Preparation method for imipenem medicine intermediate 4AA
An intermediate and penem-like technology is applied in the field of preparation of a penem-like drug intermediate 4AA, which can solve the problems of unsuitability for industrial production, high cost, environmental pollution by heavy metals, etc., and achieves mild and stable reaction conditions, and a high conversion rate. High yield, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
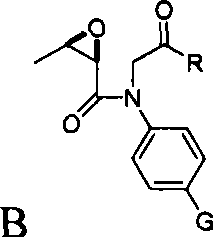
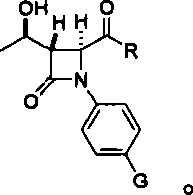
[0026] According to a typical embodiment of the present invention, the preparation method of penem drug intermediate 4AA comprises: (1) making 4-substituted aniline into intermediate A with structural formula I; (2) making L-threonine ring Oxidation makes (2R, 3R)-epoxybutyric acid, (3) coupling reaction occurs between (2R, 3R)-epoxybutyric acid and intermediate A, and obtaining structural formula is intermediate B of II; (4 ) Intermediate B undergoes a ring-closing reaction to obtain intermediate C with structural formula III; (5) carry out hydroxyl protection reaction to intermediate C to obtain intermediate D with structural formula IV; (6) pass carbonyl in intermediate D through Baeyer-Villiger reaction is oxidized to acetoxy; and (7) the oxidized product is subjected to an ozonation reaction to obtain the penem drug intermediate 4AA; wherein, the structural formula I is Structural formula II is Structural formula III is Structural formula IV is
[0027] Among them...
Embodiment 1
[0063] In Example 1 and Example 2, starting materials with different substituents were used to prepare penem drug intermediate 4AA.
[0064] Table 1
[0065]
[0066] Example 1:
[0067] (1) Substitution: Preparation of 2-(4-methoxyphenyl)amino-1-acetophenone using 4-methoxyaniline
[0068] Add main raw material 4-methoxyaniline 80kg (1.0eq) in 2000L reactor, toluene 418kg (6mL / g), triethylamine 170kg (2.6eq) and 2-bromoacetophenone 194kg (1.5eq), heat up To 100±2°C, keep warm until the reaction is complete;
[0069] After the reaction is complete, add 720 kg (9 mL / g) of water to the system at room temperature to terminate the reaction, filter, wash the filter cake with 100 kg of water and dry to obtain the product 2-(4-methoxyphenyl)amino-1-phenylethyl Ketone 148kg, yield 94.4%, HPLC purity 97.0%.
[0070] (2) Epoxidation: Preparation of (2R, 3R)-epoxybutyric acid using L-threonine
[0071] Add main raw material L-threonine 50kg (1.0eq) and concentrated hydrochloric a...
Embodiment 2
[0092](1) Substitution: Preparation of 2-(4-bromophenyl)amino-1-(3-methoxy)acetophenone
[0093] Add 10kg (1.0eq) of main raw material p-bromoaniline, 79kg (10mL / g) of acetonitrile, 13.8kg (3.0eq) of pyridine and 2-iodo-1-(3-methoxy)acetophenone successively into the 500L reactor 32kg (2.0eq), cool down to 10±2°C, keep warm until the reaction is complete;
[0094] After the reaction, add 150kg (15mL / g) of water to the system at room temperature to terminate the reaction, filter, wash the filter cake with 10kg of water and dry to obtain the product 2-(4-bromophenyl)amino-1-(3-methoxy)phenethyl Ketone 17kg, yield 91.3%, HPLC purity 96.9%.
[0095] (2) Epoxidation: Preparation of (2R, 3R)-epoxybutyric acid using L-threonine
[0096] Add 15kg (1.0eq) of L-threonine (1.0eq) and 222kg (10mL / g) of hydrobromic acid into a 500L reactor, cool down to 10±2°C, and add 58kg of sodium nitrite solution with a mass ratio concentration of 30% dropwise. (2.0eq); after dropping, keep warm at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com