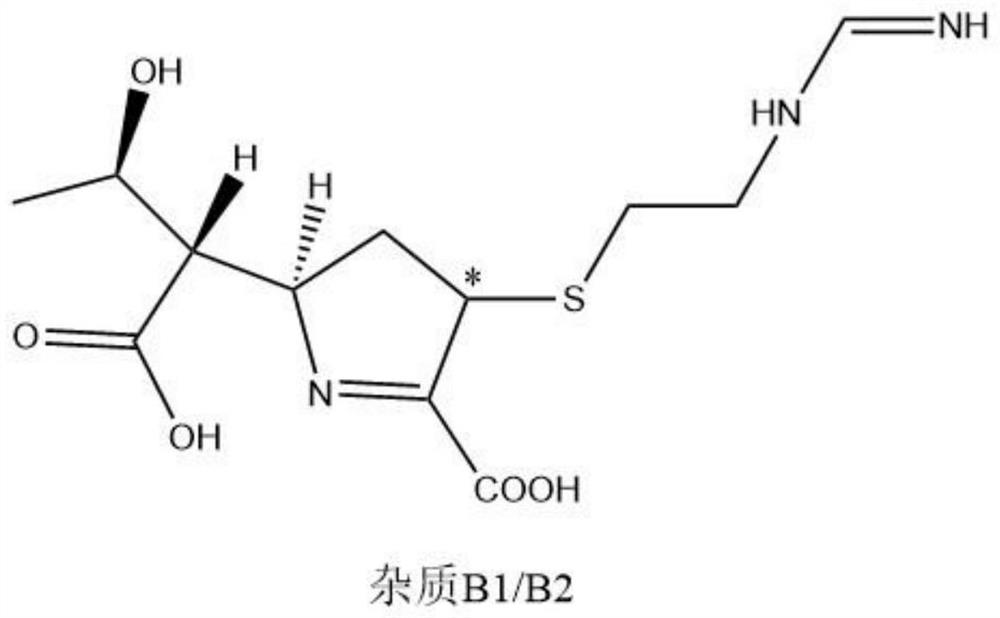
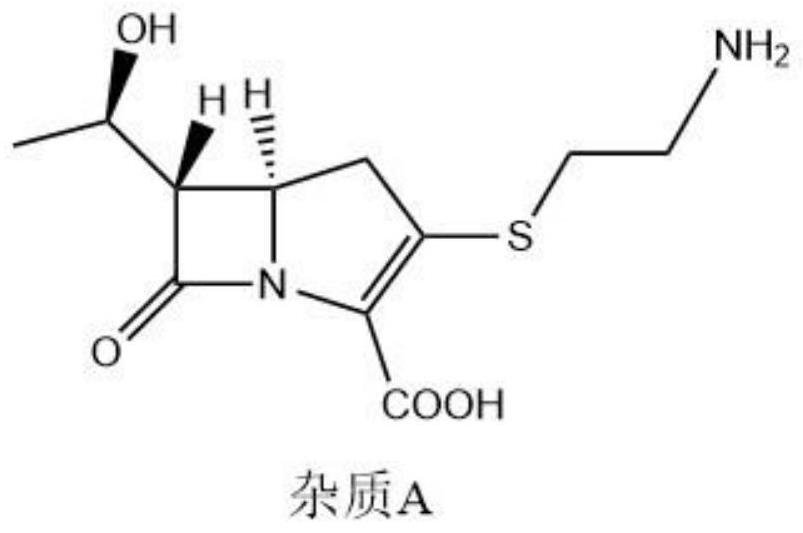
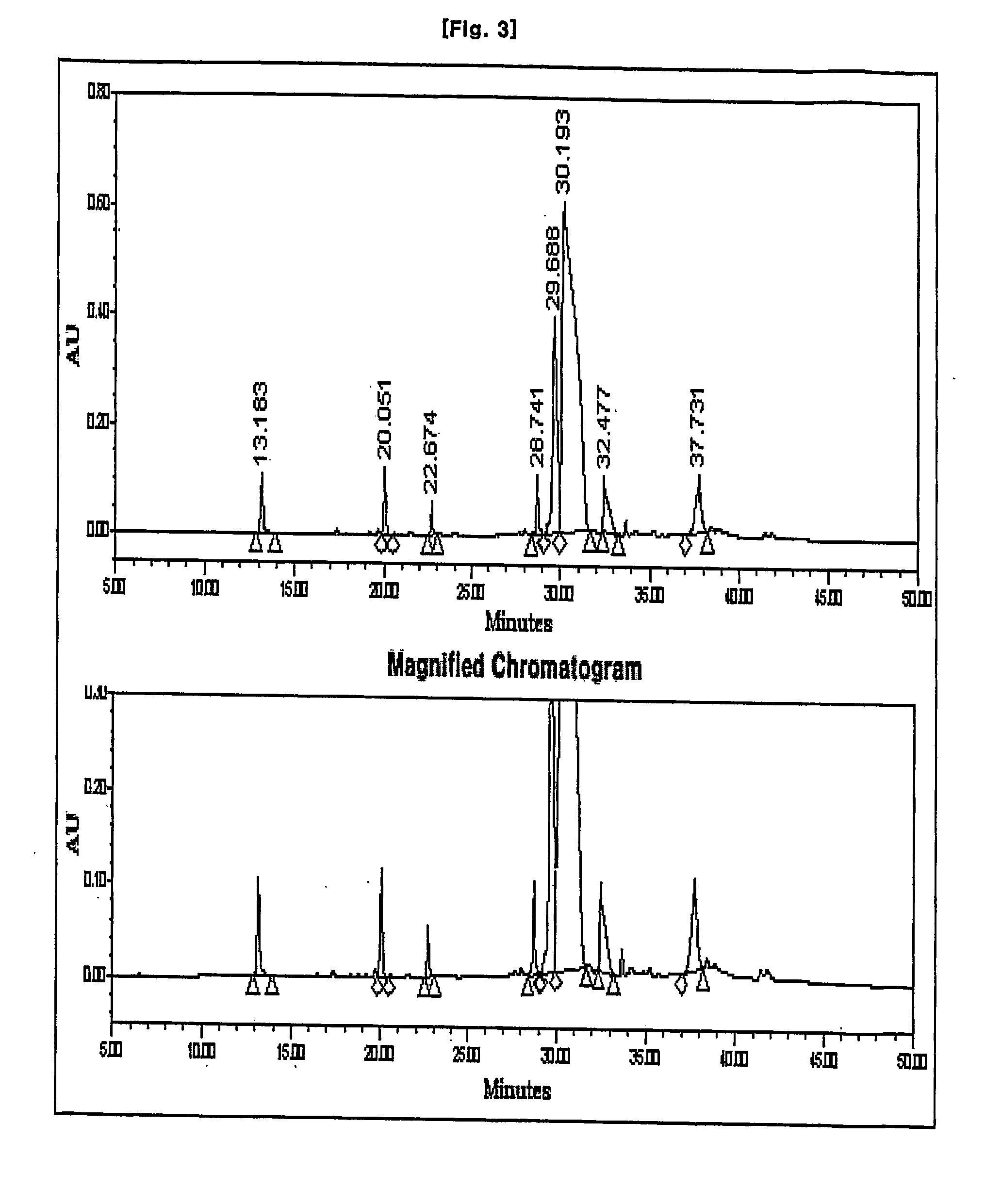
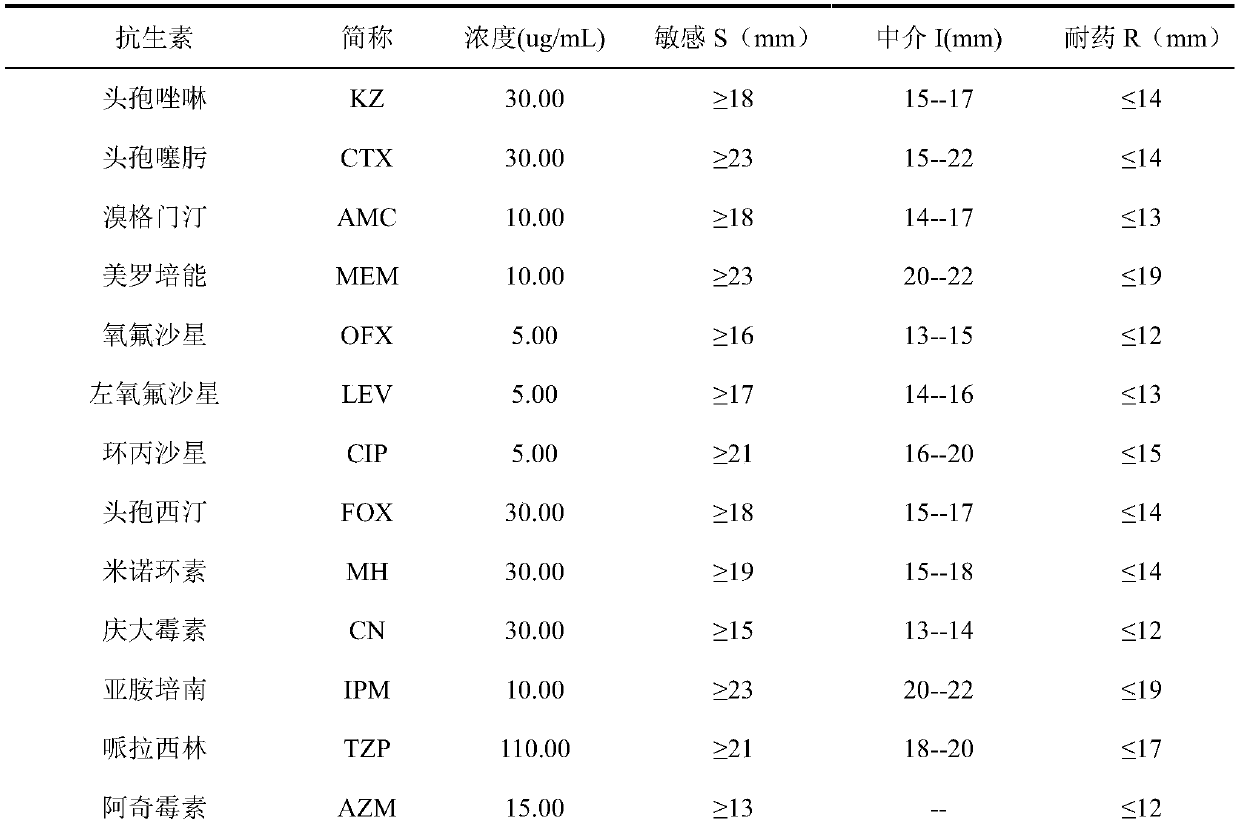
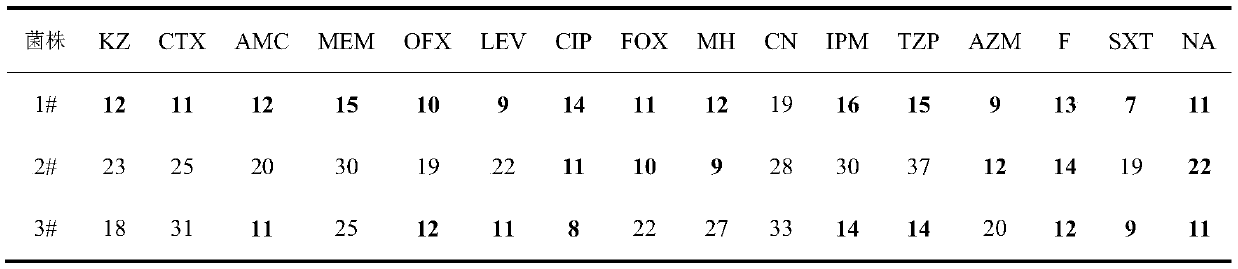
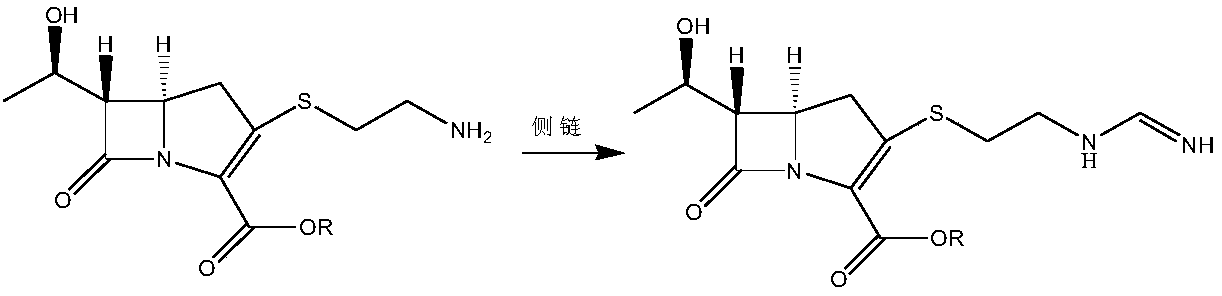
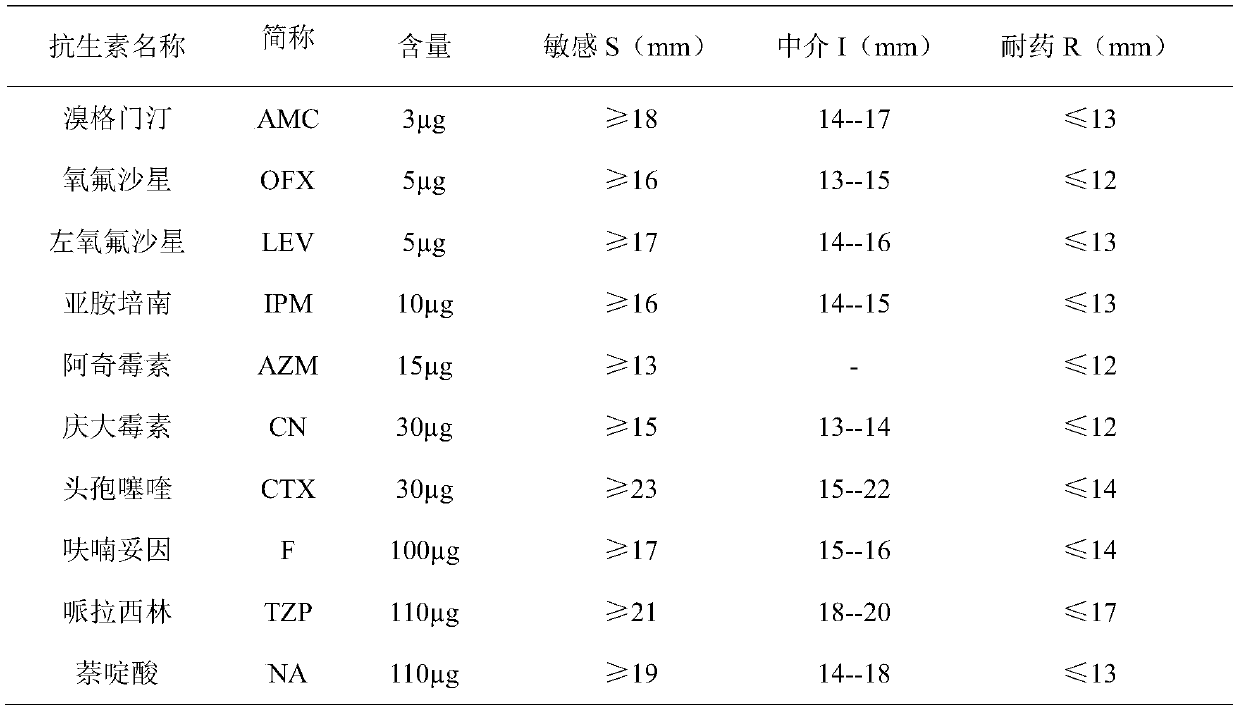
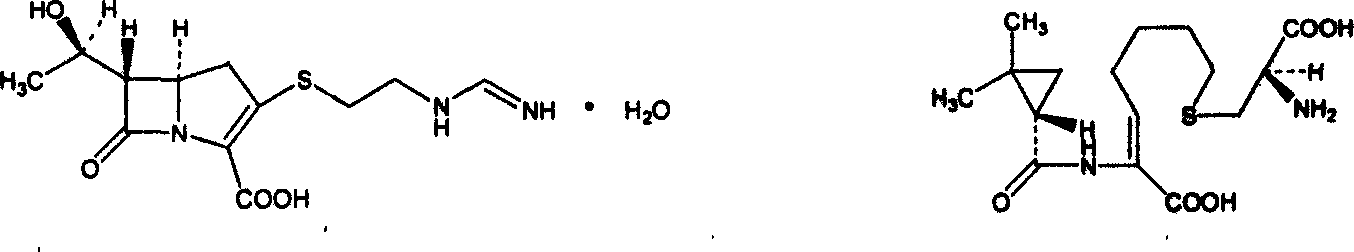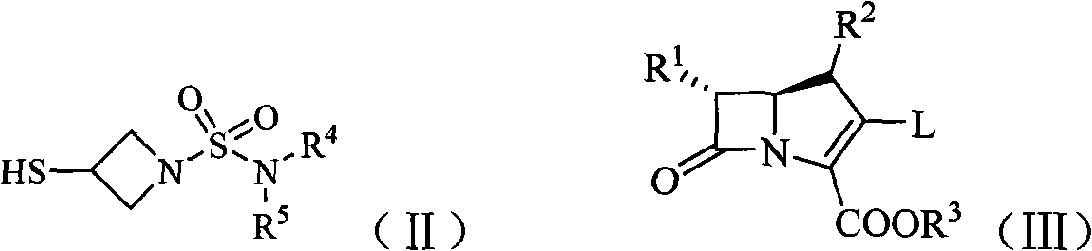Patents
Literature
89 results about "Imipenem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
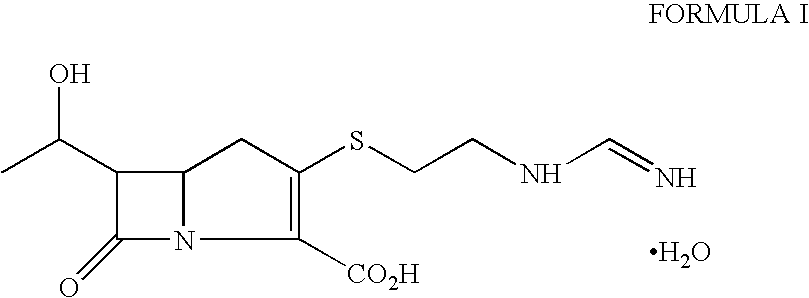
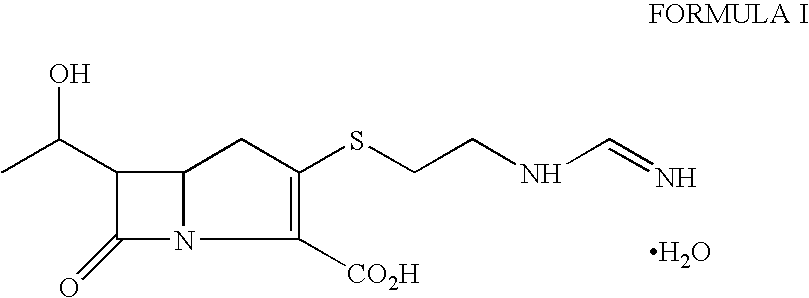
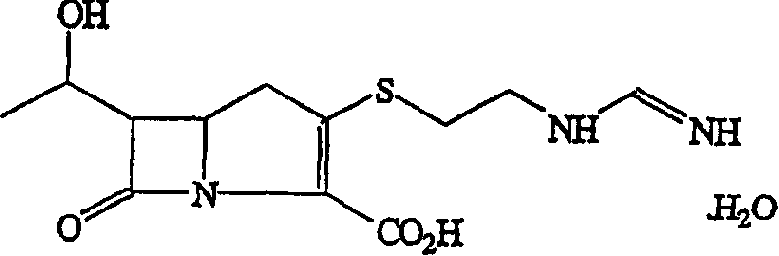
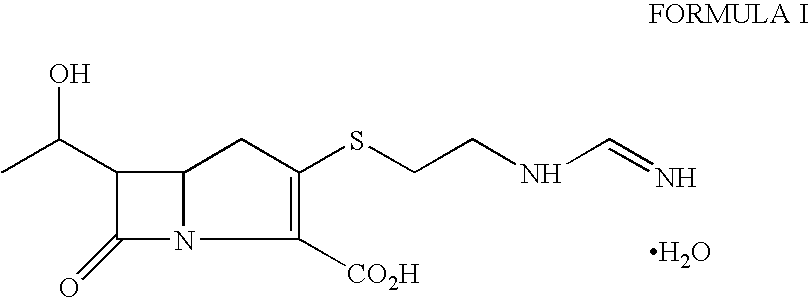
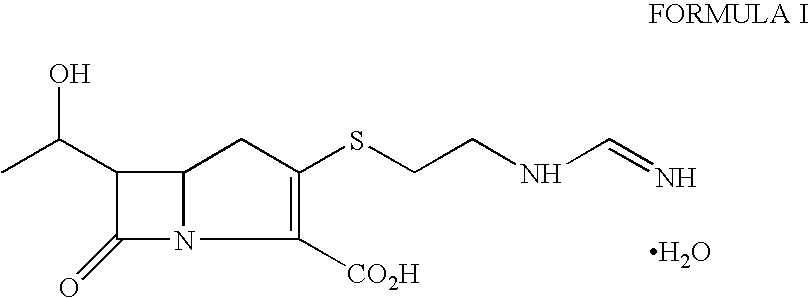
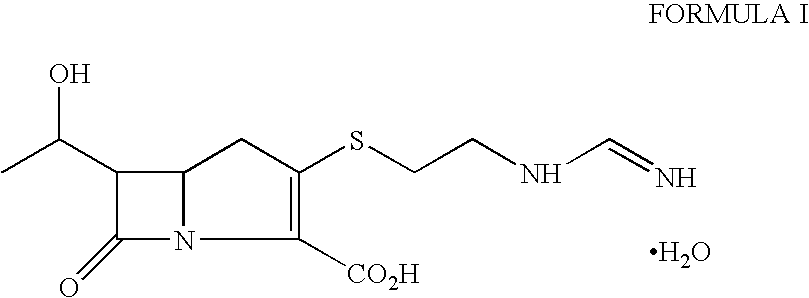
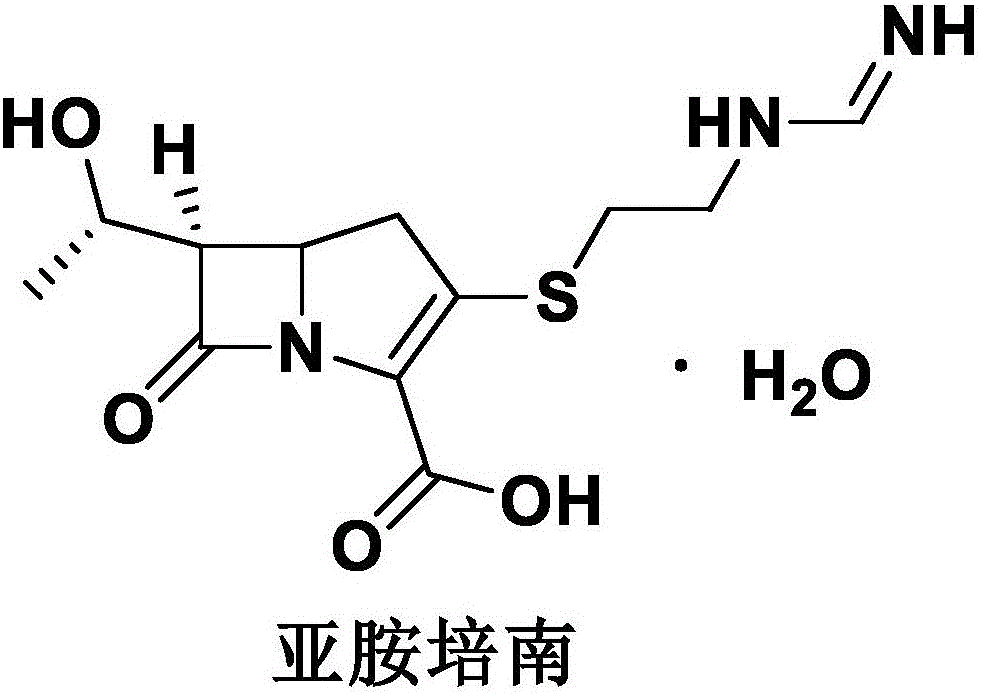
Imipenem (Primaxin among others) is an intravenous β-lactam antibiotic discovered by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s. Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria, thus play a key role in the treatment of infections not readily treated with other antibiotics.
Beta-lactamase inhibitors
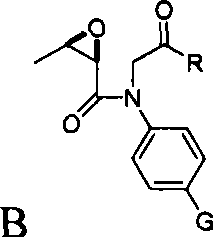
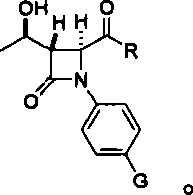
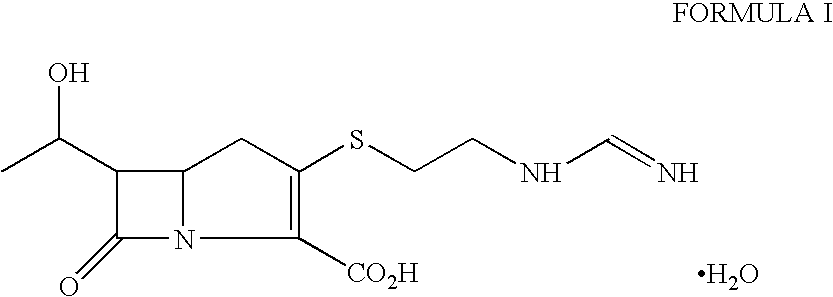
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
Β-lactamase inhibitors
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
Preparation method of high-activity palladium-carbon catalyst for synthesis of imipenem antibiotics
InactiveCN103041805AImprove effective utilizationLarge diameterOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsActivated carbonActive component
The invention relates to a preparation method of a high-activity palladium-carbon catalyst for synthesis of imipenem antibiotics. According to the preparation method of the high-activity palladium-carbon catalyst for the synthesis of the imipenem antibiotics, ammonium chloropalladite and salts thereof are taken as precursor compounds of an active component, namely palladium, powdered activated carbon is taken as a carrier, additives such as sodium citrate are added into Pd impregnation liquid, and then the Pd impregnation liquid is absorbed onto the activated carbon in a segmenting manner and subjected to wet chemical reduction to obtain the high-activity palladium-carbon catalyst; and the catalyst is used for industrial production of the synthesis of the imipenem antibiotics.
Owner:SINO PLATINUM METALS CO LTD
Process for the preparation of crystalline imipenem
The present invention relates to a cost effective and industrially advantageous process for the preparation of imipenem of high purity.
Owner:RANBAXY LAB LTD
Preparation method for imipenem medicine intermediate 4AA
InactiveCN102936262ANo pollution problemRaw materials are cheap and easy to getGroup 4/14 element organic compoundsEpoxyAniline
The invention discloses a preparation method for imipenem medicine intermediate 4AA. The preparation method comprises making 4-substituted aniline into an intermediate A, perform epoxidation on L-threonine to produce (2R, 3R)-epoxy butyric acid; enabling (2R, 3R)-epoxy butyric acid and the intermediate A to undergo a coupling reaction, obtaining an intermediate B, enabling the intermediate B to undergo a cyclization reaction, obtaining an intermediate C, enabling the intermediate C to undergo a hydroxyl protection reaction, obtaining an intermediate D, enabling the intermediate D to be oxidized to form an acetoxy group, and enabling an oxidized product to undergo an ozonation reaction, wherein G is H, F, Cl, Br, a methoxy group, oxethyl or an amino group; and R is H, straight chain alkyl of C1-C6, cyclopropyl, isopropyl, tert-butyl, a phenyl group, p-chlorophenyl, o-chlorophenyl, p-bromophenyl, o-bromophenyl, p-methoxyphenyl, o-methoxyphenyl or m-methoxyphenyl. According to the preparation method, raw materials are cheap and easy to obtain, reaction conditions are mild, the conversion rate and the yield rate are high, and the preparation method is suitable for industrial production.
Owner:ASYMCHEM LAB TIANJIN +4
Process for the preparation of imipenem
Owner:RANBAXY LAB LTD
Process for the preparation of imipenem
InactiveUS7462712B2Efficient processingObvious benefitsAntibacterial agentsOrganic active ingredientsKetoneImipenem
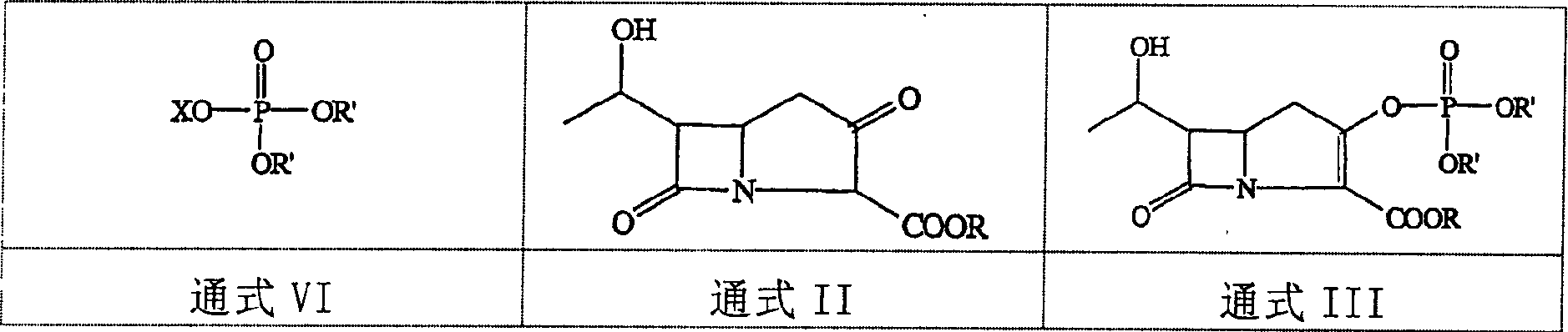
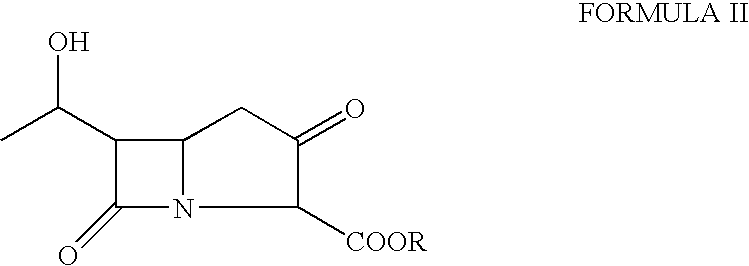
The present invention relates to an improved process for the preparation of imipenem comprising reacting a bicyclo ketone precursor of the Formula II,wherein R is a protecting group, with a phosphorohalidate in the presence of a base and a catalytic amount of dialkylaminopyridine.
Owner:RANBAXY LAB LTD
Carboxyl-containing polymer, preparation method and application thereof, as well as preparation method of supported catalyst and imipenem antibiotic intermediate
ActiveCN104262523AImprove rigidityHigh hardnessOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkPolymer science
The invention discloses a carboxyl-containing polymer, a preparation method and application thereof, as well as a preparation method of a supported catalyst and an imipenem antibiotic intermediate. The polymer is polymerized by three monomers with different structures. The carboxyl-containing polymer is a cross-linked polymer, contains a large number of benzene rings on a polymer chain, and can improve the rigidity and hardness of the polymer so as to effectively improve the mechanical properties of the polymer. Simultaneously, a carboxyl group is taken as a main functional group in the polymer and taken as a carrier, and a metal in the prepared supported metal catalyst has relatively good connection stability with the polymer through coordination reaction of the carboxyl group with the heavy metal. The stability of the supported metal catalyst can be improved through the factors in the two aspects, and the catalyst can be repeatedly used without losing catalytic activity. Simultaneously, the loss of active ingredients in the heavy metal can be reduced, and the production cost can be reduced.
Owner:ASYMCHEM LAB TIANJIN +4
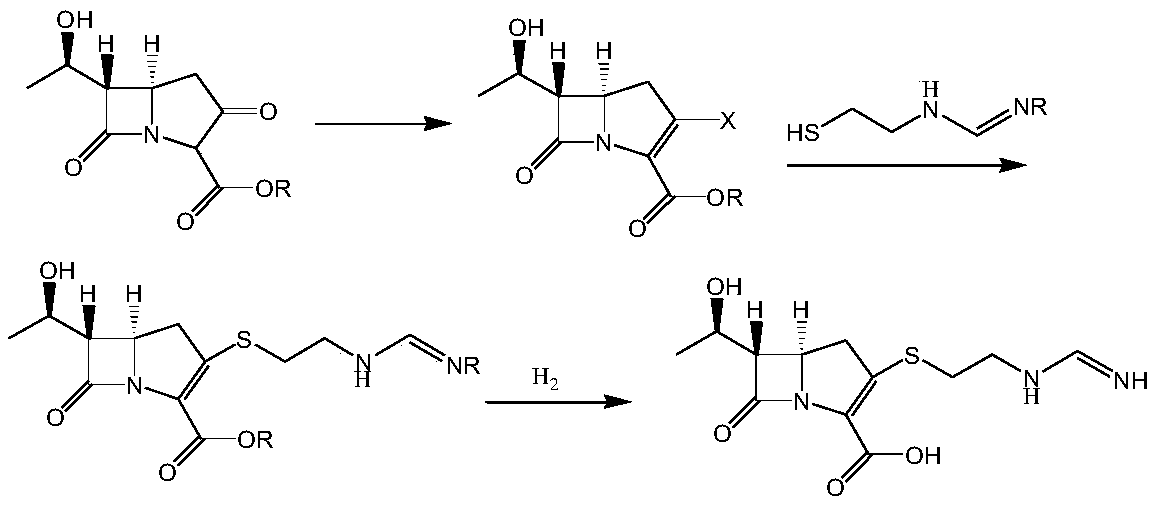
Imipenem intermediate and preparation method of imine peinan
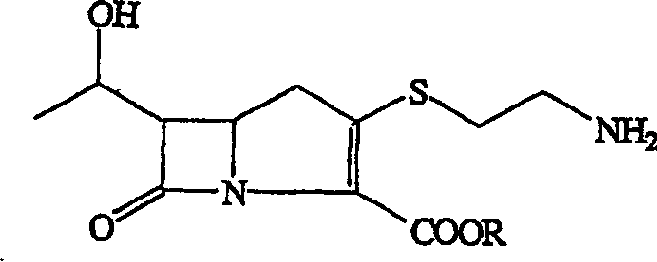
The present invention relates to a new intermediate compound for synthesizing imipenen. Said invention also provides the chemical structure formula of said intermediate compound. Besides, said invention also provides a method for preparing imipenen side chain and imipenen.
Owner:安徽巴迪生物医药科技有限公司
Preparing method of freeze-dried antibiotic formulation
InactiveCN1568975AEffective for severe infectionsNot easily oxidizedAntibacterial agentsOrganic active ingredientsImipenem/cilastatinFreeze-drying
The invention dislcloses the process for preparing Imipenem / Cilastatin Sodiun cryodesiccation powder preparation, which is prepared from imipenem, Cilastatin Sodium or the mixture of imipenem and Cilastatin Sodium.
Owner:济南久创化学有限责任公司
Method for detecting plasma protein binding rate of meropenem or imipenem by combining liquid chromatography-mass spectrometry technology with ultrafiltration technology
According to a method for detecting the plasma protein binding rate of meropenem or imipenem by combining a liquid chromatography-mass spectrometry technology with an ultrafiltration technology, the liquid chromatography-tandem mass spectrometry detection technology is combined with the ultrafiltration technology, and the total concentration of meropenem or imipenem in plasma and the free concentration of drugs in ultrafiltrate are respectively detected. And an MOPs stabilizer is added during sample treatment, so that the problem of poor drug stability is solved, and the determination result is more accurate. The method can be used for rapidly detecting the protein binding rate of the drug at high throughput, and is particularly suitable for unstable drug molecules.
Owner:PEKING UNIV THIRD HOSPITAL +1
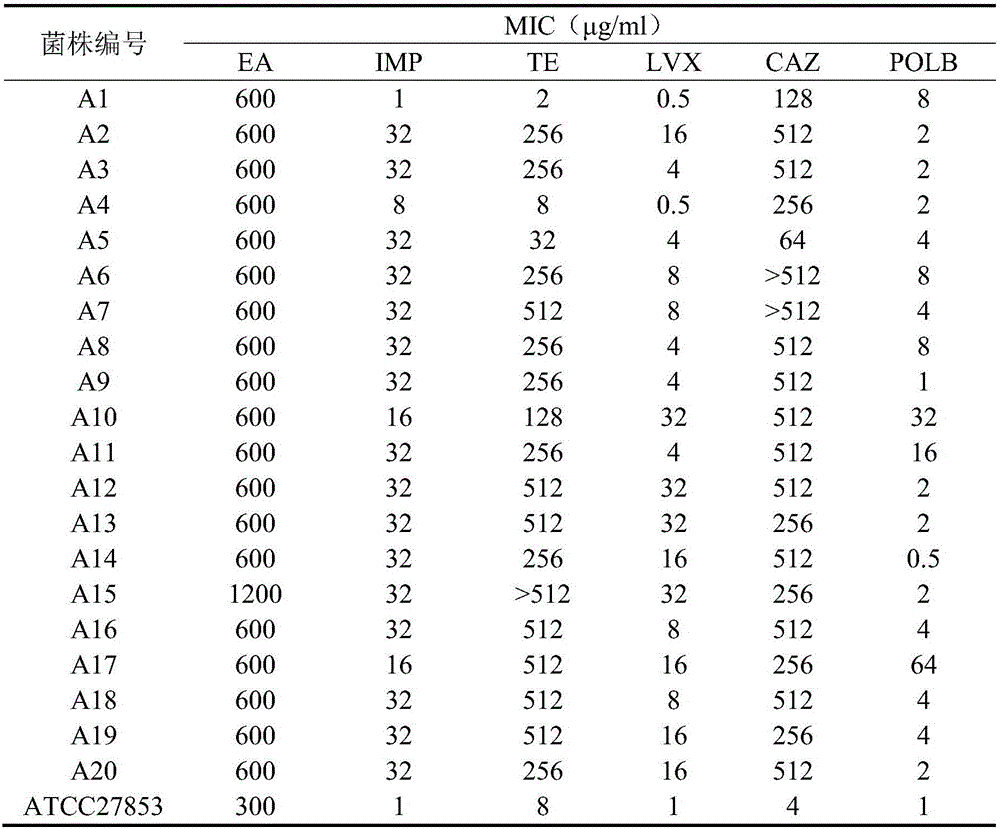
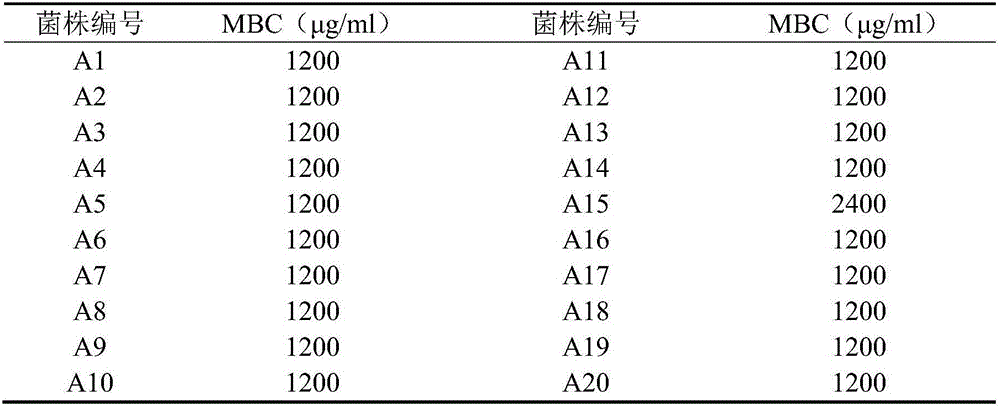
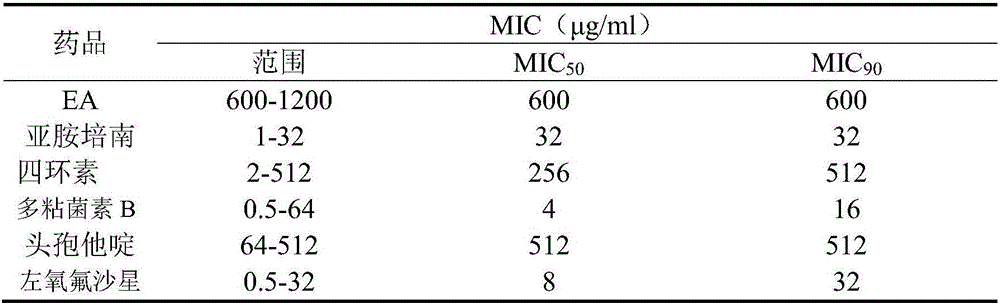
Application of pithecellobium clypearia extracts to preparation of multi-drug resistant acinetobacter baumannii medicine
ActiveCN105816511AReduce dosageAddressing drug resistanceAntibacterial agentsPlant ingredientsAntibiotic sensitivityAntibiotic Y
The invention discloses application of pithecellobium clypearia extracts to preparation of multi-drug resistant acinetobacter baumannii medicine. The pithecellobium clypearia extracts are prepared through the method includes the steps that pithecellobium clypearia coarse powder is extracted with water or an ethyl alcohol solution, the obtained extraction liquid is extracted with ethyl acetate, and the obtained extracts are target products. The antibacterial function of the pithecellobium clypearia extracts to multi-drug resistant acinetobacter baumannii and the sensitivity enhancing function of the pithecellobium clypearia extracts to similar antibiotics are disclosed for the first time. Tests prove that the pithecellobium clypearia extracts and imipenem or tetracycline or polymyxin B or ceftazidime or levofloxacin take effect together, and the use amount of antibiotics can be reduced by 50-87% compared with the mode that only the pithecellobium clypearia extracts are used. The pithecellobium clypearia extracts can serve as natural multi-drug resistant acinetobacter baumannii medicine or an antibiotic sensitivity-enhancing agent, and is applied to treatment of diseases caused by acinetobacter baumannii. A new means and alternative medicine are provided for solving the drug resistance problem of similar antibiotics.
Owner:HUACHENG PHARMA FACTORY GAUNGZHOU
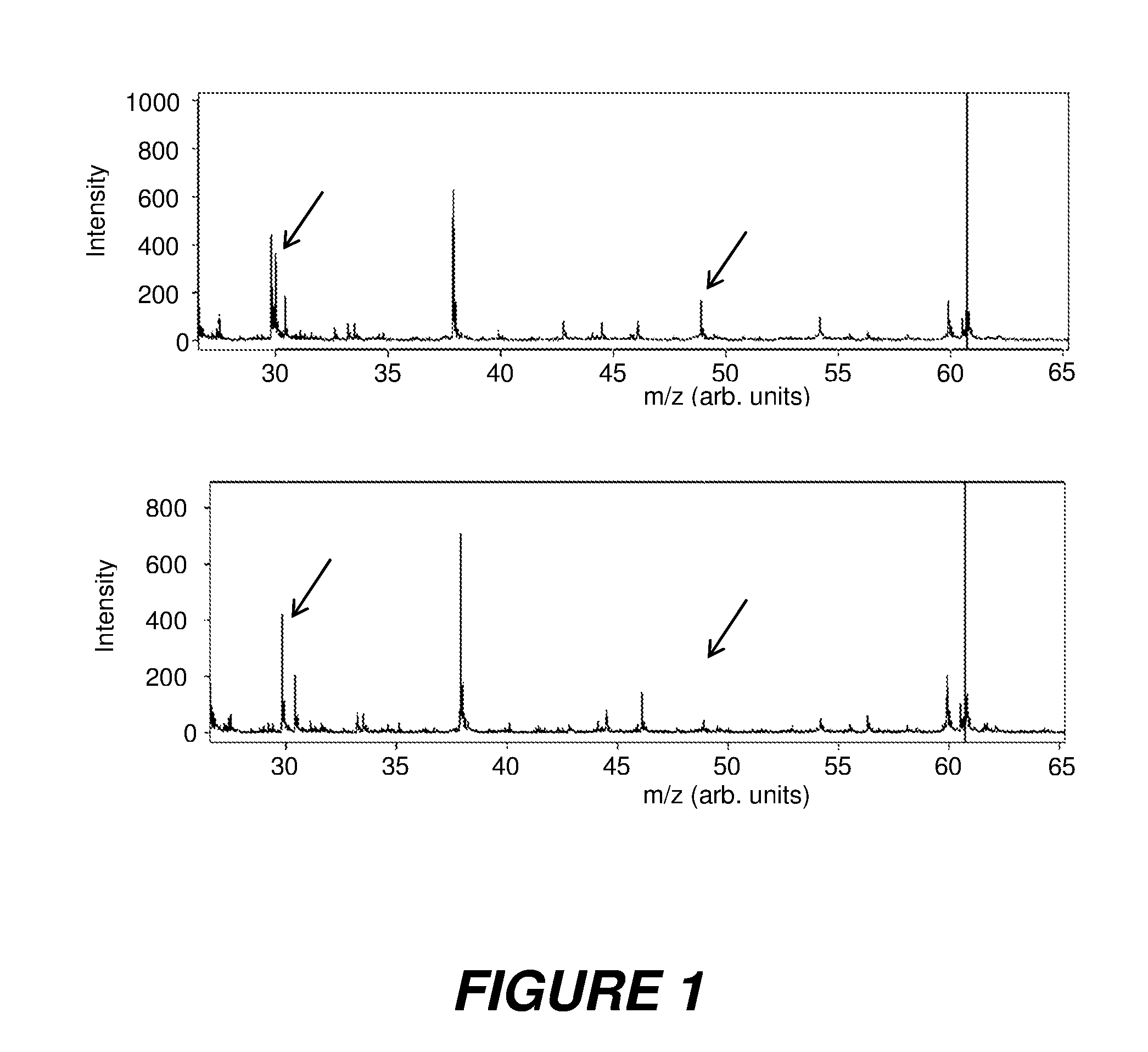
Rapid test for microbial resistances by mass spectrometry
ActiveUS20160298164A1Small volumeParticle separator tubesMicrobiological testing/measurementImipenemMicrobial resistance
The invention relates to methods and instruments for determining the resistances of microbes to antibiotics, in particular those microbes which produce beta-lactamases. The method determines the resistance of the microbes in less than an hour by incubating a tiny quantity of the microbes on a mass spectrometric sample support plate after they have been combined with a dosed quantity of a suitable antibiotic, for example the beta-lactam antibiotic imipenem, and by direct mass spectrometric measurement of the breakdown of the antibiotic by the microbial enzymes.
Owner:BRUKER DALTONIK GMBH & CO KG
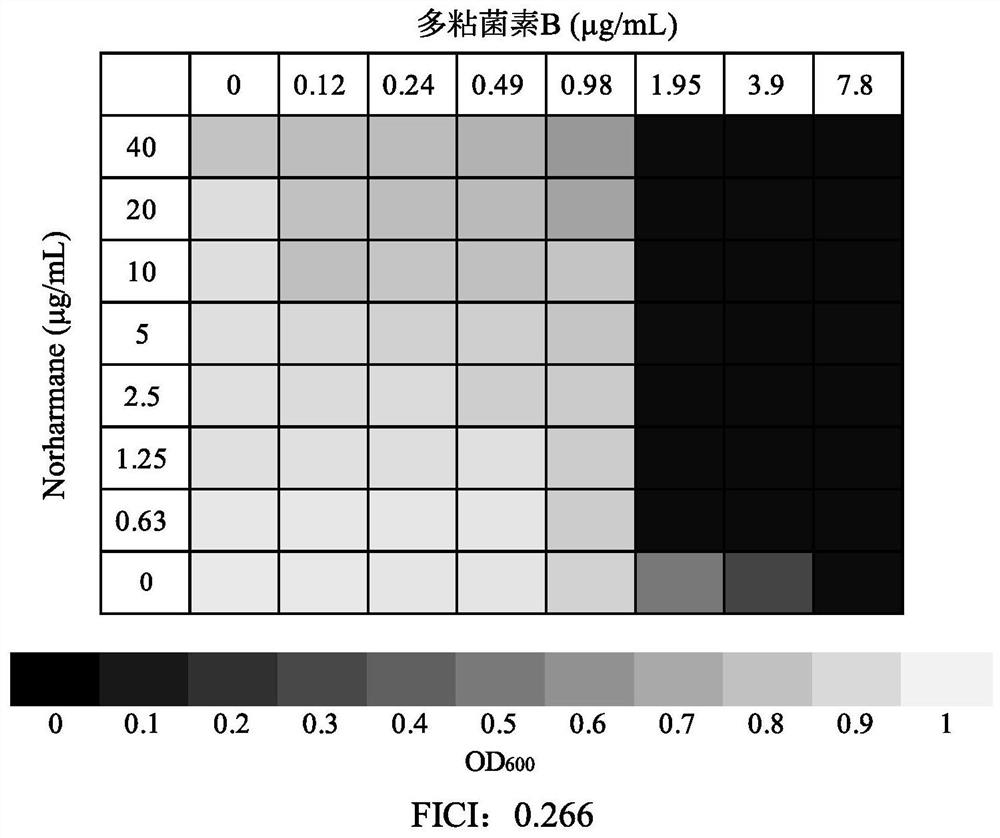
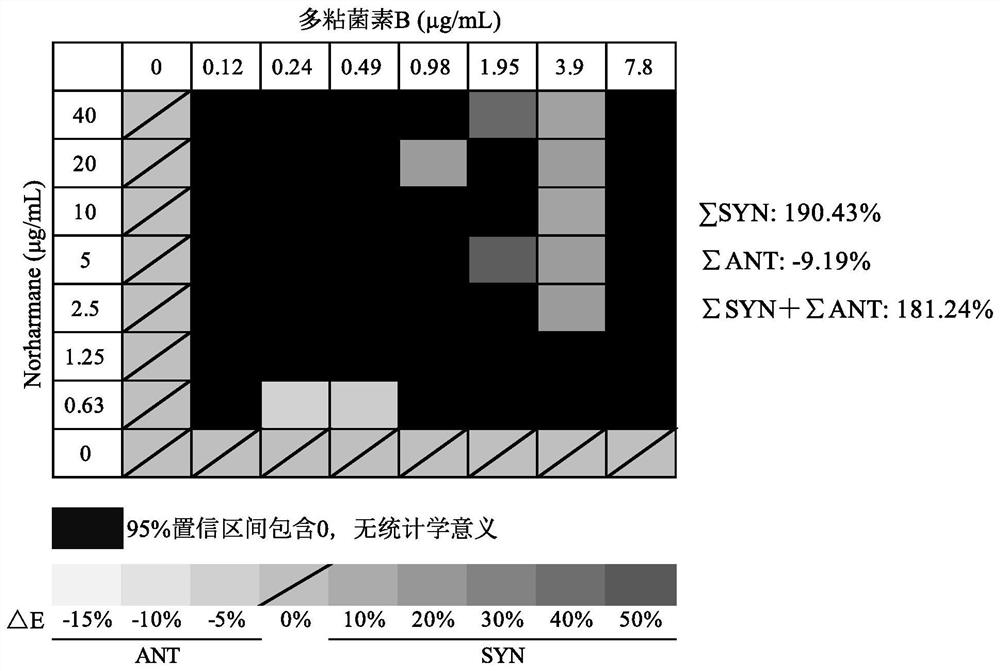
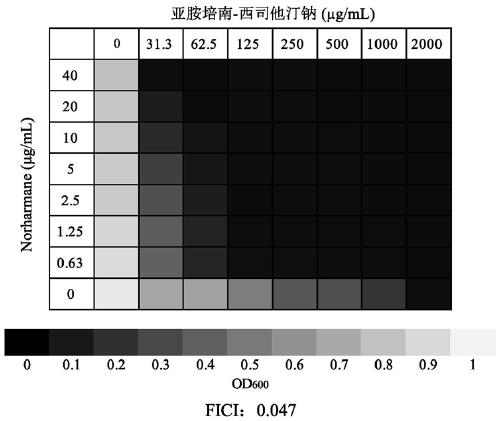
Application of norharmane in improvement of antibiotic antibacterial activity
ActiveCN111632051AReverse drug resistanceHigh antibacterial activityAntibacterial agentsOrganic active ingredientsAntibacterial activityPolymyxin B
The invention discloses an application of norharmane in improvement of antibiotic antibacterial activity. When norharmane is combined with antibiotic polymyxin B, imipenem-cilastatin sodium or levofloxacin for application, the antibacterial activity on drug-resistant pseudomonas aeruginosa can be enhanced, and a synergistic antibacterial effect can be generated. According to the method for obviously improving the killing capacity of the antibiotics on the drug-resistant pseudomonas aeruginosa, the dosage of the antibiotics required for achieving the same treatment effect is greatly reduced, and a research direction is provided for the development of new drugs and the new use of old drugs.
Owner:ZHEJIANG UNIV OF TECH
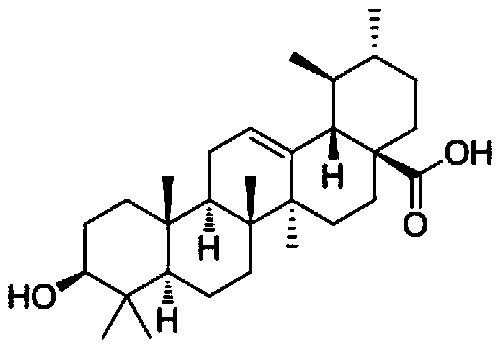
Application of ursolic acid in inhibiting growth of multi-drug-resistance enterobacter cloacae
InactiveCN110279701AMitigate or resolve drug-resistant infectionsReduce fatality rateAntibacterial agentsOrganic active ingredientsInfections problemsCase fatality rate
The invention discloses application of ursolic acid in inhibiting growth of multi-drug-resistance enterobacter cloacae. According to a good in-vitro killing function of ursolic acid upon anthropogenic multi-drug-resistance enterobacter cloacae which resists to cefazolin, cefotaxime, aupmentn, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocycline, imipenem, piperacillin, azithromycin and macrodantin, growth of multi-drug-resistance enterobacter cloacae can be inhibited, the lowest sterilization concentration is 0.6mg / mL, and the lowest antibacterial concentration is 0.3mg / mL. The invention discloses an inhibition function of the ursolic acid upon the multi-drug-resistance enterobacter cloacae, and the ursolic acid is capable of effectively alleviating or solving the drug-resistance infection problem of the multi-drug-resistance enterobacter cloacae and reducing the case fatality rate, provides new ideas for inhibiting anthropogenic multi-drug-resistance enterobacter cloacae, and has great practical significances.
Owner:SHAANXI UNIV OF SCI & TECH
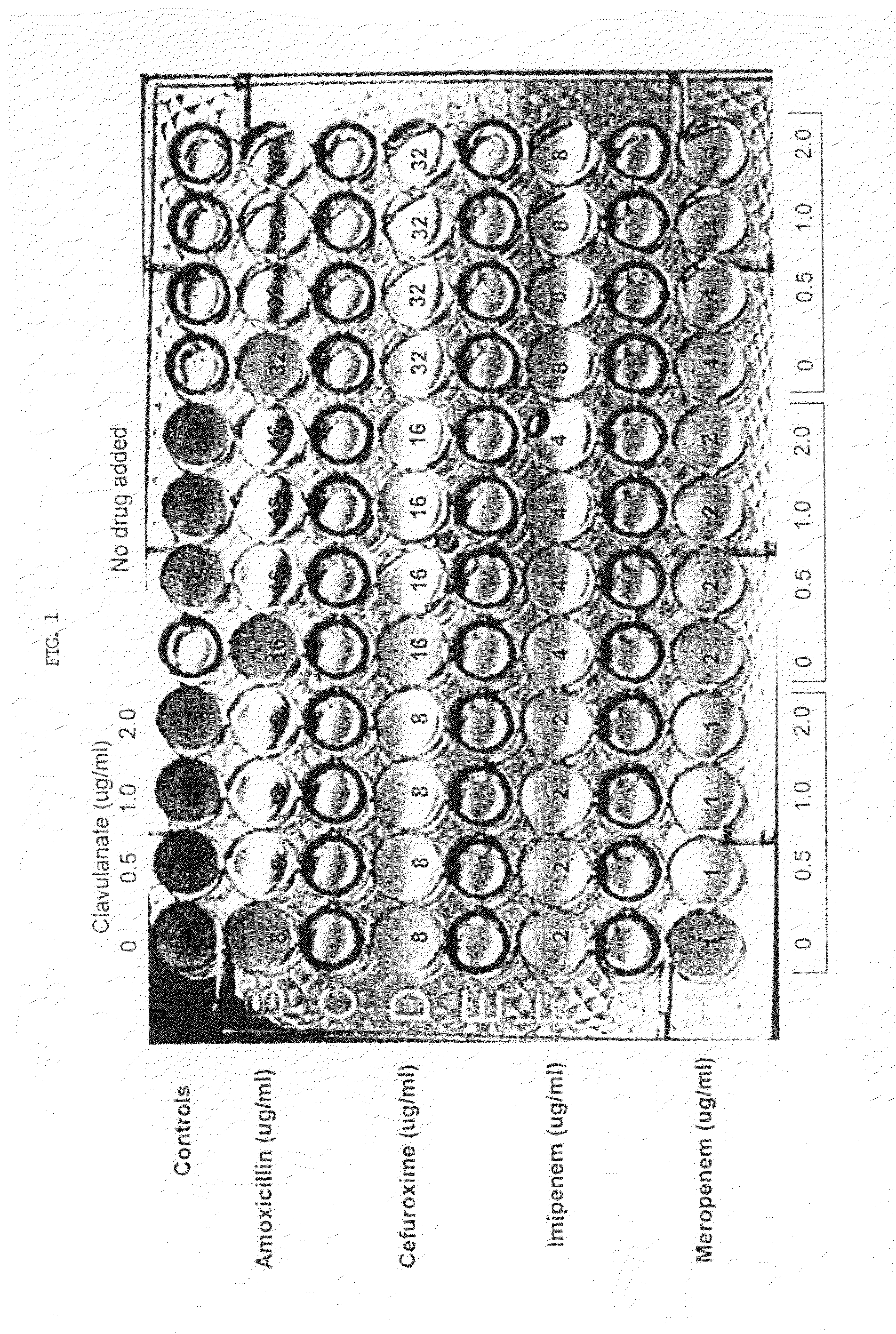
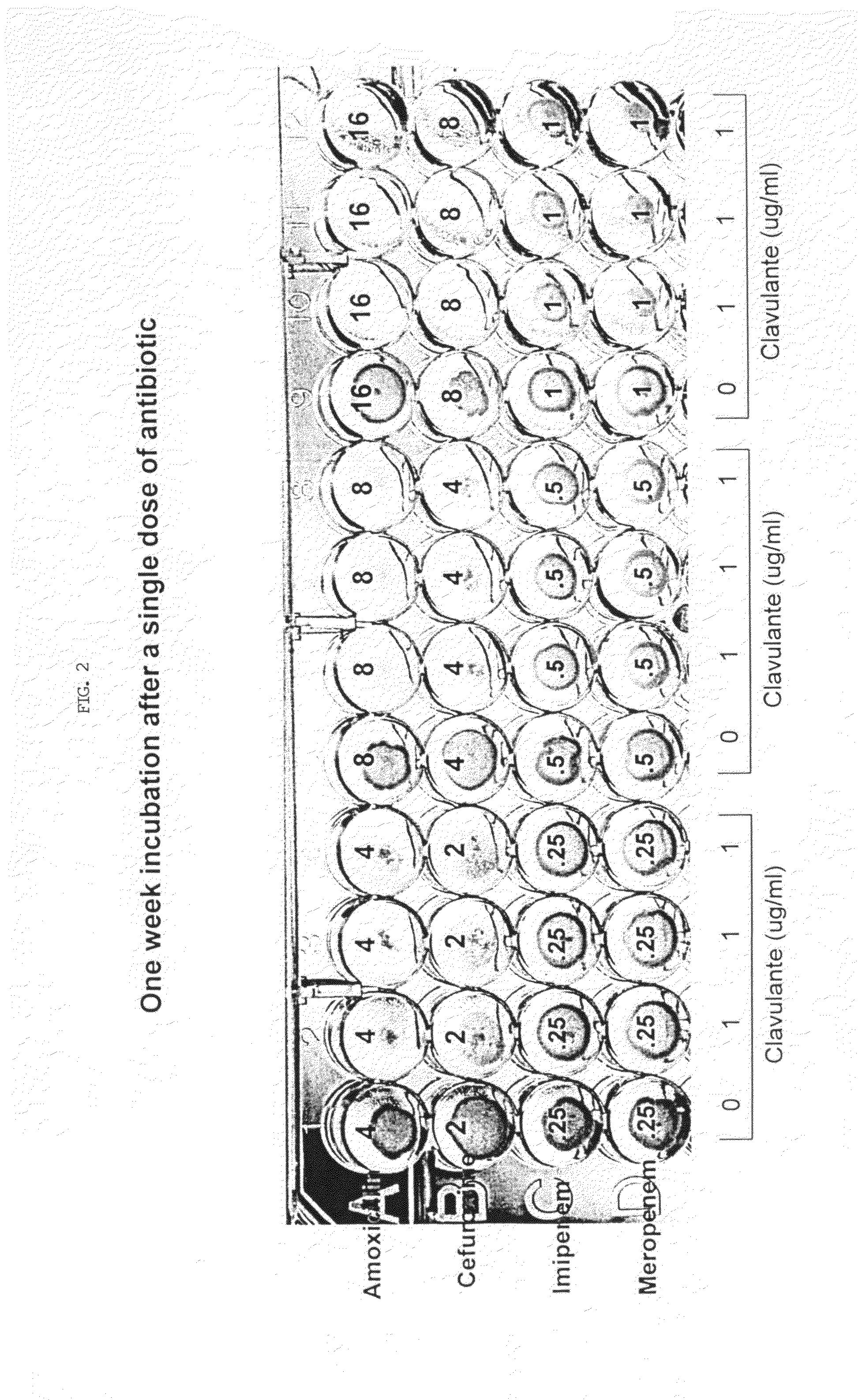
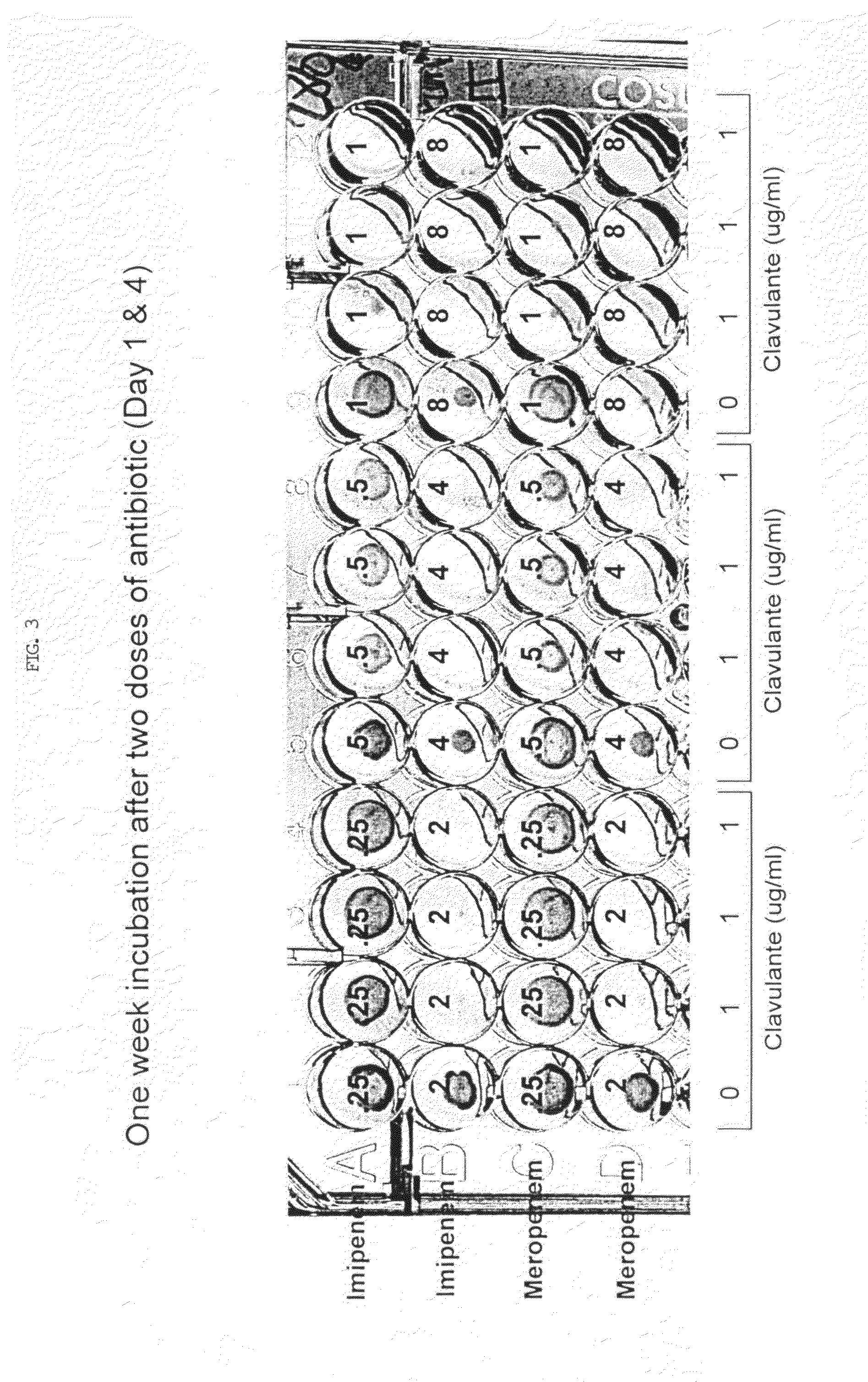
Method for treating tuberculosis
The present invention generally relates to methods for treating tuberculosis in a subject comprising administering to the subject an antibiotic in conjunction with clavulanic acid or salt thereof. The antibiotic can be carbapenem (e.g., meropenem or imipenem) or cefuroxime. The present invention also relates to related pharmaceutical compositions and methods for manufacturing said pharmaceutical compositions.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
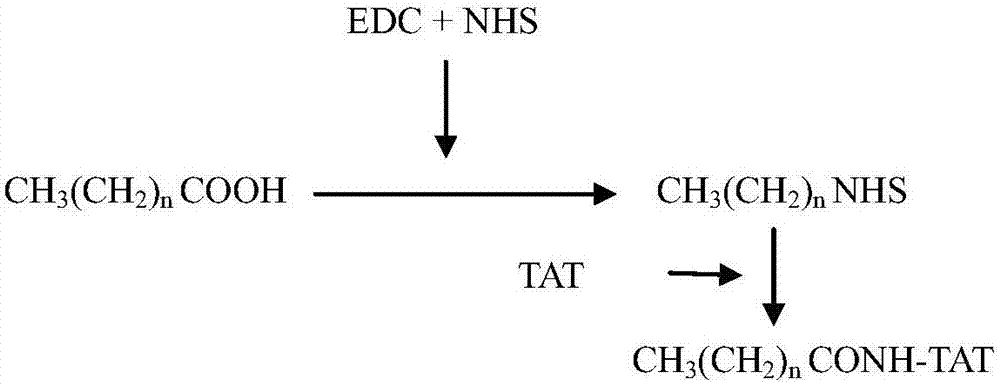
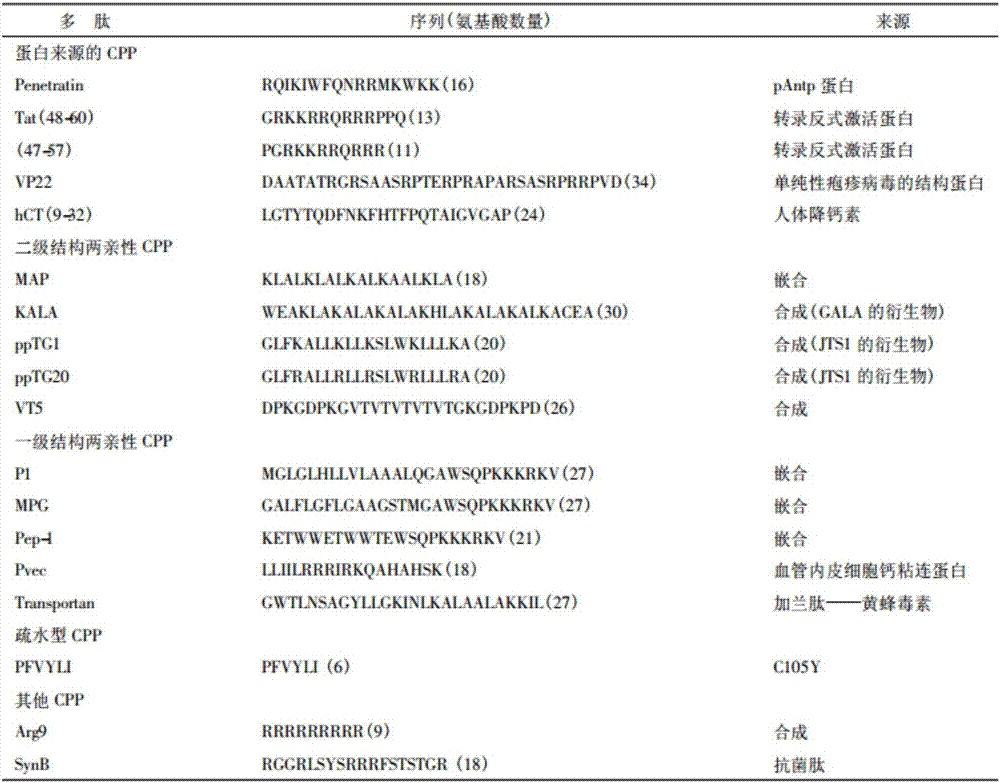
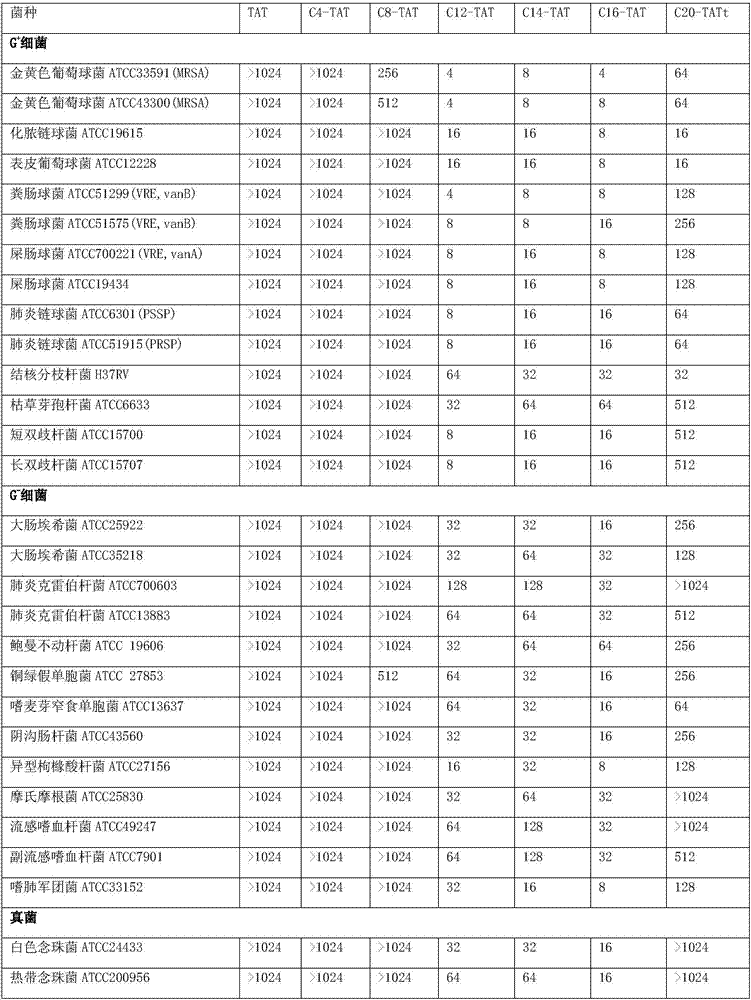
Lipophilic compound conjugate of cell penetrating peptide and its application in antibiosis
ActiveCN107236022AGood in vivo antibacterial activityEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsAntimicrobial peptidesClarithromycin
The invention discloses a lipophilic compound conjugate of cell penetrating peptide and its application in antibiosis. The lipophilic compound conjugate is formed by connecting cell penetrating peptide, and a lipophilic compound connected with an amino terminal or a carboxyl terminal of the cell penetrating peptide. The lipophilic compound conjugate of antimicrobial peptide has good inhibiting and killing effects on gram positive bacteria, gram negative bacteria and fungus, so the conjugate has broad-spectrum antibacterial effect,. On inhibition of methicillin-resistant staphylococcus aureus, C12-TAT united clarithromycin has additive effect; C12-TAT united imipenem has obvious synergistic effect. On inhibition of pseudomonas aeruginosa, C16-TAT united clarithromycin has additive effect; C16-TAT united imipenem has obvious synergistic effect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI +1
Novel inhibitors of beta-lactamase
A class of 7-oxo-2,6-diazabicyclo-[3.2.0]-heptane-6-sulfonic acid compounds substituted at the two position of the bicyclic ring with a heterocyclylaminocarbonyl group or a carbocyclylaminocarbonyl group are β-lactamase inhibitors. The compounds and their prodrugs and pharmaceutically acceptable salts are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds are suitable for use with β-lactam antibiotics (e.g., imipenem and ceftazidime) against micro-organisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME CORP
Refining method of imipenem
ActiveCN114671877ASmall and uniform particle sizeAvoid introducing microbiological risksOrganic chemistryMicroorganismChemical compound
The invention relates to the technical field of preparation of heterocyclic compounds, in particular to a refining method of imipenem. The method comprises the following steps: dissolving, filtering, sub-packaging crystal growing seeds, growing crystals, and growing the crystals again. Natural seed crystals are formed in the production process in a segmented crystallization mode, and the risk of introducing microorganisms caused by external seed crystals can be avoided. According to the invention, an anti-crystallization mode is adopted for crystallization, and the prepared crystal product is small and uniform in particle size; the process is the same as the original grinding process. Meanwhile, the process conditions are loose, operation is easy, and the method is suitable for large-scale production.
Owner:ZHUHAI UNITED LAB
An imine-resistant bacillus pyocyaneus bacteriophage and its use for treating infection therefrom
InactiveCN1699559AAnti-infection effect in vivoHost spectrum widthAntibacterial agentsViral/bacteriophage medical ingredientsIn vivoAnti-infective therapy
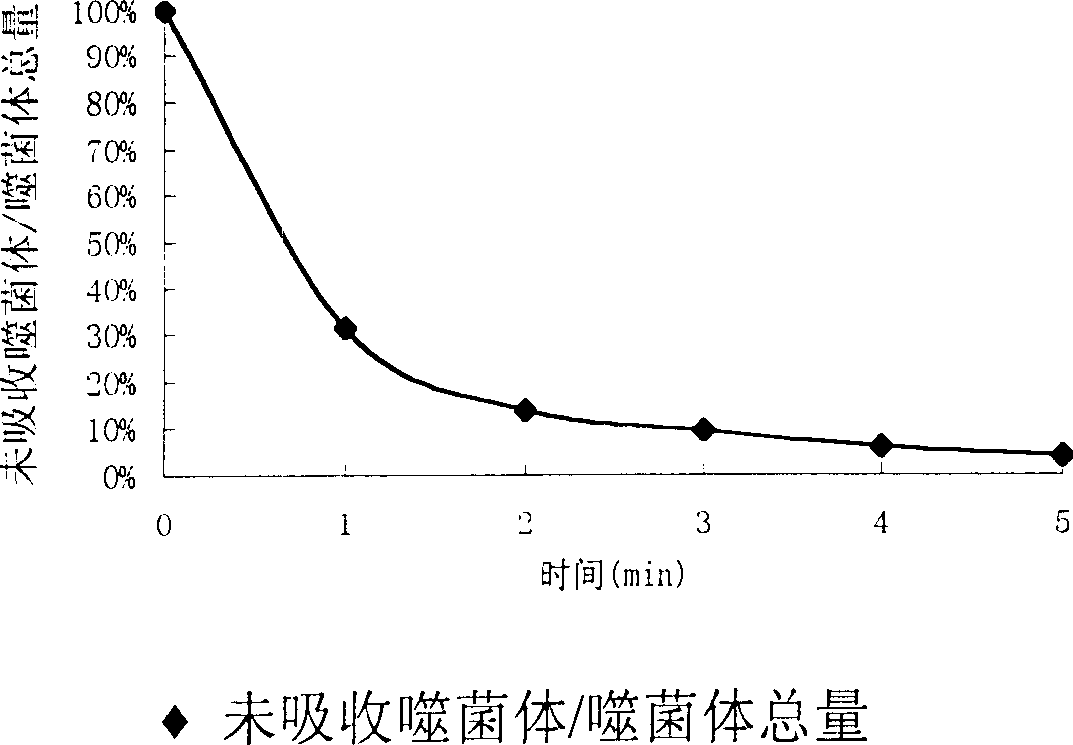
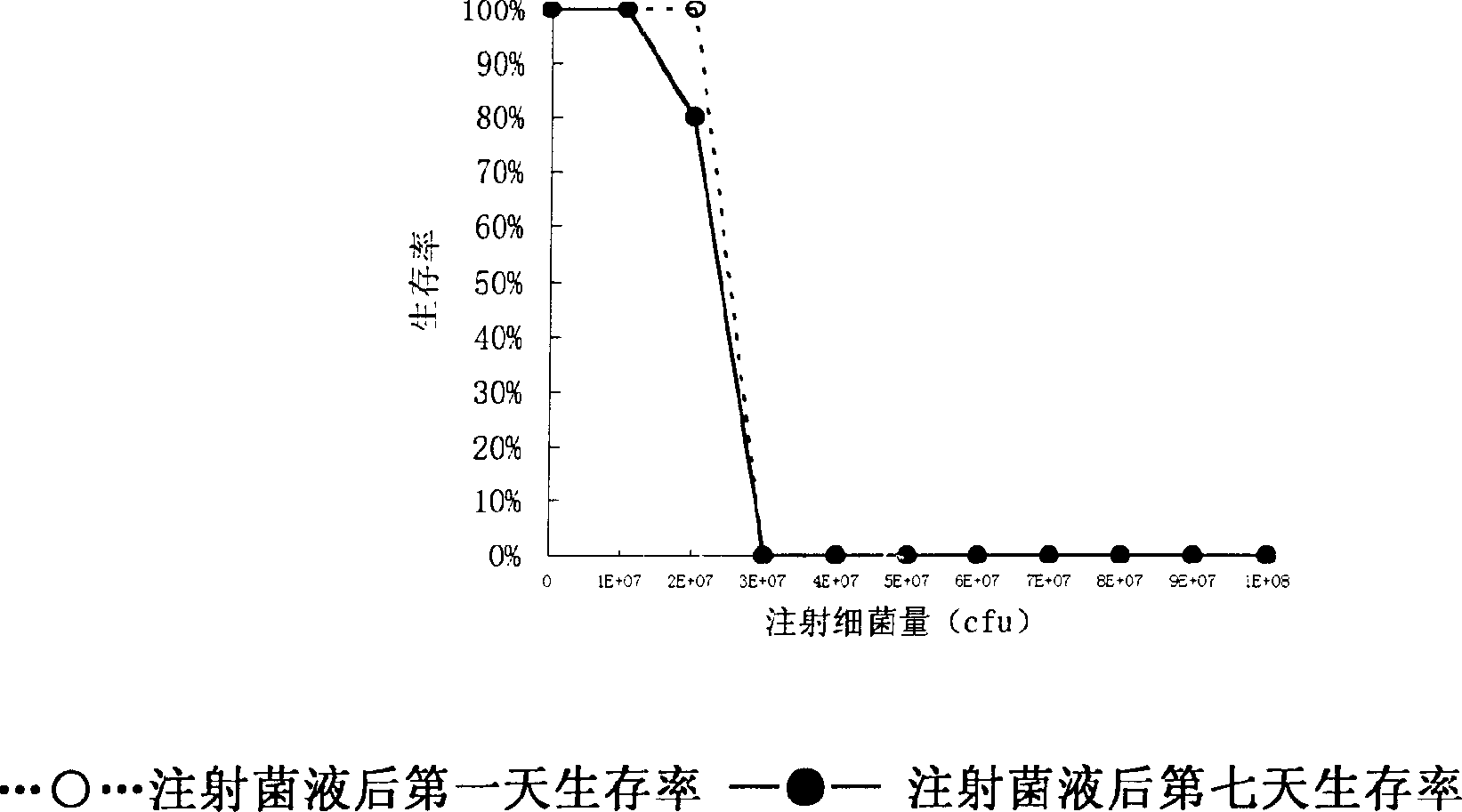
Disclosed is an imine-resistant bacillus pyocyaneus bacteriophage and its use for treating infection, wherein a stain of broad-spectrum drug-resistant bacillus pyocyaneus bacteriophage phiA392, which can effectively kill drug-resistant bacillus pyocyaneus in vivo and in vitro, the bacteriophage can decompose multiple strains of clinically segregated imipenem-resistant bacillus pyocyaneus in vivo. The invention proves the superiority and feasibility of bacteriophage as a therapeutic substance in the clinical application to the treatment of bacillus pyocyaneus resistant affection, and provides a novel therapeutic means for clinical imipenem-resistant bacillus pyocyaneus affection.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
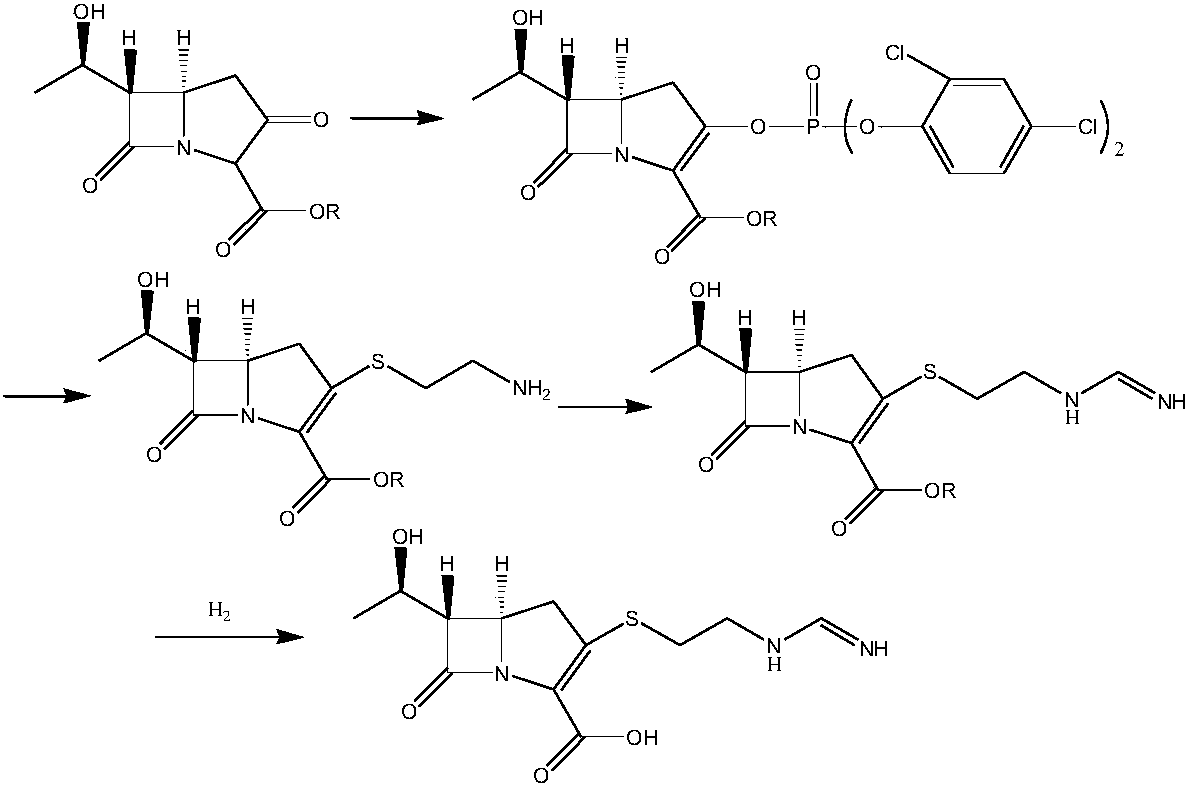
Process for preparation of imipenem
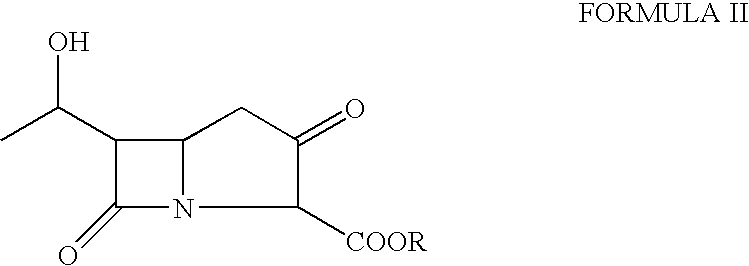
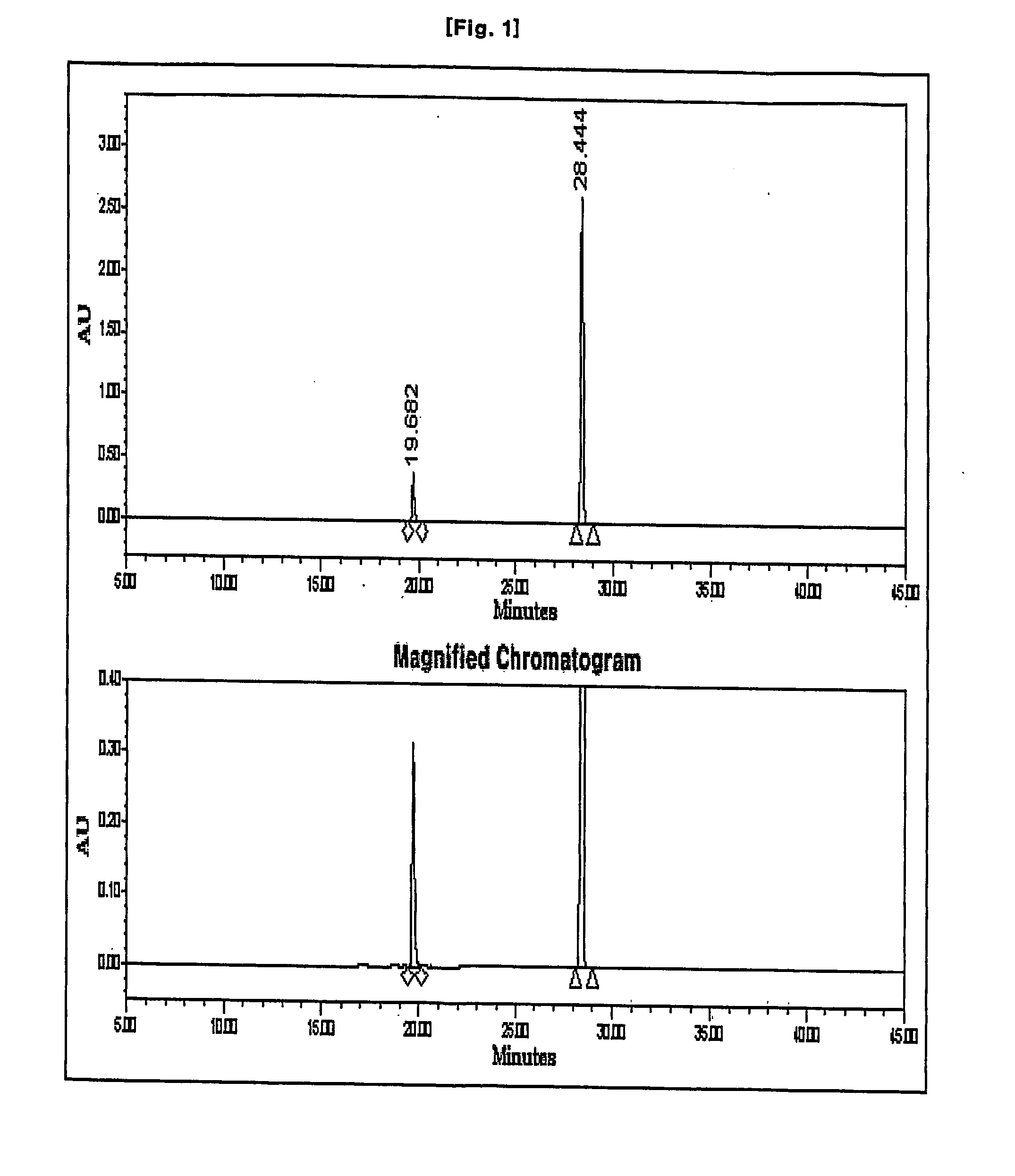
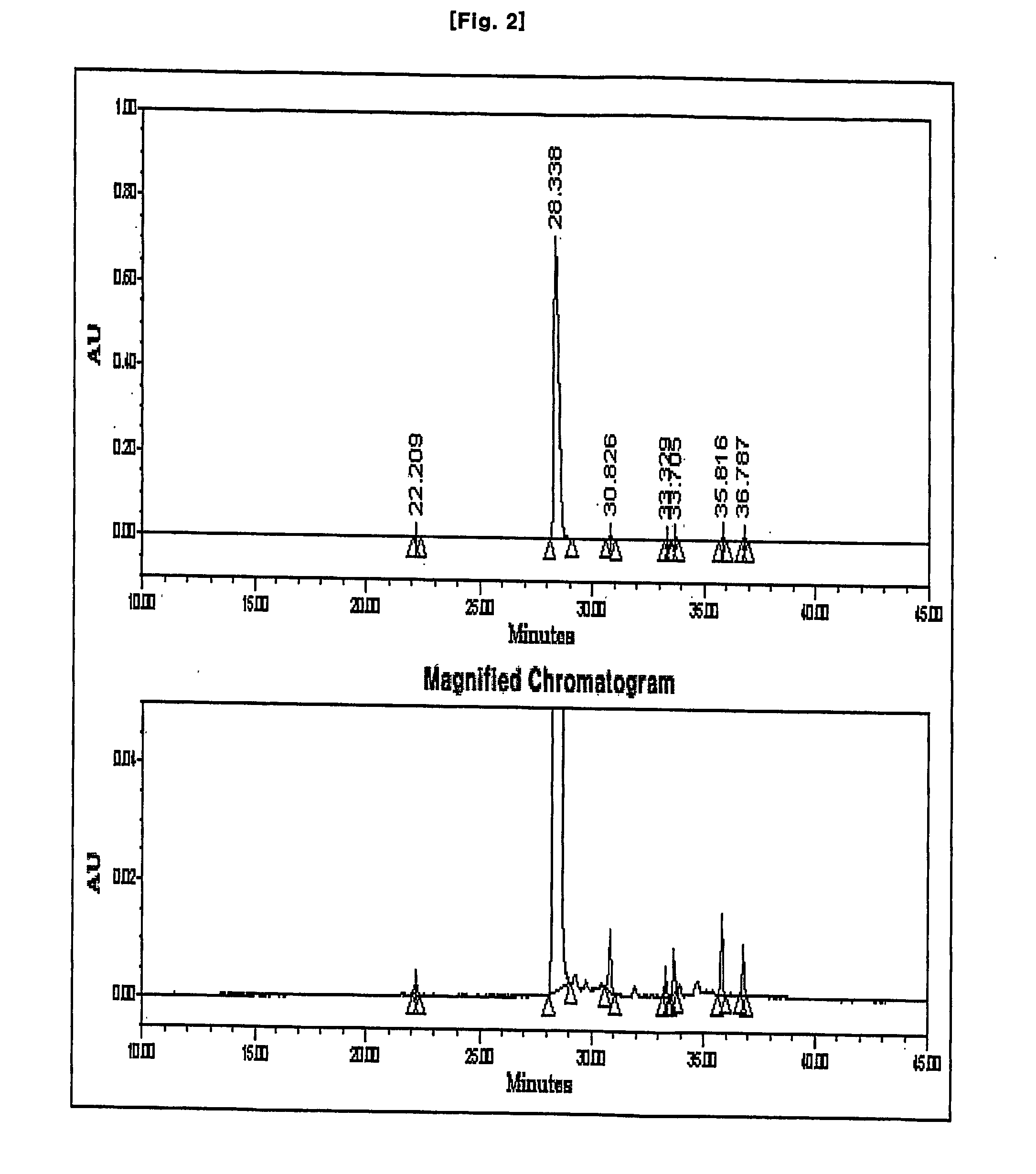
Disclosed herein is a compound of Formula II below: OH wherein R1 is a p-nitrobenzyl or p-methoxybenzyl, group; and R2 and R3 may be identical to or different from each other and are each independently a C1-6alkyl or aryl group, or a derivative thereof, and a process for preparing the compound of Formula II. Further disclosed is a process for preparing imipenem of Formula I below: by using the compound of Formula II.
Owner:JW PHARMA CORP
Application of citral in inhibiting growth of multi-drug resistant enterobacter cloacae
ActiveCN110279679AAvoid drug resistanceMitigate or resolve drug-resistant infectionsAntibacterial agentsAldehyde active ingredientsNalidixic acidCefotaxime
The invention discloses an application of citral in inhibiting growth of multi-drug resistant enterobacter cloacae. The citral can inhibit growth of multi-drug resistant enterobacter cloacae as the citral has relatively good in vitro killing action to multi-drug resistant enterobacter cloacae resisting cefazolin, cefotaxime, augmentin, meropenem, ofloxacin, levofloxacin, ciprofloxacin, cefoxitin, minocyline, imipenem, piperacillin, azithromycin, macrodantin, sulfamethoxazole and nalidixic acid. The minimum bactericidal concentration is 1.6 mg / mL and the minimal inhibitory concentration is 1.0 mg / mL. The invention provides inhibiting action of citral to multi-drug resistant enterobacter cloacae and the citral has wide application value in the field of medicine.
Owner:SHAANXI UNIV OF SCI & TECH
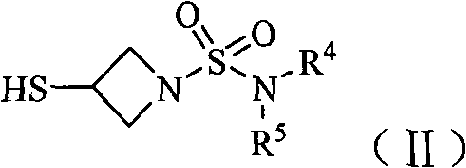
Imipenem derivant containing sulfonyl azetidine
ActiveCN101648952AHigh antibacterial activityOrganic active ingredientsOrganic chemistryImipenemAzetidine
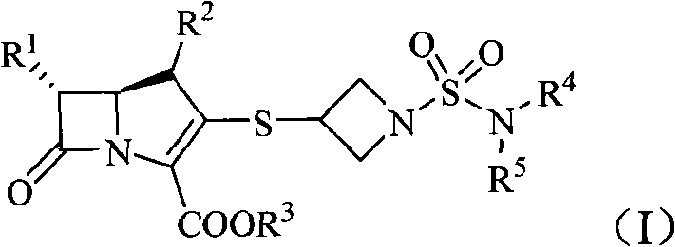
The invention belongs to the technical field of medicines, more particularly relates to imipenem derivant containing sulfonyl azetidine shown in a formula (I), acceptable salts in pharmacy, easily-hydrolyzed ester and a formula (II) of a midbody thereof, wherein R<1>, R<2>, R<3>, R<4> and R<5> are defined as a specification; and the invention also relates a preparation method of the compounds andthe midbody as well as the application of the compounds in the preparation of medicines for treating and / or preventing infectious diseases.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD
Application of buerger's lespedeza root extract in preparation of imipenem anti-pseudomonas aeruginosa sensitizer
InactiveCN113350395AGood antibacterial effectAntibacterial agentsOrganic active ingredientsPseudomona aeruginosaGINSENG EXTRACT
The invention discloses an application of a buerger's lespedeza root extract in preparation of an imipenem anti-pseudomonas aeruginosa sensitizer, and belongs to the technical field of Chinese herbal medicines. The application particularly relates to the following steps that buerger's lespedeza root is mixed with an organic solvent, extracting is carried out to obtain the buerger's lespedeza root extract, by correspondingly combining with the imipenem with 1024 [mu]g / mL and 512 [mu]g / mL of the buerger's lespedeza root extract according to 1 / 4 MIC and 1 / 8 MIC of different clinical strains, and the result shows that the buerger's lespedeza root extract can increase the sensitivity of pseudomonas aeruginosa to imipenem.
Owner:ZUNYI MEDICAL UNIVERSITY
Process for the preparation of crystalline imipenem
InactiveUS7332600B2Practical and convenientEasy to operateOrganic chemistryActivated carbonOrganic solvent
Owner:RANBAXY LAB LTD
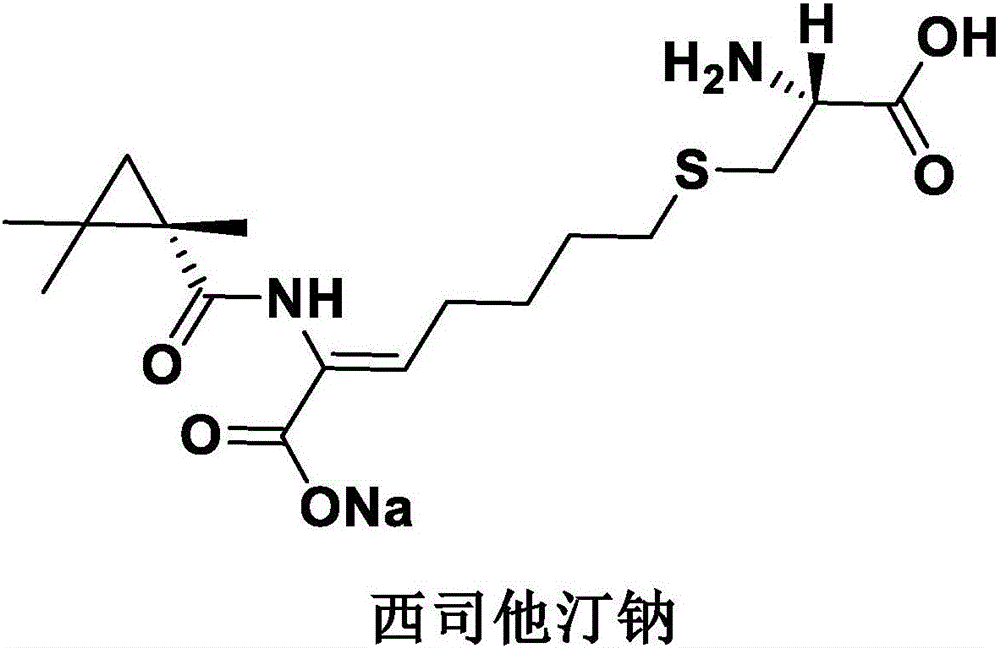
Imipenem and cilastatin sodium sterile powder preparation for injection and preparation method thereof
ActiveCN106176722AReduce adsorptionImprove solubilityAntibacterial agentsPowder deliverySolubilitySodium bicarbonate
The invention provides an imipenem and cilastatin sodium sterile powder preparation for injection and a preparation method thereof. The solubilizing effect of a pH regulator of sodium bicarbonate on imipenem is utilized; the solubility of the imipenem in a water solution is increased; antioxidants are added, so that the oxidative deterioration of the imipenem and the cilastatin sodium sterile powder preparation in production and storage processes is reduced; the use of medical auxiliary materials is reduced; the manufacturing process is simplified; the cost is reduced; meanwhile, the contents of relevant substances are reduced; the production of the stable-quality imipenem and cilastatin sodium sterile powder preparation for injection can be ensured.
Owner:泊诺(天津)创新医药研究有限公司
Rapid test for microbial resistances by mass spectrometry
ActiveUS10480020B2Small volumeSamples introduction/extractionMicrobiological testing/measurementMass spectrometry measurementAntibiotic Y
Owner:BRUKER DALTONIK GMBH & CO KG
Intermediate of synthetic imipenem medicine as well as preparation method and application thereof
ActiveCN101973915AEasy to synthesizeEasy to makeGroup 4/14 element organic compoundsSulfonic acid amide preparationSulfonyl chlorideOrganic base
The invention relates to an intermediate of a synthetic imipenem medicine as well as a preparation method and application thereof. The intermediate has a structural formula disclosed by the following formula (I), wherein R1 stands for low-carbon alkyl, nitryl, halogen or hydrogen of substituted C1-C3 in arbitrary positions; and R2 stands for aryl, alkyl or aralkyl with or without substituents. The preparation process of the intermediate comprises the following steps of: firstly reacting sulfonyl chloride compounds with aniline compounds; and then, reacting the obtained product with 2-bromopropionyl bromide under the existence of organic base. The intermediate can be used for synthesizing carbapenem antibiotics, namely an imipenem key intermediate as beta-methylazacyclo-2-ketone (shortenedfor 4-BMA). The invention has the advantages of simple synthesis, low cost, high reaction stereoselectivity and good product yield, raw materials are cheap and easy to be obtained, and a compound precursor can be repeatedly used after being recovered.
Owner:山东安弘制药有限公司
Imipenem intermediate and preparation method thereof
InactiveCN108623598AOvercoming conversion rateOvercome the defect of poor crystallinityOrganic chemistryOrganic solventFiltration
The invention provides an imipenem intermediate and a preparation method thereof. The preparation method includes: uner the organic base A, adding a compound III and diphenyl chlorophosphate in an organic solvent A for reaction to obtain an intermediate IV; under the organic base B, adding cysteamine hydrochloride to the intermediate IV, and performing stirring, suction filtration, cleaning and drying after reaction to obtain an imipenem intermediate I. In the method, under the organic base C, the imipenem intermediate I and an imine side chain are added in an organic solvent D for reaction toobtain an intermediate V; the intermediate V is subjected to separation and purification, then an organic solvent E and organic base D are added, hydrogenation is performed after pH is adjusted, andthen filtration, stirring, suction filtration, cleaning and drying are performed to obtain imipenem II; with the method, shortcomings of low conversion rate and poor crystallinity of reactants due toserious accumulation of impurities, by-products and raw materials in the hydrogenation phase in the existing preparation process of the imipenem are overcome.
Owner:重庆天地药业有限责任公司
Application of sanguinarine in inhibiting growth of multi-drug-resistant enterobacter horbiae
ActiveCN110946862AGrowth inhibitionDefinite inhibitory effectAntibacterial agentsOrganic active ingredientsNalidixic acidSanguinarine
The invention discloses application of sanguinarine in inhibiting growth of multi-drug-resistant enterobacter horbiae. The sanguinarine has a good in-vitro killing effect on the multi-drug-resistant enterobacter horbiae of piperacillin, levofloxacin, bromgrantine, imipenem, ofloxacin, furadantin, cefathiaquine, azithromycin and naphthyridine acid, and can inhibit the growth of the multi-drug-resistant enterobacter horbiae, the minimum inhibitory concentration is 0.12 mg / mL, and the minimum bactericidal concentration is 0.24 mg / mL. The invention provides the inhibition effect of the sanguinarine on the multi-drug-resistant enterobacter horbiae, and the sanguinarine has wide application value in the fields of medicines and the like.
Owner:SHAANXI UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com