Gastrodin chemical synthesis method suitable for industrialization
A technology for chemical synthesis and gastrodin, applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc., can solve the problems of high cost, pollution and low total yield, avoid hazards and pollution, and simplify purification operations , the effect of short reaction period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
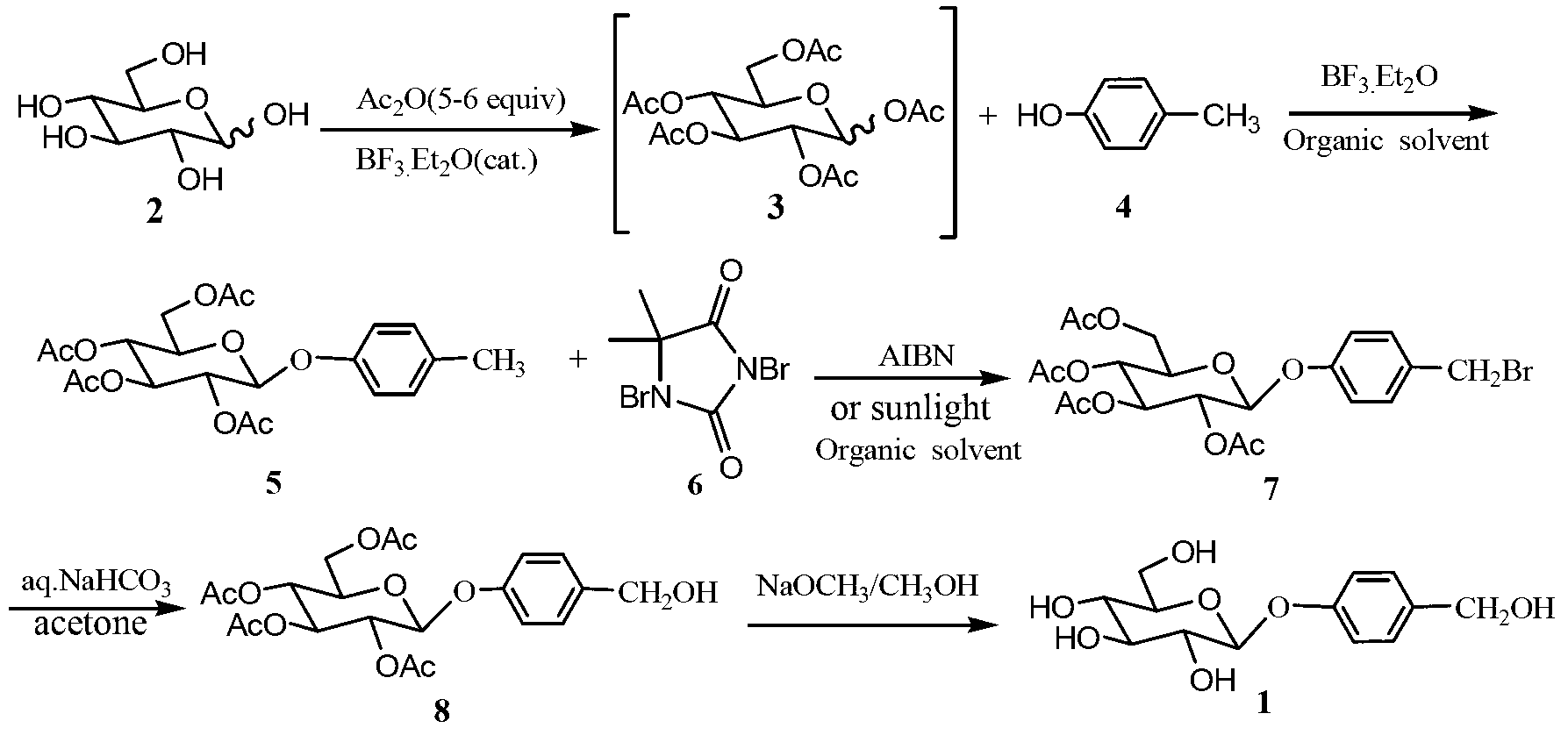
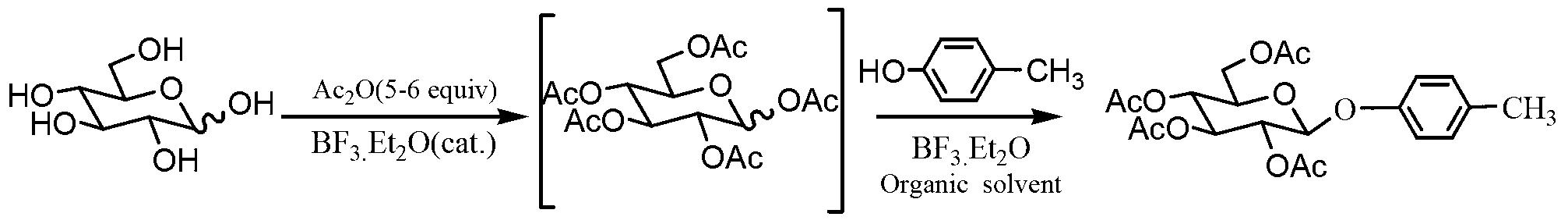
[0029]Mix D-glucose (100mmol, 18g) and acetic anhydride (500mmol, 51g), add boron trifluoride ether solution (5mmol, 0.6ml), and react at room temperature for 4 hours to obtain a transparent viscous solution, to which p-cresol was added (180mmol, 38.7g) and 150mL dichloromethane, under the protection of nitrogen, add boron trifluoride ether solution (100mmol, 10.8ml) dropwise, stir at room temperature for 4 hours, dilute the reaction solution with 100mL dichloromethane, and successively add 300mL water , 300mLx2 saturated sodium bicarbonate solution, 300mL water wash, separate the dichloromethane layer and dry it with anhydrous sodium sulfate, filter, the filtrate is concentrated under reduced pressure to give a light yellow solid, recrystallized from absolute ethanol to get white crystal 4-methylphenyl -2,3,4,6-O-tetraacetyl-β-D-glucopyranoside 25.6g, yield 59%, melting point 116-117.5°C.
[0030] 1 HNMR (500MHz, CDCl 3 )δ7.09(d,J=8.0Hz,2H),6.89(d,J=8.0Hz,2H),5....
Embodiment 2
[0041] Mix D-glucose (100mmol, 18g) and acetic anhydride (520mmol, 53g), add boron trifluoride ether solution (6mmol, 0.72ml), and react at room temperature for 4 hours to obtain a transparent viscous solution, to which p-cresol was added (160mmol, 34.4g) and 150mL dichloromethane, under the protection of nitrogen, add boron trifluoride ether solution (120mmol, 14.3ml) dropwise, stir at room temperature for 4 hours, dilute the reaction solution with 120mL dichloromethane, and successively add 300mL water , 250mLx3 saturated sodium bicarbonate solution, 300mL water washing, the dichloromethane layer was separated and dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure to obtain a light yellow solid, recrystallized from absolute ethanol to obtain white crystal 4-methylphenyl -2,3,4,6-O-tetraacetyl-β-D-glucopyranoside 27.4g, yield 63%, melting point 116-117.5°C.
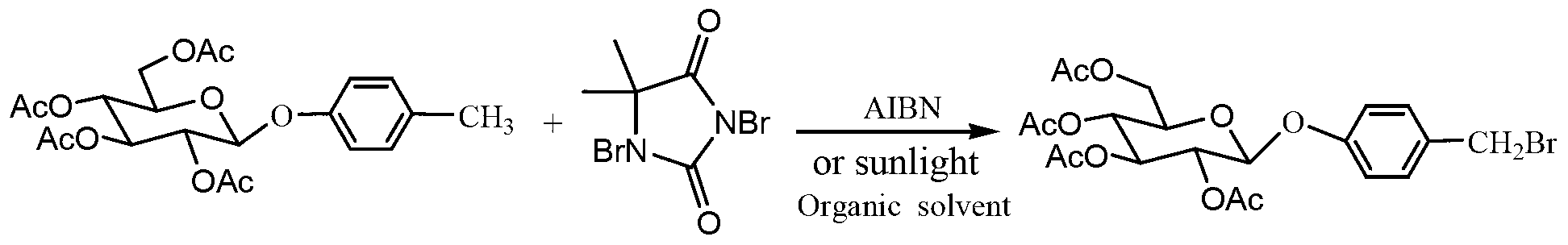
[0042] Dissolve the 4-methylphenyl-2,3,4,6-O-tetraacetyl-β-D-glucopyr...
Embodiment 3
[0046] Mix D-glucose (100mmol, 18g) and acetic anhydride (550mmol, 56g), add boron trifluoride ether solution (7mmol, 0.84ml), and react at room temperature for 4 hours to obtain a transparent viscous solution, to which p-cresol was added (150mmol, 36.7g) and 150mL dichloromethane, under the protection of nitrogen, add boron trifluoride ether solution (150mmol, 17.8ml) dropwise, stir at room temperature for 3 hours, dilute the reaction solution with 120mL dichloromethane, and successively add 400mL water , 250mLx3 saturated sodium bicarbonate solution, 400mL water wash, separate the dichloromethane layer, dry it with anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain a light yellow solid, recrystallize from absolute ethanol to obtain white crystal 4-methylphenyl -2,3,4,6-O-tetraacetyl-β-D-glucopyranoside 28.7g, yield 66%, melting point 116-117.5℃.
[0047] Dissolve the 4-methylphenyl-2,3,4,6-O-tetraacetyl-β-D-glucopyranoside (20mmol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com