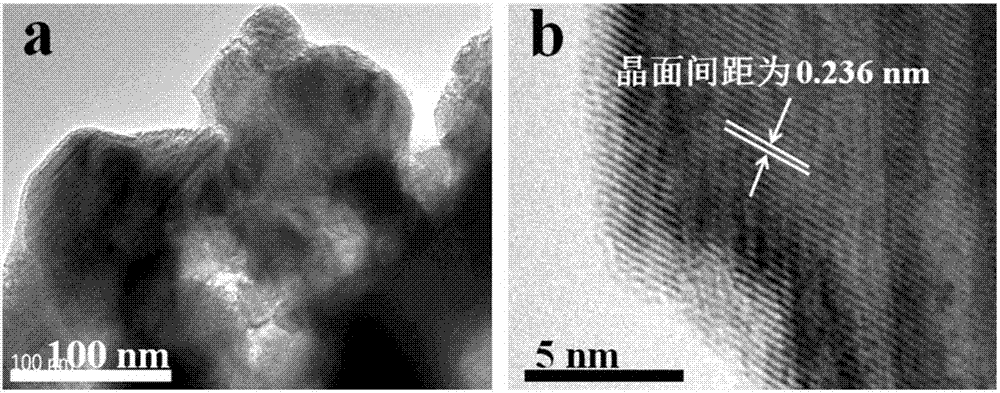
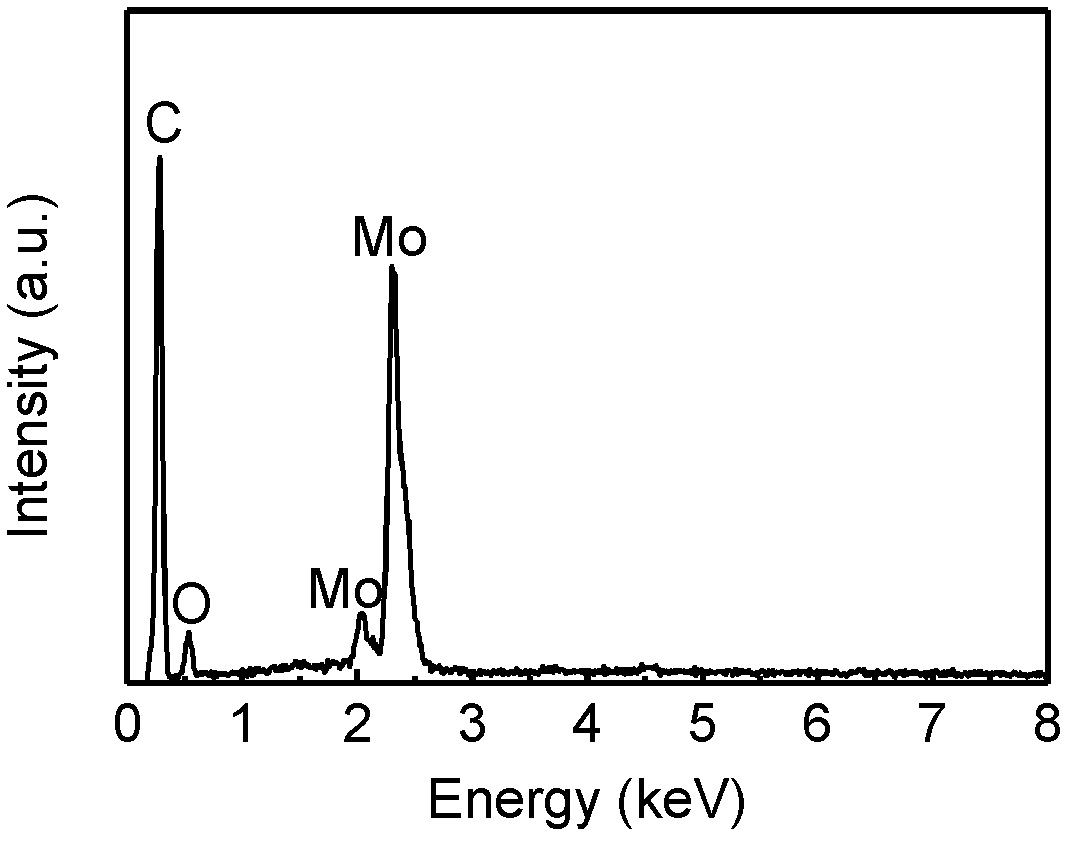
Patents
Literature
470 results about "Phosphomolybdic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphomolybdic acid, also known as dodeca molybdophosphoric acid or PMA, is a yellow-green chemical compound that is freely soluble in water and polar organic solvents such as ethanol.
Process for preparing epoxy chloropropane
InactiveCN1900071AHas reaction controlled phase transfer propertiesOrganic chemistryPhosphomolybdic acidEpoxy
In the preparation of epoxy chloropropane, chloropropene is oxidized with H2O2 aqua as oxygen source in no solvent condition and under the action of heteropoly acid catalyst of phosphomolybdic acid or phosphotungestic acid to produce epoxy chloropropane, with the catalyst being recovered for reuse. The preparation process has mild reaction condition, high epoxy chloropropane yield, single product, high selectivity and no pollution, and is suitable for industrial production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
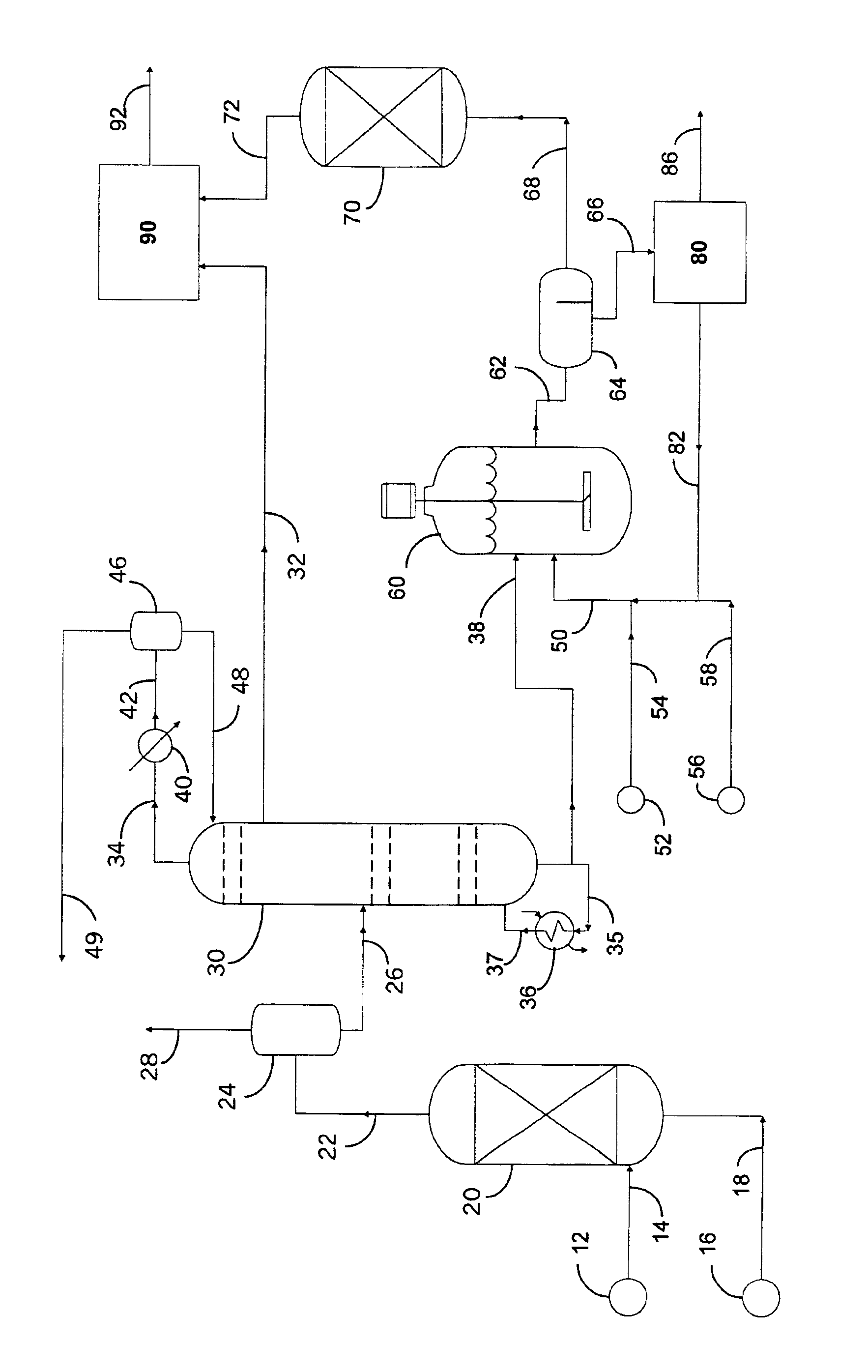
Preparation of components for transportation fuels
InactiveUS6881325B2Environmentally friendlyRefining with oxygen compoundsRefining with acid-containing liquidsPhosphomolybdic acidComponents of crude oil
Economical processes are disclosed for the production of components for refinery blending of transportation fuels by selective oxidation of feedstocks comprising a mixture of hydrocarbons, sulfur-containing and nitrogen-containing organic compounds. Oxidation feedstock is contacted with a soluble quaternary ammonium salt containing halogen, sulfate, or bisulfate anion, and an immiscible aqueous phase comprising a source of hydrogen peroxide, and at least one member of the group consisting of phosphomolybdic acid and phosphotungstic acid, in a liquid reaction mixture under conditions suitable for reaction of one or more of the sulfur-containing and / or nitrogen-containing organic compounds. Blending components containing less sulfur and / or less nitrogen than the oxidation feedstock are recovered from the reaction mixture. Advantageously, at least a portion of the immiscible acid-containing phase is recycled to the oxidation.
Owner:BP CORP NORTH AMERICA INC
Method for reclaiming carbon fiber reinforced epoxy resin composite material
ActiveCN102181071ARelieve stressImprove degradation efficiencyPlastic recyclingBulk chemical productionEpoxyPhosphomolybdic acid
The invention relates to a method for reclaiming a carbon fiber reinforced epoxy resin composite material. The conventional method is high in equipment requirement and high in reclamation cost. The method comprises the following steps of: adding a catalyst into an organic reagent to prepare supercritical CO2 composite solution; putting the carbon fiber reinforced epoxy resin composite material tobe decomposed into a reaction kettle, and adding the supercritical CO2 composite solution; and reacting for 1 to 24 hours at the temperature of between 100 and 250 DEG C under the pressure of 7.5 to 25.0MPa, cooling the product to normal temperature, washing and drying the solid product in the product to obtain carbon fibers, and performing reduced pressure distillation on the liquid product in the product to obtain phenol and derivatives thereof. The catalyst is one or two of liquid super acid, solid super acid, phosphotungstic acid, phosphomolybdic acid, acetic acid, formic acid, hydrochloric acid, sulfuric acid and nitric acid. The method has the advantages of high degradation efficiency, environmental friendliness, low cost and the like, and is a green method for reclaiming the waste and old carbon fiber reinforced epoxy resin composite material.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
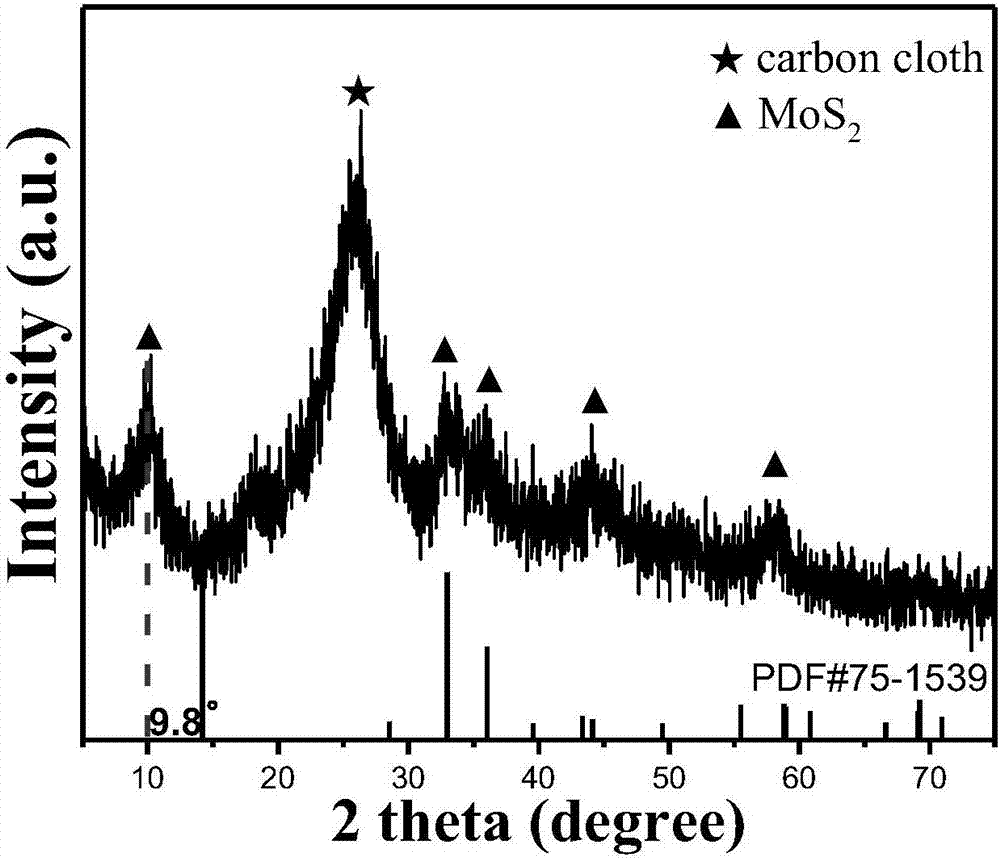
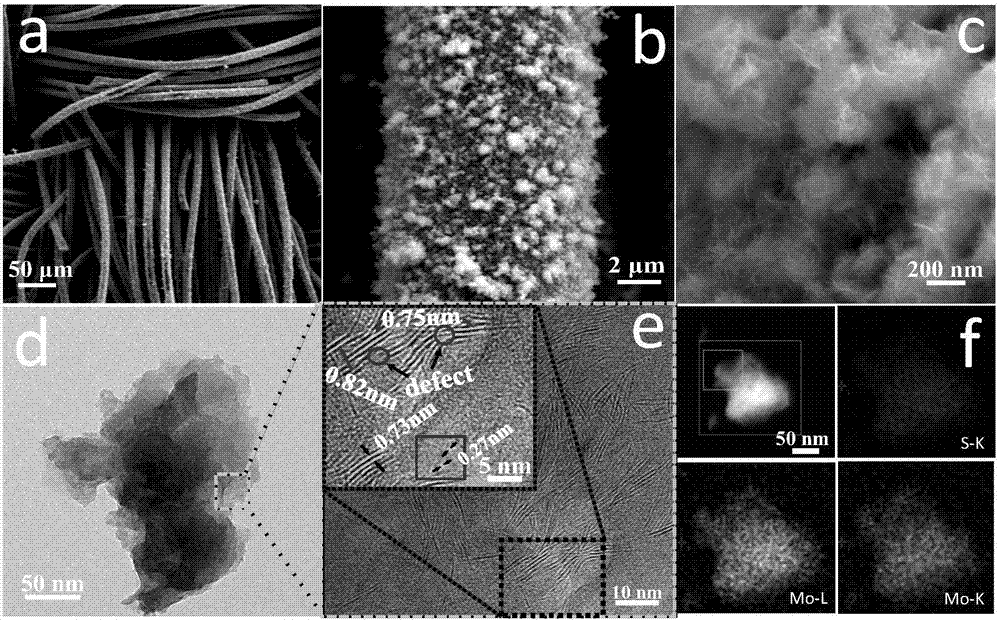
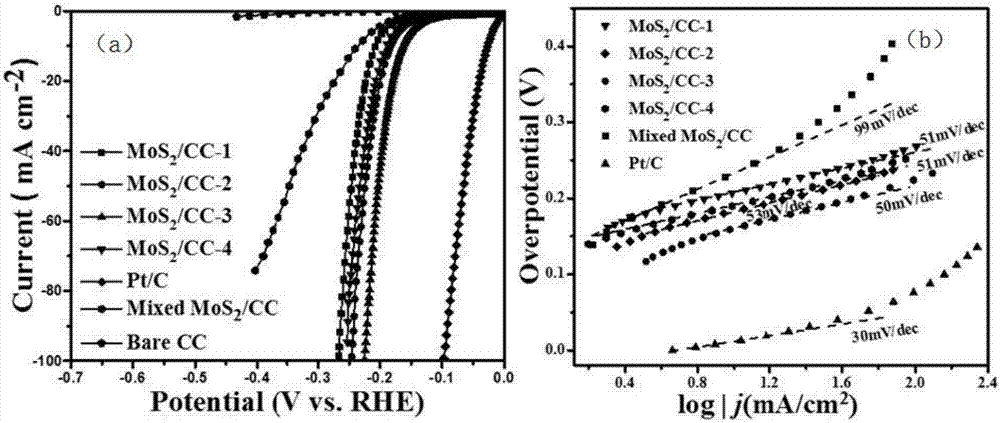
Preparation method for MoS2/CC (molybdenum disulfide/carbon cloth) composite hydrogen evolution electro-catalytic material
InactiveCN107442138AEvenly distributedNano size smallPhysical/chemical process catalystsElectrodesPhosphomolybdic acidSynthesis methods
The invention belongs to the field of electro-catalytic hydrogen evolution, and particularly relates to a preparation method for a MoS2 / CC (molybdenum disulfide / carbon cloth) composite hydrogen evolution electro-catalytic material. The preparation method comprises the following steps: washing and drying CC cut into regular shapes, placing the CC in a mixed solution of phosphomolybdic acid and thiourea, performing ultrasonic treatment to completely soak the CC in the mixed solution, then transferring the CC and the mixed solution into a reaction kettle, performing hydrothermal reaction for 24 hours under the condition of 180 DEG C, performing natural cooling to room temperature, and performing washing, centrifugation and drying to obtain an MoS2 / CC composite catalyst. Prepared MoS2 grows on the CC, and is uniformly distributed; and compared with MoS2 prepared by a conventional synthesis method, the prepared MoS2 is small in nanometric size, rich in defect and large in interlayer spacing, and the electro-catalytic hydrogen evolution activity is further improved.
Owner:JIANGSU UNIV
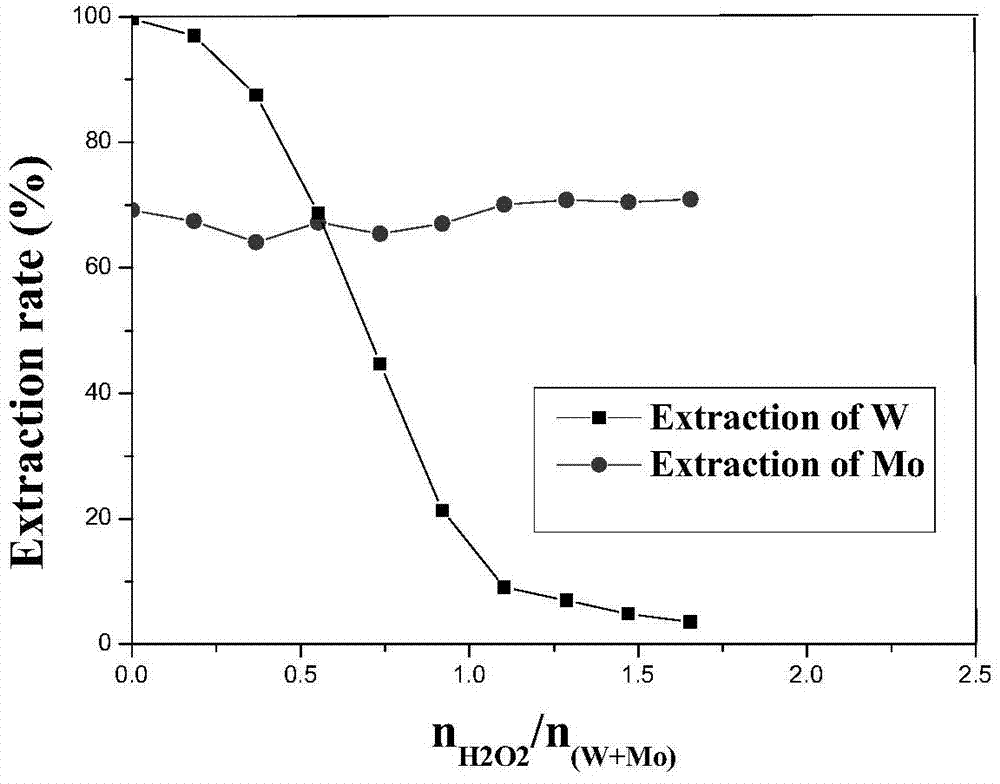
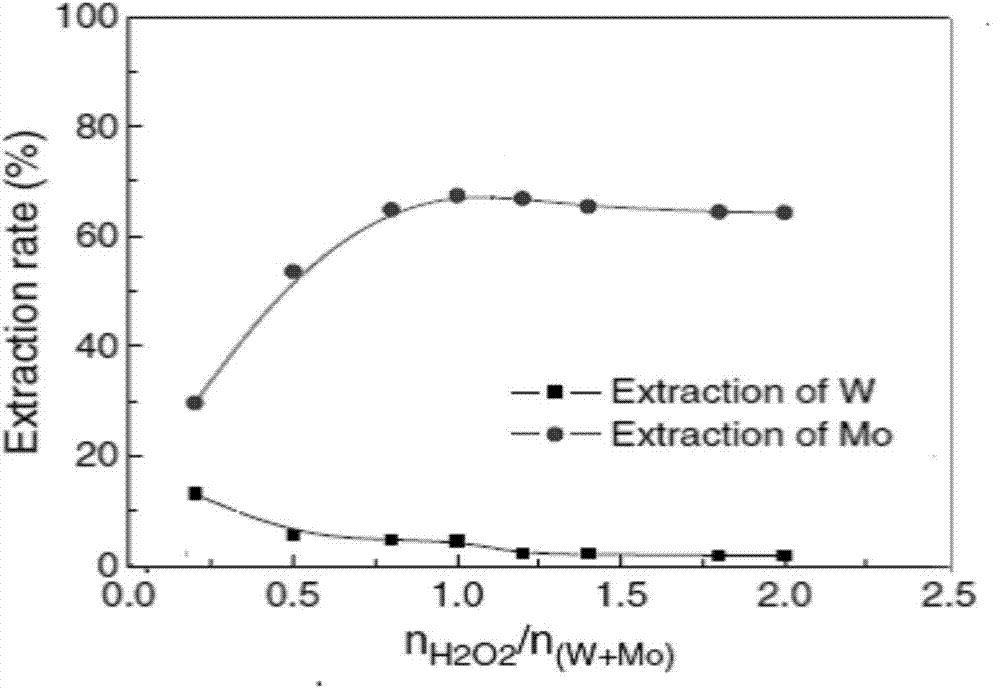
Method for extracting and separating tungsten and molybdenum in high-phosphorus mixed solution containing tungsten and molybdenum
ActiveCN104711422ARealize extraction and separationSimple methodProcess efficiency improvementPhosphomolybdic acidPhosphate
The invention relates to a method for extracting and separating tungsten and molybdenum in a high-phosphorus mixed solution containing tungsten and molybdenum. The method comprises the following steps: adding hydrogen peroxide in the high-phosphorus mixed solution containing tungsten and molybdenum for acidity adjustment and control, and converting phosphotungstic acid and phosphomolybdic acid into peroxy-phosphotungstic acid and peroxy-phosphomolybdic acid; then, taking a mixture of tributyl phosphate (TBP) and methyl phosphate dimethyl heptyl ester (P350) as an extracting agent, extracting the peroxy-phosphomolybdic acid into an organic phase, and leaving the peroxy-phosphotungstic acid in an aqueous phase; from now on, after decomposing the peroxy-phosphotungstic acid in extraction raffinate in the way of heating or ultraviolet irradiation or introduction of sulfur dioxide, extracting tungsten in the solution again with a fresh mixed extracting agent, and finally realizing extraction and separation of tungsten and molybdenum from complex material liquid. The method is small in tungsten co-extraction loss, can effectively solve the extraction and separation problem of tungsten and molybdenum in the high-phosphorus mixed solution containing tungsten and molybdenum, and is easy to realize industrialization.
Owner:CENT SOUTH UNIV
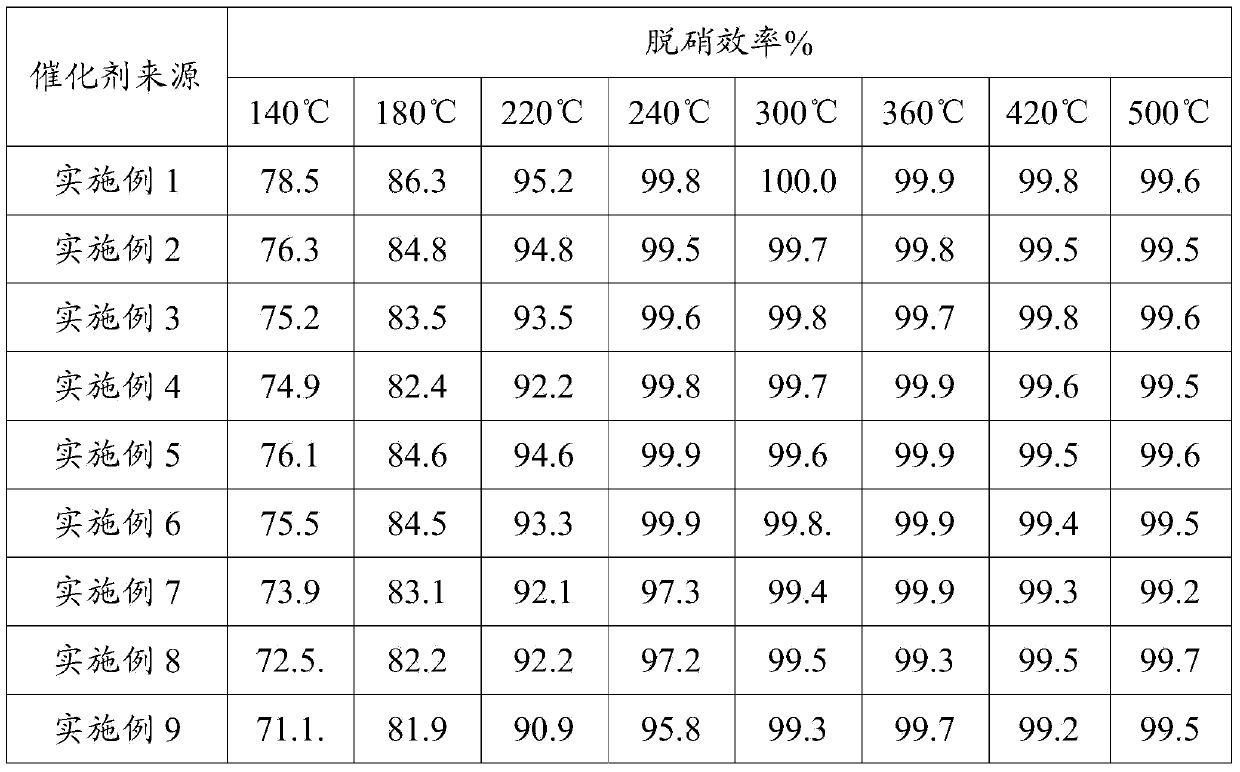
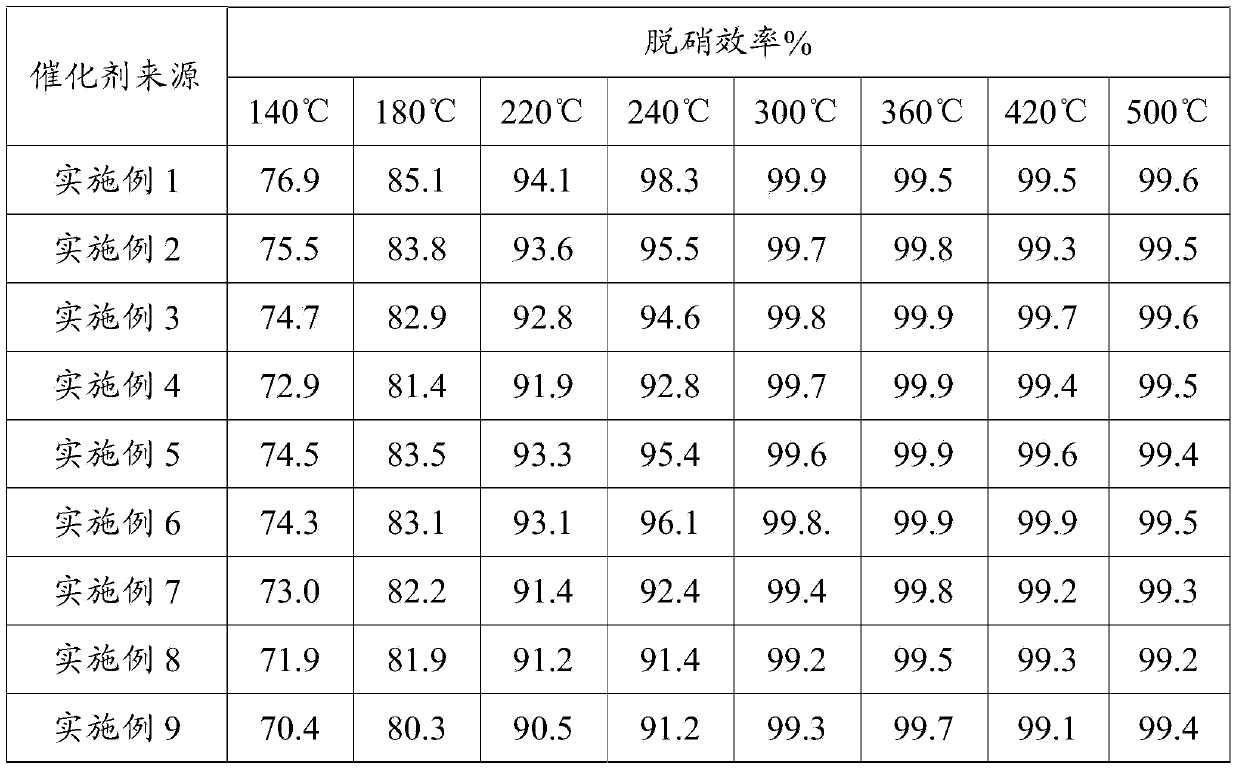
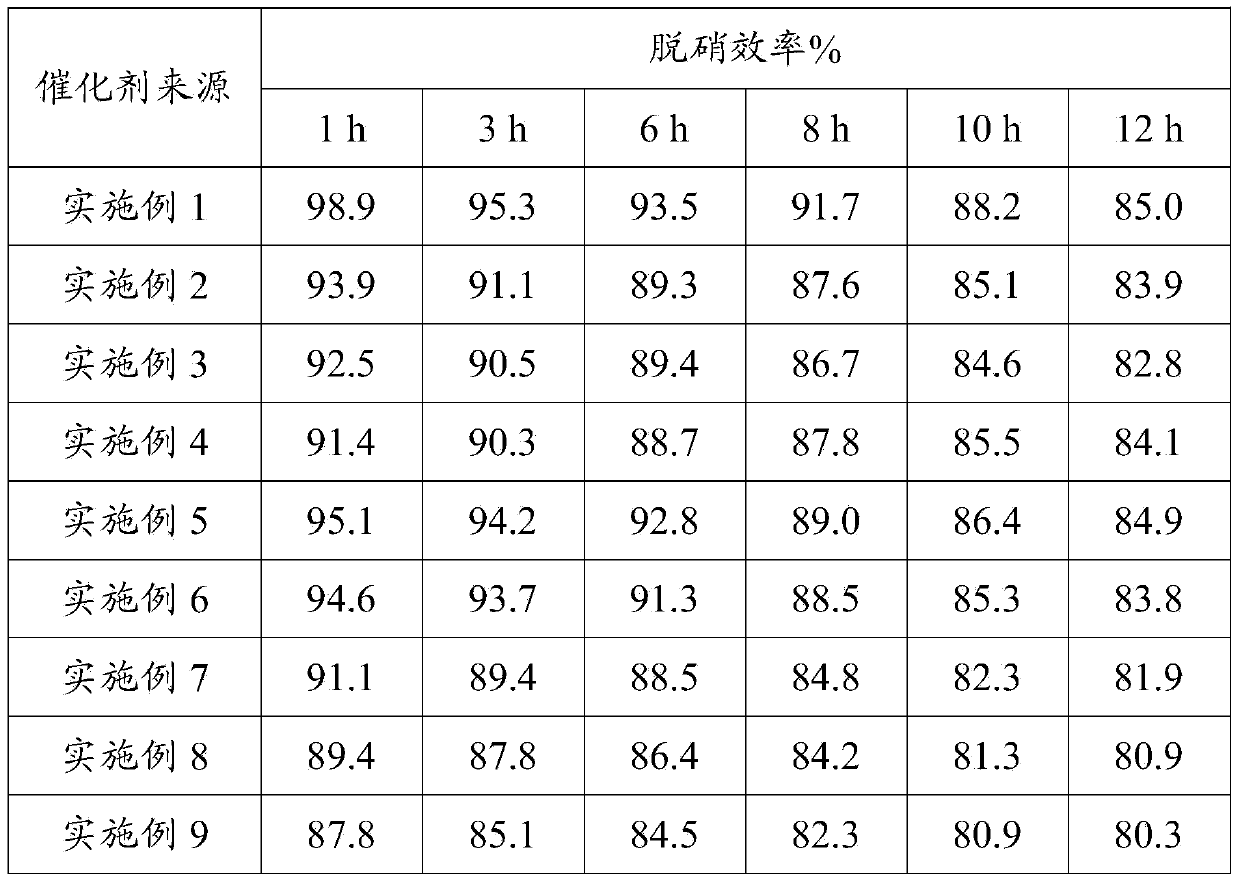
Middle and low temperature SCR denitration catalyst with anti-poisoning performance, and preparation method thereof
ActiveCN103990496AHigh activityEasy to placeOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationPhosphomolybdic acidAlkaline earth metal
The invention discloses a middle and low temperature SCR denitration catalyst with an anti-poisoning performance. The invention also discloses a preparation method of the middle and low temperature SCR denitration catalyst with an anti-poisoning performance. The preparation method comprises the following steps: 1, calcining a transition metal salt at 400-700DEG C for 1-10h to obtain a transition metal oxide; 2, mixing heteropoly acid or heteropoly salt with the prepared transition metal oxide according to a mass ratio of 1:(0.1-1), and carrying out dry mixing grinding to obtain an intermediate, wherein the heteropoly acid is phosphotungstic acid or phosphomolybdic acid, and the heteropoly salt is corresponding B salt of phosphotungstic acid or phosphomolybdic acid; and 3, calcining the intermediate obtained in step 2 at 80-500DEG C to obtain a finished product. The catalyst has an excellent middle and low temperature denitration performance, has a very excellent anti-poisoning ability on SO2, alkali metals and alkaline earth metals, and has unique advantages in the treatment of exhaust gases with high content of the alkali metals or alkaline earth metals, comprising cement kiln tail gas, biomass fuel boiler flue gas and the like.
Owner:ZHEJIANG UNIV
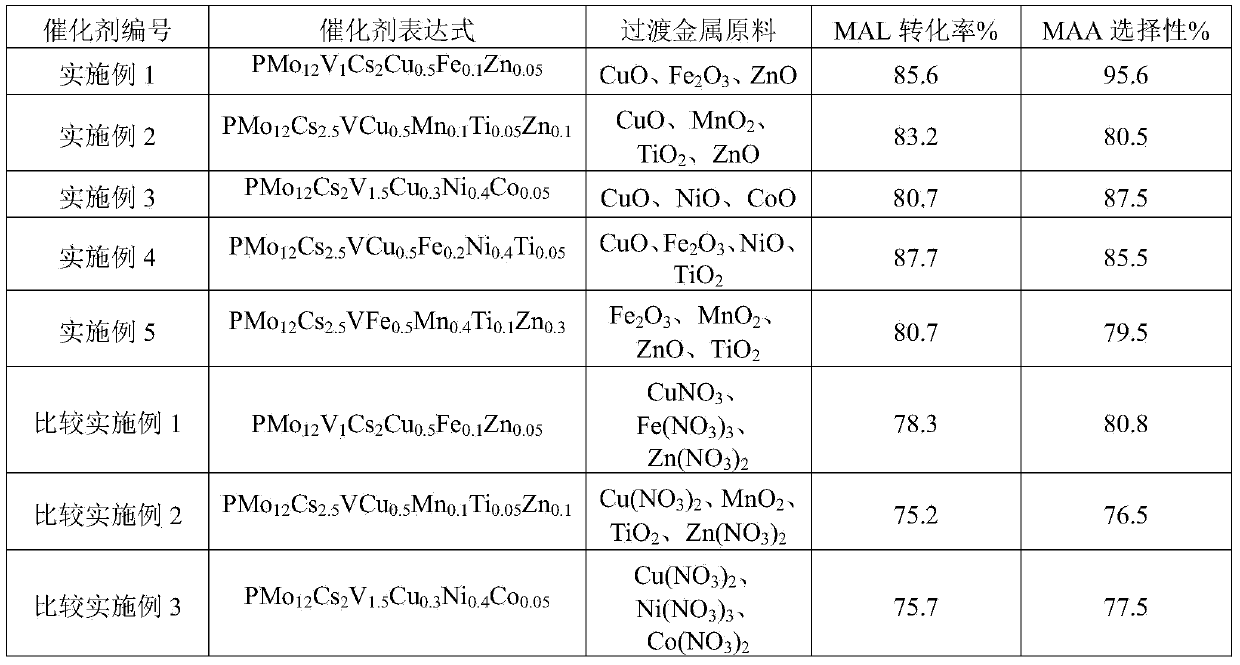
Preparation method of catalyst for preparing methacrylic acid through oxidation of methylacrolein
InactiveCN104001542AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphomolybdic acidHeteropoly acid
The invention discloses a heteropolyacid and oxide compound catalyst for preparing methacrylic acid through oxidation of methylacrolein. The preparation method of the catalyst comprises the following steps: mixing phosphomolybdic acid and metallic oxide (such as vanadium pentoxide, ferric oxide, cupric oxide, nickel oxide, manganese dioxide, cobaltous oxide, titanium dioxide and zinc oxide) in a liquid phase step by step so as to form relatively large amount of active sites on the surface of heteropolyanion; adding caesium to obtain the catalyst. The catalyst has high catalytic activity for preparing methacrylic acid through oxidation of methylacrolein and has a long catalyst service life.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
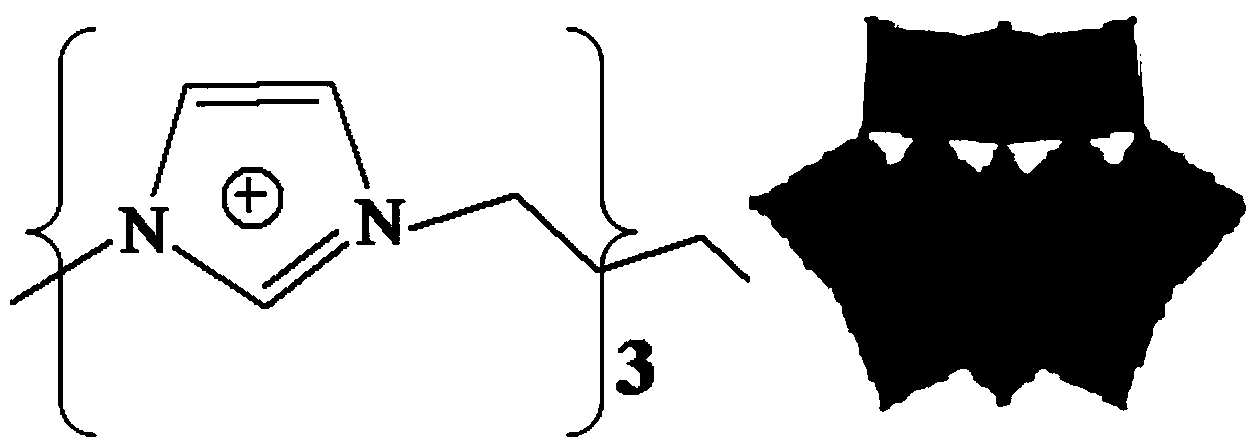
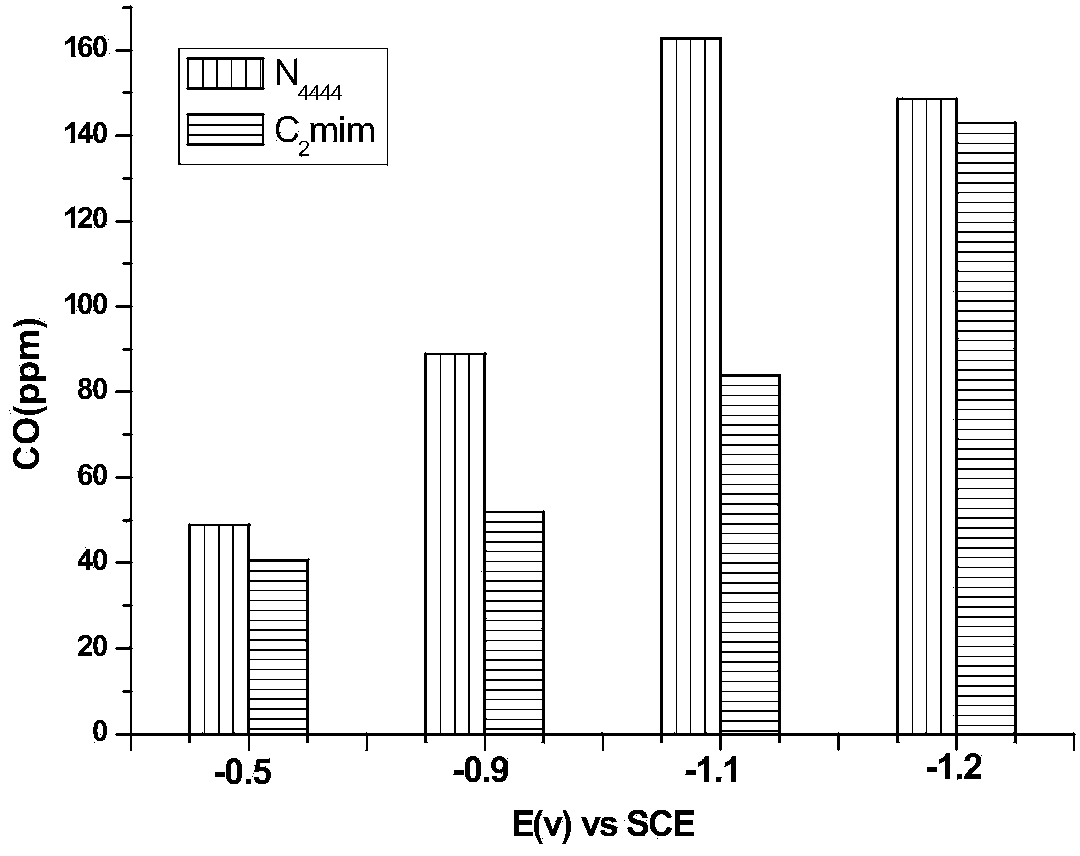
Method for electrocatalytic reduction of carbon dioxide using multi-metal oxygen cluster ionic liquid as electrocatalyst
ActiveCN104032324AEasy to synthesizeRaw materials are easy to getElectrolysis componentsOrganic-compounds/hydrides/coordination-complexes catalystsPhosphomolybdic acidResource utilization
The invention relates to a method for electrocatalytic reduction of CO2 using multi-metal oxygen cluster ionic liquid as electrocatalyst. The method is characterized in that positive ions of the multi-metal oxygen cluster ionic liquid are imidazoles [Cnmim]+(n=2, 4, 6, 8), pyridines [Cnpyr]+(n=2, 4) and quaternary ammonium salts [N4444]+, and negative ions of the multi-metal oxygen cluster ionic liquid are phosphotungstic acid negative ions (PW12O403-) and phosphomolybdic acid negative ions (PMo12O403-); and the electrocatalytic reduction reaction is a three-electrode system, wherein a glassy carbon electrode is a working electrode, a platinum sheet electrode is a counter electrode, and a saturated calomel electrode is a reference electrode. The method is simple in process and convenient in operation, performs the electrocatalytic reduction for the CO2 to form such fuels as methane and carbon monoxide, realizes the resource utilization of the CO2, reduces the atmospheric pollution, and has important significance on the environmental protection.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Environmental protection and high efficiency corrosion inhibitor for inhibiting salt water corrosion on carbon steel, preparation method and use method thereof
ActiveCN103508570AEffective corrosion inhibitionAvoid corrosionScale removal and water softeningPhosphomolybdic acidBenzoic acid
The present invention provides an environmental protection and high efficiency corrosion inhibitor for inhibiting salt water corrosion on carbon steel, a preparation method and a use method thereof, and relates to the field of chemistry. The corrosion inhibitor has characteristics of high efficiency, low cost, low toxicity and environmental protection, and is prepared through carrying out mixing stirring dissolving on 5-50% of a benzotriazole substance, 1-10% of benzoic acid and a salt thereof, 1-10% of molybdate, tungstate, phosphomolybdic acid and a salt thereof, and phosphotungstic acid and a salt thereof, 2-20% of an alcohol amine substance, 1-10% of an acid phosphate, 1-10% of a gluconate, 1-10% of an alkaline regulator, 1-10% of an oxygen scavenging agent and the balance of water. According to the present invention, the potassium dichromate or the sodium nitrite with high toxicity, and the non-environmental protection high phosphorus and high zinc can be replaced, and corrosion of calcium chloride salt water on carbon steel and copper can be significantly inhibited when the concentration of the corrosion inhibitor is 0.3-0.5%.
Owner:CHAOYANG GUANGDA CHEM
Method for preparing hexane diacid by liquid-phase catalytic oxidation of cyclohexanol
InactiveCN101302147AAvoid it happening againReduce usageOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphomolybdic acidHeteropoly acid
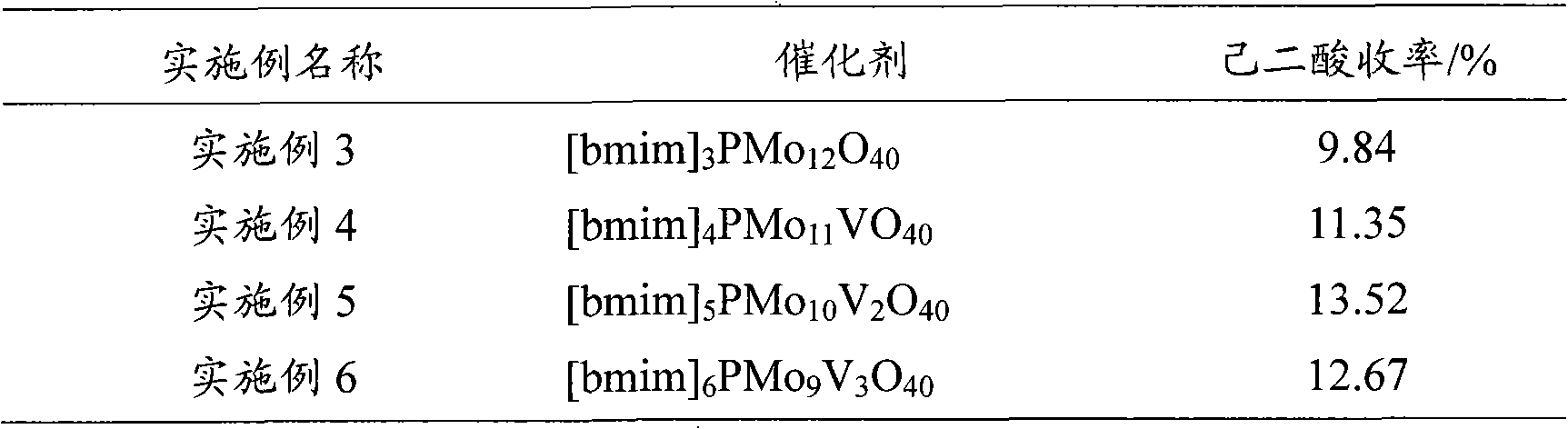
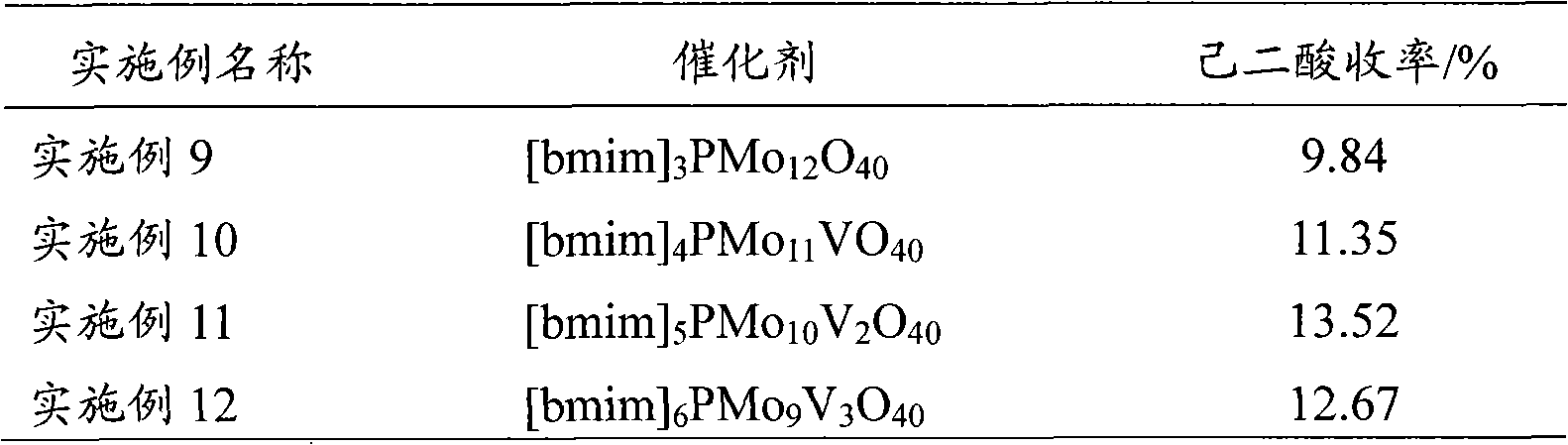
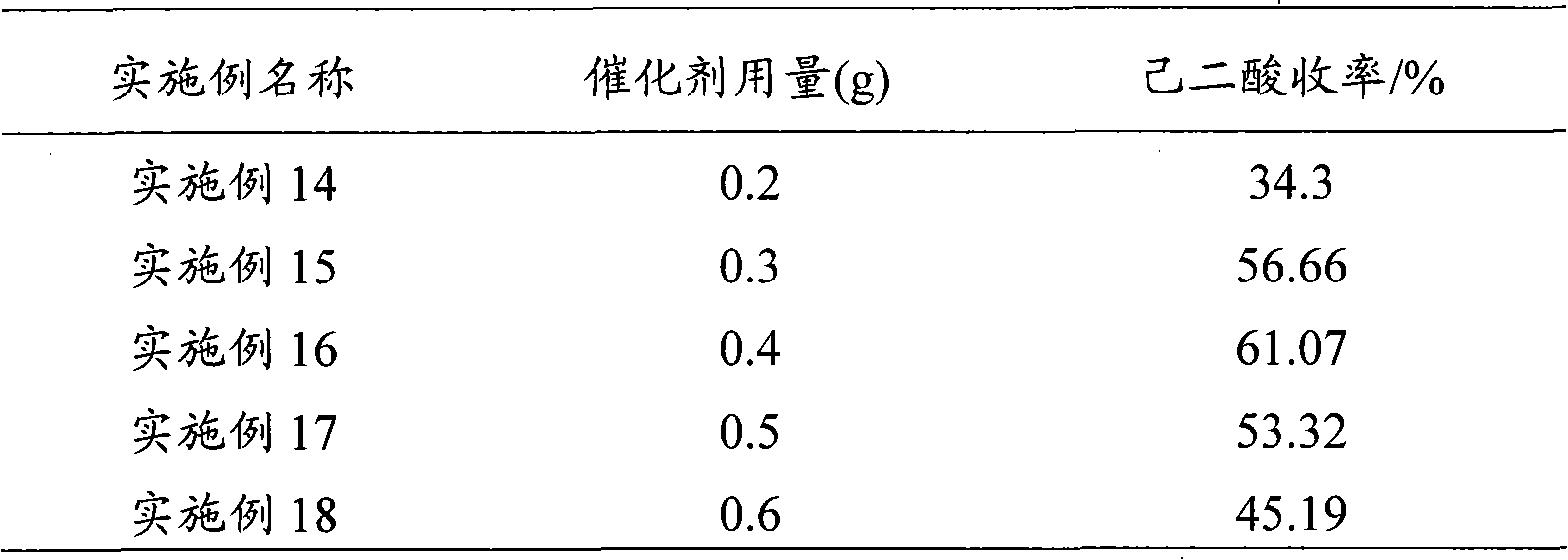
The invention provides a method for preparing adipic acid through liquid-phase catalytic oxidation of cyclohexanol. H2O2 is taken as oxidant, a heteropoly acid imidazole salt is taken as catalyst, the cyclohexanol undergoes the catalytic oxidation to prepare the adipic acid, the catalyst can be phosphotungstic acid 1-butyl-3-methylimidazolium bmim3PW12O40, phosphomolybdic acid 1-butyl-3-methylimidazolium bmim3PMo12O40, and molybdovanadophosphoric heteropolyacid 1-butyl-3-methylimidazolium bmim3+xPMo12-xVxO40 (x is equal to between 1 and 3) and so on, and the bmim represents a 1-butyl-3-methyl-imidazolium cation. Compared with the method of preparing the adipic acid by industrially using nitric acid (nitrate) to oxidize the cyclohexanol, the method can not produce poisonous oxynitride N2O, and avoids the harm to the environment; and in a reaction system, phase shift catalyst and addition agent are not used, which avoids the potential environmental pollution and has apparent economic and social significance.
Owner:ZHEJIANG UNIV OF TECH
Method for producing photochromic composite film and the product thereby
InactiveCN1566259AVisible light photochromic performance is goodEasy to operateLiquid surface applicatorsCoatingsPhosphomolybdic acidComposite film
The invention discloses a method for producing photochromic composite film which comprises, mixing the inorganic aqueous solution with organic molecular solution and agitating, forming films on clean substrates with the obtained sol by means of casting method or rotary coating method, the inorganic substance is molybdic acid, sodium molybdate, amine molybdate, phosphomolybdic acid or silicomolybdic acid, the organic molecule is N-methyl pyrrolidone, tetramethylurea, polyvinyl pyrrolidon, polyacrylamide, polyacrylic acid, or polyvinyl alcohol.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Hydrolysis method of esters
InactiveCN1541991AImprove the conversion rate of hydrolysisEasy to separateOxygen-containing compound preparationOrganic compound preparationPhosphomolybdic acidHeteropoly acid
The ester hydrolyzing method includes hydrolyzing ester in water to produce C1-C10 fatty acid or aromatic acid and C1-C4 fatty alcohol under the presence of ammonium salt of heteropoly acid as catalyst. The ammonium salt of heteropoly acid is obtained through the reaction between heteropoly acid in Keggin structure and inorganic ammonium salt or organic amine, the heteropoly acid is selected from phosphotungstic acid, silicotungstic acid, silicomolybdic acid and phosphomolybdic acid; the inorganic ammonium salt is selected from ammonium carbonate, ammonium nitrate, ammonium sulfate, ammonium phosphate and diammonium hydrochloride; and the organic amine is selected from fatty amine, alicyclic amine and arylamine. The said ester hydrolyzing method can obtain high conversion rate in relatively lower water / ester ratio and relatively short time, and the catalyst is cheap and easy to separate from the product.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for eliminating sulfur compound contained in fuel oil by catalytic oxidation of phase transition
InactiveCN101050378AAvoid pollutionAbsorptiveOrganic-compounds/hydrides/coordination-complexes catalystsRefining with oxygen compoundsPhosphomolybdic acidQuaternary ammonium cation
This invention discloses a method for removing sulfur-containing compounds from fuel oil by phase-transfer catalytic oxidation. The method comprises: preparing six types of heteropolyacid quaternary ammonium salts as the phase-transfer catalysts from quaternary ammonium salts and phosphomolybdic acid / tungstophosphoric acid, mixing the phase-transfer catalysts, fuel oil, and peroxyformic acid oxidant (prepared from H2O2 and formic acid at a weight ratio of 3.5:(1-1.5)), reacting at 25-55 deg.C under ultrasonication for 1-2 h, and separating fuel oil and peroxyformic acid to obtain desulfurized fuel oil. The method avoids adsorption and extraction procedures due to the heteropolyacid quaternary ammonium salts phase-transfer catalysts, and has such advantages as simple process, and high reaction rate. The catalyst has such advantages as no toxicity and no pollution, and is recyclable and environmentally friendly.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Spectrum-effect relationship-based propolis quality control method
ActiveCN102980952AJudging the strength of antioxidant effectJudging strengthComponent separationMaterial analysis by observing effect on chemical indicatorDPPHPhosphomolybdic acid
The invention provides a spectrum-effect relationship-based propolis quality control method. The spectrum-effect relationship-based propolis quality control method can forecast the antioxidant activity of propolis according to a propolis fingerprint. The spectrum-effect relationship-based propolis quality control method utilizes high performance liquid chromatography (HPLC) to build a propolis fingerprint and realizes evaluation of antioxidant activity according to potassium permanganate fading time, a DPPH free radical clearance rate, a phosphomolybdic acid binding rate and a hydroxyl radical clearance rate. Through analysis of a propolis fingerprint chromatograph and spectral characteristics, the spectrum-effect relationship-based propolis quality control method determines 25 characteristic peaks and 5 characteristic peak groups. Through fitting of a polynomial, the spectrum-effect relationship-based propolis quality control method builds a mathematical model for forecasting the antioxidant activity of propolis according to a propolis fingerprint. According to the characteristic peaks and the characteristic peak groups, only through the mathematical model, the spectrum-effect relationship-based propolis quality control method can directly determine strength of the antioxidant activity of a propolis drug without an anti-oxidation test thereby determining quality. The spectrum-effect relationship-based propolis quality control method has the advantages of simple processes, strong objectivity and more characteristic peaks and realizes perfect and scientific propolis quality control.
Owner:GUANGZHOU CITY TANSHAN APICULTURE
Ion sieve for extracting uranium from water body and preparation method thereof
InactiveCN103894155AOther chemical processesRadioactive decontaminationPhosphomolybdic acidHydroxylamine
The invention provides an ion sieve for extracting uranium from a water body and a preparation method thereof. The ion sieve is prepared from pyrophosphate, molybdate, zirconium oxychloride, hexadecyl trimethyl ammonium bromide, acrylonitrile, and hydroxylamine hydrochloride. The preparation method comprises the steps: preparing a hydrogen ion exchanger of zirconyl-molybdopyrophosphate polyoxometalate by using zirconium oxychloride, the molybdate and the pyrophosphate, introducing a defined amount of uranium ions to the hydrogen ion exchanger, extracting through immobilizing the uranium ions, and baking for forming; then radiating and activating, making a product obtained by radiating and activating react with hexadecyl trimethyl ammonium bromide for performing organic modification, and then adding acrylonitrile and hydroxylamine hydrochloride for performing amine oximation; finally, performing solid-liquid separation, and then performing steps of high-temperature sintering, cooling and grinding, and the like to obtain the ion sieve for extracting uranium from the water body. The prepared ion sieve has a most suitable crystal structure of receiving the uranium ions, shows an efficient selective effect, and has a chelation function and a good selectivity to the uranium ions.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Low-temperature electrolyte for LiFePO4 (lithium iron phosphate) lithium-ion batteries
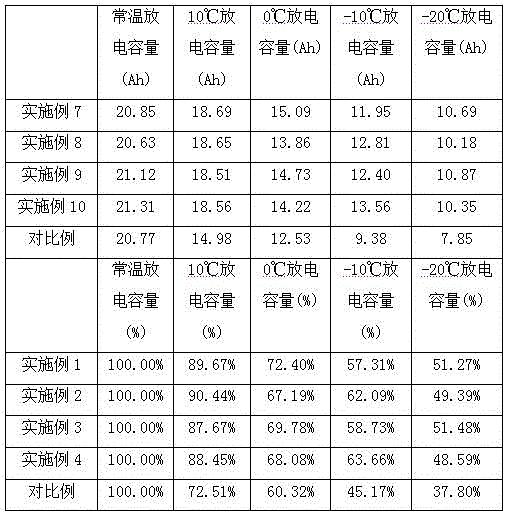
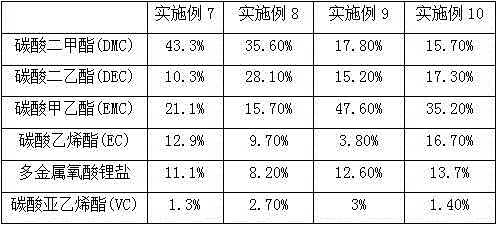
InactiveCN102983362AIncrease migration rateGood low temperature characteristicsSecondary cellsElectrolytic agentPhosphomolybdic acid
The invention relates to a low-temperature electrolyte for LiFePO4 (lithium iron phosphate) lithium-ion batteries, belonging to the technical field of low-temperature electrolytes for lithium batteries. The electrolyte comprises dimethyl carbonate, diethyl carbonate, ethyl methyl carbonate, ethylene carbonate, a film-forming additive and lithium polyoxometallate, wherein the lithium polyoxometallate refers to lithium phosphomolybdate Li3PMo12O40, lithium phosphotungstate Li3PW12O40, lithium silicotungstate Li4SiW12O40 or lithium silicomolybdenate Li4SiMo12O40. For solving the problems that in the prior art, the lithium ion transport of an electrolyte is blocked, slow in speed and low in efficiency and the electrolyte is poor in low-temperature performance, the invention provides a novel fluoride-free low-temperature electrolyte for LiFePO4 lithium-ion batteries; and by taking the non-fluoride lithium polyoxometallate with a three-dimensional skeleton structure as an electrolytic lithium salt and selectively adopting a low-viscosity carbonate solvent, through optimized proportioning, the migration rate of lithium ions is increased, and the low temperature properties of LiFePO4 batteries can be significantly improved.
Owner:中国东方电气集团有限公司
Rapid colorimetric determination method for capsaicin content of capsicum products
InactiveCN103018242AThe method is simple and easy to learnRapid responseMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsPhosphomolybdic acidFluid phase
The invention relates to a rapid colorimetric determination method for the capsaicin content of capsicum products. According to the method, a sodium carbonate-phosphotungstic acid-phosphomolybdic acid coloration liquid is prepared, capsaicin methanol liquids of different concentration gradients are then mixed with the sodium carbonate-phosphotungstic acid-phosphomolybdic acid coloration liquid and subjected to coloration, a standard colorimetric plate is manufactured, a capsaicin methanol liquid to be determined is finally mixed with the sodium carbonate-phosphotungstic acid-phosphomolybdic acid coloration liquid and subjected to coloration, the concentration of capsaicin is obtained through comparing with the standard colorimetric plate, and the capsaicin content of the capsaicin methanol liquid to be determined is determined to be 0.02-2.00 mg / mL. The method disclosed by the invention is simple, convenient and easy to learn, the reaction is quick, the determination of each time needs only 50 min, the determination is more accurate, the cost is low, the requirements on environments and personnel are low, and the deficiencies of a high-performance liquid chromatography that the cost is high, the time consumed is long (3-4 hours), the device / equipment investment is large, the requirements on fields and operators are harsh, the high-performance liquid chromatography is inconvenient, and the requirements on rapid site determination of the capsaicin content of the capsicum products cannot be met can be overcome.
Owner:SOUTHWEST UNIV
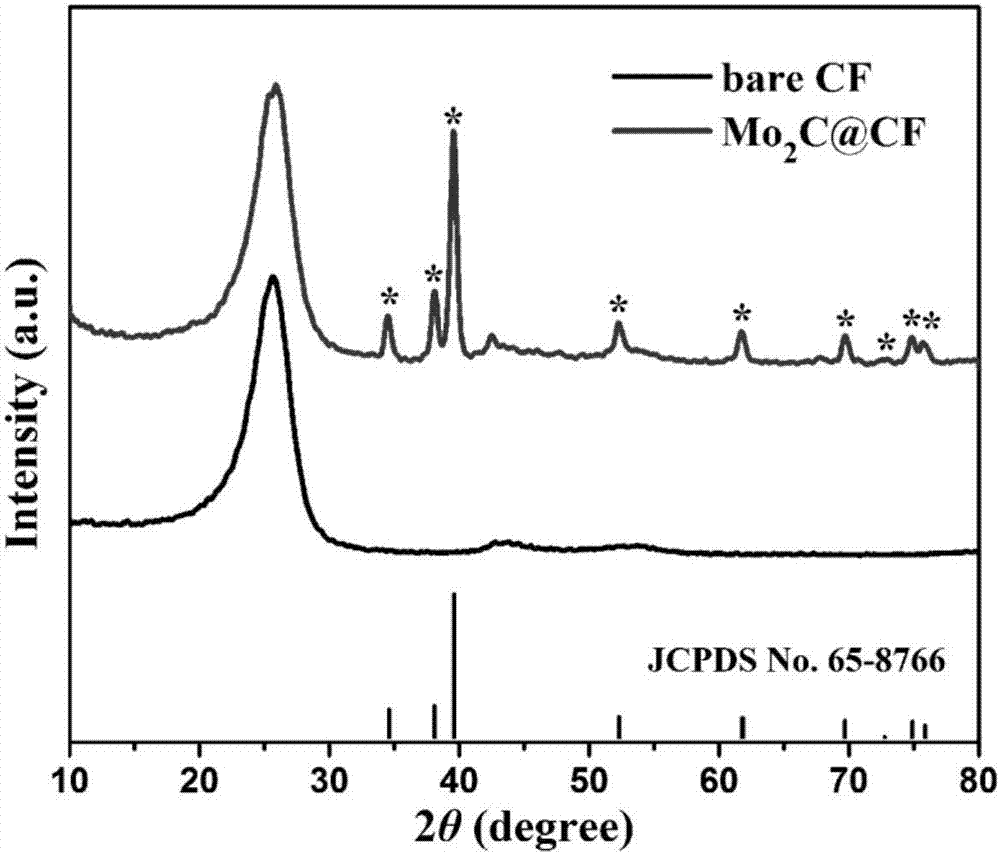
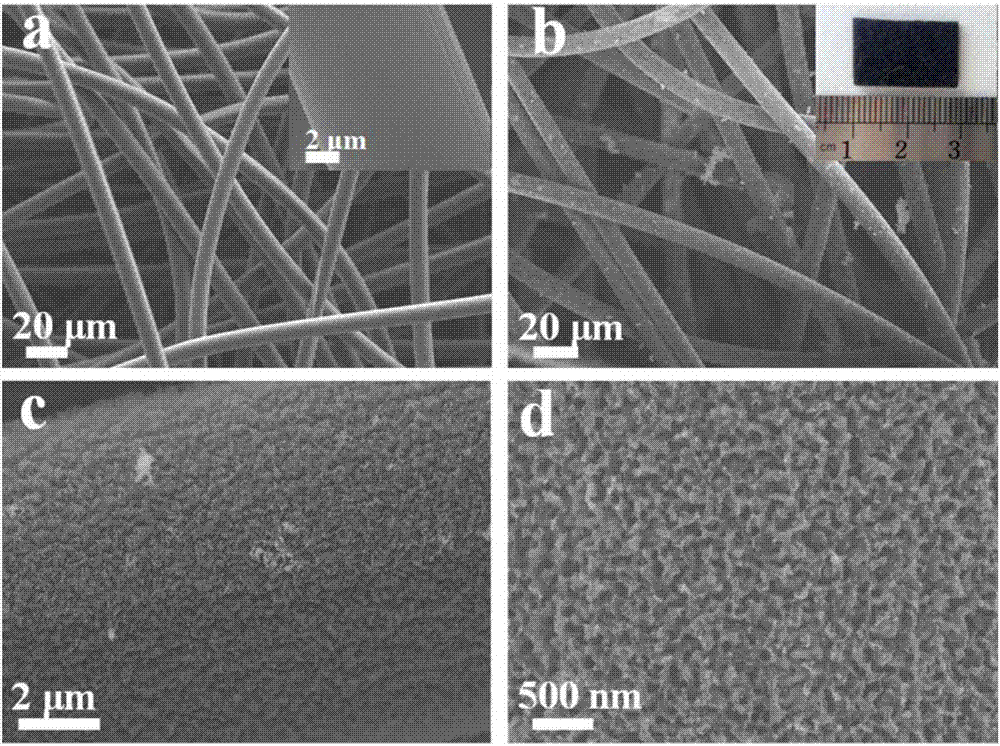
Method for in-situ modification of three-dimensional carbon microbial fuel cell by nanometer porous molybdenum carbide
InactiveCN106920982AHigh power densityImprove stabilityCell electrodesBiochemical fuel cellsPhosphomolybdic acidFiber
The invention discloses a method for in-situ modification of a three-dimensional carbon microbial fuel cell by nanometer porous molybdenum carbide. According to the method, a carbon felt electrode is used as a substrate, phosphomolybdic acid is used as a molybdenum source, the porous-structured nanometer molybdenum carbide is uniformly grown on carbon felt fiber in an in-situ way by two steps of electrostatic self-assembly of a poly diallyldimethylammonium chloride medium and high-temperature carbonization under reduction atmosphere, and the method is simple in operation step and is controllable. The prepared three-dimensional composite electrode is applied to a positive electrode of the microbial fuel cell; and compared with a non-modified carbon felt electrode, the three-dimensional composite electrode has the advantages of larger electrochemical active area and better biological compatibility, and has unique electrocatalytic activation on oxidization-reduction reaction of an electron medium, the activation overpotential of the positive electrode is reduced, and the generation power density of the microbial fuel cell is remarkably improved.
Owner:JIANGXI NORMAL UNIV
Preparation method of loaded solid catalyst used in deacidification of high-acid-value grease
InactiveCN102343273AHigh activityEasy to separate and recycleMolecular sieve catalystsLiquid hydrocarbon mixture productionPhosphomolybdic acidMolecular sieve
The invention relates to a preparation method of a loaded solid catalyst, in particular to a preparation method of a loaded solid catalyst used in deacidification of high-acid-value grease. In the method, a SiO2-based mesoporous molecular sieve with a dual-model duct structure is taken as a carrier; one of super acids such as phosphotungstic acid, phosphomolybdic acid, zirconium phosphate, phosphorus niobate and the like is loaded for serving as an active ingredient; in the deacidification process of high-acid-value waste grease, the acid value can be lowered from initially 30-100 mg KOH / mL to 5-10 mg KOH / mL; and the catalyst has the characteristics of high catalytic activity, easiness for separating and recovering, no corrosion of equipment by a reaction system, environmental friendliness and the like.
Owner:BEIJING UNIV OF TECH
Process for producing high capacity molybdenum dioxide/carbon cathode materials
InactiveCN102623677AEasy to shapeIncrease capacitySecondary cellsNon-aqueous electrolyte accumulator electrodesFiberCarbon composites
The invention discloses a process for producing high capacity molybdenum dioxide / carbon cathode materials. The process includes the steps of 1) immersing cotton fiber fabric of a certain size in an ethanol solution of phosphomolybdic acid and stirring; 2) drying and ageing the immersed cotton fiber fabric; and 3) subjecting the dried and aged cotton fiber fabric to heat treatment in mixed gases, and obtaining the molybdenum dioxide / the carbon (MoO2 / C) composite materials. The invention further discloses cathode materials which are produced by the process, an electrode slice which is made of the cathode materials and a button cell which comprises the electrode slice. According to the molybdenum dioxide / the carbon composite materials produced by the process, the specific capacity is high, the multiplying power property and the cycling stability are good, and the coulomb efficiency is high. The process is simple and suitable for large-scale production.
Owner:HUAZHONG UNIV OF SCI & TECH
Preparation method of ABC dry powder extinguishing agent
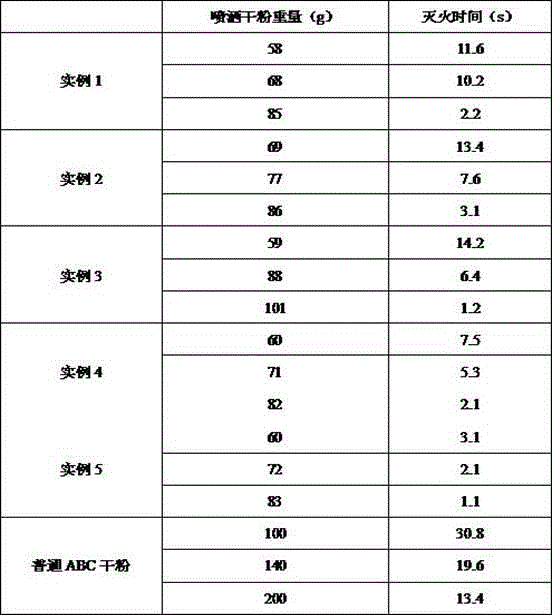
InactiveCN104436510AHigh fire extinguishing efficiencyImprove efficiencyFire extinguisherPhosphomolybdic acidPyridine phosphate
The invention discloses a preparation method of an ABC dry powder extinguishing agent. The preparation method comprises the following steps: mixing monoammonium phosphate, ammonium sulfate, nano-silica, bentonite, mica, melamine, carbon nano tubes, graphite, ammonium polyphosphate and a coupling reagent in a weight ratio; heating a mixture to 70-90 DEG C, and spraying silicon oil into the mixture; adding manganese hyphosphate, phosphomolybdic acid, diphosphopyridine nucleotide and bismuthyl nitrate, and stirring at 60-90 DEG C for 30-50 minutes, as to obtain a product D; crushing the product D by virtue of a continuous modified coating machine until the particle size of over 80% of particles is smaller than 30 microns, stirring at 60 DEG C for 20-40 minutes at the speed of 300-1,000rpm, and drying the obtained product at 90-100 DEG C; and cooling to 30 DEG C for 10-20 minutes, so as to obtain the ABC dry powder extinguishing agent. The obtained dry powder extinguishing agent is high in efficiency and low in smoke emission during extinguishment.
Owner:袁福德
Hydroxyl phenylalanine tyrosine urine detecting kit
InactiveCN105181687AMaterial analysis by observing effect on chemical indicatorPhosphomolybdic acidPhenylalanine+Tyrosine
The invention provides a hydroxyl phenylalanine tyrosine urine detecting kit, and the detecting kit is at least mixed from an acidic solution, phospho molybdic acid and / or a soluble salt of phosphomolybdic acid; the acidic solution at least contains H+, Ni2+, Hg+ and NO3-, and the concentration of phosphomolybdic acid and / or the soluble salt of phosphomolybdic acid in the detecting kit is 45-900 mmol / L. The detecting kit has a higher detection accuracy.
Owner:朱建华
Method for synthesizing crotonic acid by oxidizing croton aldehyde selectively
InactiveCN101003472AEasy to manufactureLow costPhysical/chemical process catalystsOrganic compound preparationPhosphomolybdic acidCrotonaldehyde
This invention relates to a method for synthesizing crotonic acid by selective oxidation of crotonaldehyde. The method adopts crotonaldehyde as the raw material, acetone, acetic acid, benzene or toluene as the solvent, phosphomolybdic acid as the main catalyst, and V2O5 as the cocatalyst, and reacts at 30-100 deg.C and 0.3-0.9 MPa in the presence of O2 to obtain crotonic acid. Phosphomolybdic acid can be supported by such carriers as active carbon, SiO2, gamma-Al2O3 or molecular sieve. Therefore, the catalyst can be separated from the reaction solution by filtration, and dried in air at 120 deg.C for recycling. The supported catalyst has such advantages as simple preparation, high catalytic activity, high selectivity, and high stability, and can be used repeatedly.
Owner:SINOPEC YANGZI PETROCHEM
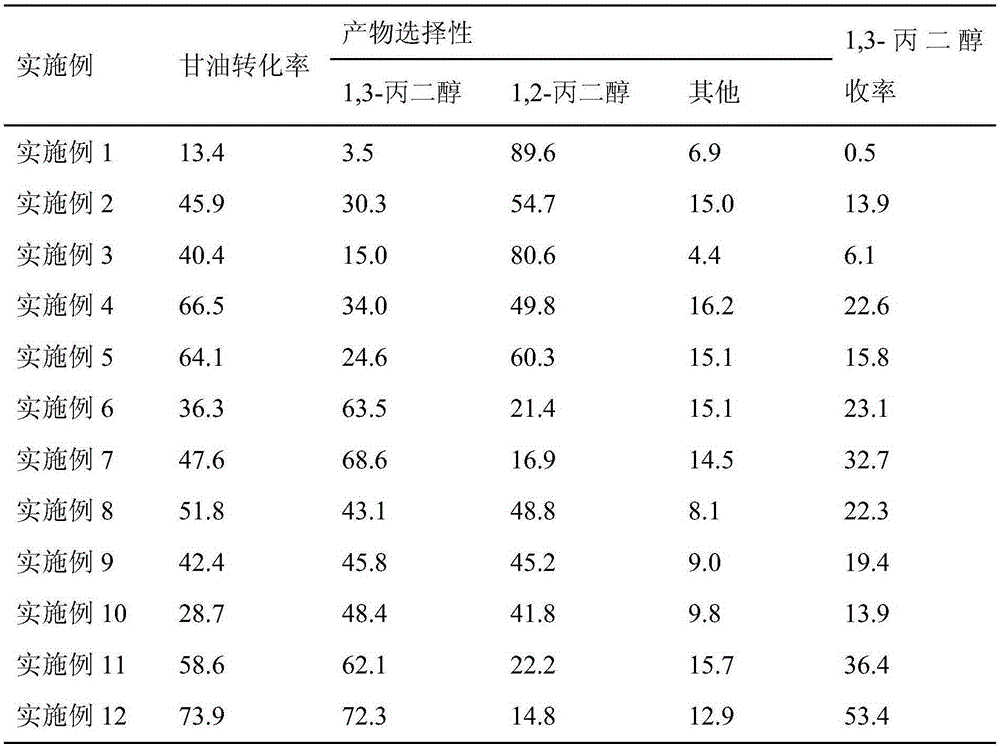
Catalyst for preparing 1,3-propanediol through glycerine hydrogenolysis
InactiveCN105344357AReduce manufacturing costReduce usageHeterogenous catalyst chemical elementsPreparation by OH group eliminationPhosphomolybdic acidAdjuvant
The invention discloses a catalyst for preparing 1,3-propanediol through glycerine hydrogenolysis. According to the catalyst, alumina serves as a carrier, cobalt metal serves as an active ingredient, and adjuvants comprise silicotungstic acid, phosphotungstic acid, phosphomolybdic acid and the like, wherein the content of the active ingredient is 30% the mass of the carrier, and the content of the adjuvants is 5% the mass of the catalyst. The catalyst has the advantages of good catalytic activity, high 1,3-propanediol yield and the like, and can achieve high-selectivity glycerine-hydrogenolysis preparation of 1,3-propanediol at the reaction temperature of 150-300 DEG C and the hydrogen gas pressure of 1-10MPa. Raw materials for preparing the catalyst are rich and cheap, the process is simple, and the catalyst is reusable.
Owner:SOUTHEAST UNIV
Ionic liquid-functionalized snowman-shaped anisotropic composite material and preparation method and application thereof
The invention relates to an ionic liquid-functionalized snowman-shaped anisotropic composite material and a preparation method and application thereof. The preparation method comprises the steps that Janus snowman-shaped composite particles are prepared and then modified with ionic liquid, and imidazolinyl-modified Janus snowman-shaped composite particles are prepared; chlorobutane is added to obtain Cl-based ionic liquid-functionalized Janus snowman-shaped composite particles, and anion exchange is performed on the Cl-based ionic liquid-functionalized Janus snowman-shaped composite particles and multiple inorganic salts such as KPF6 and silicotungstic acid or phosphomolybdic acid and the like to prepare the PF6-based or silicotungstic acid-based or phosphomolybdic acid-based ionic liquid-functionalized Janus snowman-shaped composite particles. According to the material, not only is the morphology partitioned, but also the surface chemical property is partitioned, the polystyrene side is oleophylic, the silicon dioxide side is adjustable, and when the silicon dioxide side uses hydrophilic anions, the characteristics obtained when a general Janus material is used for stabling Pickering emulsion are achieved.
Owner:LIAONING UNIVERSITY
Process for preparing acrylic aldehyde by using selective glycerol dehydration and preparation method of catalyst thereof
InactiveCN101879456AHigh activityIncrease reaction rateOrganic compound preparationCarbonyl compound preparationPhosphomolybdic acidFixed bed
The invention discloses a process for preparing acrylic aldehyde by using selective glycerol dehydration and a preparation method of a catalyst thereof. Heteropolyacid / a carrier is disclosed in the expression of the catalyst, wherein the heteropolyacid is one of silicotungstic acid, phosphotungstic acid and phosphomolybdic acid, and the carrier is one of activated aluminium oxide, kieselguhr, activated carbon, rutile type titanium dioxide and kaoline. The catalyst is prepared by using a constant-pore volume dipping method. When the catalyst is used in a fixed-bed miniature reaction device, the transformation rate of glycerol is 13.5%-80.6%, and the selectivity of the acrylic aldehyde is 49.0%-90.5%. When used for carrying out the process for preparing acrylic aldehyde by using glycerol dehydration, the catalyst has the advantages of high catalyst activity, high reaction speed, high selectivity for the acrylic aldehyde and the like, and the main byproduct is hydroxyl acetone.
Owner:JIANGSU UNIV
Charge-transporting material and charge-transporting varnish
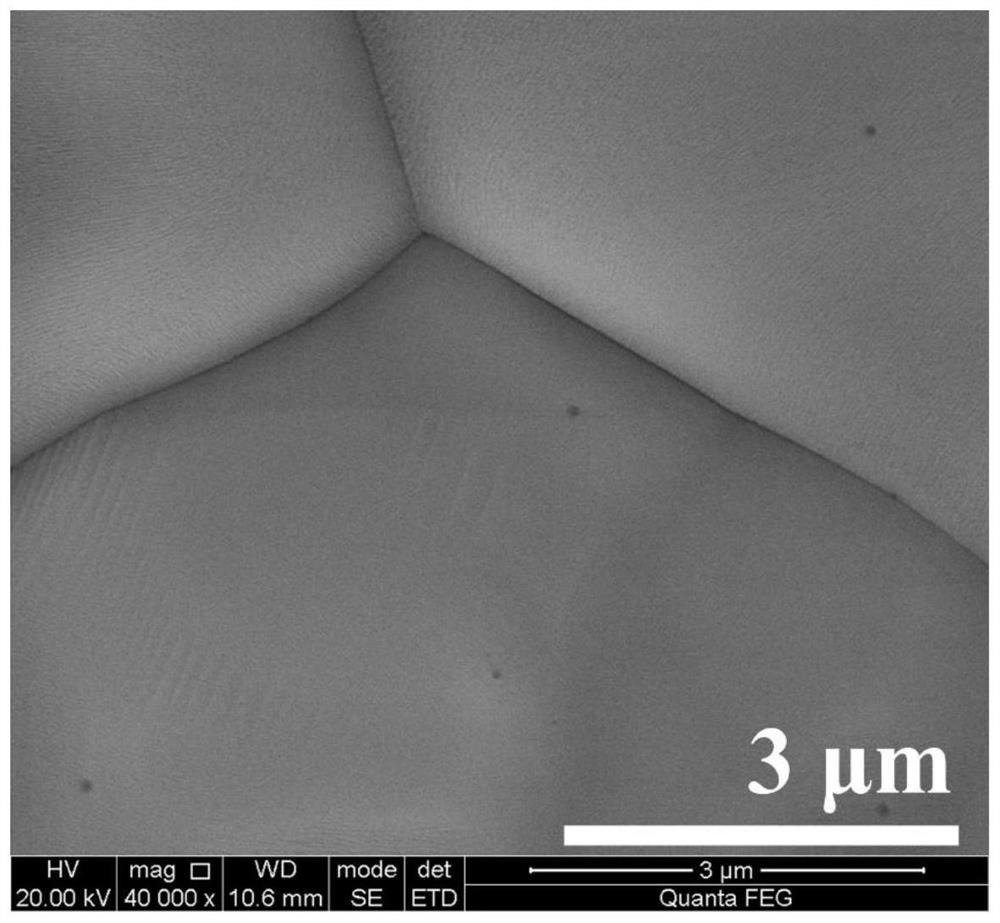
InactiveUS20110220853A1Improve solubilityImprove flatnessPhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesPhosphomolybdic acidSolubility
Disclosed is a charge-transporting material comprising a heteropoly acid compound such as phosphomolybdic acid as a charge-transporting substance. Also disclosed is a charge-transporting varnish comprising the charge-transporting material and an organic solvent, wherein the charge-transporting substance is dissolved in the organic solvent. It becomes possible to provide a charge-transporting material which comprises a substance having high solubility in an organic solvent, charge-transporting properties, and an ability to oxidize hole transport materials, and a charge-transporting varnish comprising the charge-transporting material.
Owner:NISSAN CHEM IND LTD
Method for preparing bisphenol F
InactiveCN103936562ASimple preparation processShorten the production cycleOrganic chemistryOrganic compound preparationPhosphomolybdic acidHeteropoly acid
The invention discloses a method for preparing bisphenol F. The method is characterized in that phenol and formaldehyde serving as raw materials and a solid acid catalyst serving as a catalyst are used to prepare the target product bisphenol F, wherein the solid acid catalyst is prepared from heteropoly acid modified clay, and the mass dosing ratio of the catalyst to formaldehyde is (0.01-0.6):1; and the heteropoly acid is one or more of phosphotungstic acid, phosphomolybdic acid, silicotungstic acid, silicomolybdic acid and the like, wherein the clay is one or more of bentonite, kaoline, montmorillonoid, metakaolin, diatomaceous earth and the like. The method has the prominent advantages that the yield of the product bisphenol F is up to 93 percent, the selection to bisphenol F is 96 percent, the catalyst is friendly to environment, the preparation process is simple, the production cycle is short, the catalyst activity and catalytic selection are high, separation and reclamation are easy, recycling is available, and industrialization is facilitated.
Owner:HUNAN UNIV
Preparation method of foamed nickel-loaded amorphous phosphorus-doped nickel molybdate bifunctional electrocatalytic electrode and application thereof
ActiveCN111663152ASimple preparation processMild conditionsCatalyst activation/preparationElectrolytic organic productionPhosphomolybdic acidElectrolysis
The invention provides a preparation method of a foamed nickel-loaded amorphous phosphorus doped nickel molybdate bifunctional electrocatalytic electrode and an application thereof, and belongs to thetechnical field of hydrogen energy and fuel cells. The preparation method comprises the following steps: soaking foamed nickel into an HCl solution, removing metal oxide on the surface, soaking the pickled foamed nickel into a phosphomolybdic acid aqueous solution, carrying out a spontaneous oxidation-reduction reaction, taking out, washing with deionized water, and drying for later use, therebyobtaining the foamed nickel-loaded amorphous phosphorus-doped nickel molybdate nanostructure. The foamed nickel is etched by using the phosphomolybdic acid aqueous solution, and can be directly used after drying, the material can be used as a bifunctional electrode material for urea electrooxidation and water and electricity reduction; a urea electrooxidation reaction with low oxidation potentialis used for replacing an electrocatalytic oxygen evolution reaction, a two-electrode urea auxiliary electrolysis hydrogen production system based on a bifunctional electrocatalyst is constructed in analkaline solution, low-cost, low-energy-consumption and stable electrochemical hydrogen production is achieved, and the method is suitable for large-scale industrial electrochemical hydrogen production application.
Owner:HARBIN INST OF TECH
Method for synthesizing crotonic acid by using byproduct of croton aldehyde
InactiveCN101003473AIncrease valuePromote cleaner productionPhysical/chemical process catalystsOrganic compound preparationPhosphomolybdic acidCrotonaldehyde
This invention relates to a method for synthesizing crotonic acid from crotonaldehyde byproduct. The method comprises: distilling 20-40% crotonaldehyde byproduct by controlling the boiling point of the distillate on the top of the column at 25-65 deg.C to remove light components and obtain 45-60% crotonaldehyde in the solution in distillation kettle, adopting the solution in distillation kettle as the raw material, acetone, acetic acid, benzene or toluene as the solvent, phosphomolybdic acid as the main catalyst, and V2O5 as the cocatalyst, and reacting at 30-100 deg.C and 0.3-0.9 MPa in the presence of O2, separating the catalyst, distilling to remove the solvent, and recrystallizing with deionized water for twice to obtain crotonic acid with a purity higher than 99%. Phosphomolybdic acid can be supported by such carriers as active carbon, SiO2, gamma-Al2O3 or molecular sieve. Therefore, the catalyst can be separated from the reaction solution by filtration, and dried in air at 120 deg.C for recycling. The method has such advantages as simple process and high crotonic acid yield.
Owner:SINOPEC YANGZI PETROCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com