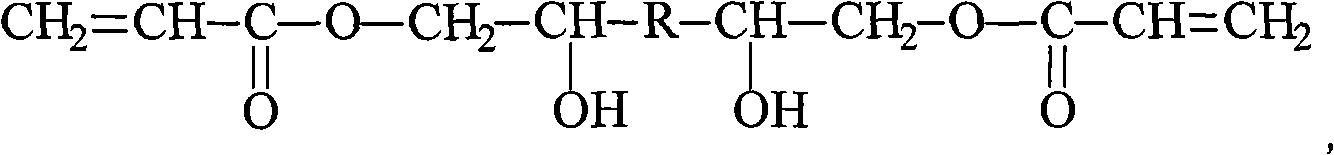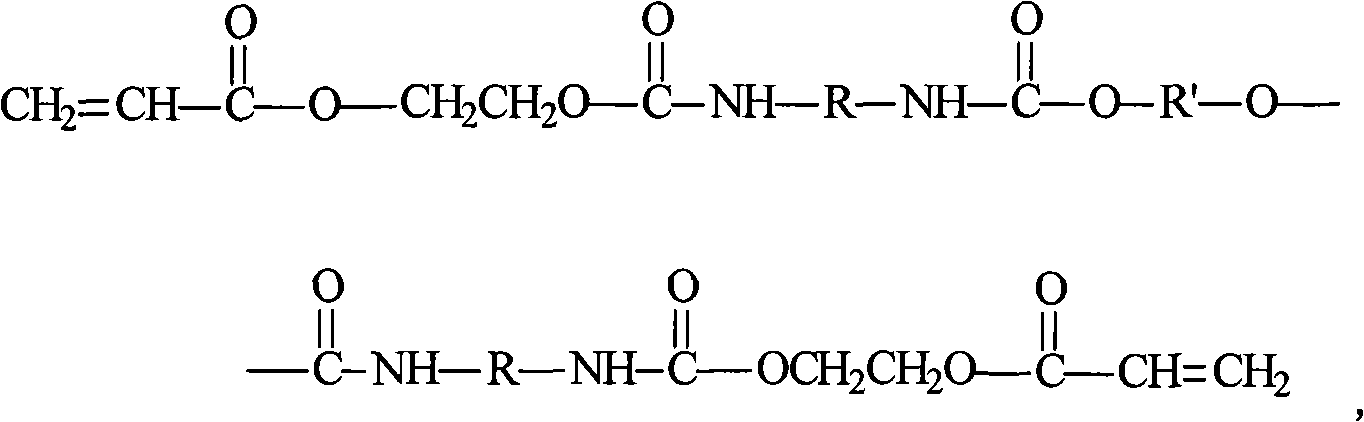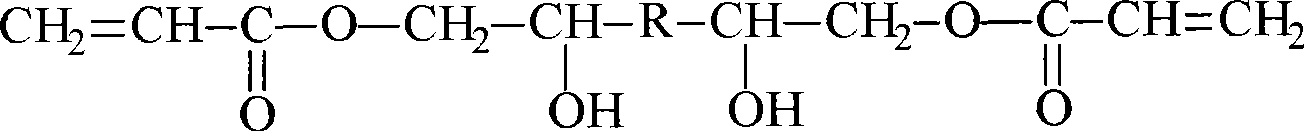Patents
Literature
453results about How to "No pollution problem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
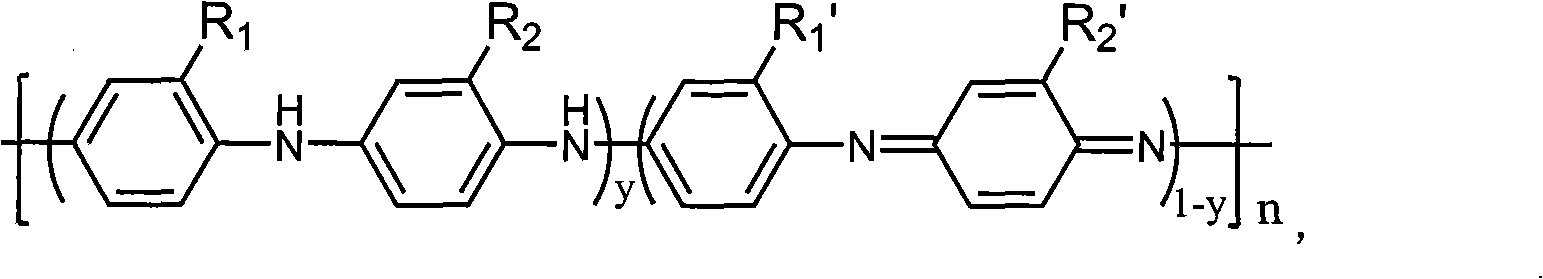
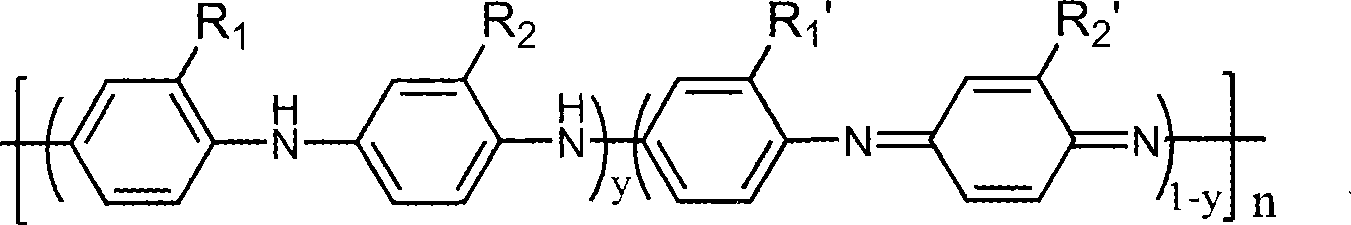
UV curing polyaniline anti-corrosive paint and preparation thereof
InactiveCN101407650AImprove anti-corrosion performanceTo achieve anti-corrosion effectAnti-corrosive paintsPhotoinitiatorChemistry
The invention provides an ultraviolet curing polyaniline anticorrosive coating and a preparation method thereof. The coating adopts polyaniline as a main anticorrosive material, an oligomer, a reactive diluent, the polyaniline, a filler, a photoinitiator and an auxiliary agent are mixed according to a formula, stirred, ground, filtered, and the like, thereby obtaining the ultraviolet curing polyaniline anticorrosive coating. The prepared anticorrosive coating has good anticorrosive effect on different metals, such as steel, copper, aluminum, etc. The coating has stronger corrosion resistance property of acid, base and other media, thereby being applicable to the use in extremely harsh environmental conditions and being particularly applicable to the corrosion resistance in marine environment. The coating does not contain Pb, Cr, Zn and other heavy metals, and the formula does not contain any organic solvents. Therefore, the coating does not have the environmental pollution problem during the production and the using processes and is a completely green environment-friendly coating.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
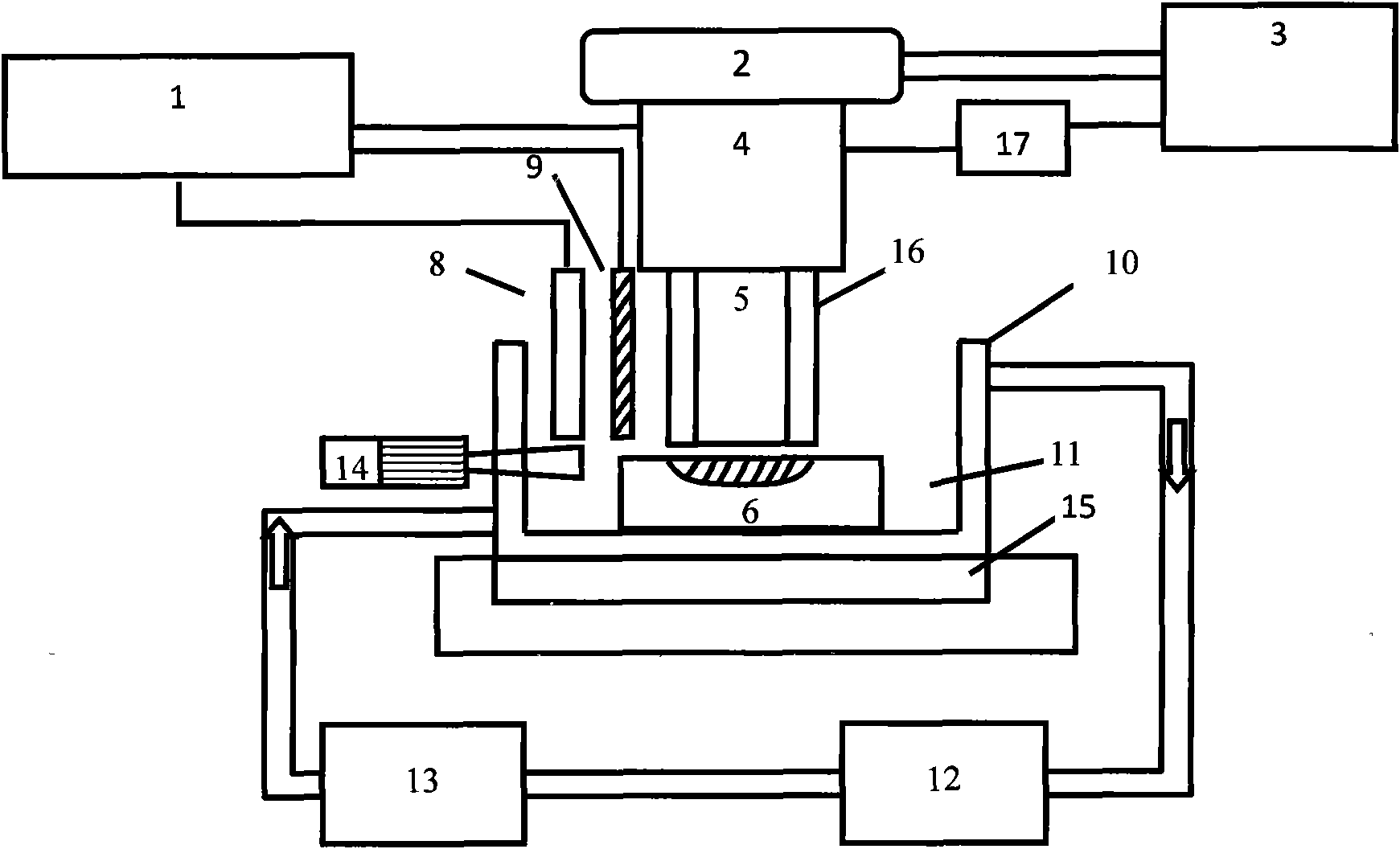
Electrochemical levelling and polishing processing method with nanometer precision and device thereof
ActiveCN101880907ANo mechanical damageNo pollution problemElectrolysis componentsSemiconductor/solid-state device manufacturingElectrochemical responseLiquid layer
The invention provides an electrochemical levelling and polishing processing method with nanometer precision and a device thereof, relating to an electrochemical etching levelling and polishing technology. The device is provided with a cutter with nanometer levelling precision, an electrochemical reaction control system capable of accurately controlling the thickness of an etching levelling agent liquid layer in nanoscale, a solution circulating device, a solution thermostat and an automatic control system. The method is implemented by the following steps: preparing the cutter with nanometer levelling precision to serve as an electrochemical working electrode, and placing the cutter at the bottom of a container together with a workpiece; immersing the cutter in a solution, starting an electrochemical system, generating the etching levelling agent on the surface of the cutter, compressing the etching levelling agent liquid layer on the cutter surface into a nanoscale thickness, and regulating and controlling the thickness of the etching levelling agent liquid layer; driving a tri-dimensional micro-drive device, leading the cutter to approach the workpiece gradually, and regulating and controlling the distance and parallelism between the workpiece surface and the cutter; and leading the cutter to move toward the workpiece surface, and enabling the constraint etching levelling agent liquid layer on the cuter surface to contact with the workpiece surface until the whole workpiece is etched, leveled and polished.
Owner:XIAMEN UNIV
Litsea cubeba oil microcapsule and preparation method thereof
The invention discloses a litsea cubeba oil microcapsule and a preparation method thereof, and belongs to the technical field of mouldproof and insecticidal capsules. The litsea cubeba oil microcapsule is characterized by being prepared by the complex coacervation of litsea cubeba oil taken as a core material, sodium alginate taken as a main wall material and an aqueous solution which contains 1% to 5% of chitosan and 2% to 5% of anhydrous calcium chloride and is taken as a coagulating bath solution, wherein the mass ratio of the sodium alginate to the litsea cubeba oil is 1:(1-4). The preparation method mainly comprises the following steps: firstly preparing an emulsion A of the sodium alginate and the litsea cubeba oil; then preparing the aqueous solution of the chitosan and the anhydrous calcium chloride as the coagulating bath solution B; dripping the emulsion A into the coagulating bath solution B; and solidifying the mixture for certain time and then filtering the mixture, thereby obtaining the litsea cubeba oil microcapsule. Thus, the litsea cubeba oil microcapsule which is high in litsea cubeba oil content, long in service time and good in mouldproof and insecticidal effect and the preparation method of the litsea cubeba oil microcapsule are provided. The litsea cubeba oil microcapsule is mainly used for performing mouldproof and insecticidal functions on grains as well as on clothes.
Owner:JIAYING UNIV +1
Ultraviolet-heat dual curing polyaniline anti-corrosive paint and preparation method thereof
ActiveCN101418146ANo emission issuesNo pollution problemAnti-corrosive paintsPolyurea/polyurethane coatingsUltravioletChromium
The invention provides an ultraviolet-thermal double curing polyaniline anti-corrosive coating and a preparation method thereof. In the coating, polyaniline is adopted as a main anti-corrosive material, and oligomer, a reactive diluent, polyurethane polylol, polyaniline, filler, light trigger and an auxiliary agent are used for preparing filtrate of the coating; and the filtrate and a polyurethane curing agent are stored respectively and uniformly mixed in proportion for use. The anti-corrosive paint has good anti-corrosive effect on different metals, not only can perform ultraviolet curing crosslinking but also can perform thermal curing crosslinking, well overcomes the defect of incomplete curing of an ultraviolet curing anti-corrosive paint, has the characteristics of resisting acid medium corrosion and alkali medium corrosion, is suitable to be used under the condition of a severe environment and particularly suitable for corrosion protection under the condition of a marine environment, does not contain heavy metals such as plumbum, chromium and zinc, and simultaneously does not contain any organic solvent in a formula. Therefore, the coating does not have the problem of environmental pollution during the production process and the use process, and is a completely environment-friendly coating.
Owner:中科应化(长春)科技有限公司
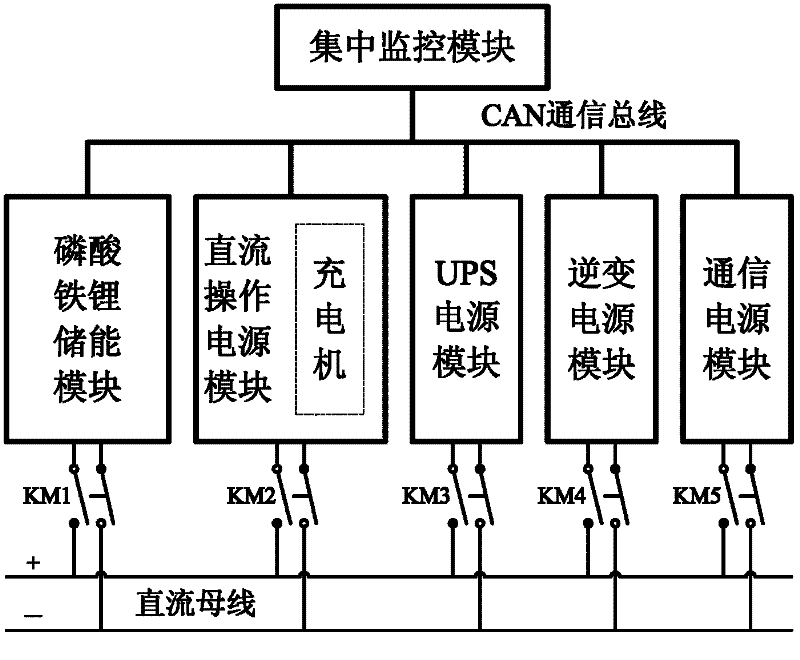
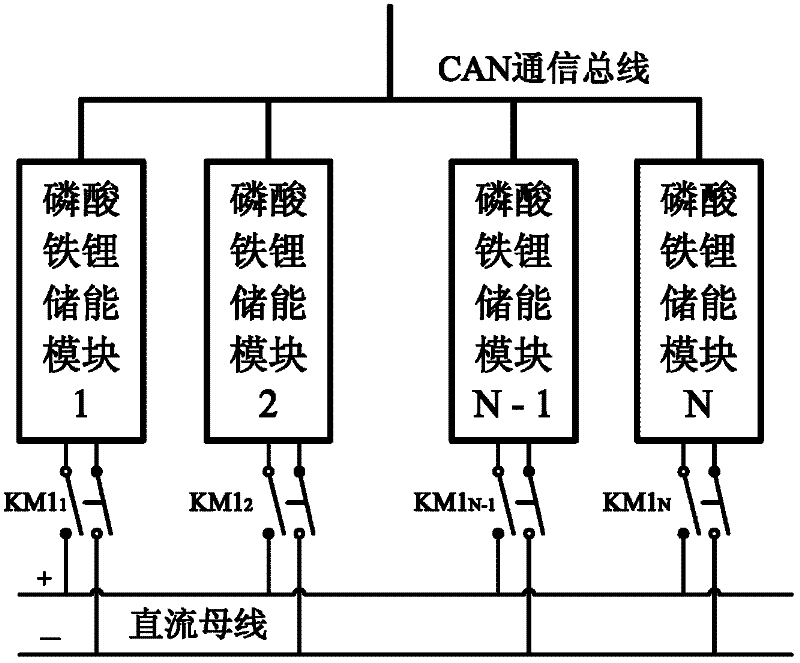
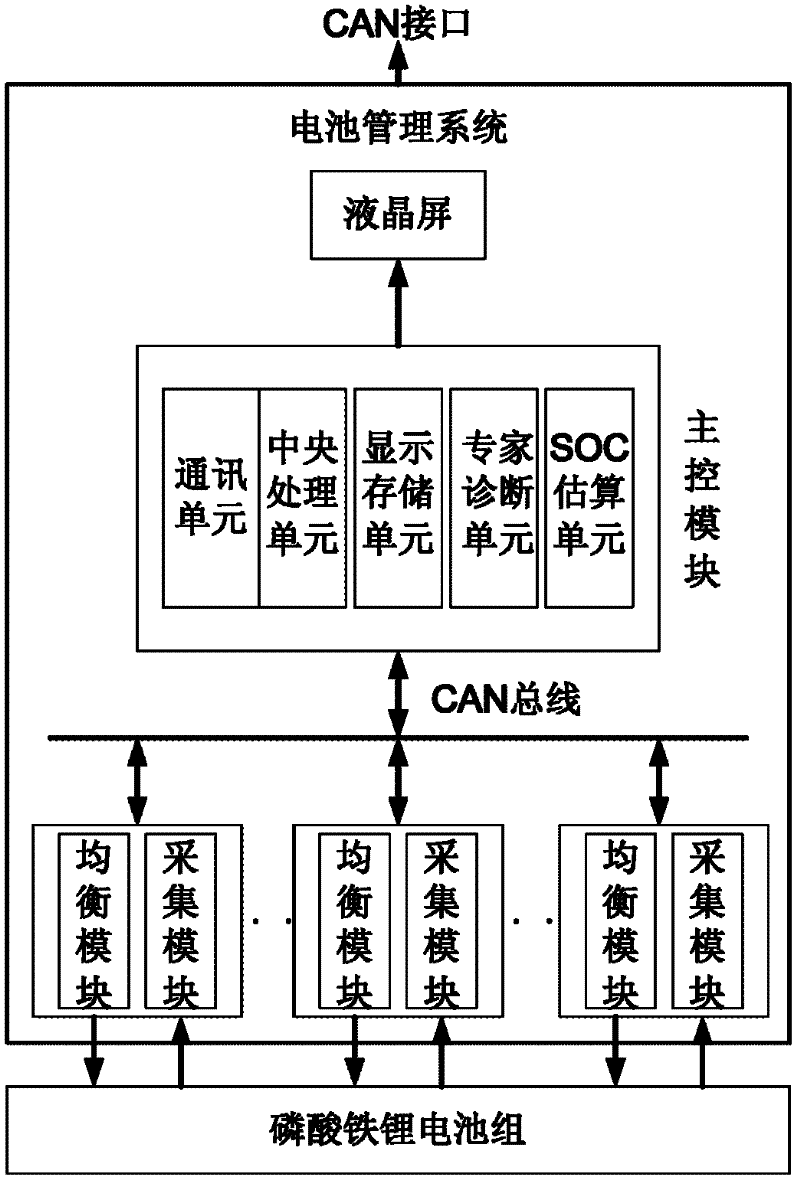
A DC power supply system for substation and its control method
InactiveCN102270878AImprove charge and discharge efficiencyIncrease charge and discharge rateBatteries circuit arrangementsElectric powerArea networkElectrical battery
The invention provides a direct current power supply system for a transformer substation and a control method thereof. The direct current power supply system comprises a centralized monitoring module, a direct current operation power module, an uninterruptible power supply (UPS) power module, an inversion power supply module, a communication power supply module and a lithium iron phosphate energy storage module; each module of the direct current power supply system is connected to a common direct current bus through a relay switch, and redundancy backup of each module is realized in a mode that each module is connected in parallel to the common direct current bus through a multi-submodule; the modules of the direct current power supply system are connected with the centralized monitoring module through a controller area network (CAN) communication bus; the centralized monitoring module collects the running state information of each module and manages the modules of the system uniformly; and a battery is charged or discharged slightly through intelligent coordination control, and on-line automatic maintenance of the battery is realized. The invention has the advantages that: the system has high reliability, high shock load resistance, no maintenance, small floor area, energy conservation and environmental friendliness; and the service life of the battery is prolonged and the performance of the battery is improved by the intelligent coordination control method.
Owner:南瑞(武汉)电气设备与工程能效测评中
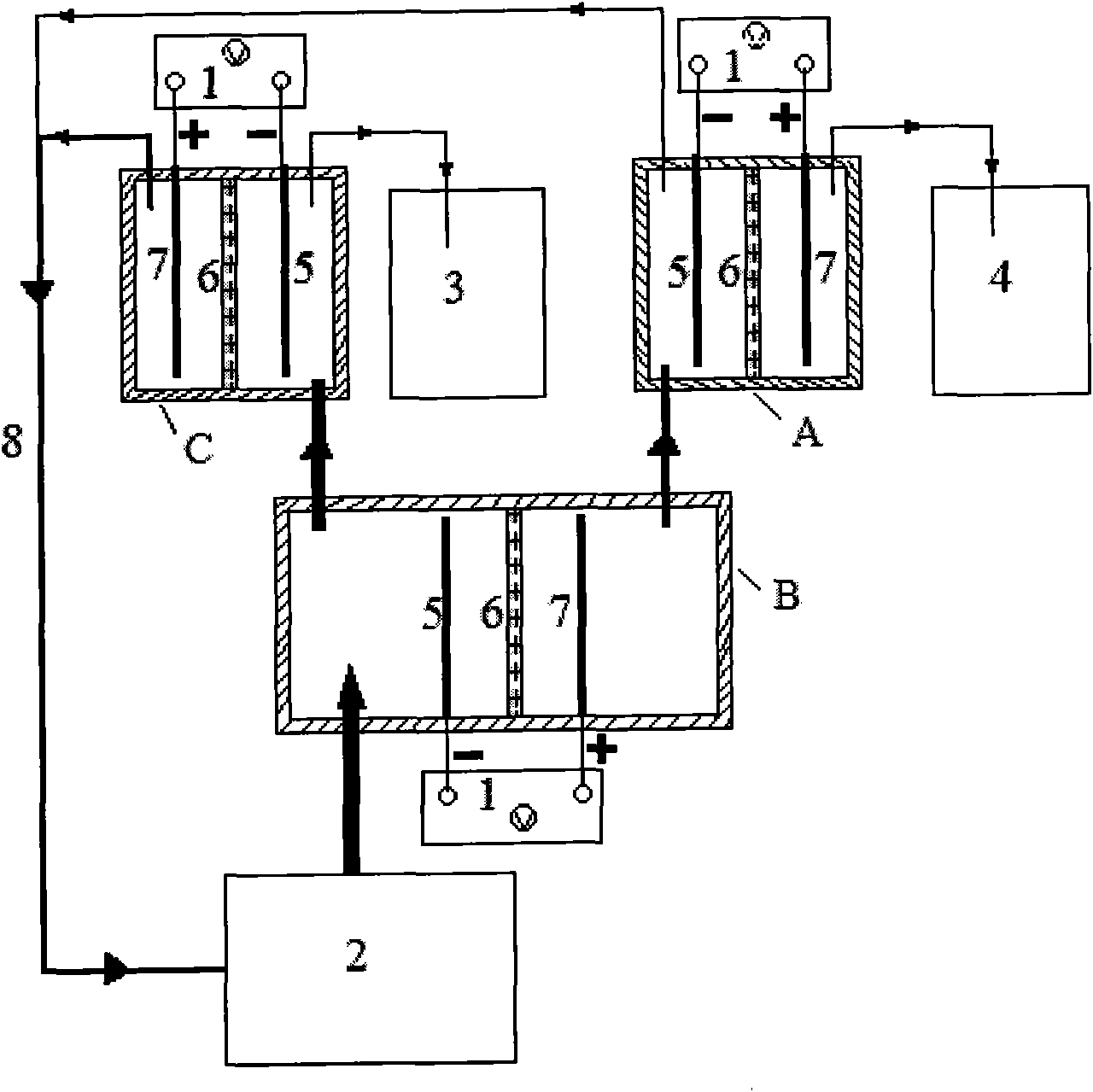
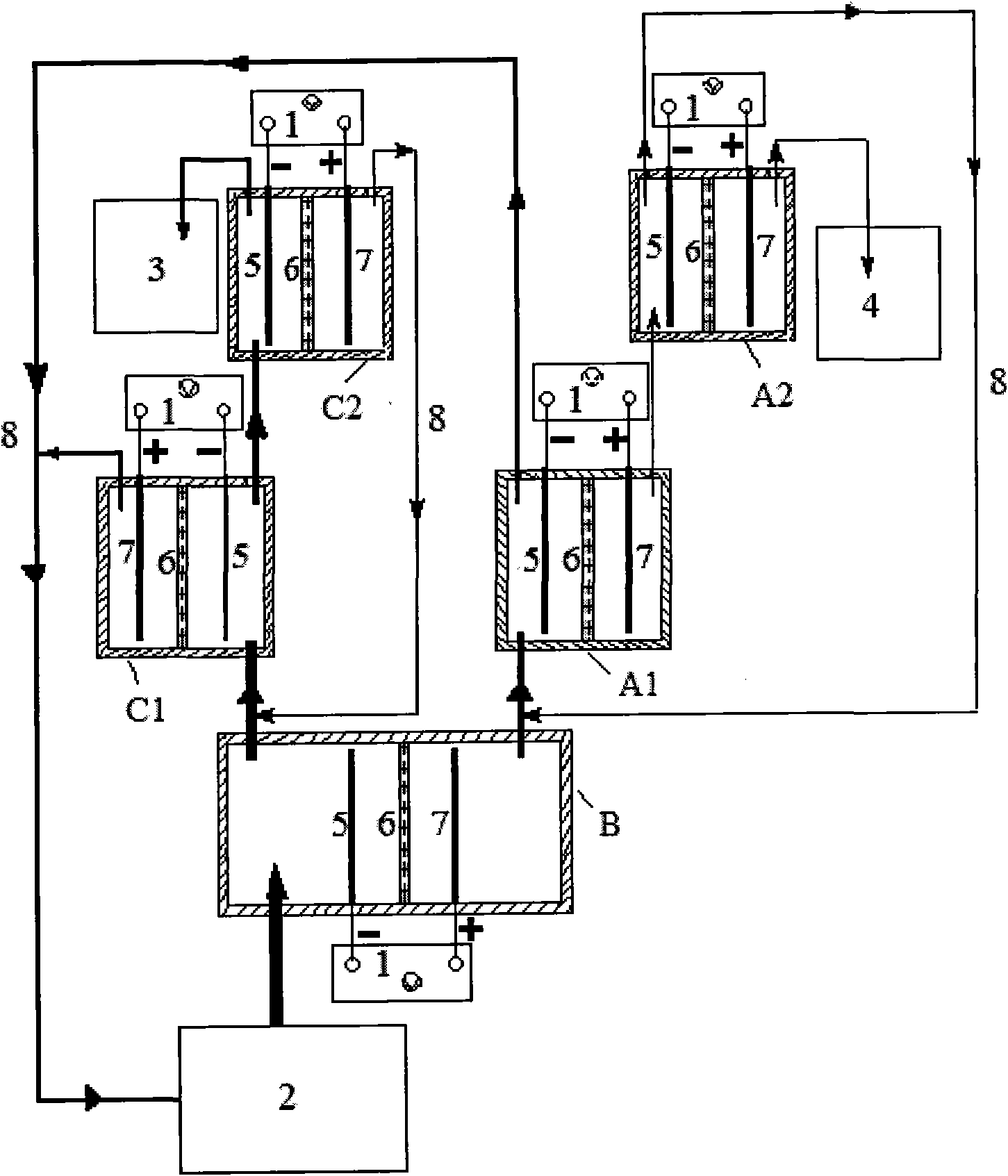
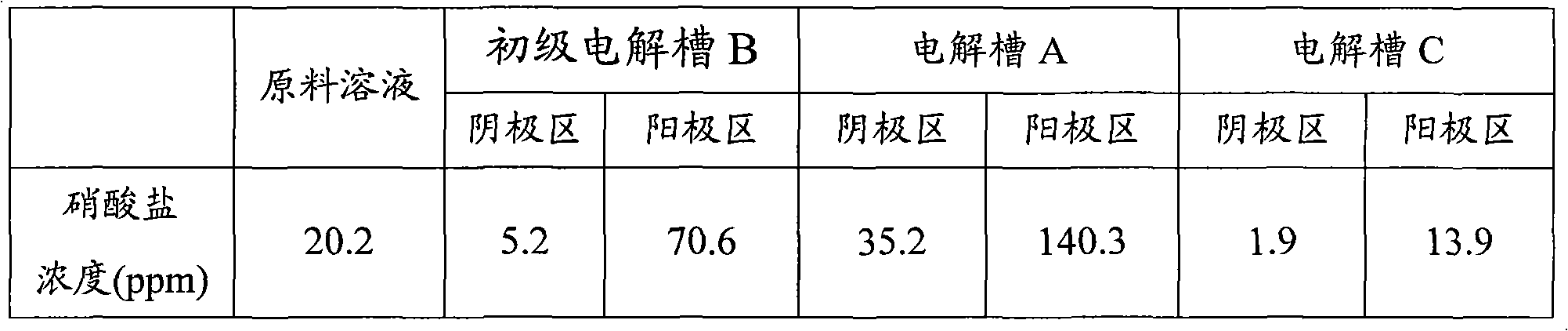
Electrochemical device for wastewater treatment and method for treating wastewater by using same
InactiveCN101967017AImprove degradation efficiencyReduce energy consumptionWater/sewage treatmentPhosphateChloride
The invention relates to an electrochemical device for wastewater treatment and a method for treating wastewater by using the same. The electrochemical device consists of a power supply, a primary electrolytic bath B, an electrolytic bath A, an electrolytic bath C, a cathode, a permeable isolation material part, an anode, a raw water tank, a purified water collector and a concentrated solution collecting tank. The electrochemical device can simultaneously remove common negative ions such as nitrate radicals, phosphate radicals, chloride ions, fluorine ions and the like and organic substances, does not need to add any agent, can avoid second pollution to the effluent, is convenient to operate, and has high treatment efficiency and low energy consumption.
Owner:BEIJING UNIV OF CHEM TECH
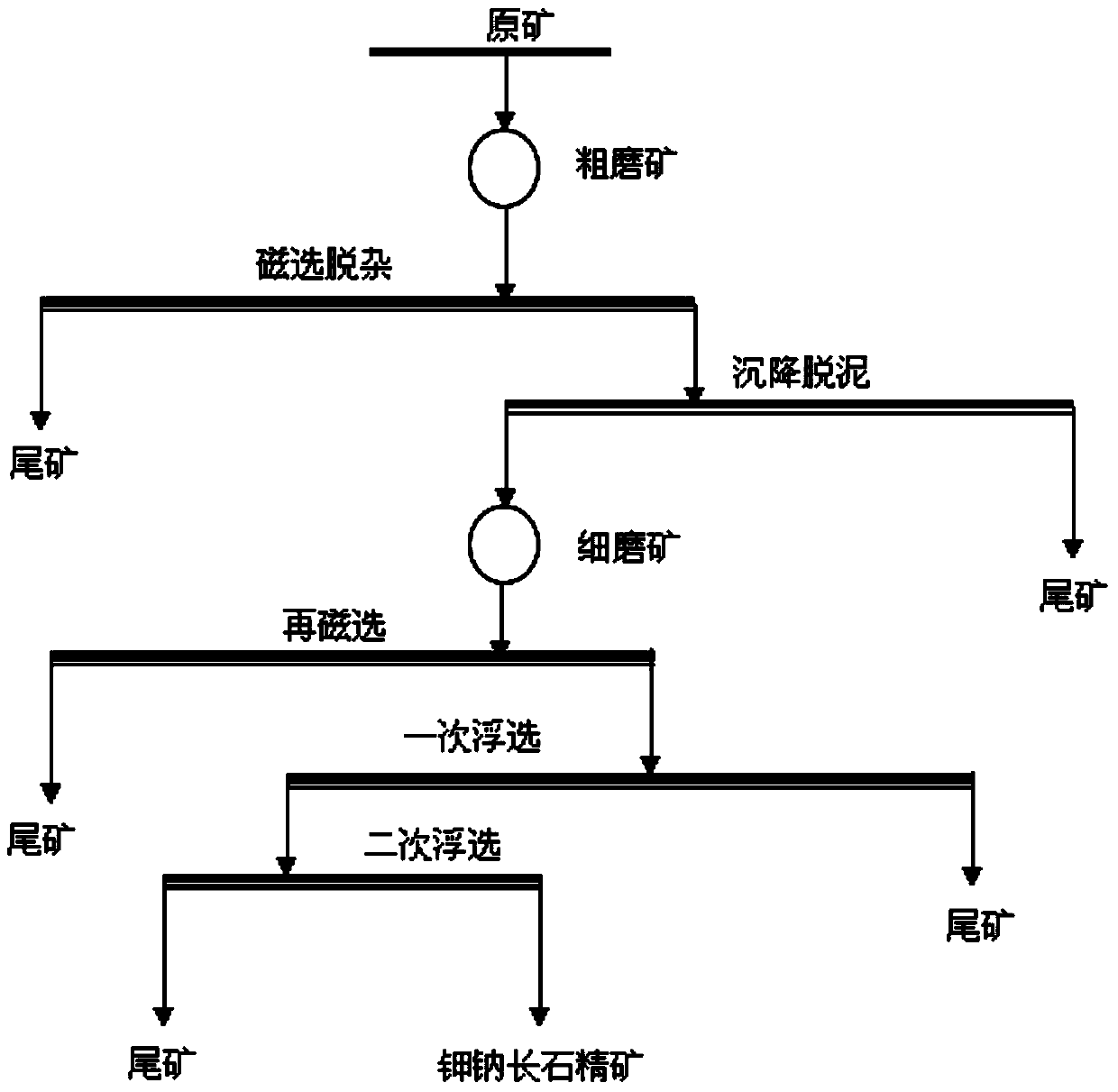
Method for separating potassium feldspar and soda feldspar from potassium-soda feldspar ore
The invention provides a method for separating potassium feldspar and soda feldspar from potassium-soda feldspar ore and belongs to the technical field of mineral separation. The method includes procedures of double grinding of ore, double magnetic-separation of ore, double flotation of concentrates and the like, is a simple and low-cost floatation separating process, causes no environmental pollution, has the effects of improving quality and recovery rate of concentrates of potassium feldspar and soda feldspar remarkably, also has effects of high impurity removal speed and high finished-product recovery rate, and is suitable for industrial application.
Owner:广西弘耀祥科技有限公司
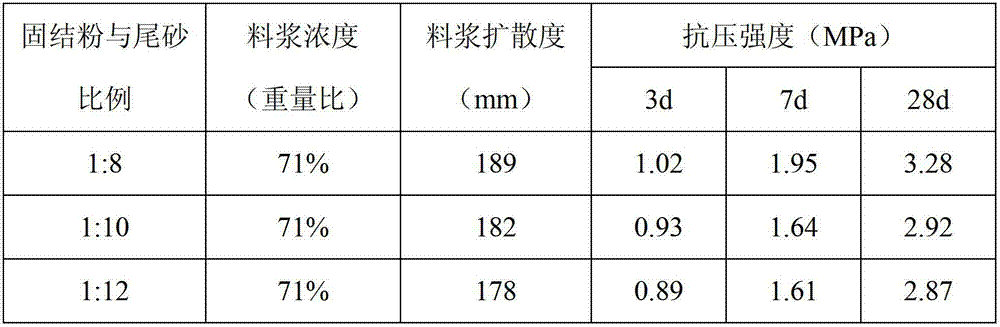
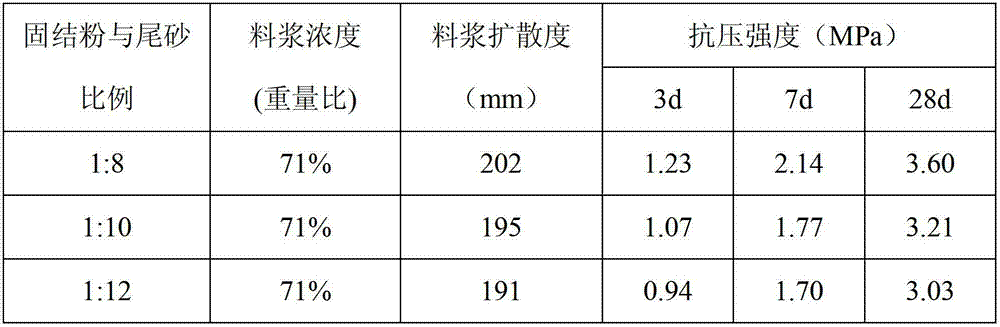
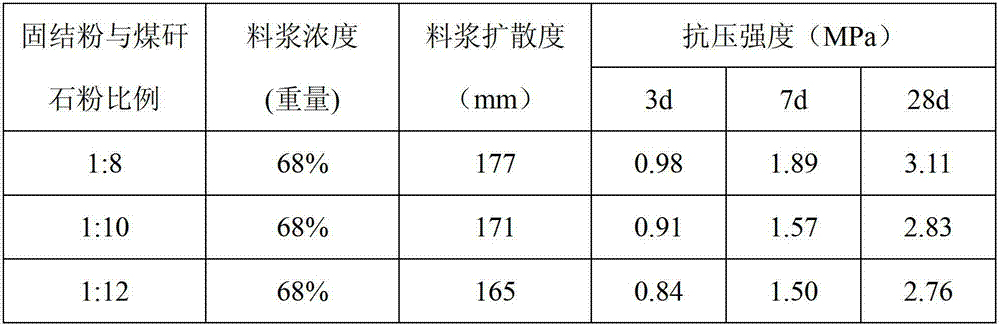
Filling and consolidating powder for mining purposes and use thereof
The invention discloses filling and consolidating powder for mining purposes and a use thereof. The filling and consolidating powder comprises the following raw materials in percentage by weight: 3-5% of portland cement clinker, 8-10% of metakaolin, 5-15% of carbide slag, 18-24% of phosphogypsum, 18-22% of fly ash, 9-14% of red mud, 15-19% of blast furnace slag, 4-10% of limestone, 0.8-1.2% of polycarboxylic acid type water reducing agent and 0.4-0.7% of sodium hydroxide and is prepared by uniformly mixing and grinding to get fine powder with 400 meshes-600 meshes. When the filling and consolidating powder is used, mining tailing or coal gangue is firstly mixed with water according to a proportion in a mixing machine for forming uniform slurry, and then the consolidating powder is further added, uniformly mixed and then conveyed to a downhole place by a pipeline for filling. The cost of the filling material blended by the consolidating powder disclosed by the invention is 50-70 yuan / ton, while the cost of the similar filling and consolidating powder is 90-110 yuan / ton; and compared with the prior art, the cost is greatly reduced and the filling effect is good.
Owner:ZIBO GEOLOGICAL MINE TECH SERVICE CENT
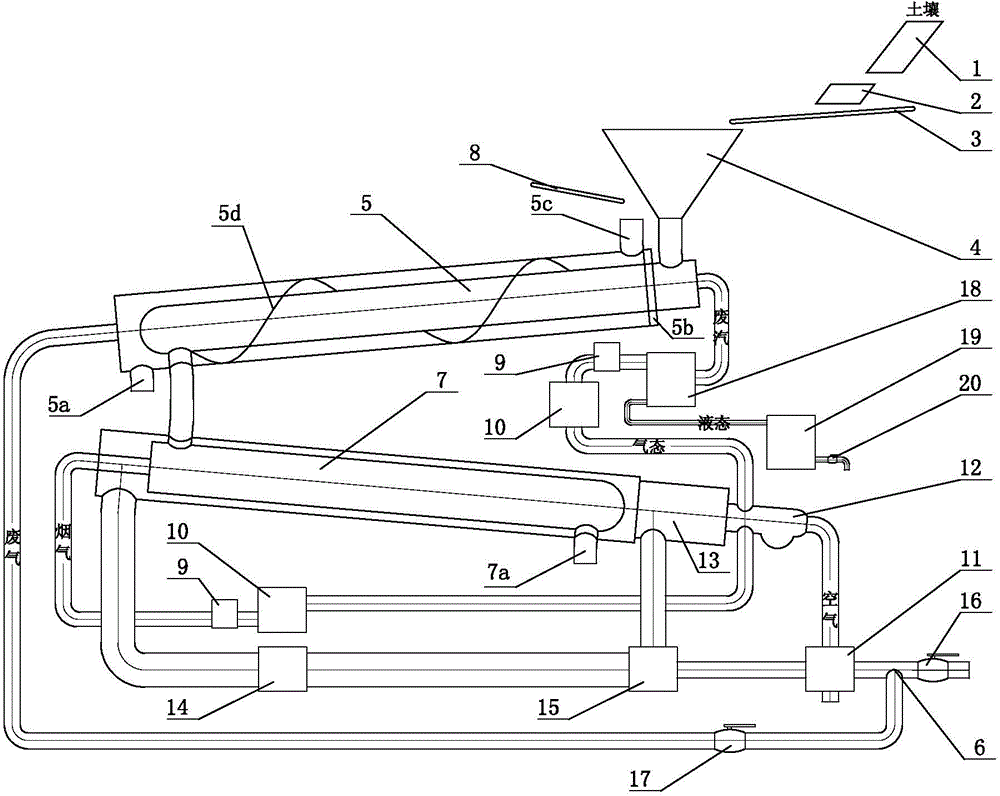
Contaminated soil remediation system
ActiveCN104785512AImpact emissionsNo pollution problemContaminated soil reclamationCombustion chamberCombustor
The invention discloses a contaminated soil remediation system. Soil is sequentially fed into the inner container of a waste heat rotary kiln through a feeding cabin, the inner container of a heat desorption rotary kiln, and the interlayer of the waste heat rotary kiln through a first conveyer belt, and is finally discharged out through a first soil outlet; air is fed into a combustion chamber for combustion through a combustor; generated fume is fed into the interlayer of the heat desorption rotary kiln to make straight line movement, is further accessed to the interlayer of the waste heat rotary kiln through a pipeline to make spiral movement, and is finally discharged out through a fume outlet formed in the shell of the waste heat rotary kiln; the waste gas generated in the inner container of the heat desorption rotary kiln is accessed to the combustor for incineration; the waste gas generated in the inner container of the waste heat rotary kiln is accessed to a condensation separator through a pipeline; the gas separated in the condensation separator is accessed to the combustion for incineration through another pipeline; a liquid separated in the condensation separator is discharged out through another pipeline. The contaminated soil remediation system has the characteristics of low energy consumption, high efficiency, low manufacturing cost, high easiness to implement, good remediation effect and the like, and has wide market prospect.
Owner:重庆化医太湖锅炉股份有限公司 +1
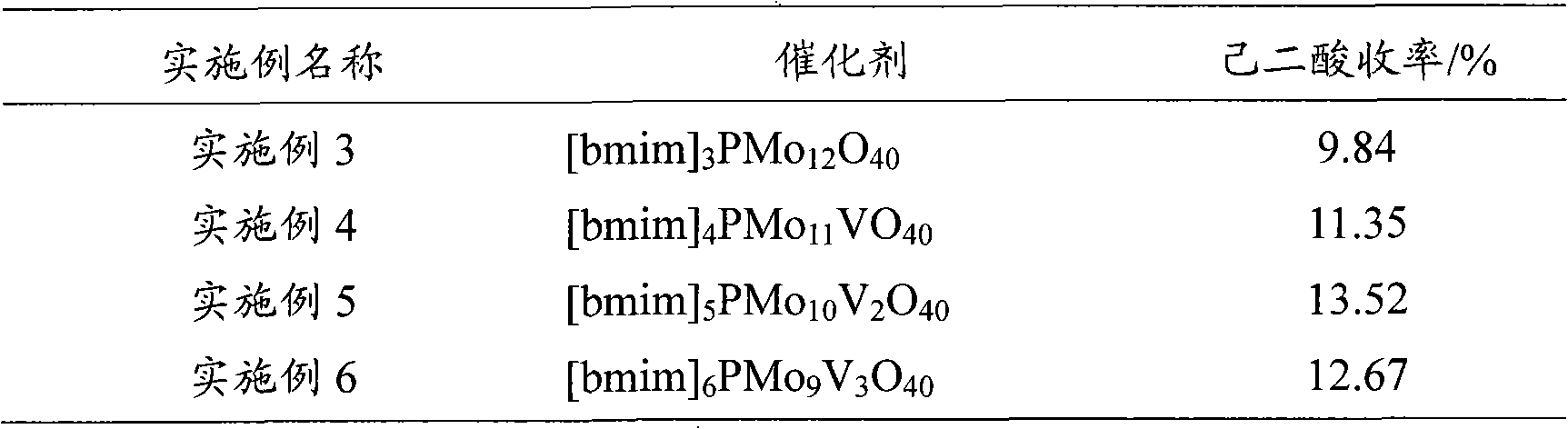
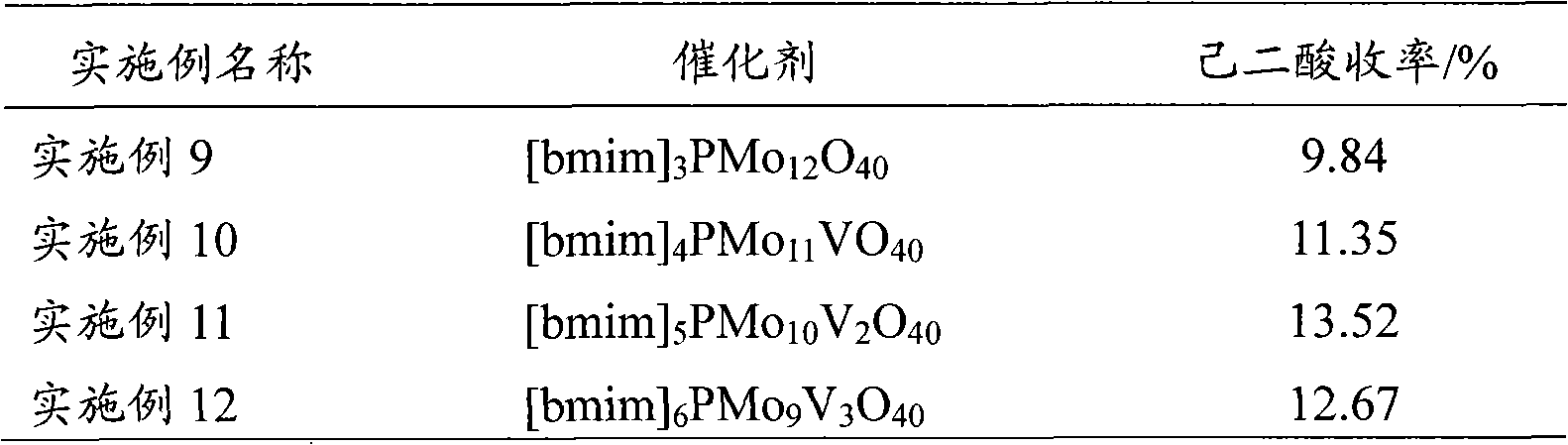
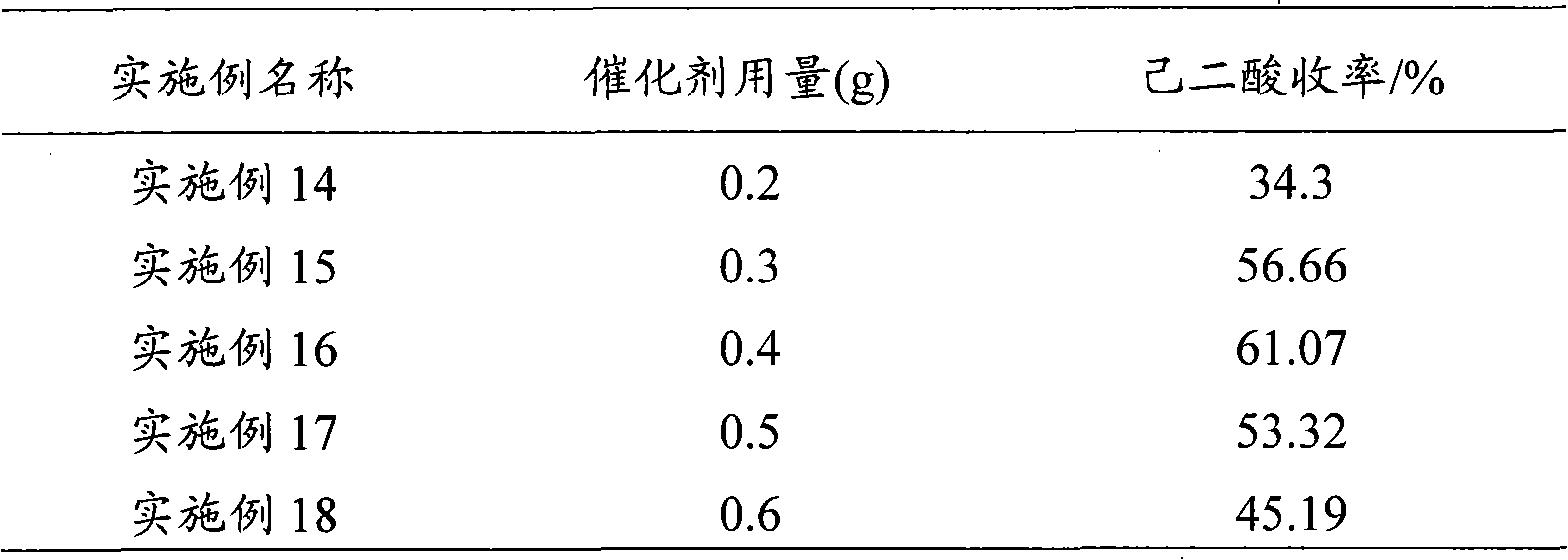
Method for preparing hexane diacid by liquid-phase catalytic oxidation of cyclohexanol
InactiveCN101302147AAvoid it happening againReduce usageOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphomolybdic acidHeteropoly acid
The invention provides a method for preparing adipic acid through liquid-phase catalytic oxidation of cyclohexanol. H2O2 is taken as oxidant, a heteropoly acid imidazole salt is taken as catalyst, the cyclohexanol undergoes the catalytic oxidation to prepare the adipic acid, the catalyst can be phosphotungstic acid 1-butyl-3-methylimidazolium bmim3PW12O40, phosphomolybdic acid 1-butyl-3-methylimidazolium bmim3PMo12O40, and molybdovanadophosphoric heteropolyacid 1-butyl-3-methylimidazolium bmim3+xPMo12-xVxO40 (x is equal to between 1 and 3) and so on, and the bmim represents a 1-butyl-3-methyl-imidazolium cation. Compared with the method of preparing the adipic acid by industrially using nitric acid (nitrate) to oxidize the cyclohexanol, the method can not produce poisonous oxynitride N2O, and avoids the harm to the environment; and in a reaction system, phase shift catalyst and addition agent are not used, which avoids the potential environmental pollution and has apparent economic and social significance.
Owner:ZHEJIANG UNIV OF TECH
Plane button type packing technology of integrated circuit or discrete component and its packing structure
ActiveCN1725460ASmooth productionImprove yieldSolid-state devicesSemiconductor/solid-state device manufacturingIntegrated circuitEngineering
This invention relates to a plane salient point encapsulation technology and its structure of IC or discrete components, among which, the technology includes: taking a base plate, dry-film layers are pasted to the front and back side of the base plate, removing part of the upper layer for the preparation of forming a basic island and pins, plating metal layers on the front, removing the rest dry film on the upper layer of the base plate, semi-etching it, removing the dry film on the back, implanting the chip, wiring, packaging with plastic capsule, pasting a dry film on the back of the base plate again, etching the dry film at the back and the rest of the metal of the semi-etched zone again so as to enable the back of the basic island and the pin projecting over the plastic capsule, removing the rest dry film, plating metal layers on the surface, pasting film on the front of the plastic capsule then cutting.
Owner:长电科技管理有限公司
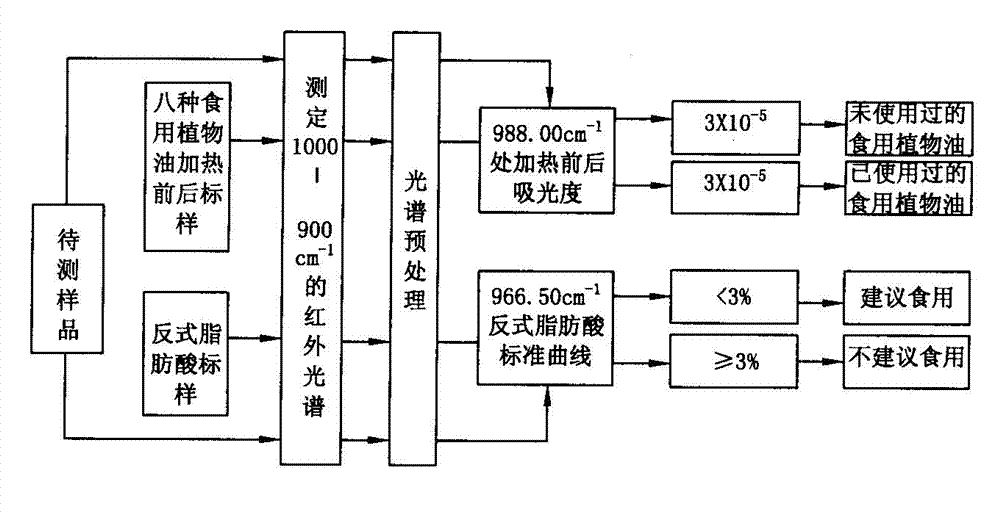
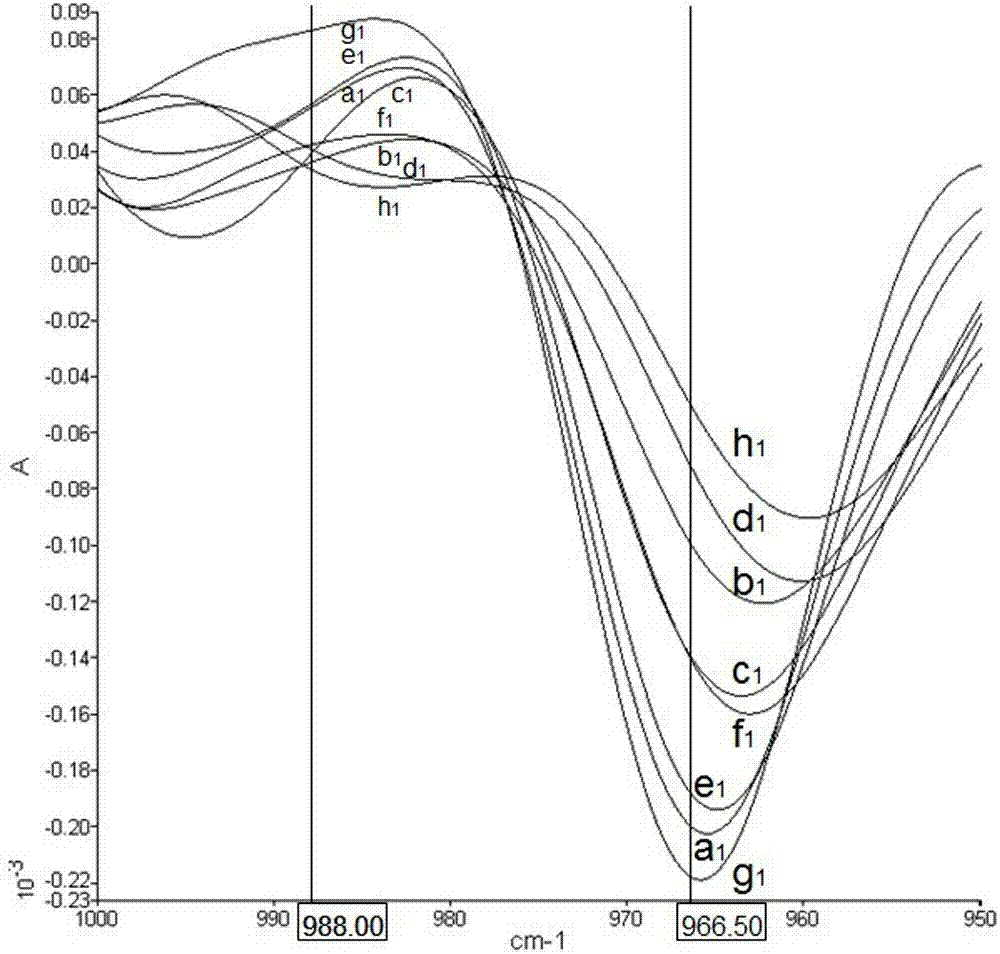
Quick detecting method of edible vegetable oil quality
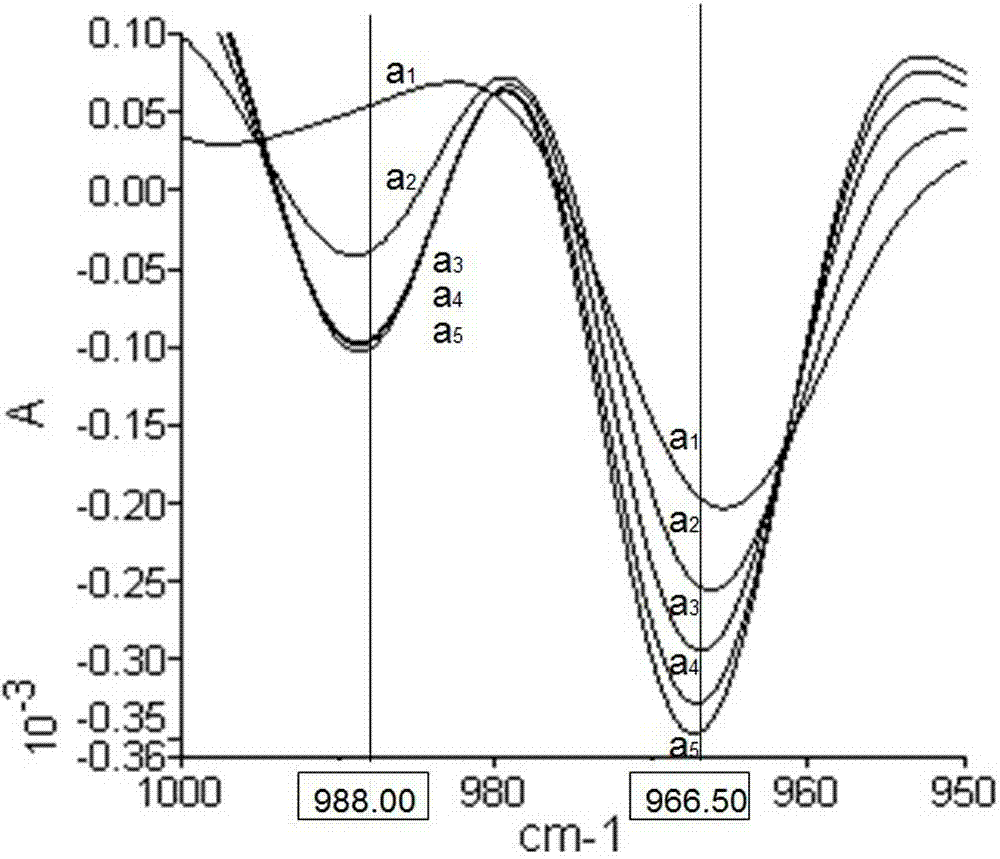
ActiveCN103245628ANot harmful to healthNo pollution problemPreparing sample for investigationColor/spectral properties measurementsVegetable oilHeating time
The invention discloses a quick detecting method of the edible vegetable oil quality through using an attenuated total reflection Fourier infrared spectroscopy, and the problems that the current detecting method can not verify whether the edible vegetable oil is repeatedly heated at high temperature or whether swill-cooked dirty oil is mixed in fresh edible vegetable oil can be solved. The method comprises the steps of: indicating the change of oil ingredients by fully utilizing the changes of the peak shape and the peak height of an infrared second derivative spectrometry; measuring the content of trans-fatty acids in the edible vegetable oil through absorbance of a characteristic absorption peak at 966.50cm<-1>; judging whether the edible vegetable oil is heated through the change of the characteristic peak at 988cm<-1>, and calculating the heating time; and determining whether the edible vegetable oil is swill-cooked dirty oil through the change of the characteristic absorption peak at 988cm<-1>. The quick detecting method of the edible vegetable oil quality is simple in operation, low in cost, free of pollution, high in detecting precision, and quick and accurate and can be capable of judging an edible vegetable oil sample to be detected which is repeatedly heated at the high temperature, the swill-cooked dirty oil and the swill-cooked dirty oil mixed in the fresh edible vegetable oil.
Owner:辽宁省分析科学研究院
Rail guided vehicle (RGV)
The invention discloses a rail guided vehicle (RGV). The rail guided vehicle comprises a driving device fixed on a RGV main body; the driving device is provided with driving wheels, a driving frame and a powder unit; the driving wheels are fixed on the power unit; the power unit is fixed on the driving frame; the driving frame is fixed on the RGV main body; the driving wheels are contacted with a rail platform, and are driven to rotate by the powder unit; and the motion of the RGV on the rail platform is driven by the friction driving force generated between the driving wheels and the rail platform generated by rotation. The RGV is capable of moving fast between a feeding position and an assembly location, utilization ratio of the RGV is increased, the number of RGV is reduced, cost is reduced, and no noise pollution or oil stain pollution is caused.
Owner:HUBEI HUACHANGDA INTELLIGENT EQUIP
Water generating method by utilizing separating membrane to enrich air water vapor and device thereof
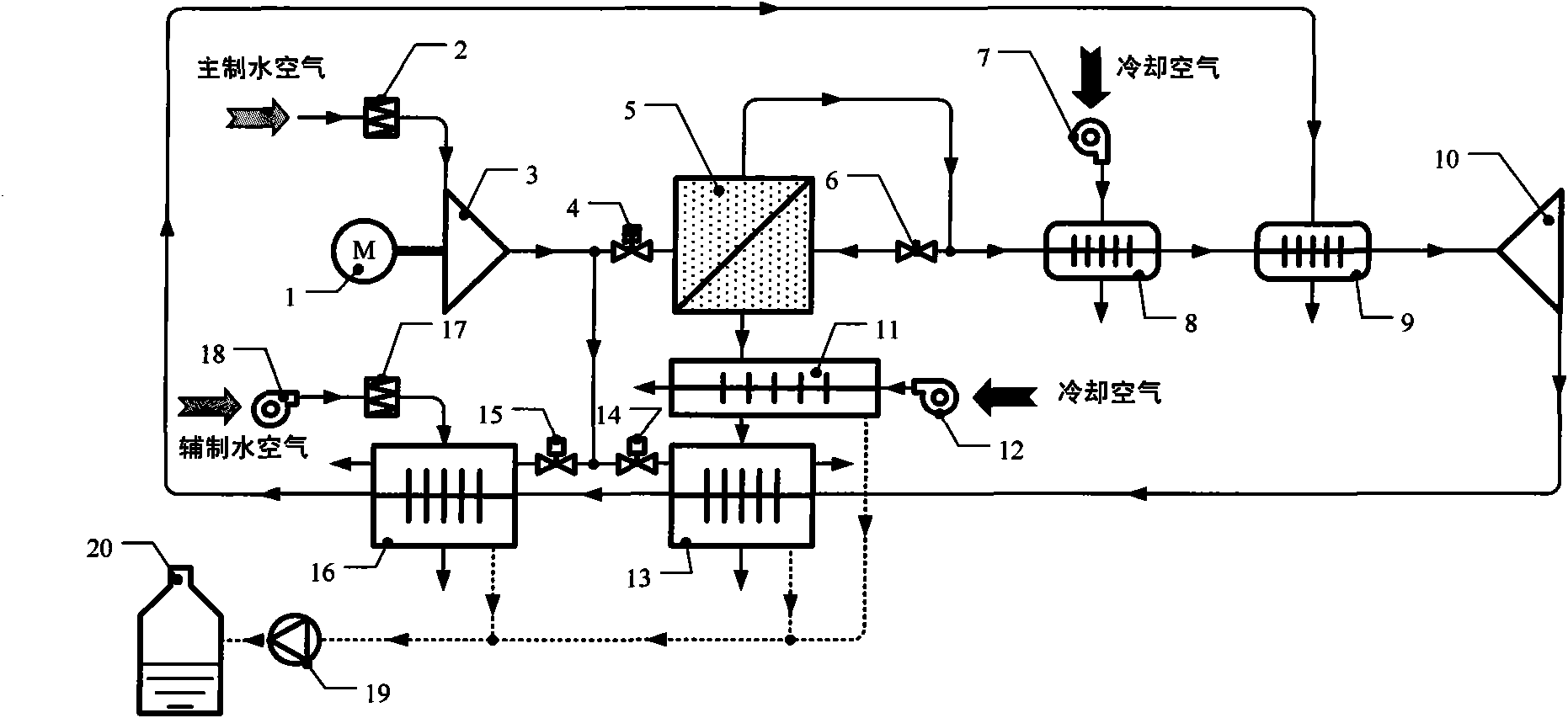
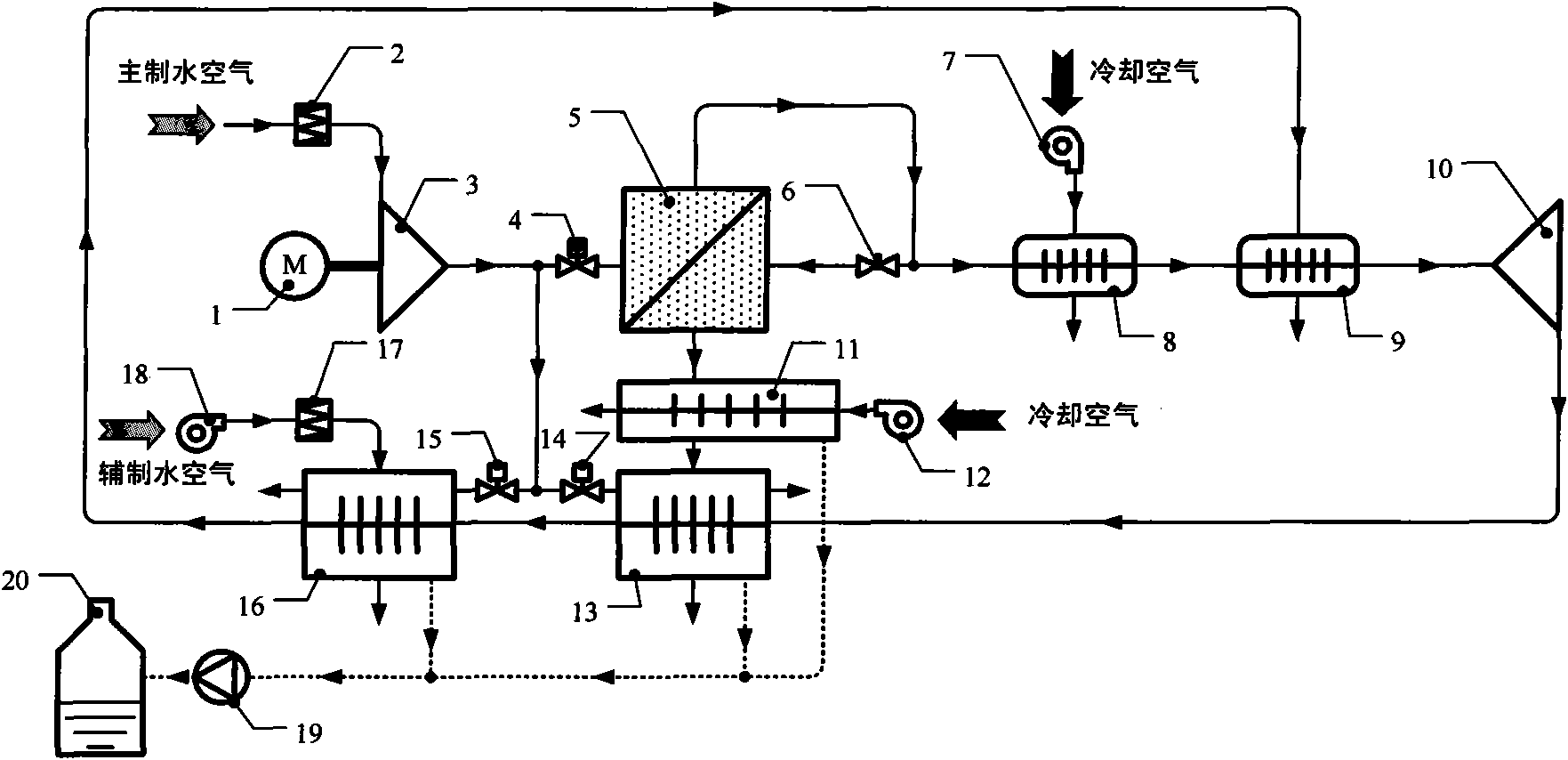
InactiveCN101851946AAchieve the purpose of enrichmentContinuously workingDispersed particle separationVapor condensationWater vaporWater production
The invention discloses a water generating method by utilizing separating membrane to enrich air water vapor and device thereof. The water generating method includes that membrane dehumidification assembly and compression method are adopted to carry out air dehumidification treatment, then sweep gas is input into the water vapor side of the membrane dehumidification assembly by virtue of pipeline to realize enrichment of water vapor, and finally condensing is carried out on the high humidity sweep gas for enriching water vapor, thus obtaining water in liquid state or solid state. Compared with absorption method, the invention has the advantages of continuous operation process, no corrosion problem, easy maintenance and low energy consumption; the adopted refrigeration working medium is air, no pollution problem is produced, and compressor and expansion machine are all centrifugal machineries, rotating speed is high, thus equipment volume is small and weight is light under unit water production rate; and meanwhile the low temperature water vapor cooling medium at the outlet of the cold side passage of the main water generating device is led into the cold side passage of the secondary water generating device, so as to cool the air from atmosphere environment and obtain extra water yield.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Method of preparing water-soluble mold core
InactiveCN101229574ANo pollution in the processGood water solubilityFoundry moulding apparatusSolubilityResin matrix
The invention pertains to a casting moulding technical field, which relates to improvement of a technical mold core for casting and molding resin matrix composites. The steps in the invention are the following: preparing aqueous solution of a bonder-adding aggregate-adding plaster powder-defoaming-casting moulding- drying- post-processing. Solidification has advantages of room temperature curing and good temperature endurance, good water solubility at room temperature and no environmental pollution.
Owner:BEIJING AVIATION MATERIAL INST NO 1 GRP CORP CHINA AVIATION IND
Mixed gas separation, mass storage, pressure rise and energy storage device and method and utility system
ActiveCN105299945AGuaranteed uptimeReduce emergency start timeCompression machinesSteam engine plantsBiomassMixed gas
The invention belongs to the field of energy storage, and discloses a mixed gas separation, mass storage, pressure rise and energy storage device and a method and a utility system, and a heat exchange pressure equalizer. The separation of mixed gas, the pressure rise, the energy storage, the heat exchange and the conversion of gas and liquid are finished in one device, so that the equipment is simplified, the efficiency is improved, and the heat energy is reduced; the cold energy is lost; the energy storage and the energy release can be separately or synchronously performed, so that the emission of carbon dioxide is reduced; and a nitrogen oxide, a sulfur oxide, heavy metal [pm 2.5] and the like are collected for storage and utilization to change harmful substances to useful substances. The invention discloses a gas (air and smoke) condensation, separation and energy storage power station, a wind-electricity plant air separation and energy storage power station, a photovoltaic power generation plant air separation and energy storage power station, a solar heat power air energy storage power station, a wind-electricity solar heat power complementary air energy storage power station, a nuclear power air separation and energy storage power station, a ire coal combustion biomass power generation plane smoke separation and energy storage power station, a gas power generation smoke separation and energy storage power station and an industrial kiln smoke separation and energy storage power station.
Owner:江洪泽
Cordyceps militaris water extract as well as preparation method and application thereof
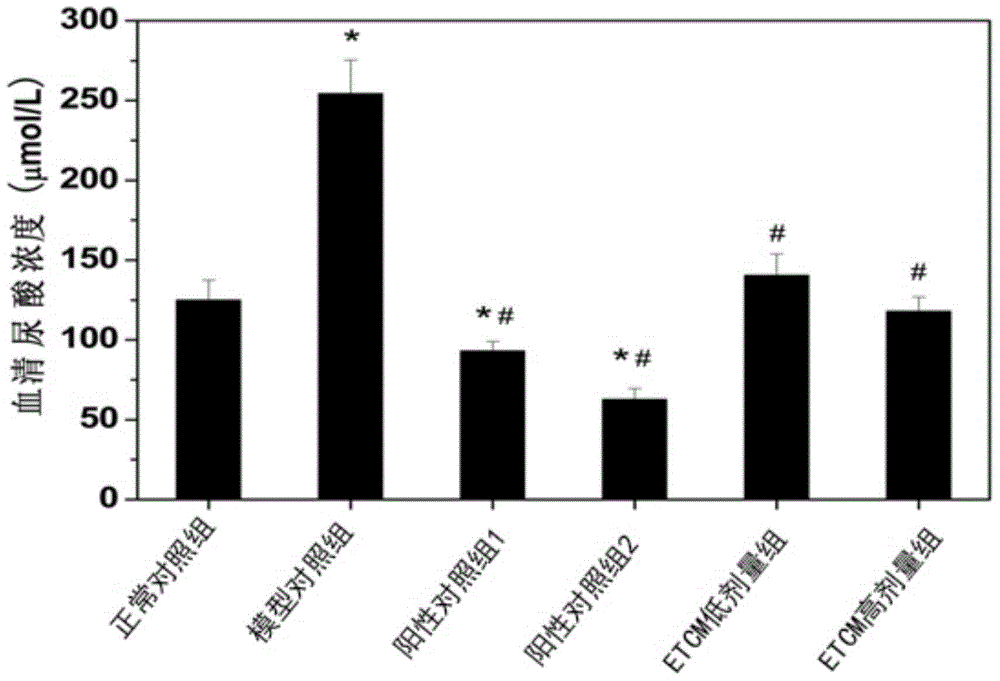
The invention provides a method for preparing a cordyceps militaris water extract. Water which is rich in resources and low in price serves as a solvent, and the aims of reducing the production cost and avoiding pollution of organic solvents are achieved. The cordyceps militaris water extract prepared by the method can be used for preparing medicines, health care products or auxiliary agents for reducing the uric acid level and improving the gout diseases.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY
Gastrodin chemical synthesis method suitable for industrialization
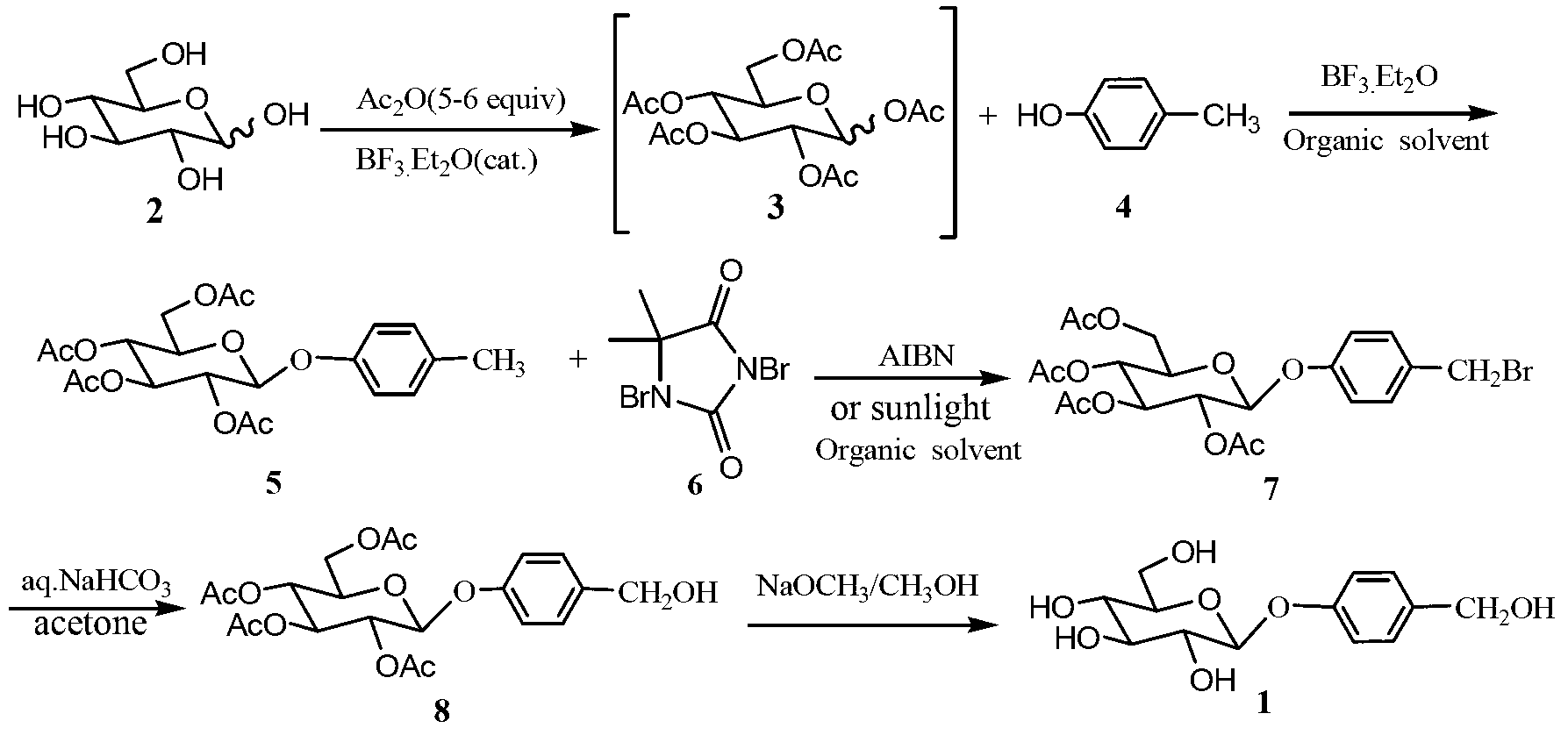
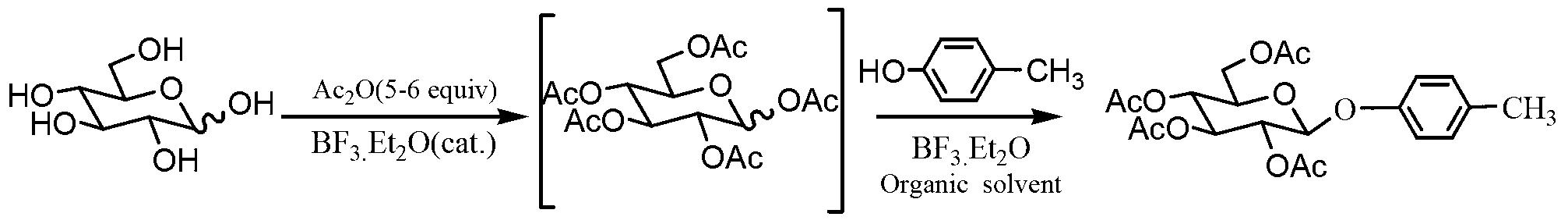
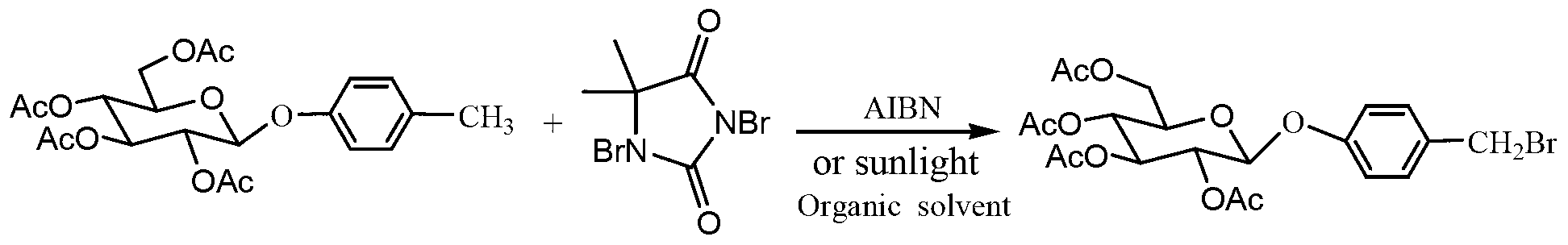
InactiveCN103275146AAvoid Pollution and HazardsEasy to prepareSugar derivativesSugar derivatives preparationChemistryChemical synthesis
The invention discloses a gastrodin chemical synthesis method. The method comprises the steps as follows: under Lewis acid catalysis, D-glucose reacts with acetic oxide, pentacetyl-D-glucose is obtained and not subjected to separation and purification; the pentacetyl-D-glucose and the p-cresol are catalyzed by Lewis acid and subjected to a glycosylation reaction directly in an organic solvent, so that 4-methylphenyl 2,3,4,6-O-tetracetyl-b-D-glucopyranoside is generated; then the 4-methylphenyl-2,3,4,6-O-tetracetyl-b-D-glucopyranoside reacts with 1,3-dibromo-5,5-dimethylhydantoin under the action of an initiator or in solar radiation, so that 4-bromomethyl-2,3,4,6-0-tetracetyl-b-D-glucopyranoside is obtained; then under the alkalescence condition, 4-bromomethyl-2,3,4,6-0-tetracetyl-b-D-glucopyranoside is hydrolyzed selectively, so that 4- Hydroxymethyl phenyl-2,3,4,6-O-tetracetyl-b-D-glucopyranoside is obtained; and finally an acetyl protecting group is removed from a methyl alcohol-sodium methoxide system, so that the gastrodin is obtained. Compared with a conventional method, the gastrodin chemical synthesis method has the advantages as follows: raw materials are easy to obtain, the cost is low, reaction products in all steps can be prepared through recrystallization, the process is simple, the reaction period is short, the yield is high, and the method is more suitable for preparing the gastrodin in large scale.
Owner:QINGDAO AGRI UNIV
Catalyst for polymerization or copolymerization of propylene and its preparation and application
The active component of the catalyst is prepared through dissolving magnesium halide in organic epoxide, organic phosphide and inert diluent to form homogeneous solution, mixing the solution with titanium tetrahalide or its derivative to separate solid matter in the presence of separate assistant, and washing the solid matter with the same inert solvent as that in forming magnesium halide solution, rather than more poisonous ethane dichloride. The preparation has no trouble of two kinds of solvent to form hard to separate azeotrope during solvent recovery, and has short technological process, high solvent recovering rate and low catalyst producing cost.
Owner:CHINA PETROCHEMICAL CORP +1
Method for producing recombinant human epidermal growth factor acceptor 2 cytoplasmic region with methylotrophy yeast
InactiveCN101492675AEfficient expressionOptimizing Dissolved Oxygen LevelsFungiMicroorganism based processesYeastFermentation
The invention relates to a method for producing, purifying and appraising protein, in particular to a method for expression of recombinant human her2 / neu ICD in Pasteur yeast as well as optimized large scale industrialized fermentation production and purification of the recombinant human her2 / neu ICD.
Owner:吉林圣元科技有限责任公司
Special molybdenum powder used for automatic forming and preparation method thereof
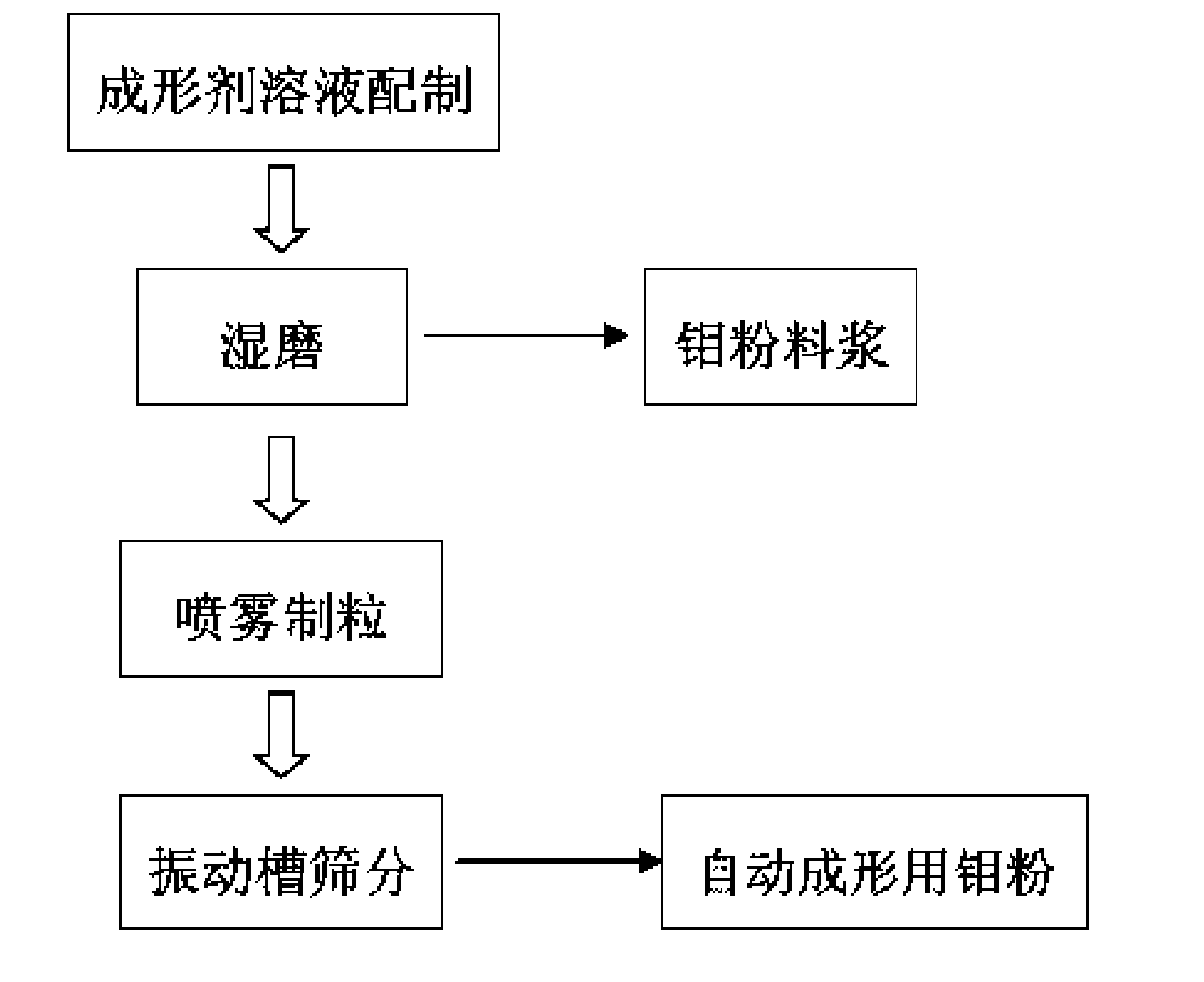
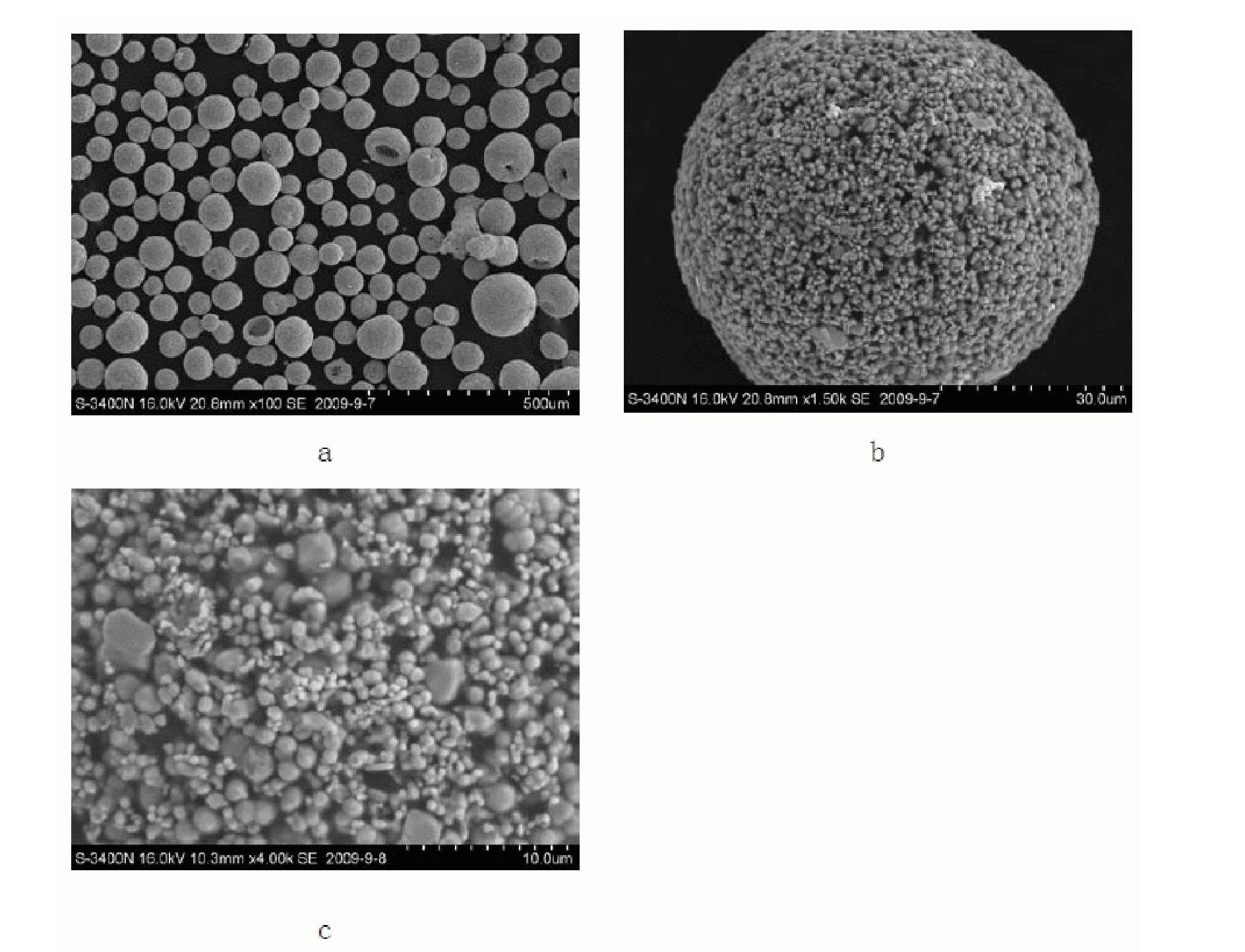
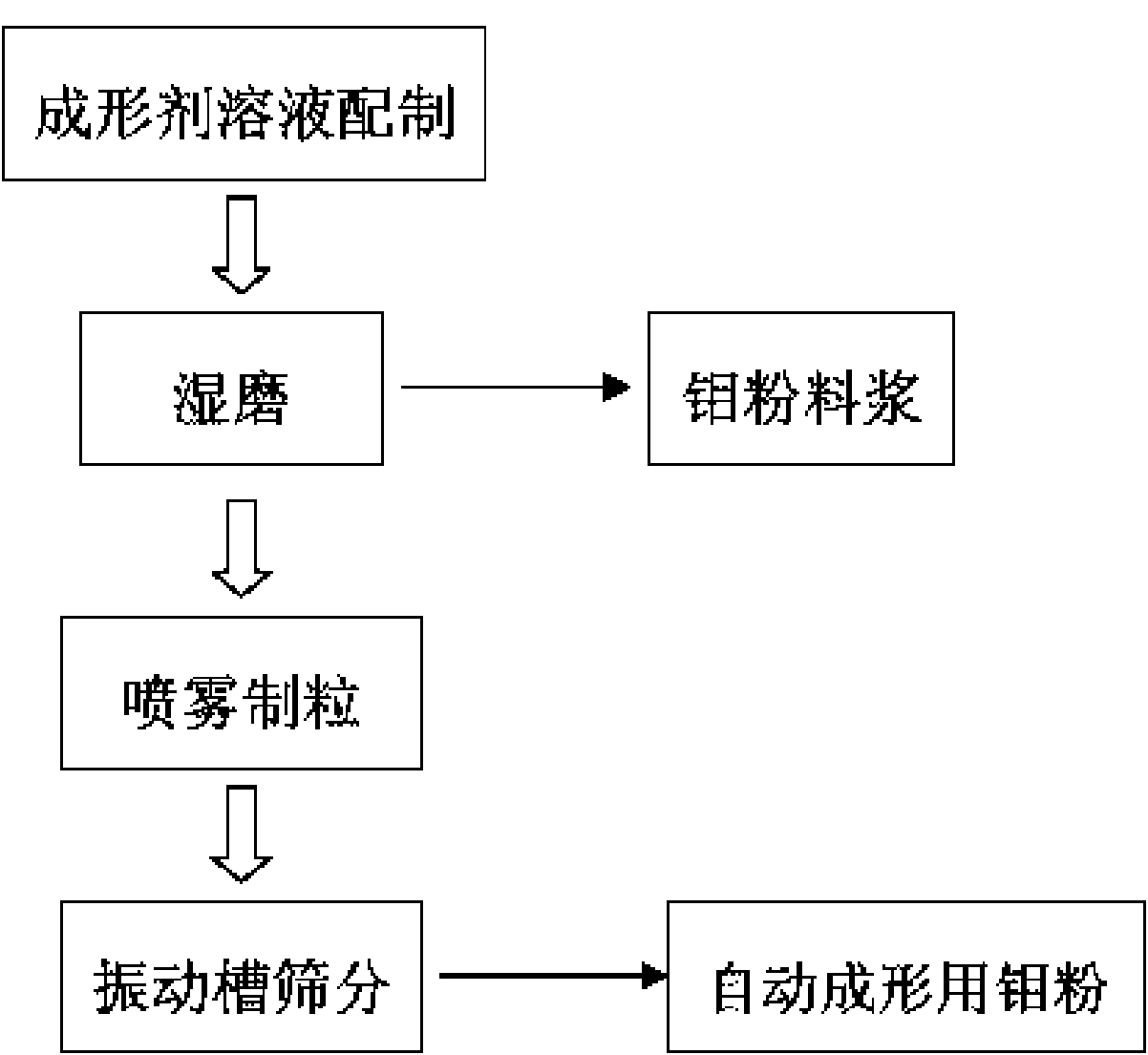
The invention discloses a special molybdenum powder which is directly used for the automatic forming of precision devices of the machinery industry and the electronic industry. The invention is characterized in that the molybdenum powder comprises the following materials according to the parts by mass: 96.6-99.5 parts of primary molybdenum powder, 0.5-2.4 parts of polyvinyl alcohol, 0-0.4 part of polyethylene glycol, 0-0.5 part of metallic stearate and 0-0.15 part of ammonium polyacrylate. The molybdenum powder has good flowability, high apparent density and appropriate particle size distribution and has the self-lubricating action in the process of the automatic forming. The invention also discloses a preparation method of the special molybdenum powder, which comprises the processes: (1) forming agent solution is prepared by the polyvinyl alcohol, the polyethylene glycol, water and other additives; (2) wet grinding is carried out on the primary molybdenum powder, metallic balls and the forming agent solution by proportion in a ball milling tank to prepare molybdenum powder slurry; (3) spray drying is carried out on the prepared molybdenum powder slurry for granulation; (4) the prepared molybdenum powder is collected by a vibrating screen arranged at the discharge hole of a spray drying tower. The preparation method has the greatest advantages of the special molybdenum powder used for the automatic forming can be prepared by one step, the efficiency is high, the cost is low, and the industrialization and no pollution can be realized.
Owner:XIAMEN HONGLU TUNGSTEN MOLYBDENUM IND CO LTD
Environment-friendly process for producing dihydromyrcenol by using dihydromyrcene hydration reaction
ActiveCN101684064ASimple processReduce energy consumptionOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by hydroxy group additionHydration reactionFixed bed
The invention discloses an environment-friendly process for producing dihydromyrcenol by using a dihydromyrcene hydration reaction. The process adopts an integrated system consisting of a jet reaction device, an oil-water separation device and a rectification device. A high-speed injector is adopted in a reactor for reinforcing heat transfer and mass transfer of a reaction process; an acid (containing sulphuric acid, phosphoric acid and p-toluenesulfonic acid) is used as a catalyst and is in closed cycle; and an oil-water separator is arranged in the process flow to reduce the heat load of a rectification tower, and meanwhile the catalyst and the water phase in the oil-water separator are cycled and used in the system together so as to avoid the environment problems caused by the discharge of acid waste water. The process of the invention can improve the conversion rate of dihydromyrcene, and reduce the energy consumption and the production cost. Compared with a mechanical stirring reactor or a fixed bed reactor with the same scale, the process can improve the conversion rate of the dihydromyrcene by 1.2 to 4 times, and reduce the energy consumption of a dihydromyrcenol product per ton by more than 55 percent.
Owner:厦门中坤化学有限公司 +1
Manufacturing method for honeycomb structure made of composite material and equipment used for manufacturing honeycomb structure made of composite material
PendingCN107244087AThe production process is environmentally friendlyRealize automated productionDomestic articlesHoneycomb structureFiber
The invention provides a manufacturing method for a honeycomb structure made of a composite material. The manufacturing method comprises the following steps of: step 1, soaking fiber cloth with a resin material to prepare prepreg; step 2, performing mould pressing on the prepreg obtained in step 1 to form corrugated sheets with needed shapes; and step 3, stacking and jointing the corrugated sheets obtained in step 2 to form the honeycomb structure made of the composite material. The invention further provides equipment used for manufacturing the honeycomb structure made of the composite material. According to the manufacturing method for the honeycomb structure made of the composite material and the equipment used for manufacturing the honeycomb structure made of the composite material, the fiber cloth is sequentially soaked with resin and cured by the equipment, the resin adopts thermosetting resin or thermoplastic resin, viscidity of the resin can be reduced by a heating mode, and the resin is changed into a flowable state and is conveniently soaked onto the fiber cloth. In a production process, a solvent does not need to dilute, so that the pollution problem due to absence of the solvent is avoided, and therefore, the production process is environmentally friendly.
Owner:苏州云逸航空复合材料结构有限公司
Method and device for hot assembling large-diameter bearing
InactiveCN101920431AReduce consumptionShort preparation timeMetal working apparatusEngineeringBottle
The invention discloses a method and a device for hot assembling a large-diameter bearing. The method comprises the following steps of: cleaning the interior and exterior of a bearing to be heated, and manufacturing a heating device; determining the dimension of a bottom plate, arranging a heating coil under the bottom plate, arranging a plurality of gas nozzles on the heating coil, connecting the heating coil with a gas bottle, and making the preparation work before heating ready; determining the heating temperature of the bearing; hanging the bearing in a sealed cavity of the heating device; igniting the gas nozzles of the heating device, and heating the bearing to the heating temperature required by the process; and hanging the bearing to a mounting position and assembling according to the requirement. In the method for hot assembling the large-diameter bearing, air in the sealed space is heated by coal gas and then directly heats the bearing, the energy consumption is low, the preparation time required is short, and the construction period can be shortened; and the oil heating is not adopted, the treatment for the waste oil is avoided, and the problem of environmental pollution caused by oil splashing and oil emission is avoided.
Owner:CHINA METALLURGICAL CONSTR ENG GRP
Method of manufacturing carbon nano tube composite film on glass substrate surface
The invention discloses a making method of carbon nanometer pipe composite film on the glass base surface, which comprises the following steps: immersing glass base in the Pirahan solution to dispose for 1h at 90 deg.c; cleaning; drying; immersing in the mercapto silicane solution; stewing 6-8h; washing; blowing through nitrogen; placing in the nitric acid solution; obtaining the glass base with sulfosilicane film on the surface; placing base into carbon nanometer suspension liquid modified by rare earth; stewing for 4-20h under 20-80 deg.c; fetching; washing through large amount of deionized water; blowing through nitrogen; obtaining the product.
Owner:SHANGHAI JIAO TONG UNIV
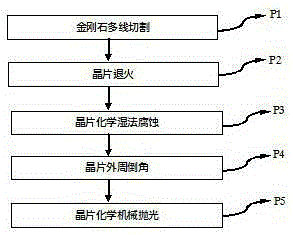
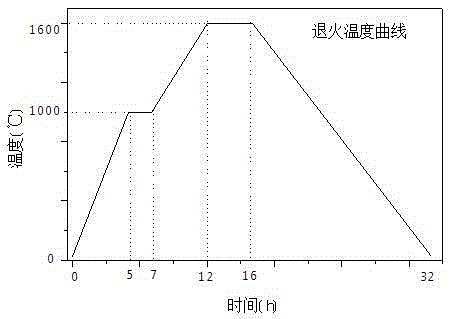
Processing method for double-sided polished sapphire wafers
ActiveCN105313234AEasy to control speedIncrease controlEdge grinding machinesAfter-treatment detailsWaferingEtching
The invention provides a processing method for double-sided polished sapphire wafers. The main technological process comprises the steps of multi-wire cutting, wafer annealing, wafer chemical wet etching, wafer periphery chamfering and wafer chemical-mechanical polishing. Since the sapphire wafers are processed through the method, the operation processes are simplified, operation is easier, and the processing cycle can be efficiency shortened. On the precise of guaranteeing the thickness and surface quality of the wafers, the reserved processing allowance for subsequent processing can be reduced in the slicing process, the utilization rate of crystals can be increased, and therefore the production and manufacturing cost of the sapphire wafers is reduced.
Owner:哈尔滨秋冠光电科技有限公司
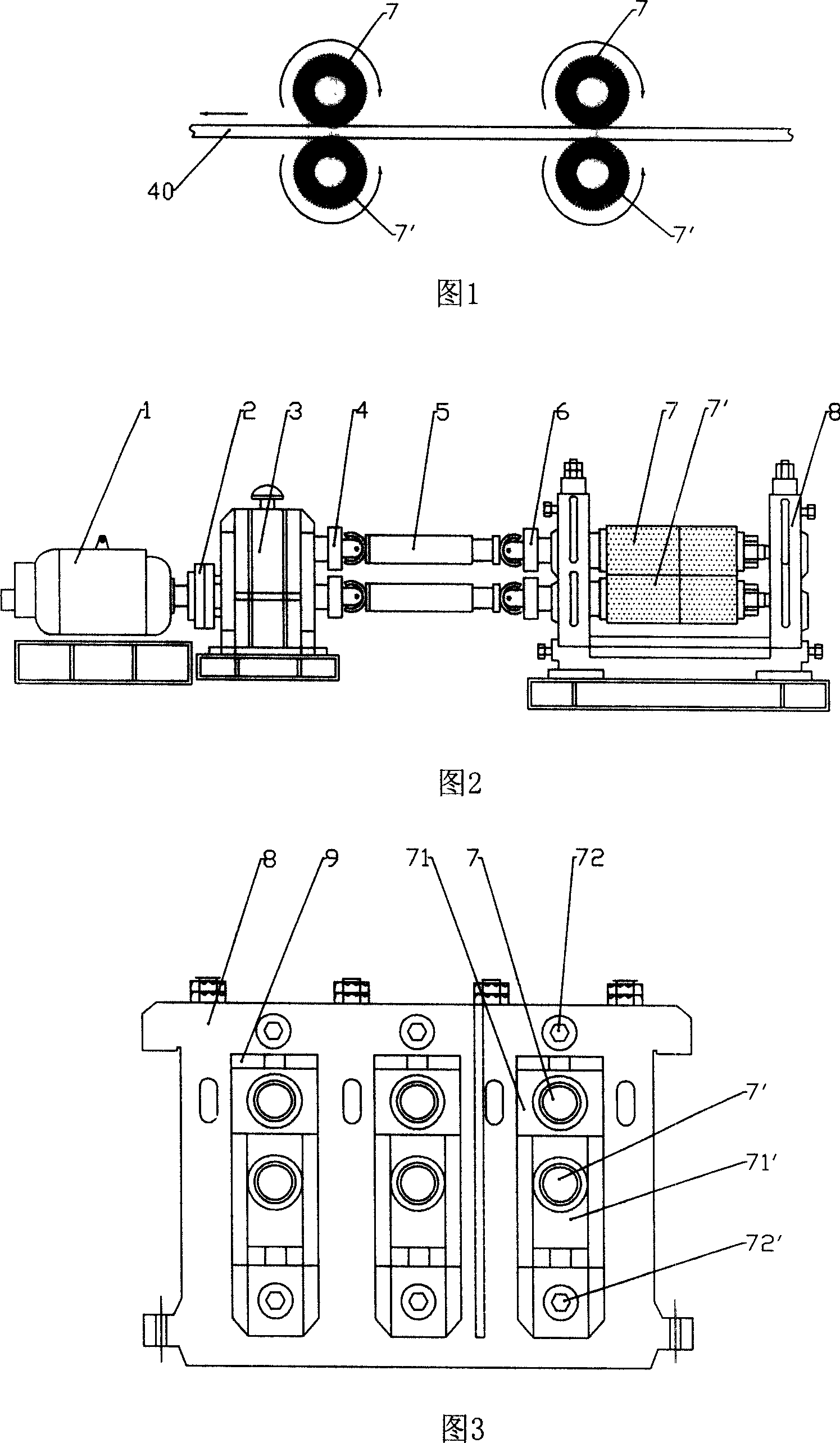
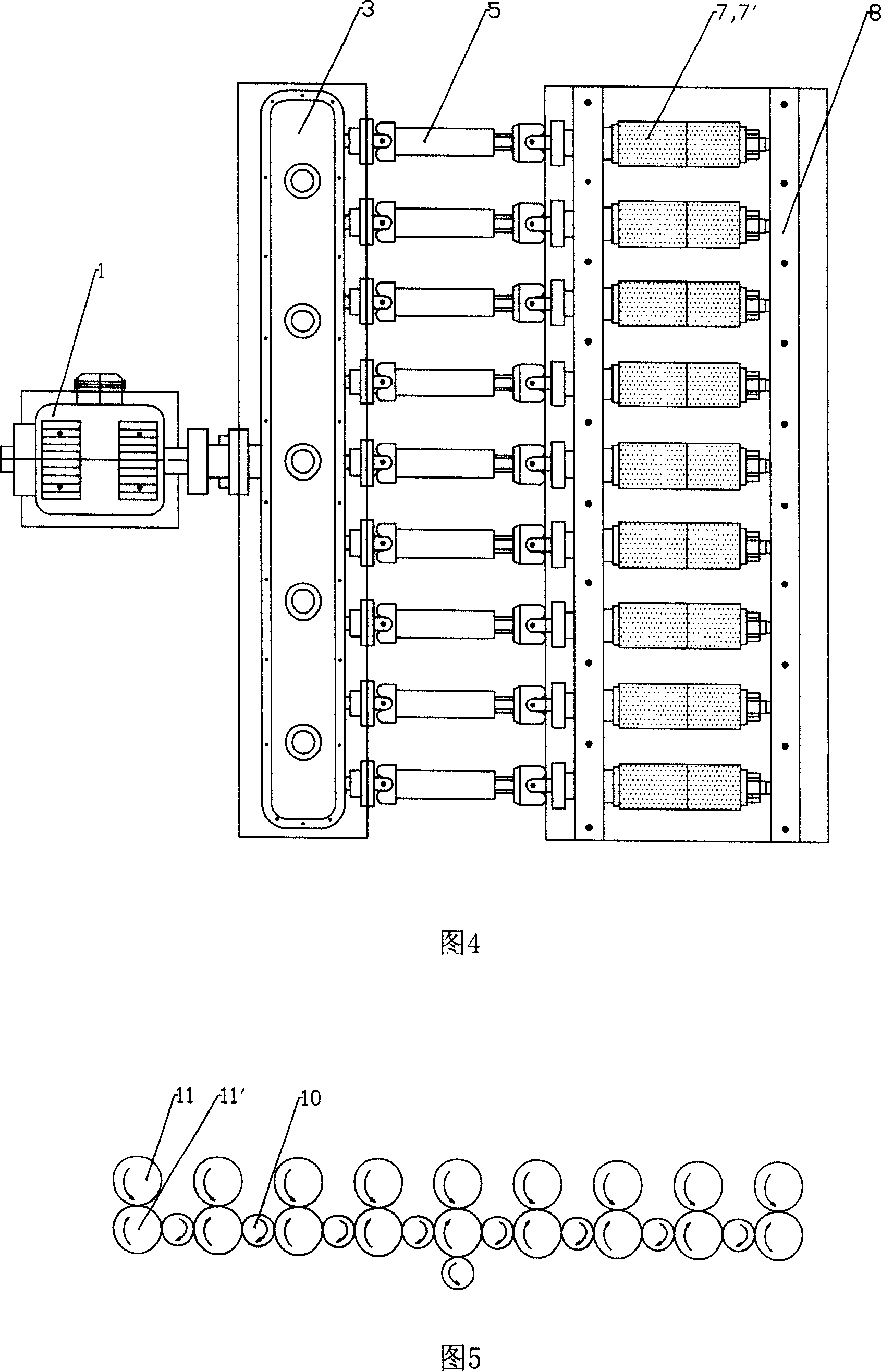
Derusting and descaling machine for belt steel
The invention discloses a band steel descaling machine, comprising a frame via a bearing parallel arranged with several couples of steel wire wheels, wherein two steel wire wheels of each couple are up and down parallel with a space between to pass steel plate. The invention also comprises a power device for rotating the steel wire wheels via a transmitter, all upper steel wire wheels rotate in same direction, while all lower steel wire wheels rotate reversed to the upper wheels. The invention can save acid material and cost or the like.
Owner:翟文海 +1
Water-soluble core mold material for forming filament-wound case and preparation method of water-soluble core mold material
InactiveCN102827445AExcellent high temperature mechanical propertiesMeet the requirements of curing molding processFiberTemperature resistance
The invention relates to a water-soluble core mold material for forming a filament-wound case and a preparation method of the water-soluble core mold material. The water-soluble core mold material for forming the filament-wound case comprises composite binder aqueous solution and core mold packing, and the composite binder aqueous solution comprises polyvinyl pyrrolidone binder aqueous solution and polyvinyl alcohol binder aqueous solution. The polyvinyl pyrrolidone binder aqueous solution accounting for 20-80Wt% of the total mass of the composite binder aqueous solution is mixed with the polyvinyl alcohol binder aqueous solution to prepare the composite binder aqueous solution. The addition quantity of the water-soluble high-polymer binder aqueous solution is 0.05-0.5 time of the weight of the core mold packing. The preparation method includes steps of preparing the polyvinyl pyrrolidone binder aqueous solution; preparing the polyvinyl alcohol binder aqueous solution; preparing the composite binder aqueous solution; preparing the packing; mixing the materials; filling and forming; drying and solidifying; and demoulding and machining. The density and the compressive strength of a core mold prepared by the method can be adjusted according to service conditions, and the core mold is good in temperature resistance, room-temperature water-soluble collapsibility and fracture toughness, and is free of environmental pollution.
Owner:中国航天科工集团第六研究院41所
High-and-low-temperature supercritical carbon dioxide waste heat utilization system
ActiveCN105443170AEfficient use ofTake advantage ofSteam engine plantsGas compressorCycle efficiency
The invention provides a high-and-low-temperature supercritical carbon dioxide waste heat utilization system which comprises a high-temperature turbine, a low-temperature turbine, a waste heat recoverer, a high-temperature reheater, a low-temperature reheater, a condenser and a gas compressor. A gas inlet of the high-temperature turbine is sequentially connected with the waste heat recoverer, the high-temperature reheater, the gas compressor and the condenser. A gas inlet of the condenser is further sequentially connected with a gas outlet of the high-temperature reheater and a gas outlet of the high-temperature turbine, so that a high-temperature turbine circulation loop is formed. A gas inlet of the low-temperature turbine is sequentially connected with the waste heat recoverer, the low-temperature reheater, the gas compressor and the condenser. A gas inlet of the condenser is further sequentially connected with a gas outlet of the low-temperature reheater and a gas outlet of the low-temperature turbine, so that a low-temperature turbine circulation loop is formed. According to the high-and-low-temperature supercritical carbon dioxide waste heat utilization system, exhausted high-temperature gas of a gas turbine or industrial waste heat / lost heat serves as a heat source, high-grade heat energy is utilized efficiently, and low-grade heat energy is utilized, so that energy sources are utilized sufficiently, and the overall circulation efficiency is improved.
Owner:SHANGHAI TURBINE
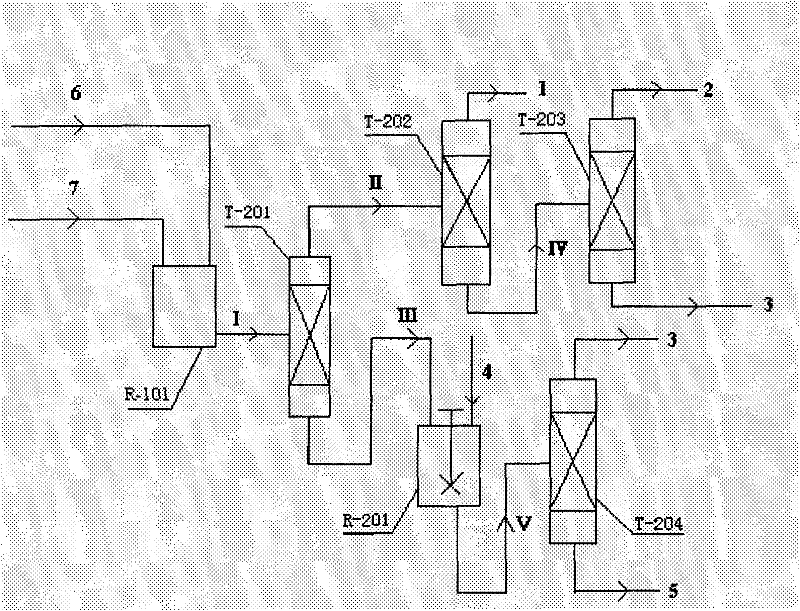
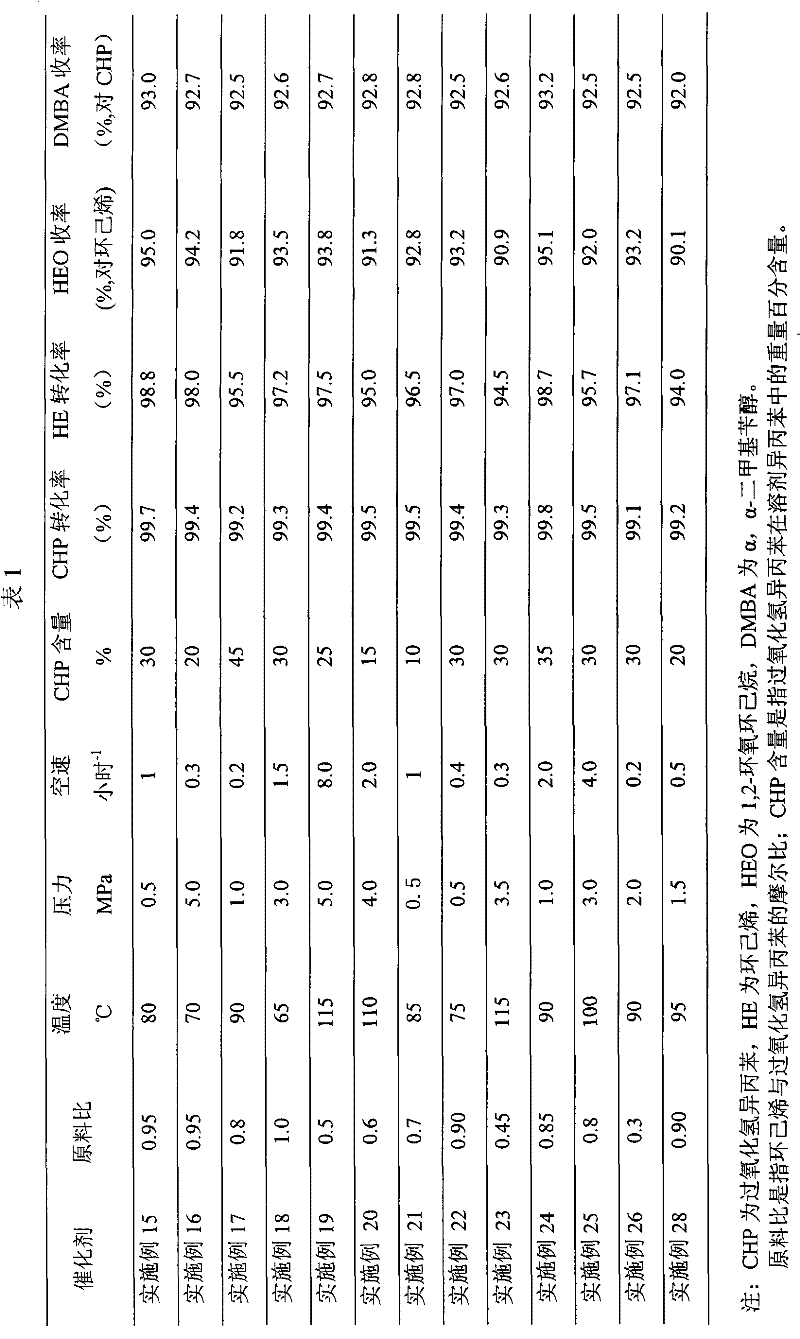
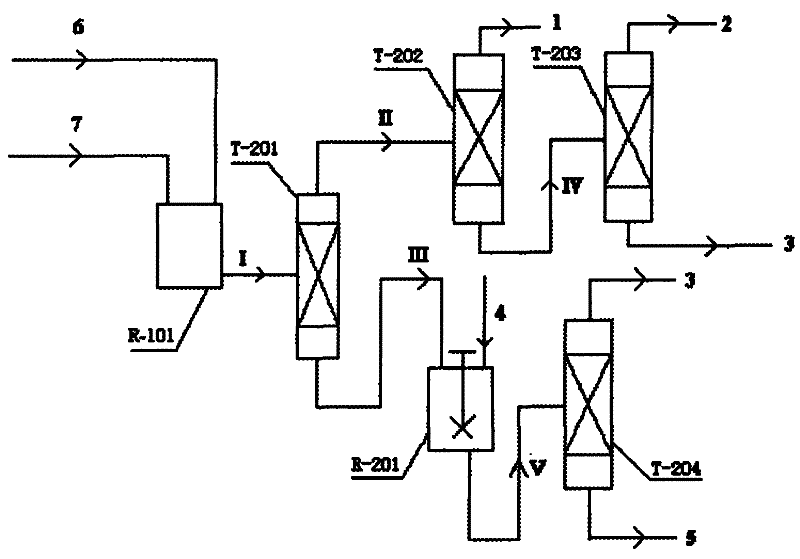
Coproduction method of 1,2-epoxycyclohexane and α,α-dimethylbenzyl alcohol
The invention relates to a method for producing 1,2-epoxy cyclohexane and alpha, alpha-dimethyl benzyl alcohol. The method mainly solves the problems of serious production process pollution, poor product quality and high production cost when the 1,2-epoxy cyclohexane and the alpha, alpha-dimethyl benzyl alcohol are separately produced in the prior art. Isopropyl benzene hydroperoxide and cyclohexene undergo oxidation-reduction reaction on a titanium-containing porous silicon dioxide catalyst under the mild reaction condition, wherein the isopropyl benzene hydroperoxide is reduced into the alpha, alpha-dimethyl benzyl alcohol, and the cyclohexene is oxidized into the 1,2-epoxy cyclohexane; and meanwhile, by adjusting the molar ratio of the cyclohexene to the isopropyl benzene hydroperoxide in the raw materials, the cyclohexene is totally converted or has little residue in the reaction process. According to the technical scheme, the problems are well solved, and the method can be used for industrial production of producing the 1,2-epoxy cyclohexane and the alpha, alpha-dimethyl benzyl alcohol.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com