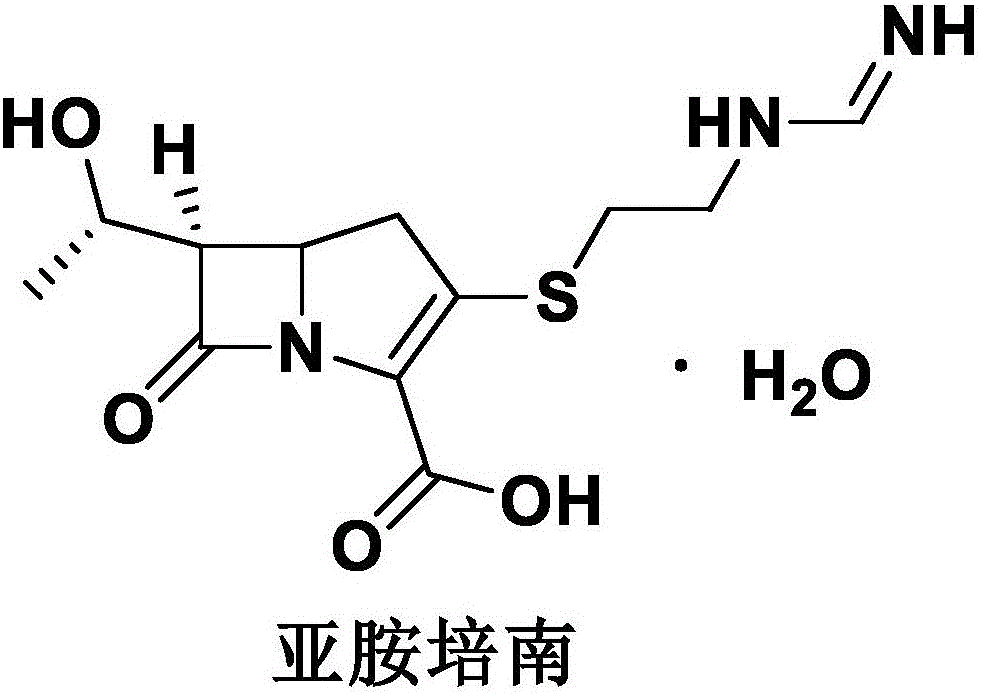
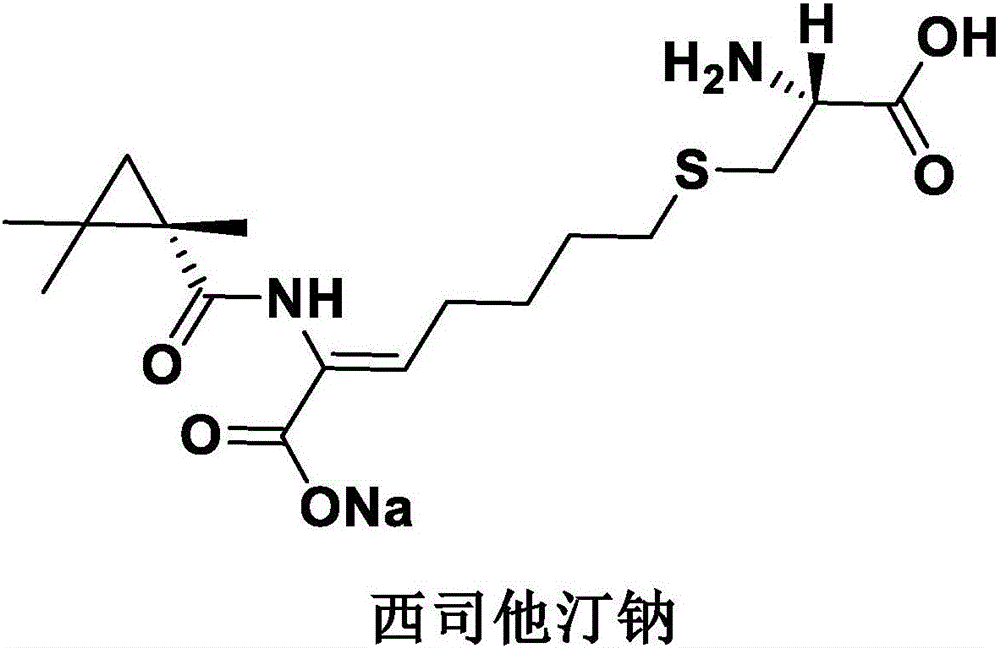
Imipenem and cilastatin sodium sterile powder preparation for injection and preparation method thereof
A technology of cilastatin and imipenem, applied in the field of medicine, can solve the problems of difficulty in powder injection for injection, increase the dosage of auxiliary materials, increase the risk of hemolysis, etc., and achieve the effects of reducing toxic side effects, increasing solubility, and avoiding organic solvent residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Imipenem cilastatin sodium sterile powder preparation for injection
[0030] Prescription (1000 bottles):
[0031]
[0032] Preparation Process:
[0033] (1) Under sterile conditions, dissolve 10 g of sodium bicarbonate in 500 mL of water for injection to make a pH regulator for use.
[0034] (2) Dissolve 20 g of sodium bicarbonate in 500 mL of water for injection under sterile conditions, add 100 g of imipenem and 100 g of cilastatin sodium, and stir to dissolve. Add 5g of sodium sulfite, stir to dissolve, and add the pH regulator obtained in (1), pH = 7.1.
[0035] (3) The solution obtained in (2) was filtered with a 0.2 μm filter membrane, and then equally divided into 1000 sterilized vials.
[0036] (4) Place the vial in a freeze dryer, pre-freeze at -40±5°C for 3 hours, then adjust the temperature to -10±5°C for sublimation drying, and then raise the temperature to 5±5°C for re-drying after 5 hours , a white lyophilized product was obtained after 3 hours. S...
Embodiment 2
[0037] Example 2 imipenem cilastatin sodium sterile powder preparation for injection
[0038] Prescription (1000 bottles):
[0039]
[0040] Preparation Process:
[0041] (1) Under sterile conditions, dissolve 30 g of sodium bicarbonate in 400 mL of water for injection to make a pH regulator for use.
[0042] (2) Dissolve 70g of sodium bicarbonate in 600mL of water for injection under sterile conditions, add 250g of imipenem and 250g of cilastatin sodium, and stir to dissolve. Add 12g of sodium sulfite, stir to dissolve, and add the pH regulator obtained in (1), pH = 7.7.
[0043] (3) The solution obtained in (2) was filtered with a 0.2 μm filter membrane, and then equally divided into 1000 sterilized vials.
[0044](4) Place the vial in a freeze dryer, pre-freeze at -40±5°C for 3 hours, then adjust the temperature to -10±5°C for sublimation drying, and then raise the temperature to 5±5°C for re-drying after 6 hours , a white lyophilized product was obtained after 3 hou...
Embodiment 3
[0045] Example 3 imipenem cilastatin sodium sterile powder preparation for injection
[0046] Prescription (1000 bottles):
[0047]
[0048]
[0049] Preparation Process:
[0050] (1) Under sterile conditions, dissolve 80g of sodium bicarbonate in 800mL of water for injection to make a pH regulator for use.
[0051] (2) Dissolve 170g of sodium bicarbonate in 2000mL of water for injection under sterile conditions, add 500g of imipenem and 500g of cilastatin sodium, and stir to dissolve. Add 25g of sodium sulfite, stir to dissolve, and add the pH regulator obtained in (1), pH = 7.9.
[0052] (3) The solution obtained in (2) was filtered with a 0.2 μm filter membrane, and then equally divided into 1000 sterilized vials.
[0053] (4) Place the vial in a freeze dryer, pre-freeze at -40±5°C for 5 hours, then adjust the temperature to -10±5°C for sublimation drying, and then raise the temperature to 5±5°C for re-drying after 8 hours , a white lyophilized product was obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com