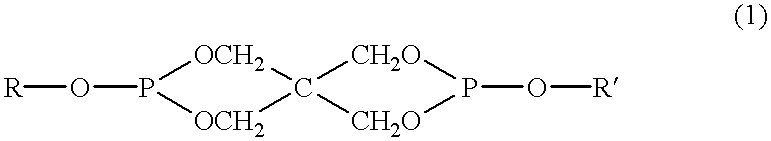
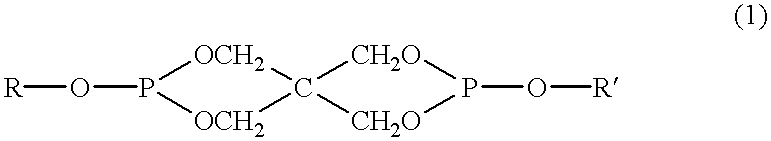
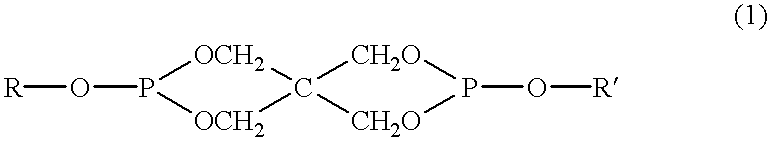
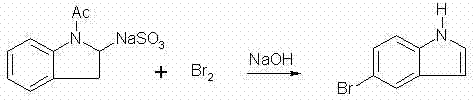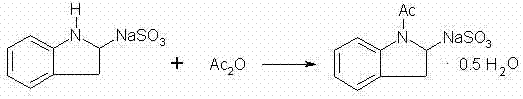Patents
Literature
238 results about "Acetic oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of 4,4'-(Hexafluoroisopropylidene) diphthalic anhydride
ActiveCN101696199AOvercoming the Factors Affecting Industrial Safety ProductionHigh yieldOrganic chemistryAlkyl transferO-Xylene
The invention discloses a preparation method of 4,4'-(Hexafluoroisopropylidene) diphthalic anhydride (6-FDA), belonging to the technical field of liquid crystal materials. The main technical scheme is achieved as follows: o-xylene and 2,2-dichloro-hexafluoropropane undergo alkylation reaction in an ionic liquid under the catalytic action of Lewis acids (AlCl3, ZnCl2) to obtain 4,4'-(Hexafluoroisopropylidene) di(2-xylene); then the 4,4'-(Hexafluoroisopropylidene) di(2-xylene) is oxidized by permanganic acid TEBA triethylbenzylammonium salts to obtain 4,4'-(Hexafluoroisopropylidene) diphthalandione; and finally, the 4,4'-(Hexafluoroisopropylidene) diphthalandione is dehydrated by acetic oxide to obtain the 4,4'-(Hexafluoroisopropylidene) diphthalicanhydride (6-FDA). The preparation method has the advantages of mild reaction condition, less three-waste emission and high yield.
Owner:天津众泰材料科技有限公司
Method for synthesizing 6-methyl-17alpha-acetoxyl-19-norpregnane-4,6-diene-3,20-diketone
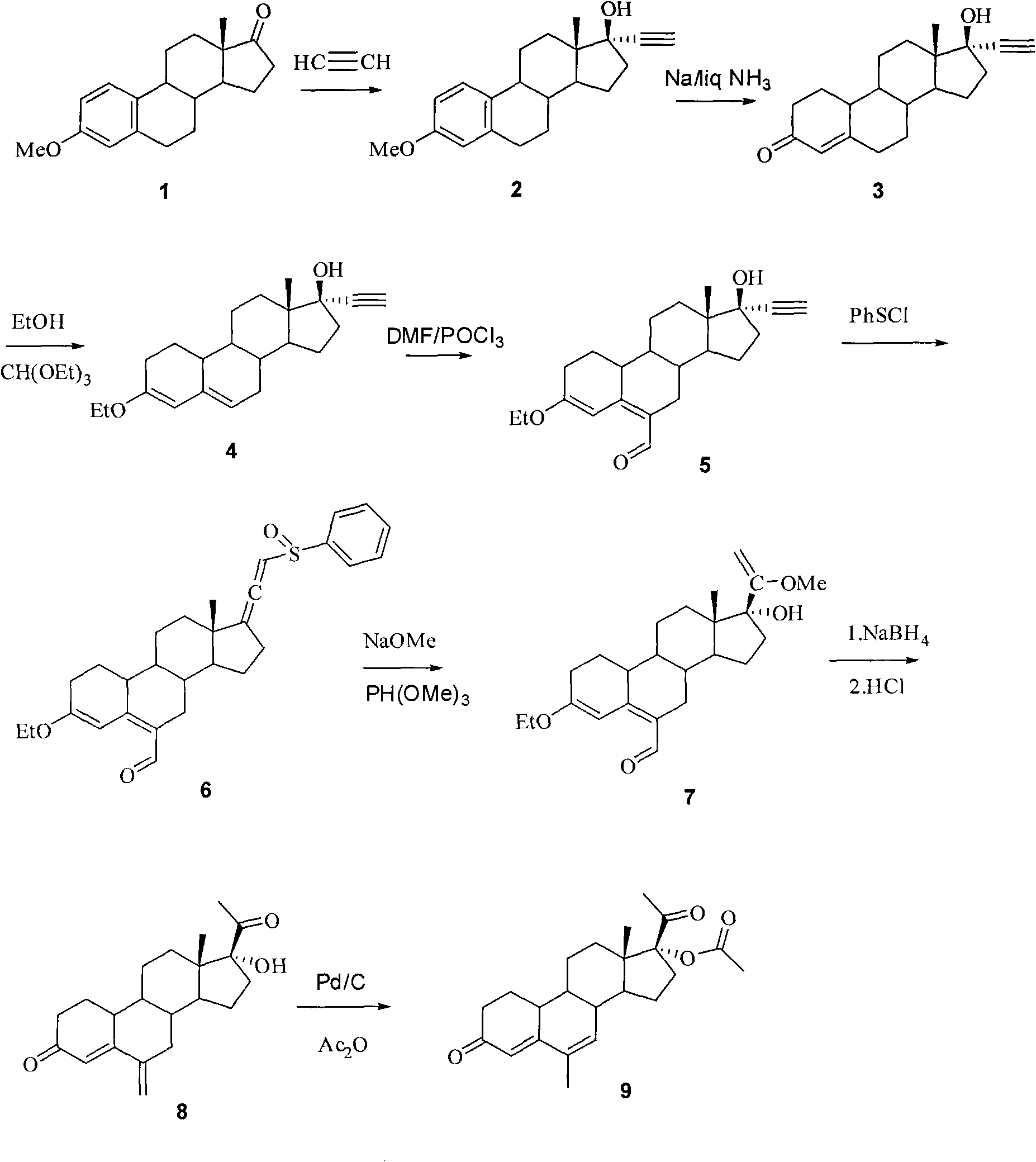
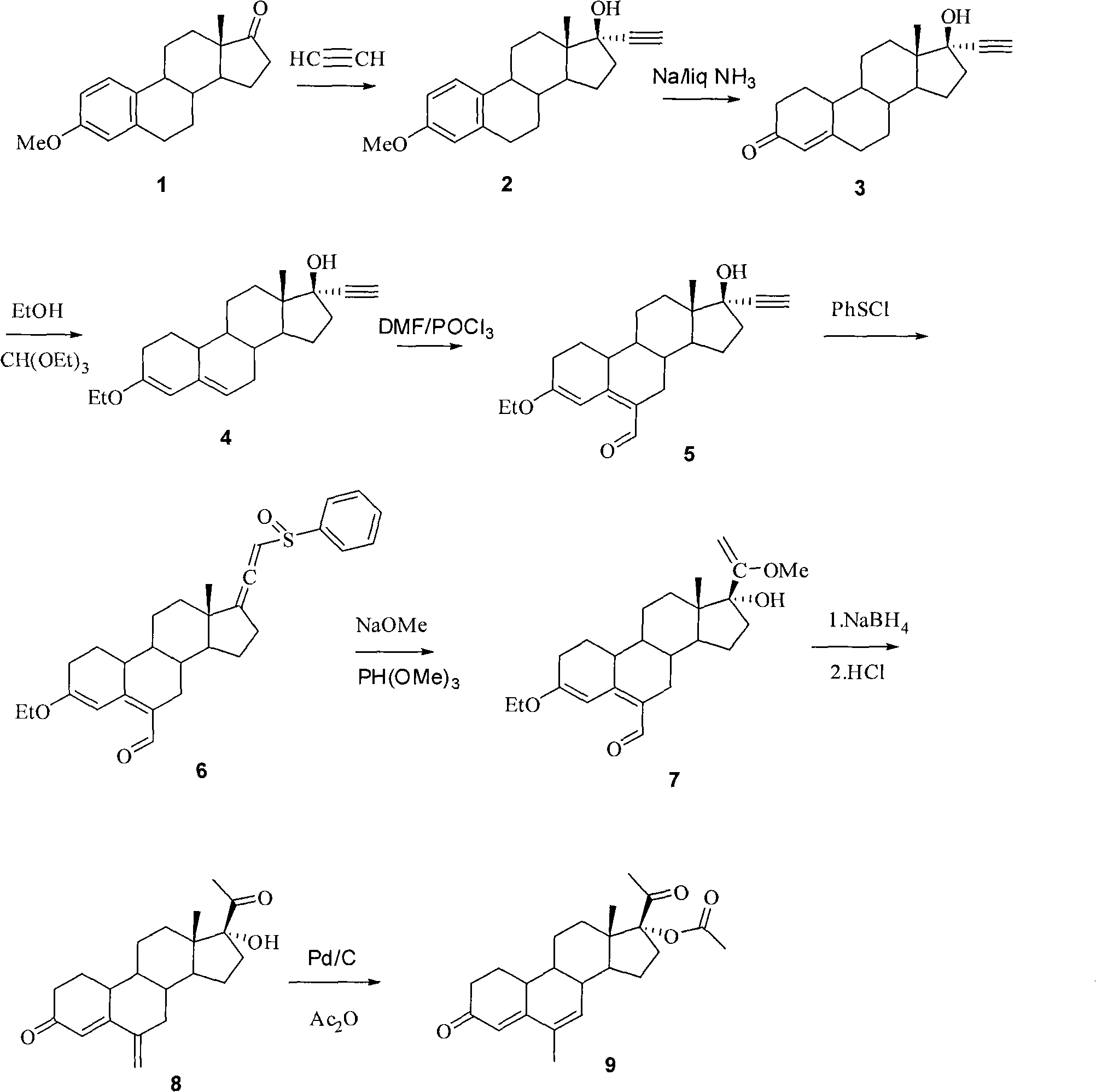
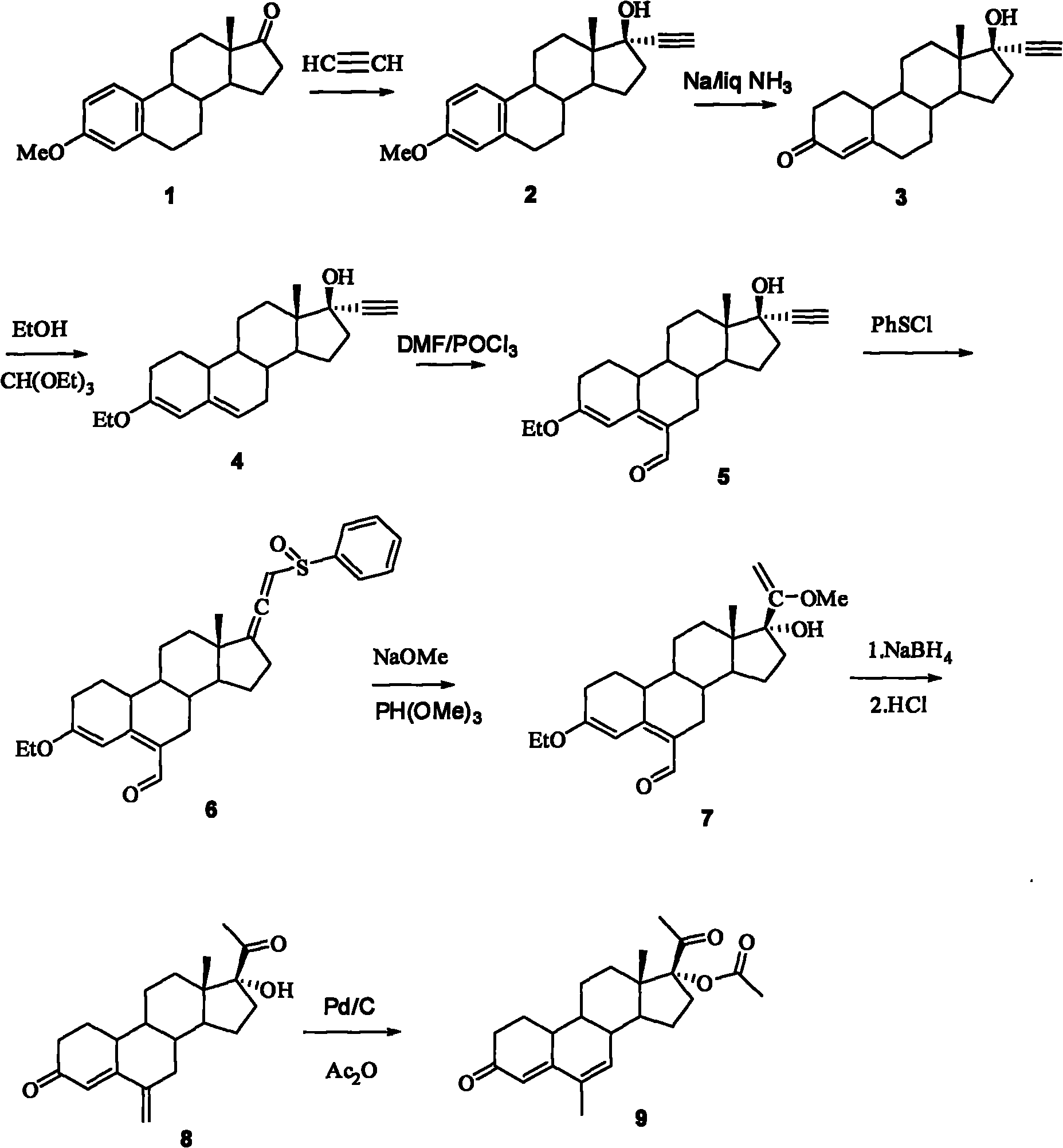
The invention relates to a method for synthesizing 6-methyl-17alpha-acetoxyl-19-norpregnane-4,6-diene-3,20-diketone. The method comprises the following steps: 1) reacting acetylene with estrone-3-methyl ether so as to obtain 17alpha-acetenyl estrone-3-methyl ether; 2) carrying out Birich reaction at a low temperature; 3) carrying out alkene etherification on the product obtained in the step 2); 4) carrying out a Vilsmeier reaction on the product obtained in the steps 3); 5) reacting the product obtained in the step 4) with benzene sulfenyl chloride; 6) reacting the product obtained in the step 5) with sodium methoxide and trimethyl phosphate; 7) reducing the formoxyl at the 6-position of the product obtained in the step 6); and 8) reacting the product obtained in the step 7) with Pd-C / cyclohexene, then reacting with acetic oxide so as to obtain the target product 6-methyl-17alpha-acetoxyl-19-norpregnane-4,6-diene-3,20-diketone. By using the method, the operation is simplified, and theproduction cost is reduced; and the reactions involved in the invention are simple to operate, and the yield is high.
Owner:黄云生 +1
Bromotetraacetylglucose, synthetic method and use thereof
ActiveCN101671375AThe method is simple and efficientHigh yieldEsterified saccharide compoundsSugar derivativesAcetylationGlucose polymers
The invention relates to a synthetic method of bromotetraacetylglucose, bromotetraacetylglucose and use thereof. The invention comprises the steps of taking glucose as raw materials, carrying out acetylation reaction by acetyl oxide as an acylating agent, then carrying out bromination reaction by phosphorous tribromide and hydrobromic acid as a brominating agent, and then carrying out recrystallization by diethyl, and at last obtaining bromotetraacetylglucose. The invention has the advantages of simple and efficient method, high yield and high purity reaching above 98.9%.
Owner:YANCHENG CITY CHUNZHU AROMA
Synthetizing method of lacosamide
InactiveCN103113256AHighlight substantive featuresSignificant progressOrganic compound preparationCarboxylic acid amides preparationPtru catalystAmmonium chloride mixture
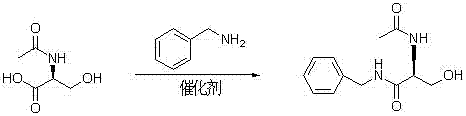
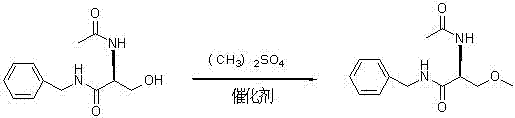
The invention provides a synthetizing method of lacosamide. The method comprises the steps of: based on D-serine as a raw material, performing an acylation reaction with acetic anhydride and then performing a condensation reaction with benzylamine; and finally, performing a methylation reaction with dimethyl sulfate, thereby obtaining lacosamide, wherein N,N' dicyclohexylcarbodiimide (DCC) or N,N' carbonyl diimidazole (CDI) is used as a catalyst in the condensation reaction; and a phase transfer catalyst including triethyl benzyl ammonium chloride (TEBA), tetrabutylammonium chloride (TBAC), tetrabutylammonium bromide (TBAB) or tetrabutylammonium hydrogen sulfate (TBAHS) is adopted in the methylation reaction. The method has the advantages of being simple in synthetizing process, moderate in reaction condition, simple in after-treatment, high in yield and high in product purity.
Owner:SUZHOU HONGRUI MEDICAL TECH
Method for preparing ultra-high heat resistant polyimide polymer
The invention discloses a method for preparing ultra-high heat resistant polyimide polymer. The method includes the following steps: dissolving aromatic-diamine containing carborane component and traditional aromatic dianhydride or aromatic dianhydride containing the carborane component and traditional aromatic-diamine into DMF or DMAc, carrying out ring opening polymerization to obtain polyamic acid; adding proper amounts of acetic oxide and pyridine into the obtained polyamic acid solution, stirring at room temperature for appropriate time, adding a proper amount of methyl alcohol to precipitate solid, and drying the solid to obtain polyimide containing the carborane component. According to the method provided by the invention, the 5% weight loss temperature of the prepared polyimide in nitrogen is greater than 600 DEG C, and that in the air is greater than 1000 DEG C, which are higher than those of the currently developed polyimide materials.
Owner:GENERAL ENG RES INST CHINA ACAD OF ENG PHYSICS
Catalyst system for synthesizing acetic acid and acetic anhydride from carbonyl compound and its uses
ActiveCN1876239AImprove performanceGuaranteed stabilityCarboxylic preparation from carbon monoxide reactionMetal/metal-oxides/metal-hydroxide catalystsIodised saltAcetic anhydride
The related catalyst system comprises Rh compound as catalyst, all of alkyl iodide, heteropoly salt and alkali iodide together as promoter. This invention is fit to prepare acetyl oxide by carbonylating methyl acetate or acetic acid by methanol.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Method of producing thermotropic liquid crystalline copolyester, thermotropic liquid crystalline copolyester composition obtained by the same method, and molding made of the same composition
InactiveUS6268419B1Suppress generationSmall amountThin material handlingLiquid crystallineAcetic anhydride
A method of producing a thermotropic liquid crystalline copolyester having an extremely small amount of out-gases comprising the steps of: (1) charging in a reactor 5-100 mol % of aromatic hydroxycarboxylic acid, 0-47.5 mol % of aromatic dicarboxylic acid and 0-47.5 mol % of aromatic diol, so that the sum of mol % of each material is 100 mol % and the mol % of aromatic dicarboxylic acid and that of aromatic diol are substantially equal; (2) adding acetic anhydride of an amount which satisfies the formula, (B-C) / A>=1.04, "A" representing the total molar number of the hydroxy group in a reaction system, "B" representing the molar number of acetic anhydride to be added, and "C" representing the molar number of water present in the reaction system prior to addition of acetic anhydride; (3) acetylation; (4) melt polymerization; and (5) solid-phase polymerization.
Owner:NEC ELECTRONICS CORP +1
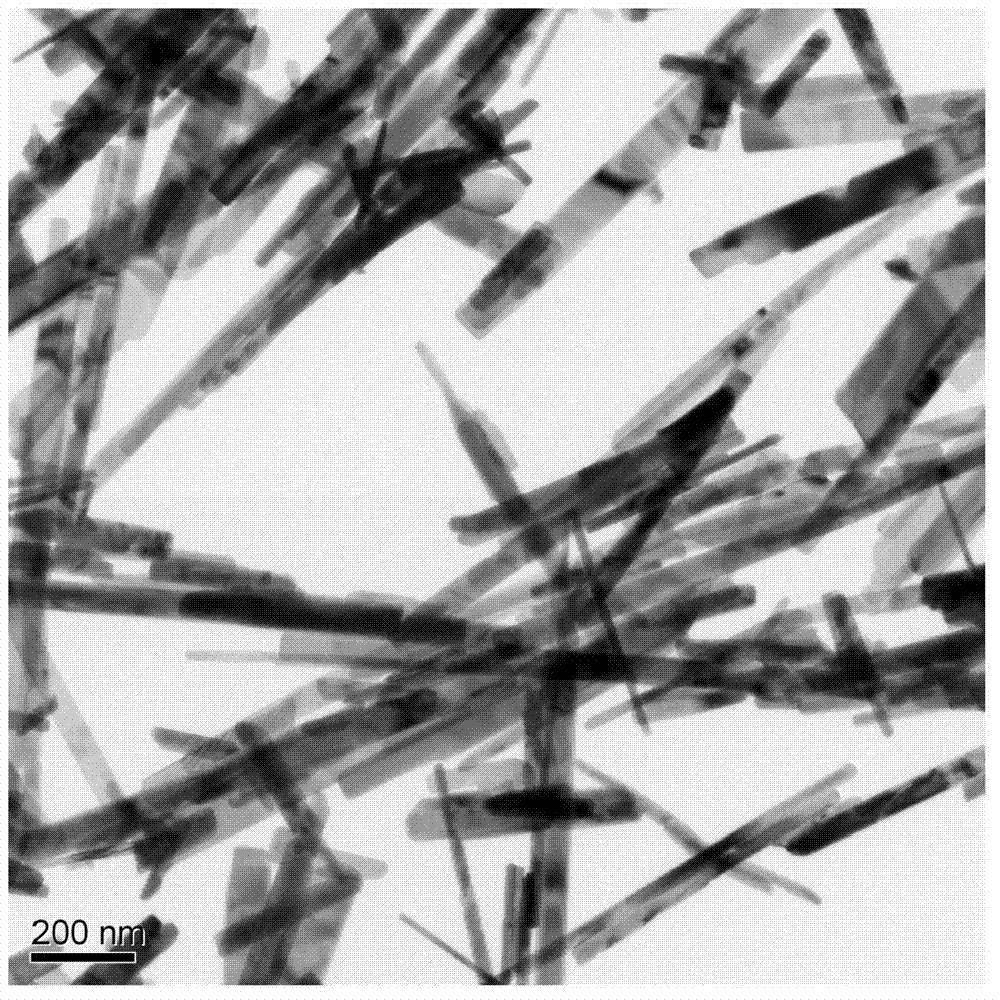
Preparation method of molybdenum dioxide nanorod
InactiveCN102815749ALow costControllable sizeMaterial nanotechnologyMolybdenum oxides/hydroxidesDecompositionAcetic oxide
The invention discloses a preparation method of a molybdenum dioxide nanorod. The preparation method comprises the following steps of: by taking sodium molybdate as a molybdenum source, acetic oxide as an additive, dimethylformamide as a solvent, concentrated hydrochloric acid as an acidifier, and tetrabutylammonium bromide as a settling agent, through a simple experiment device and reaction steps, controlling concentration of molybdate suspension, an additive amount of the additive, a pH value of the solution and the amount of the settling agent, recrystalizing and purifying to obtain precursor tetrabutyl ammonium hexamolybdate; and thermally treating the precursor tetrabutyl ammonium hexamolybdate in a quartz tube under an inert atmosphere to have decomposition to obtain a monoclinic phase molybdenum dioxide nanorod. The method, prepared by the invention, has the advantages of low cost and high reaction yield, and capability of overcoming the vices of expensive instrument device, rigorous reaction conditions and reduction at high temperature with H2 with safety hidden trouble of the conventional preparation method.
Owner:XI'AN POLYTECHNIC UNIVERSITY
Synthetic method of perfume o-tert-butylcyclohexyl acetate
InactiveCN103193638AExpand sourceLow costOrganic compound preparationCarboxylic acid esters preparationTert butyl phenolAcetic anhydride
The invention relates to a chemical synthetic method, and concretely relates to a synthetic method of a perfume o-tert-butylcyclohexyl acetate. The synthetic method comprises the following steps: reacting a raw material phenol with isobutene under the catalysis of anhydrous aluminum trichloride to obtain o-tert-butyl phenol, carrying out catalytic hydrogenation of o-tert-butyl phenol under the catalysis of Raney nickel to obtain o-tert-butyl cyclohexanol, and carrying out an esterification reaction of o-tert-butyl cyclohexanol and acetic anhydride under the catalysis of anhydrous sodium acetate to obtain o-tert-butylcyclohexyl acetate. The method has the advantages of mild reaction conditions, safe and convenient operation, high reaction yield, fine and strong fragrance of the obtained product, raw material source enlargement and raw material cost saving because of the adoption of industrially cheap phenol and isobutene as initial raw materials, total synthetic reaction yield improvement, suitableness for the industrial adoption, and good economic benefit.
Owner:南昌洋浦天然香料香精有限公司
Process and device for synthesizing peroxyacetic acid
InactiveCN103204793AAvoid the danger of strong irritationSynthetic safetyOrganic compound preparationChemical/physical/physico-chemical processesAcetic anhydridePhysical chemistry
The invention discloses a process and a device for synthesizing peroxyacetic acid. The process includes: enabling acetic anhydride and hydrogen peroxide to be respectively and intensively mixed in a micro-mixer, entering a micro-channel reactor with the length of 1-10m and the inner diameter of 0.5-2.0mm, enabling the reaction temperature to be 10-70 DEG C and the retention time to be 2-10min, and synthesizing to obtain the 5-30wt% peroxyacetic acid. The synthesis device comprises a constant flow pump, the micro-mixer, the micro-channel reactor and a water bath, wherein the constant flow pump is connected with the micro-mixer which is connected with the micro-channel reactor, and the micro-channel reactor is disposed in the water bath.
Owner:JIAXING UNIV +2
Acetic acid triethanolamine ester as well as preparation and application thereof
InactiveCN101665439ASimple methodLow costOrganic compound preparationAmino-hyroxy compound preparationAcetic anhydrideAcetic oxide
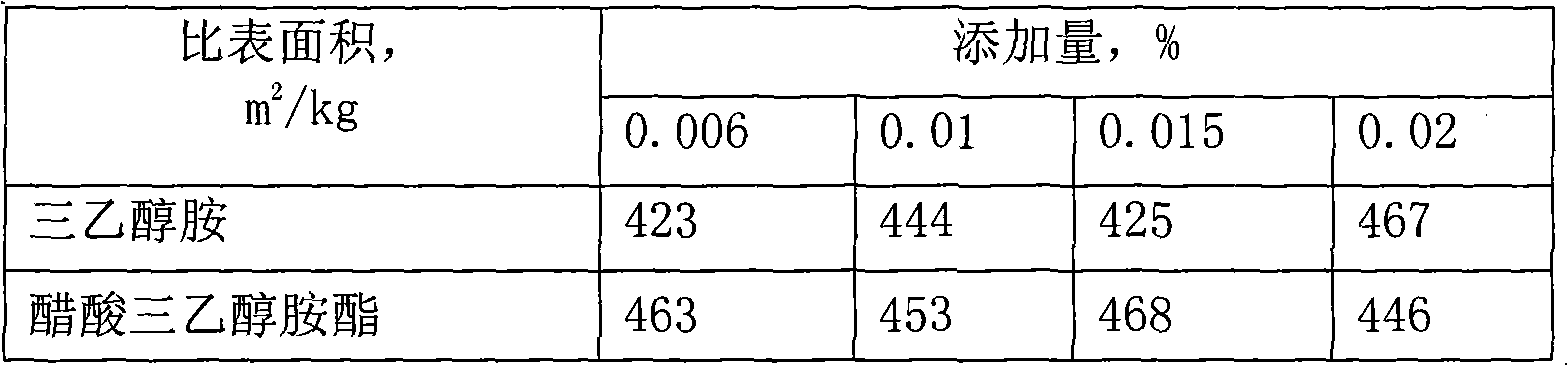
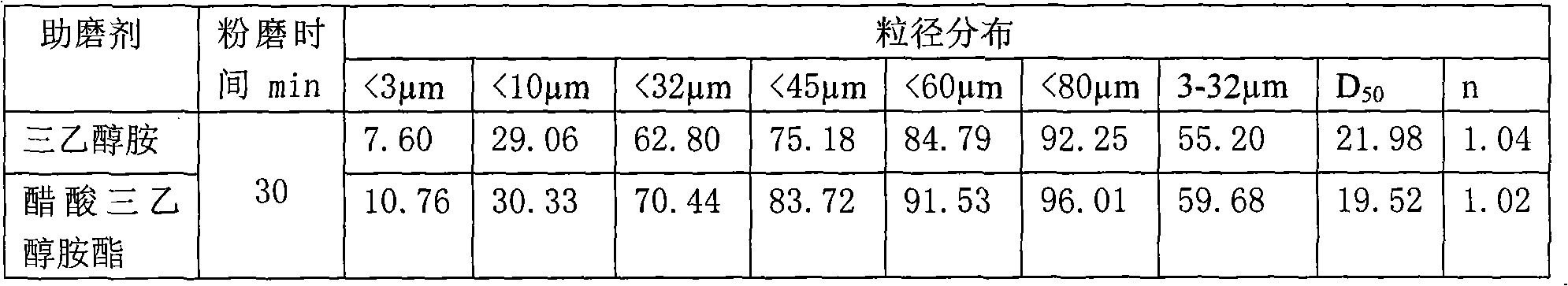
The invention discloses acetic acid triethanolamine ester which comprises monoacetic acid triethanolamine ester, diacetic acid triethanolamine ester and triacetic acid triethanolamine ester. The acetic acid triethanolamine ester is prepared by taking triethanolamine and acetic acid (acetic anhydride) as raw materials to carry out dehydration reaction at 100-140 DEG C under the action of a catalyst. The invention also discloses an application of the acetic acid triethanolamine ester as a cement grinding assistance reinforcing agent in the grinding production of cement, and the adding amount ofthe acetic acid triethanolamine ester is 0.006-0.02 percent of the total weight of the cement. The acetic acid triethanolamine ester has favorable grinding assistance reinforcing effect on the cement,has less dosage, no corrosion and low cost and can effectively improve the quality of the cement and further reduce the production cost of the cement; compared with the common triethanolamine grinding assistance agent, the acetic acid triethanolamine ester can obviously increase the specific surface area of the cement and improve the strength of the cement under the same condition.
Owner:SHANDONG UNIV
Banana cellulose crystallite/polylactic acid aerogel and preparation method and use thereof
ActiveCN105017541ARealize comprehensive utilizationEasy to handlePaper material treatmentCelluloseNitrogen
The invention discloses a preparation method of a banana cellulose crystallite / polylactic acid aerogel. The preparation method comprises the following steps: step one, taking banana cellulose crystallite and ionic liquid and blending the banana cellulose crystallite and the ionic liquid, then performing reflux condensation for 3 hours under the protection of nitrogen at the constant temperature of 100 DEG C, then adding acetic oxide for modification, and then continuously stirring for 3 hours at the temperature of 90 DEG C under the protection of nitrogen to obtain a banana cellulose crystallite hydrosol; and step two, firstly taking polylactic acid and dichloromethane in the mass-to-volume ratio of 1.8: 10, dissolving the polylactic acid in the dichloromethane, then putting the banana cellulose crystallite hydrosol obtained in the step one into the dichloromethane dissolved with the polylactic acid, and then blending to obtain a crude banana cellulose crystallite / polylactic acid aerogel product. The invention also discloses the banana cellulose crystallite / polylactic acid aerogel. The invention also discloses use of the banana cellulose crystallite / polylactic acid aerogel for oil absorption.
Owner:GUANGXI TEACHERS EDUCATION UNIV
Metal-porphyrin polymer catalyst and preparation and application thereof
ActiveCN102658202AEasy to makeImprove stabilityPreparation by oxidation reactionsOrganic compound preparationFiltrationPorphyrin
The invention relates to a metal-porphyrin polymer material and preparation and the application thereof. 4-amino-phenyl-porphyrin and pyromellitic dianhydride react under the 60-DEG C room temperature with N-methyl-pyrrolidone as a solvent and pyridine and acetic oxide as catalysts under protection condition of nitrogen, the obtained solution is poured into a carbinol solution, separated solid is porphyrin polymer, suction filtration is conducted, carbinol and water are respectively used for washing the solid till the filtering liquid is colorless, drying is conducted, and metal ions Ru3+, Pd2+, Fe2+, Mn2+, Cu2+, Co2+, Ni2+ or Zn2+ and the porphyrin polymer are solved in the N-methyl pyrrolidone, react under 60-DEG C temperature and are poured into carbinol solvent to separate out the metal-porphyrin polymer material. The catalyst is mild in reaction condition, free of pollution, high in selection of being used in thioether and arene conversion rate and sulfoxide and simple in post-processing and can be recycled by simple filtering.
Owner:PETROCHINA CO LTD
Preparation method and application of efficient corn straw adsorbent
InactiveCN103877947AWide variety of sourcesSimple production processOther chemical processesWater/sewage treatment by sorptionAcetic acidAcetic anhydride
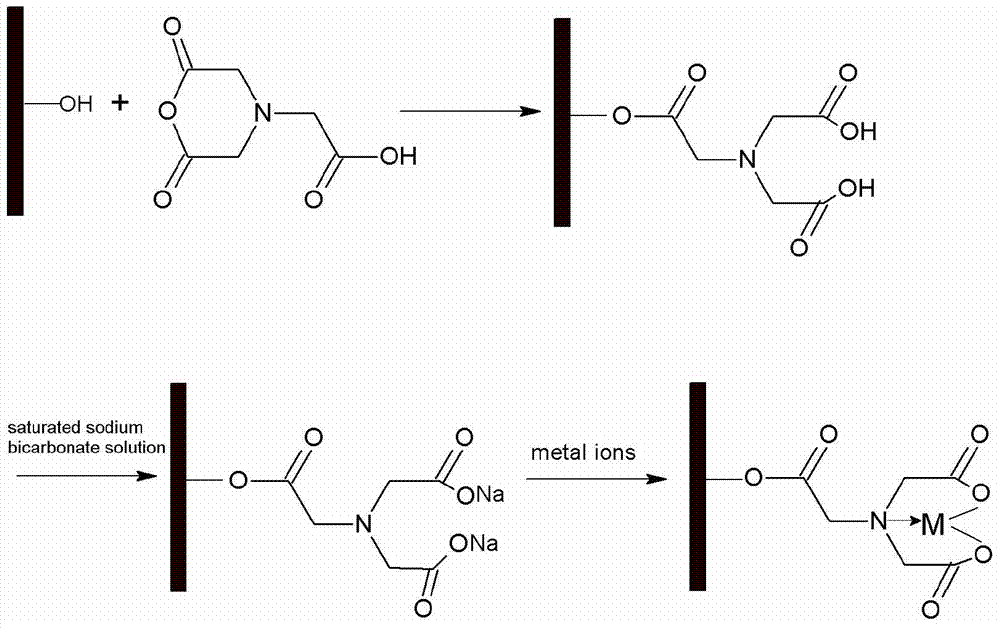
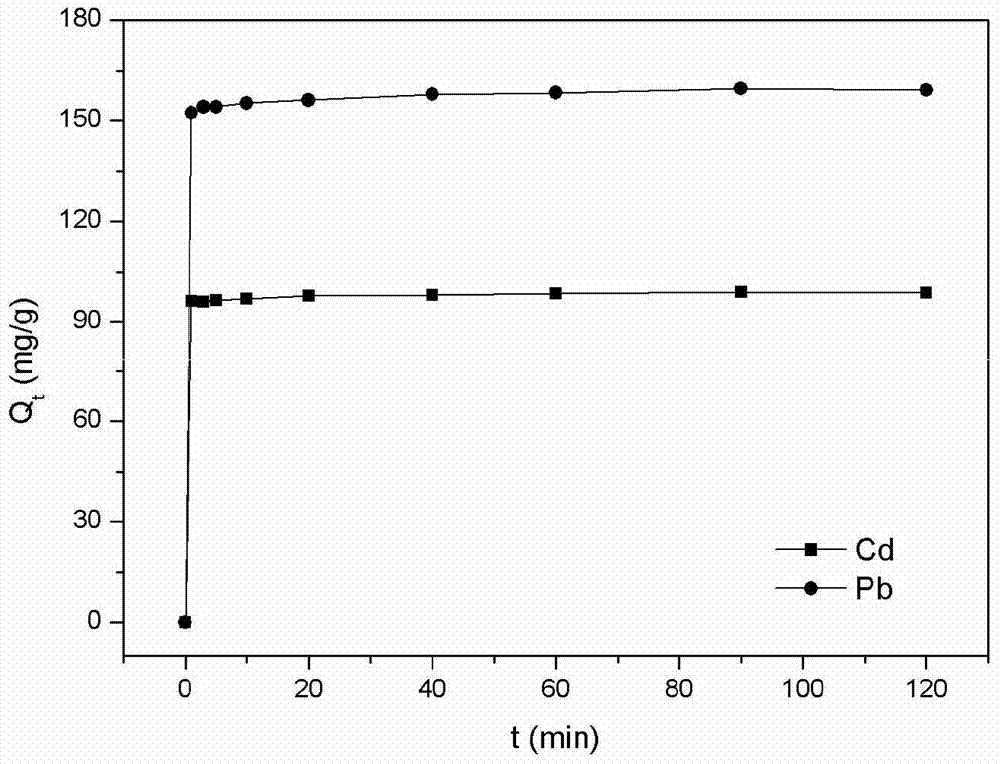
The invention relates to a preparation method and application of an efficient corn straw adsorbent. The preparation method of the efficient corn straw adsorbent particularly comprises the following steps: cleaning corn straw, drying, crushing, sieving and dewaxing; then adding nitrilotriacetic acid into a mixed organic solution containing acetic anhydride and an activator, stirring at 60-70 DEG C, adding the corn straw after reaction, stirring at 70-80 DEG C, and separating, washing and drying after reaction so as to obtain the efficient modified corn straw adsorbent. The adsorbent can be used for removing heavy metal ions such as Cd<2+> and Pb<2+> in wastewater, has the absorbing capacity reaching up to 100mg / g and 160mg / g respectively for heavy metal ions of Cd<2+> and Pb<2+>, has the characteristics of simple preparation method, high absorbing capacity, reusability, adsorption stability and no secondary pollution, and also achieves resource utilization of biomass resources.
Owner:HUNAN UNIV
Thermally-induced liquid crystal polymer and preparation method and application thereof
ActiveCN109824876AImprove melt tensionLight colorLiquid crystal compositionsAcetic anhydrideLiquid-crystal display
The invention discloses a preparation method of thermally-induced liquid crystal polymer. The preparation method includes following steps: S1, in a first reactor, allowing hydroxyl-containing aromaticmonomer and acetic anhydride to be in acetylation reaction; S2, in a second reactor, allowing a reactant obtained after acetylation reaction to be in melt polycondensation reaction or to be in melt polycondensation with aromatic diacid monomer to obtain the thermally-induced liquid crystal polymer. The preparation method can effectively improve melt tension, and the prepared TLCP is light in color. The preparation method is simple in operation, easy-to-obtain in product and suitable for industrial production, and a TLCP thin film can be prepared stably by using the prepared TLCP through a film blowing method. The invention further discloses the TLCP and application thereof in thin film preparation.
Owner:KINGFA SCI & TECH CO LTD +1
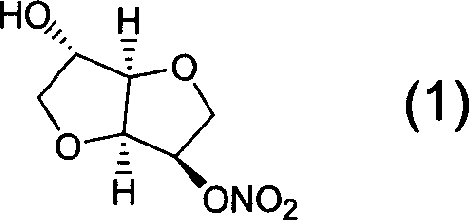
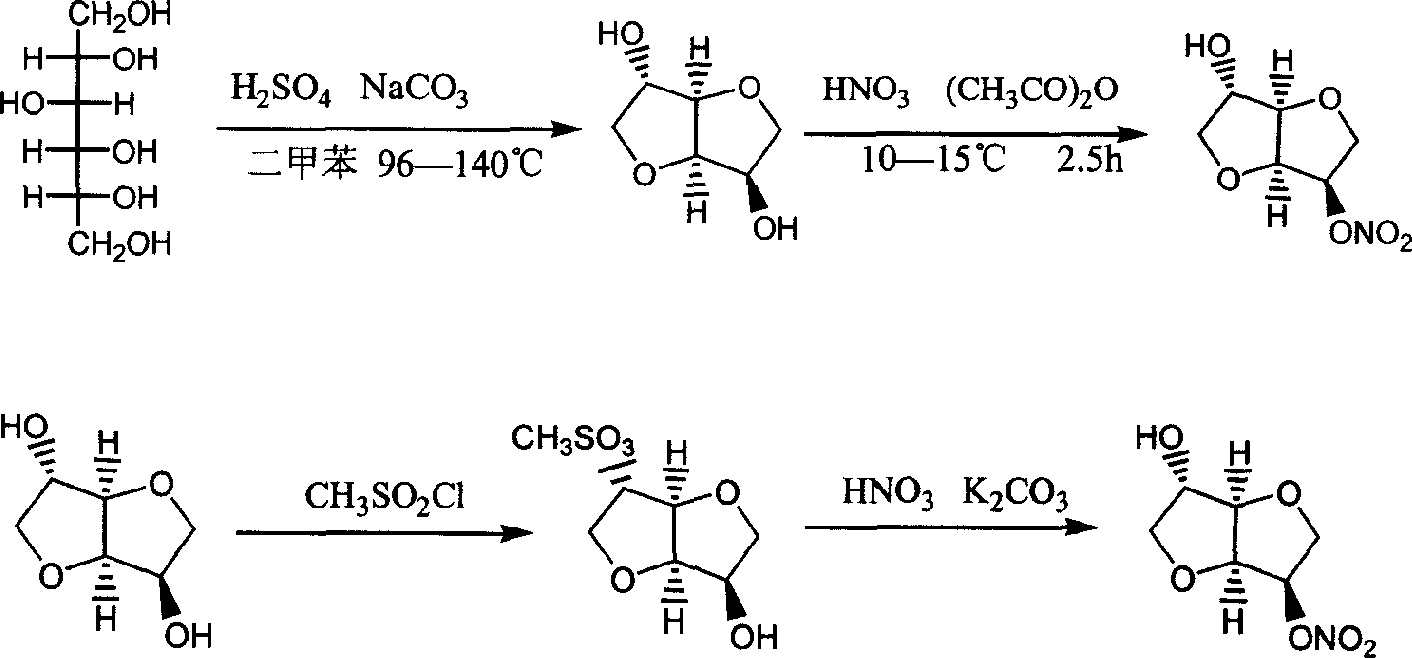
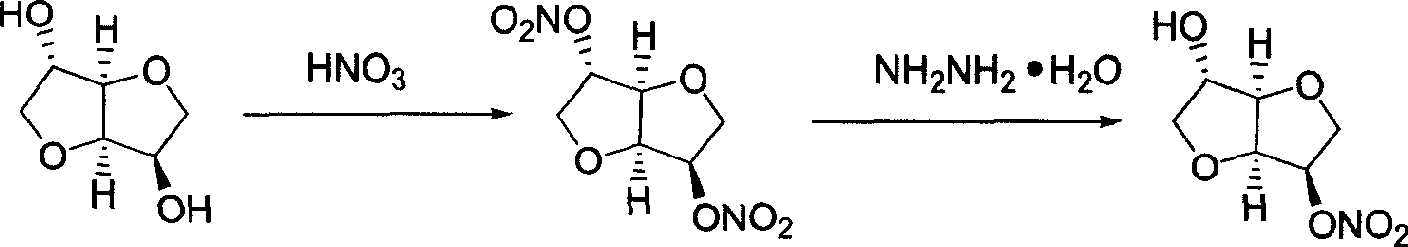
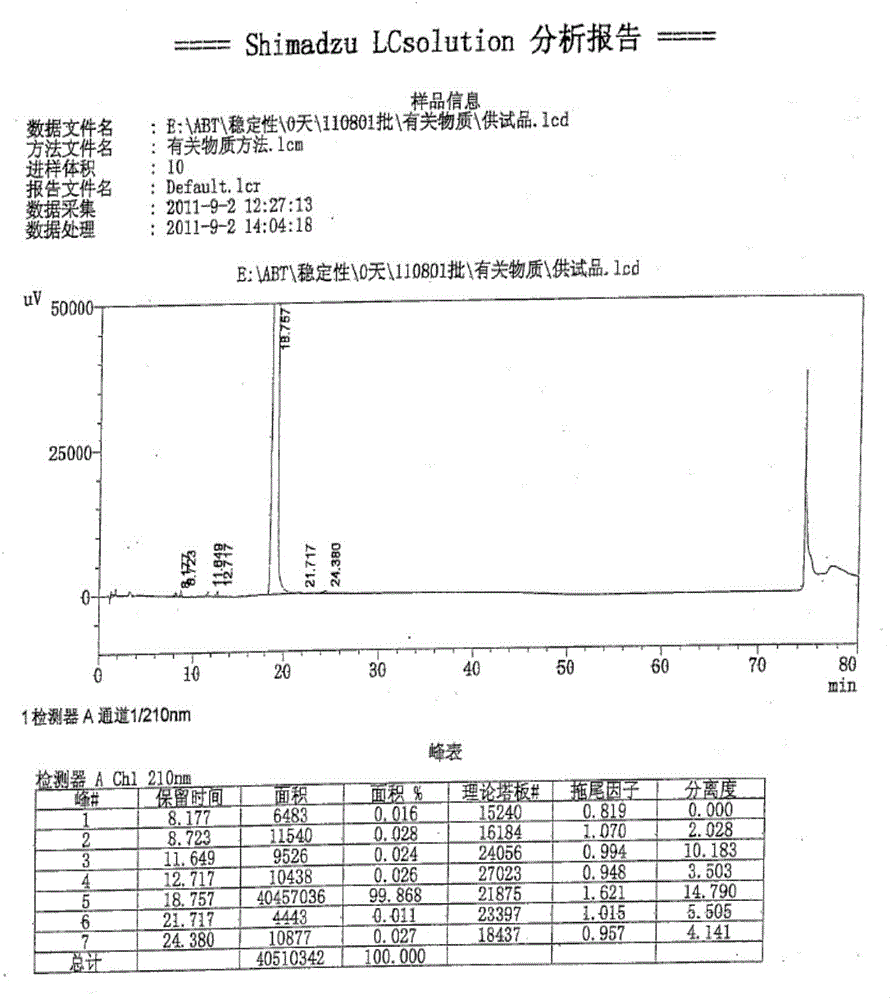
Preparation process of isosorbide mononitrate
InactiveCN1618798AHarsh reaction conditionsLow yieldOrganic chemistryCardiovascular disorderAnginaAcetic oxide
A process for preparing the isosorbide mononitrate from sorbitol includes dewatering sorbitol by p-methylphenyl sulfonic acid to obtain anhydrosorbitol, protecting by acetic oxide under existance of N,N-dimethylaminopyridine, nitrating by nitric acid / acetic oxide / acetic acid system, and removing protection by potassium carbonate-methanol system. It can be used to treat angina pectoris.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for purifying abiraterone acetate
ActiveCN104447934ASolve processing problemsSuitable for industrial productionSteroidsAcetic anhydrideOrganic base
The invention belongs to the technical field of medicine synthesis, and provides a method for purifying abiraterone acetate. The method comprises the following steps: in the presence of acetic anhydride and organic bases, dissociating abiraterone acetate salt into free abiraterone acetate, wherein the high-performance liquid chromatography (HPLC) purity of dissociated abiraterone acetate is more than 99%; and then refining abiraterone acetate by virtue of acetonitrile recrystallization to obtain abiraterone acetate which has the HPLC purity of more than 99.7% and can meet the medical standards, and ensuring that the content of hydrolysis impurity abiraterone is less than 0.02%. By adopting the method provided by the invention, the problem that inevitable hydrolysis impurity abiraterone exists in a salt dissociation process of abiraterone acetate can be solved, a purification process can be simplified, and reagents can be saved; and moreover, the method is easy to operate, and is suitable for industrial production.
Owner:SHENZHEN KEXING PHARM CO LTD
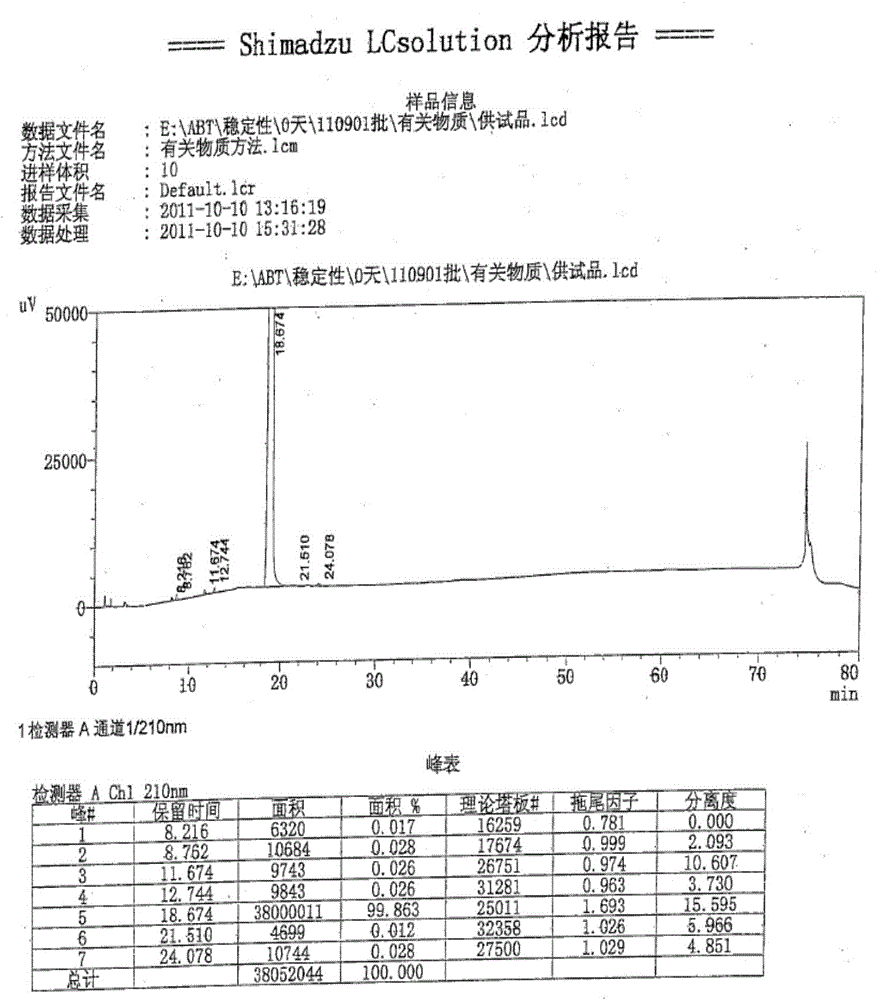
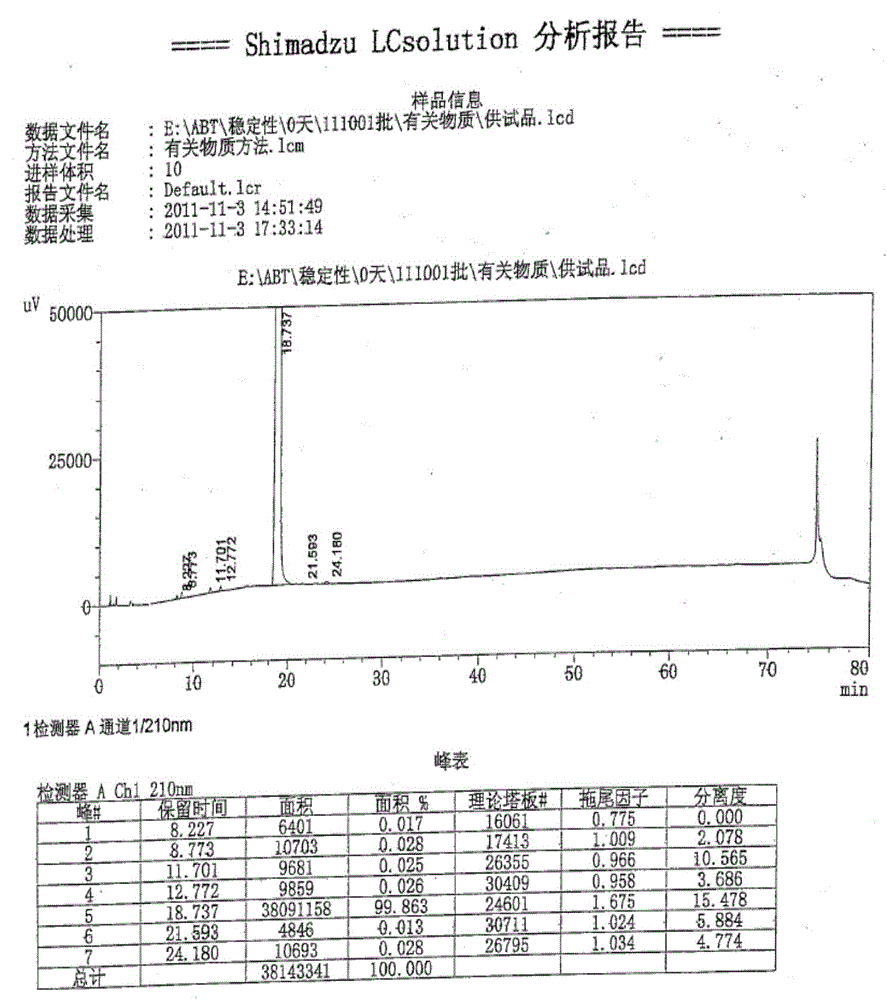
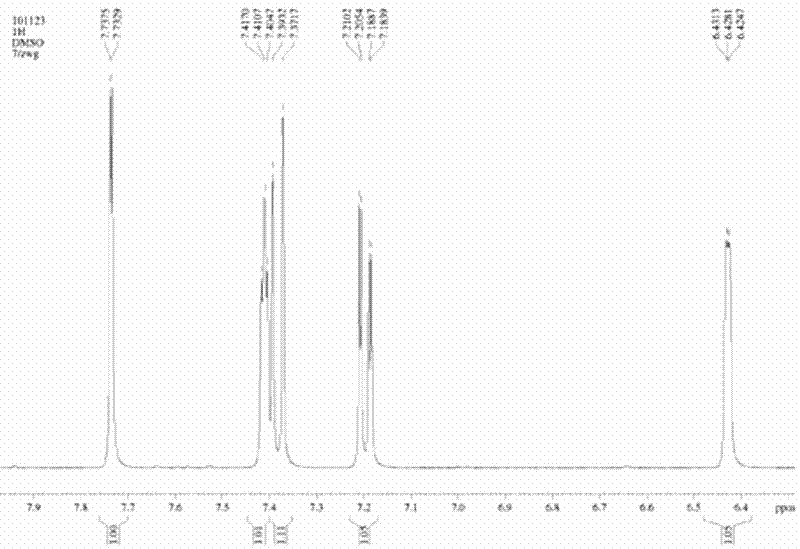
Method for preparing 5-bromoindole
The invention relates to a method for synthesizing 5-bromoindole. The method comprises the following steps of: (1) dissolving indole into alcoholic organic solvents, adding an aqueous solution of sodium hydrogensulfite or potassium hydrogensulfite, reacting for 15 to 20h, filtering a reaction solution, washing, and drying to obtain an intermediate I; (2) mixing the intermediate I and acetic anhydride, raising the temperature to 68 to 75 DEG C, reacting for 2 to 3h, adding ester or benzene organic solvents, reacting for 0.5 to 1h, cooling to room temperature after the reaction is finished, filtering, washing, and drying to obtain an intermediate II; and (3) dissolving the intermediate II into water, adding bromine at the temperature of between 0 and 5 DEG C, keeping the reaction for 1 to 3h, raising to room temperature, continuing to react for 1 to 2h, adding the aqueous solution of sodium hydrogensulfite or potassium hydrogensulfite, reacting for 10 to 30min, adding an aqueous solution of sodium hydroxide or potassium hydroxide, refluxing for 12 to 18h, cooling a reaction solution, precipitating crystals, filtering, washing, and drying to obtain a product, namely the 5-bromoindole. The method is low in cost consumption and is simple, and the quality of the product is high.
Owner:河南凯美克制药有限公司 +1
Method of producing thermotropic liquid crystalline copolyester, thermotropic liquid crystalline copolyester composition obtained by the same method, and molding made of the same composition
InactiveUS20010014708A1Suppress generationSmall amountGroup 5/15 element organic compoundsSynthetic resin layered productsLiquid crystallinePolymer science
Owner:NIPPON PETROCHEMICAL CO LTD
Preparation of acetoxylsilane
ActiveCN101323625AEffective isolationAvoid it happening againGroup 4/14 element organic compoundsNaphthaOrganic solvent
The invention discloses a preparation method of organoacetoxysilane, which adopts organochlorosilane and acetic acid as raw materials. The method comprises the following steps: the organochlorosilane and an organic solvent are added into a reaction vessel and the organic solvent is selected from one of solvent naphtha, hexane, hexamethylene, toluene, xylene and dichlorethane. The acetic acid is slowly added into the reaction vessel and reacts with the organochlorosilane and boiled for eight hours to obtain organoacetoxysilane solution; the organic solvent in the organoacetoxysilane solution is distilled at ordinary pressure and then recycled. Matters with low boiling point, remaining in the reaction vessel, are removed by distillation at reduced pressure to obtain colorless and transparent finished products. As the acetic acid has lower cost than acetic oxide and the preparation method of the invention optimizes each technological parameter, the preparation method of the invention is characterized by low cost, high efficiency, good quality, etc. In the finished product obtained, the effective content of organoacetoxysilane is more than 93 percent while the content of chloridion is less than 50PPM.
Owner:湖北环宇化工有限公司
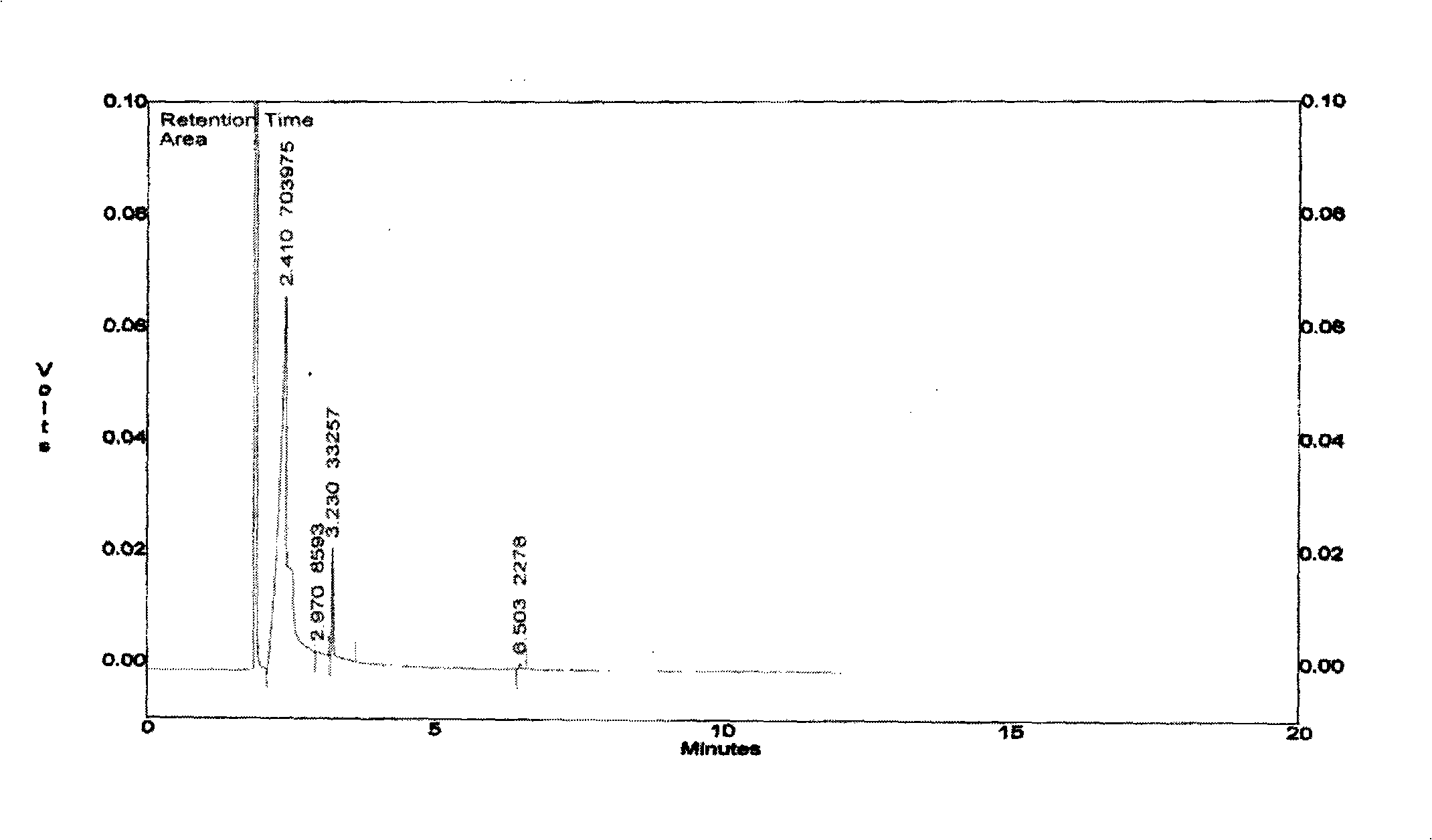
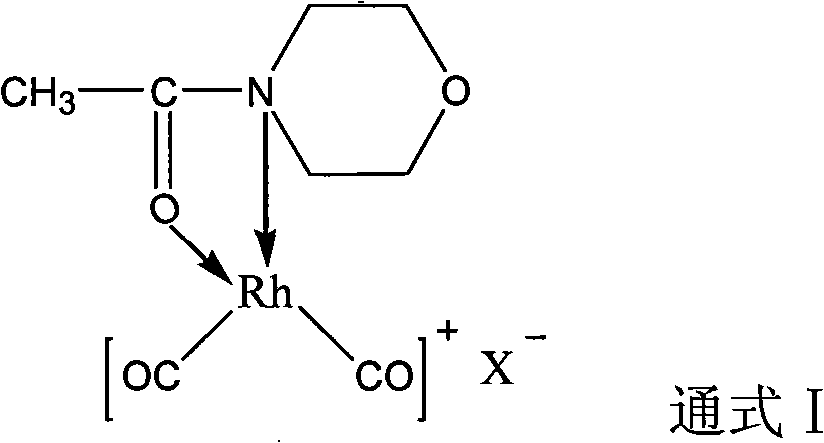
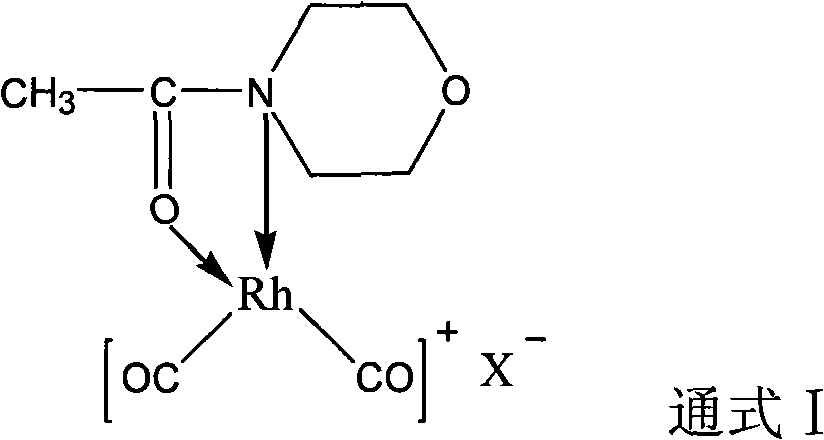
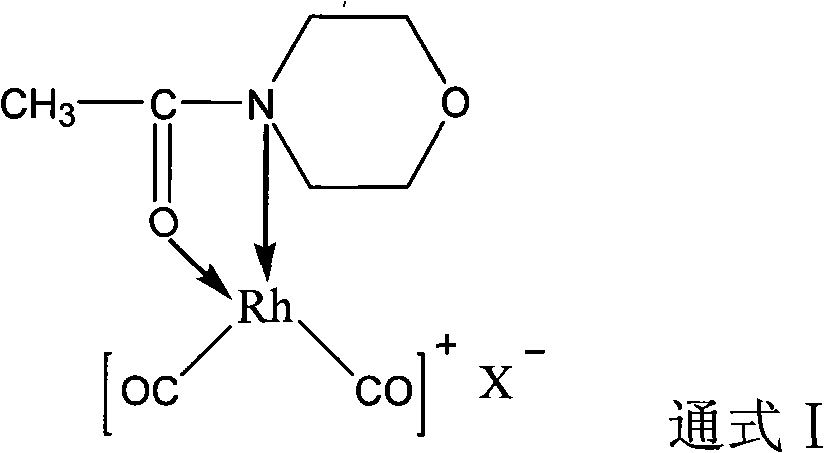
Rhodium catalyst for acetic oxide carbonyl synthesis from methyl acetate and preparation thereof
ActiveCN101279294AHigh activityImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsMetal/metal-oxides/metal-hydroxide catalystsSolubilityAcetic anhydride
The present invention discloses a rhodium catalyst for synthesizing acetic anhydride by acid methyl carbonyl, which is a cis-dicarbonyl rhodium cation structural catalyst formed by carbonyl rhodium and ligand function; the catalyst is a chelate square plane complex; the cation part of the catalyst is cis-dicarbonyl rhodium cation and the ligand is acetyl morpholine. The catalyst not only shows excellent thermal stability, but also keeps excellent catalyzing activity during the reaction of synthesizing acetic anhydride by acid methyl carbonyl; simultaneously the solubility of the catalyst species can also be increased in a reaction system, thus avoiding the addition of acetic acid and / or acetic anhydride as a catalyst solvent in the industrial production, thus effectively reducing the load of flash evaporation and evaporation and improving the manufacture efficiency of acetic anhydride synthesis by acid methyl carbonyl. The present invention also discloses a preparation method of synthesizing acetic anhydride by acid methyl carbonyl by the rhodium catalyst..
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
High selectivity method for synthesizing moxifloxacin
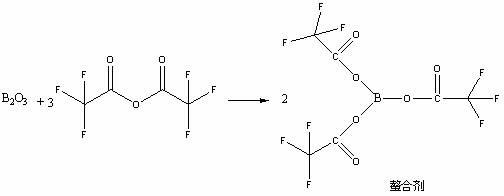
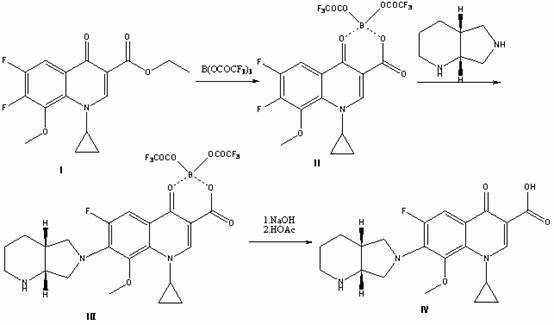
ActiveCN102351858AAvoid it happening againSimple processing methodOrganic chemistryAcetic anhydrideIce water
The invention discloses a high selectivity method for synthesizing moxifloxacin. The method comprises the following steps of: reacting boric anhydride with trifluoro acetic anhydride to obtain a chelant; reacting 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester with the chelant, cooling to room temperature, adding ice water, performing suction filtration, and washing a filter cake with water until neutrality to obtain a 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate; and reacting the 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate with (S,S)-2,8-diazabicyclo[4,3,0]nonane to obtain a 1-cyclopropyl-6-fluoro-7-([S,S]-2,8-diazabicyclo[4,3,0]nonane-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid ethyl ester trifluoroacetic anhydride boronized chelate, recycling a solvent under reduced pressure, adding alkali, refluxing, discoloring, filtering, freezing, performing suction filtration, and drying a filter cake. The method is simple, mild in conditions, and high in selectivity, avoids difficultly separated impurities, is high in reaction yield and product purity, and is suitable for industrial production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Integrated process for synthesizing acetyl tributyl citrate (ATBC) from active carbon solid-carried sulphuric acid catalyst
ActiveCN102304045AEasy to separateAvoid pollutionPhysical/chemical process catalystsOrganic compound preparationActivated carbonAcetic anhydride
The invention relates to an integrated process for synthesizing acetyl tributyl citrate (ATBC) from an active carbon solid-carried sulphuric acid catalyst. The integrated process comprises the following steps of: (1) preparing the catalyst: immersing wooden active carbon in a 5-20 times of sulphuric acid solution, stirring and solid-carrying at normal temperature, and obtaining the active carbon solid-carried sulphuric acid catalyst after filtering and drying; (2) preparing TBC (Tributyl Citrate): reacting (hydrate) citric acid, n-butyl alcohol and the catalyst at 130-160 DEG C for 3.0-6.0 h, and dealcoholizing; and (3) preparing the ATBC: adding acetic anhydride into the TBC, reacting at 70-90 DEG C for 0.5-1.5 h, filtering a reaction solution after cooling, adding water, recycling acetic acid, and post-processing to obtain the ATBC finished product. According to the method disclosed by the invention, the catalyst is low in use amount and easy for being separated and recycled and also has decolourization function; after being dealcoholized, the TBC is unnecessary to be separated and the acylation reaction is directly carried out; the steps of refining the TBC, decolourizing and the like are saved; the three wastes (waste gas, waste water and industrial residue) are reduced obviously; the cost is reduced; the product yield is increased; and the integrated process is applied to industrial application.
Owner:NANJING UNIV OF TECH
Lithium pyridine carboxylate-rhodium acetate complex catalyst for synthesizing acetic acid and acetic anhydride through carbonylation, and preparation method and application thereof
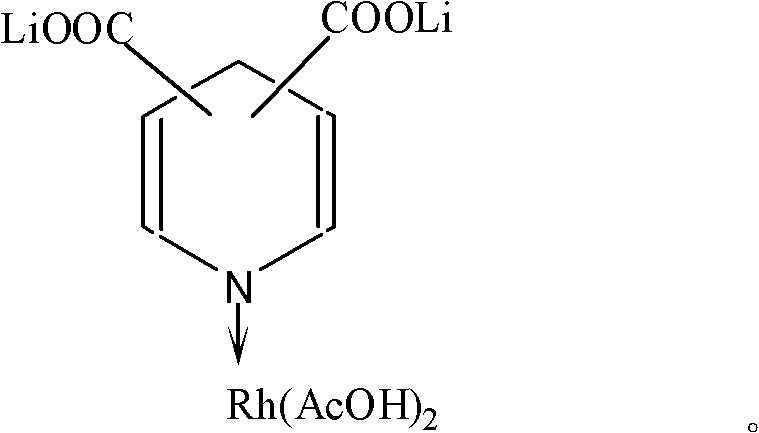
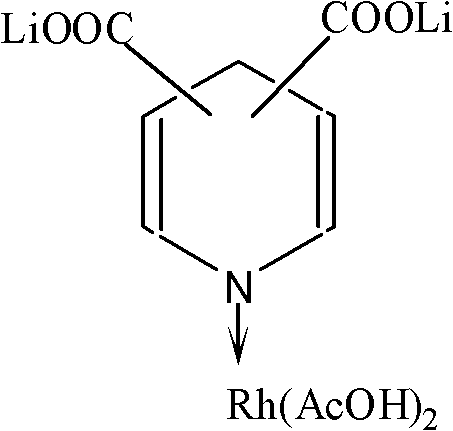
InactiveCN102218343AGood activity and stabilityImprove reaction stabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationLithiumAcetic anhydride
The invention belongs to the field of synthesis of acetic acid and acetic anhydride through carbonylation, and relates to a lithium pyridine carboxylate-rhodium acetate complex catalyst for synthesizing acetic acid and acetic anhydride through carbonylation of methanol, and a preparation method and application of the catalyst. When the catalyst is used for catalyzing carbonylation reaction of the methanol to prepare the acetic acid and catalyzing carbonylation reaction of methyl acetate to prepare the acetic anhydride, the catalyst has good catalytic activity and reaction stability. Under the relative mild conditions, the catalyst can catalyze the carbonylation of the methanol to prepare the acetic acid and catalyze the carbonylation of the methyl acetate to prepare the acetic anhydride at high speed and high selectivity. The catalyst has an active ingredient of rhodium; and a coordination structure formed by the rhodium active ingredient and the lithium pyridine carboxylate is shown as the specifications.
Owner:INST OF CHEM CHINESE ACAD OF SCI
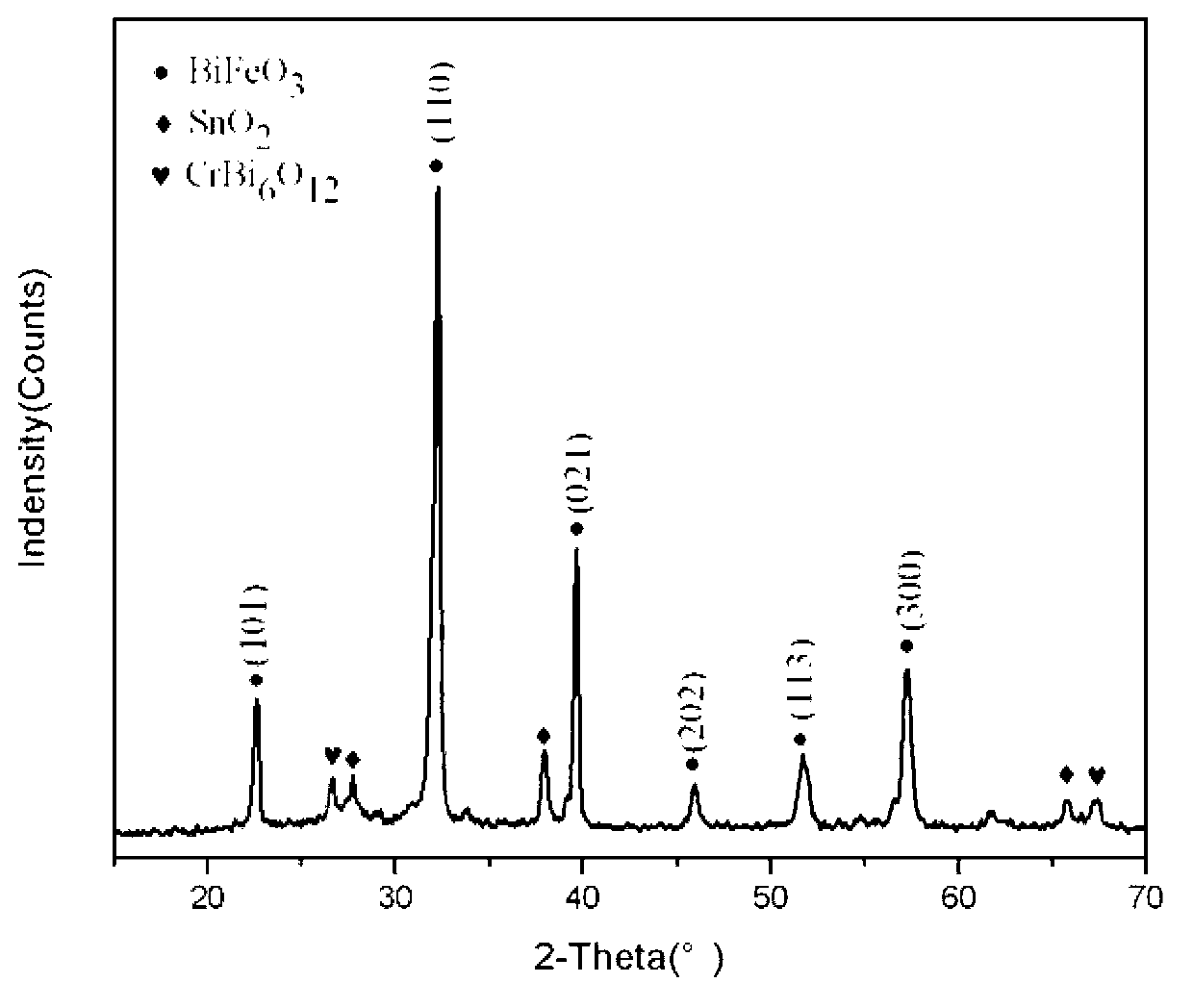
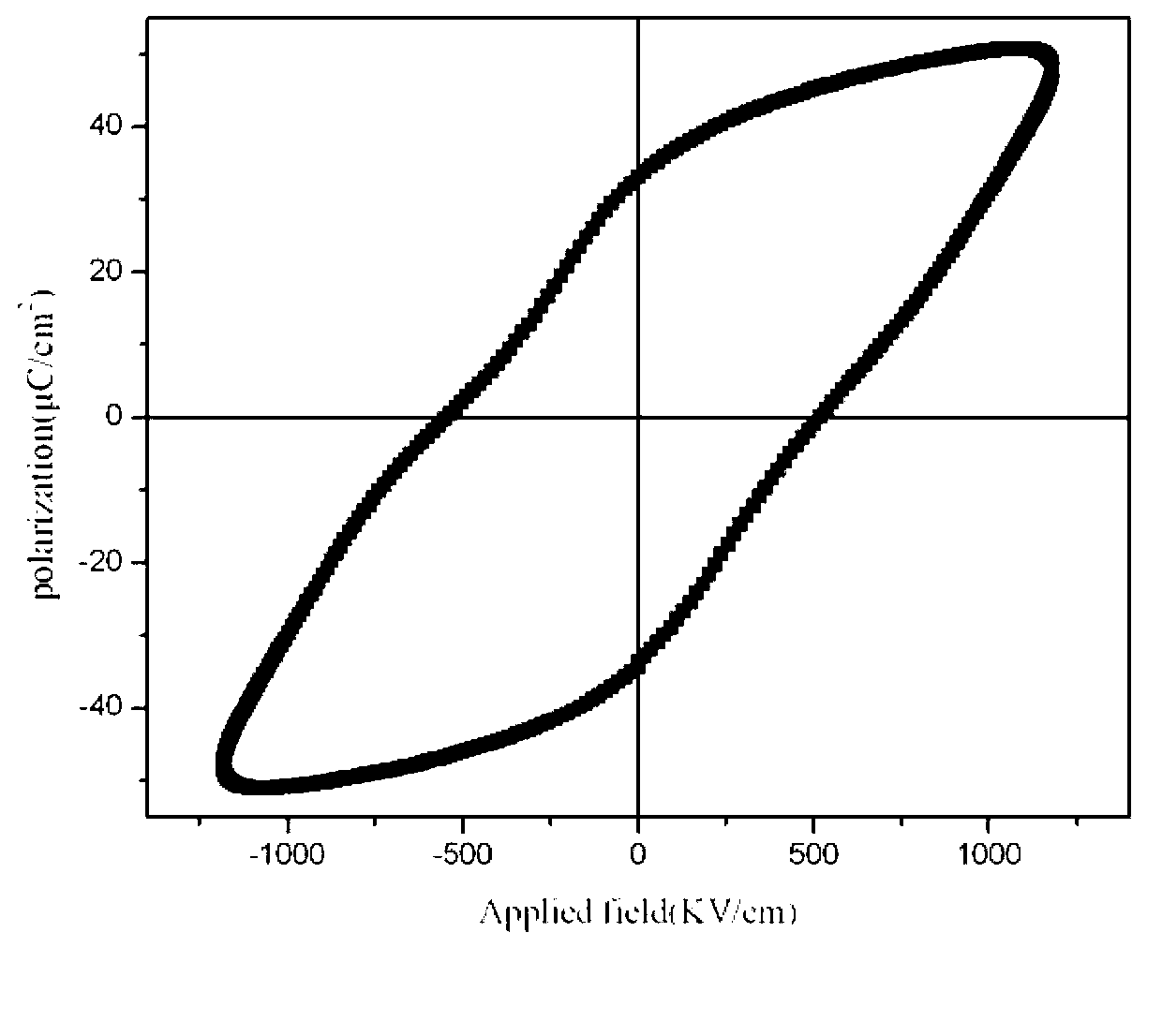
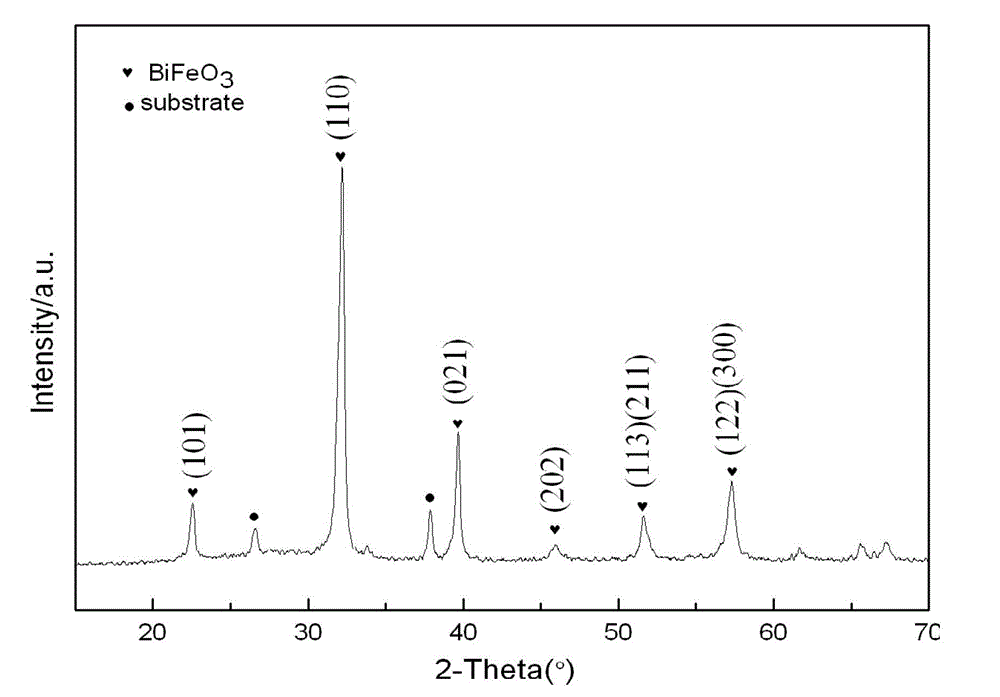
Method for preparing BiFe1-xCrxO3 ferroelectric film by using sol-gel method
The invention provides a method for preparing a BiFe1-xCrxO3 ferroelectric film by using a sol-gel method. The method comprises the following steps of: dissolving bismuth nitrate, ferric nitrate and chromic nitrate according to a molar ratio of 1.05: (1-x): x into a mixed solution containing ethylene glycol monomethyl ether, acetic oxide and cholamine, and magnetically stirring to obtain a stable BiFe1-xCrxO3 precursor with the metal ion concentration of 0.003-0.3mol / L, wherein x is equal to 0.00-0.03, and the volume ratio of the ethylene glycol monomethyl ether to the acetic oxide to the cholamine is 14: 5: 1. A film is prepared by spin-coating the BiFe1-xCrxO3 precursor on an FTO (Fluorine-doped Tin Oxide) / glass substrate by using a spin-coating method, and a crystalline BiFe1-xCrxO3 film is obtained by adopting a layer-by-layer annealing process. The method disclosed by the invention has simple requirements on equipment, and experimental conditions are easy to achieve; the prepared film is better in uniformity, and the doping amount is easy to control; and the ferroelectric property of the BiFeO3 film is improved through B-bit Cr doping.
Owner:盐城青墩津邦水务有限公司
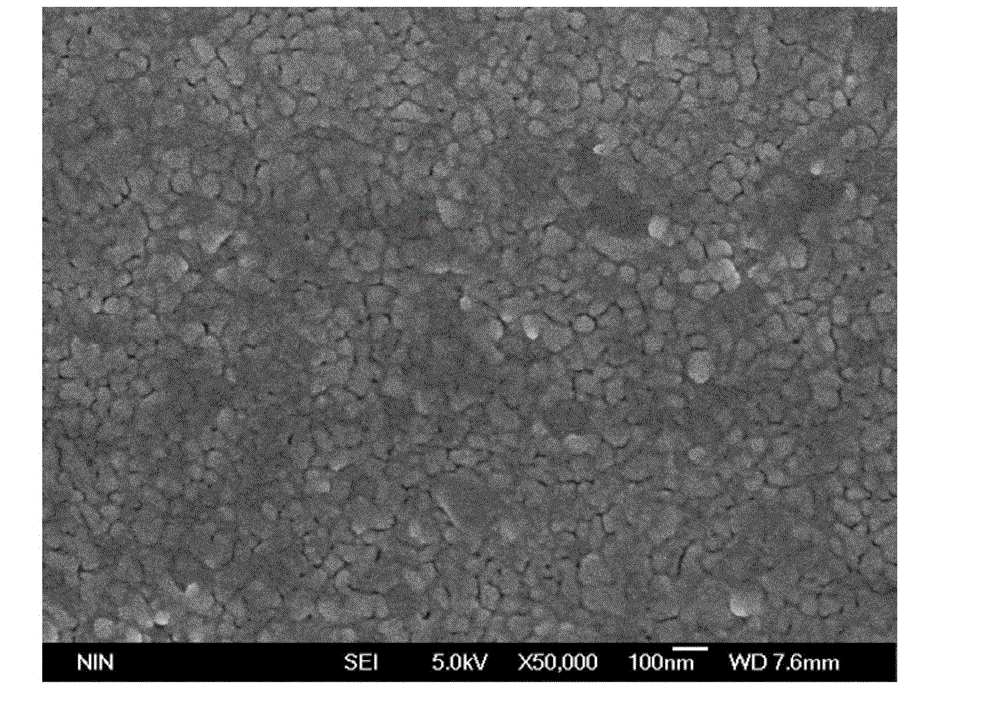
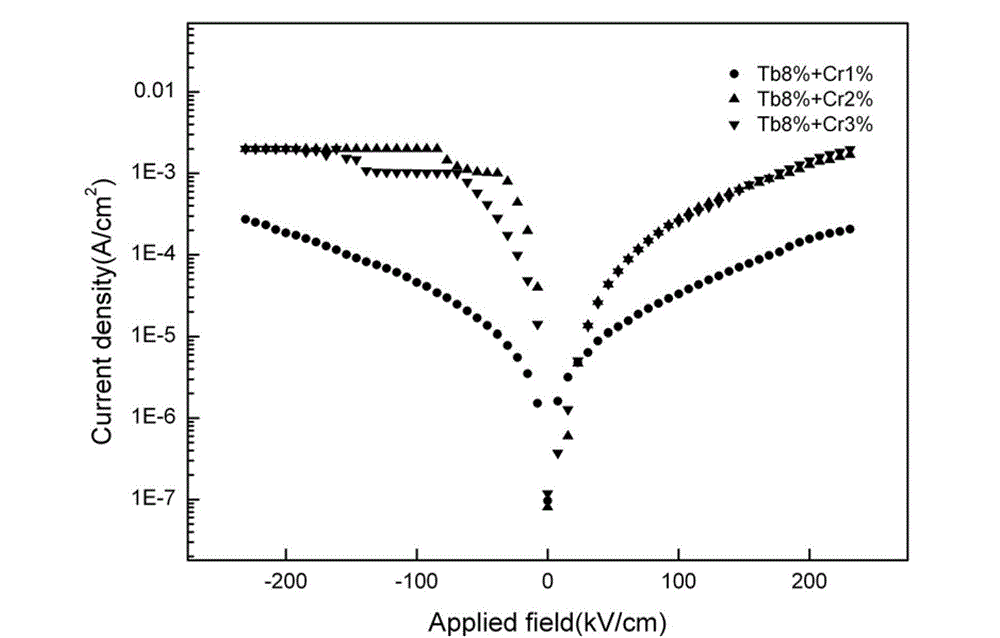
Preparation method of low-leakage current Bi0.92Tb0.08Fe(1-x)CrxO3 film
ActiveCN102976764AImprove multiferroic propertiesImprove electrical performanceAcetic anhydrideLow leakage
The invention provides a preparation method of a low-leakage current Bi0.92Tb0.08Fe(1-x)CrxO3 film. The method comprises the following steps of: dissolving bismuth nitrate, ferric nitrate, terbium nitrate and chromic nitrate at a molar ratio of 0.97:(1-x):0.08:x into the mixed solution of ethylene glycol monomethyl ether and acetic anhydride to form a mixed solution; adding ethanolamine into the mixed solution to adjust the viscosity and the complexing degree to obtain a stable BiFeO3 precursor liquid; and preparing a Tb and Cr co-doped crystalline BiFeO3 film by a spin-coating method and layer-by-layer annealing technology. The film is a crystalline Bi0.92Tb0.08Fe0.99Cr0.01O3 film, wherein the leakage current density is still kept below 10<-4>A / cm<2> in a 150kv / cm test electric field. According to the method provided by the invention, the requirements on equipment are simple, the experimental conditions are easy to realize, the prepared film has good uniformity, and the leakage current of the film is reduced through the co-doping of Tb and Cr.
Owner:SHAANXI UNIV OF SCI & TECH
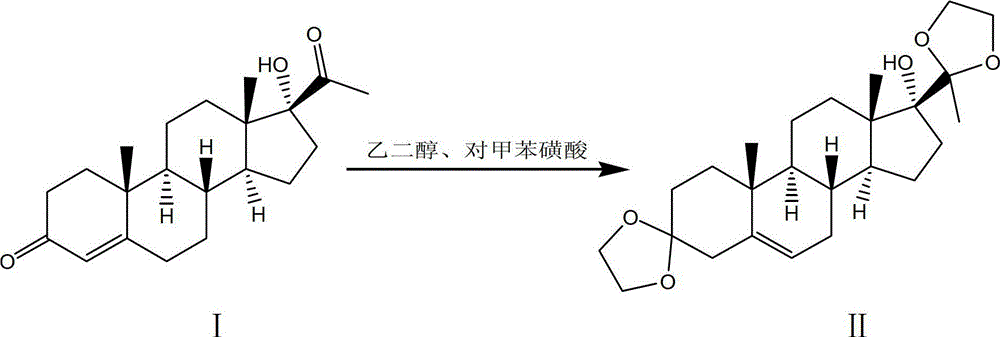
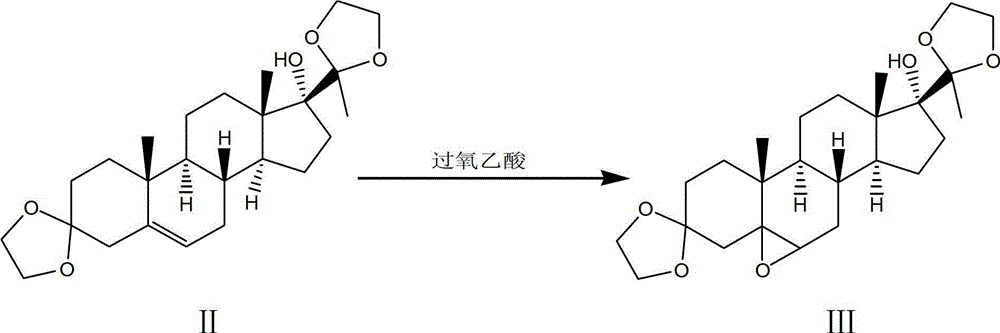
Synthesis method of medroxyprogesterone acetate
The invention discloses a synthesis method of medroxyprogesterone acetate. The method comprises the following steps of: 1) enabling 17 alpha-hydroxyprogesterone and ethylene glycol to perform ketalation under the catalysis of para-toluenesulfonic acid to obtain a ketal; 2) enabling the ketal to perform epoxidation reaction under the action of a peroxyacetic acid solution of anhydrous sodium acetate to obtain an epoxide; 3) enabling the epoxide and methylmagnesium bromide to perform Grignard reaction, and then performing hydrolysis reaction by dilute sulphuric acid to obtain a Grignard matter; 4) performing hydrolysis on the Grignard matter by glacial acetic acid to perform deprotection so as to obtain 5 alpha, 17 alpha-dihydroxy-6 beta-methyl progesterone; 5) performing hydrogenation translocation reaction under the action of hydrogen chloride to obtain 6 alpha-methyl-17 alpha-hydroxyprogesterone; and 6) enabling the 6 alpha-methyl-17 alpha-hydroxyprogesterone to perform acetylation reaction with acetic acid and acetic anhydride so as to obtain the medroxyprogesterone acetate. During the reaction process of the method disclosed by the invention, the use of a precious metal catalyst, namely palladium on carbon is avoided, the cost of auxiliary materials and the recovery cost are greatly reduced, the reaction conditions in each step are mild, the violent heat release and deflation phenomena are avoided, and the safety in production is good.
Owner:BAOJI KANGLE BIOTECH
Preparation method of modified starch biodegradable film
The invention discloses a preparation method of a modified starch biodegradable film and belongs to the technical field of environmental science. The preparation method includes: using sweet potato starch as a raw material, crosslinking with epoxy polypropylene as a crosslinking agent to obtain crosslinked starch, esterifying the crosslinked starch with acetic anhydride to obtain crosslinked esterified starch, adding deionized water, an enhancer polyvinyl alcohol, a water repellent urea, a crosslinking agent glyoxal and a plasticizer glycerol into the crosslinked esterified starch, and subjecting the crosslinked esterified starch to swelling, pasting and crosslinking reaction to obtain a substance of certain viscosity, spreading the substance to polyester flakes, drying, and taking down a film to obtain the modified starch biodegradable film. The film prepared herein has good biodegradability, is free of environmental pollution, good in water resistance and high in stability, and is good in water tolerance and high in mechanical strength.
Owner:陈建峰
Preparation method of biodegradable cellulose acetate film
The present invention relates to preparation process of biodegradable cellulose acetate film. Ramie cellulose is swelled first with water and then with glacial acetic acid, and reacted with acetic anhydride under catalysis of sulfuric acid to obtain yellow cellulose acetate gel. The obtained cellulose acetate gel is dissolved in acetone, and the solution is filtered and added with different kinds of plasticizer to compound film casting liquid. The film casting liquid is cast in mold to form cellulose acetate film of certain thickness, and after volatilizing the solvent, the cellulose acetate film is separated from the mold in a water bath. The biodegradable cellulose acetate film may be used in preserving soil moisture and may be biodegraded without destroying soil structure and polluting environment.
Owner:SHAANXI NORMAL UNIV
Preparing method for electrostatic spinning lignin/cellulose acetate nanometer composite material
InactiveCN105040146ARealize comprehensive utilizationImprove adsorption capacityConjugated cellulose/protein artificial filamentsNon-woven fabricsCellulose acetateAcetic oxide
The invention relates to a preparing method for an electrostatic spinning lignin / cellulose acetate nanometer composite material and belongs to the field of adsorption material preparation. According to the method, barnyard grass straw is used as a raw material, pretreatment such as smashing and activating is carried out on the barnyard grass straw to remove lignin on the surface of the straw, acetic acid is used as solvent, acetic oxide is used as an acetylation agent, sulfuric acid is used as a catalyst for carrying out acetylation treatment on the barnyard grass straw, then solvent extraction is used for separation to obtain cellulose acetate, the cellulose acetate is mixed with the lignin, the acetic acid and tetrahydrofuran uniformly to prepare a spinning solution, and a high-voltage static spinning device carries out spinning and curing on the spinning solution to obtain the electrostatic spinning lignin / cellulose acetate nanometer composite material finally. Harmful plant barnyard grass is utilized as the raw material, waste is utilized, and the prepared composite material is excellent in adsorption performance, biodegradable and free from pollution to environment.
Owner:JIANGSU JINYU ENVIRONMENTAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com