Method for preparing hexane diacid by liquid-phase catalytic oxidation of cyclohexanol
A technology of liquid-phase catalysis and ring oxidation, which is applied in the production of adipic acid, chemical instruments and methods, and the preparation of organic compounds, etc. It can solve the problems such as the decline in the yield of adipic acid, and meet the requirements of low reaction energy consumption and reaction equipment Low, the effect of low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of phosphomolybdovanadic acid:
[0029] 3.58g Na 2 HPO 4 12H 2 O dissolved in 50mL distilled water, 24.2g Na 2 MoO 4 2H 2 O was dissolved in 60mL distilled water, the two solutions were mixed, heated to boiling, and reacted for 30min; then 1.58g NaVO 3 2H 2 O was dissolved in 20mL of distilled water, and the solution was added to the above mixture under stirring, and reacted at 90°C for 30min; stop heating; add 1:1 H while stirring 2 SO 4 until the pH of the solution = 2.0, and continue to stir to room temperature, add 50mL ether to the mixture, shake it well, then add 1:1H 2 SO 4 Continue to vibrate until the solution is divided into three layers after standing still, and the bright red oil in the middle layer is heteropolyacid ether compound. The middle layer was taken, and ether was removed under vacuum at room temperature to obtain 16.4 g of phosphomolybdenum-vanadate.
[0030] According to the above method steps, 1.58g NaVO 3 2H 2 O is repl...
Embodiment 2
[0035] In a 100mL three-necked flask equipped with magnetic stirring and a reflux condenser, add 4.5g (45mmol) of cyclohexanol, 30% H 2 o 2 Oxidant 25mL, heating up to 60°C, adding 0.2g of phosphotungstic acid 1-butyl-3-methylimidazolium salt ([bmim] 3 PW 12 o 40 ) catalyst, stirring reaction 5h. After filtration, the filtrate was cooled to -5°C and kept for 12 hours. Then filter, and vacuum-dry the solid at room temperature for 2 hours to obtain 0.7 g of a white crystalline adipic acid product with a purity of 98% and a yield of adipic acid of 10.15%.
Embodiment 3~6
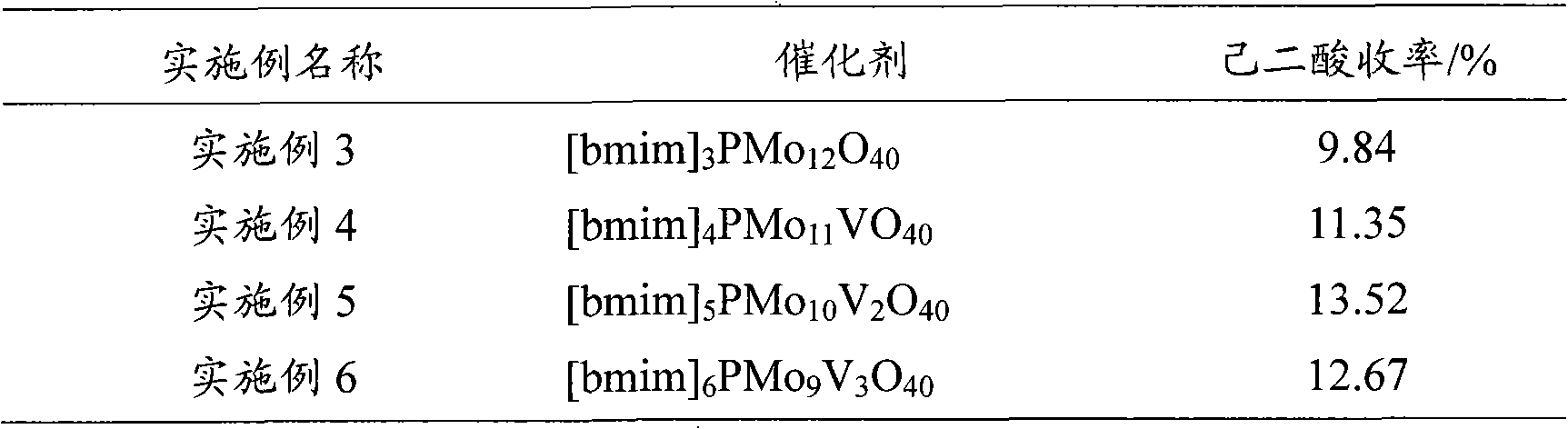
[0037] The 1-butyl-3-methylimidazolium phosphotungstate catalyst described in Example 2 was replaced by different imidazolium salt heteropolyacids, and other conditions were the same as in Example 2. The test results are listed in Table 1.
[0038] Table 1
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com