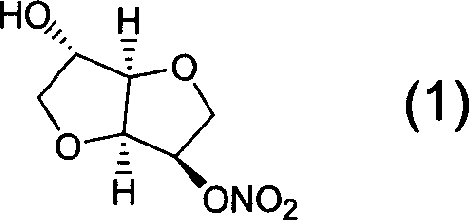
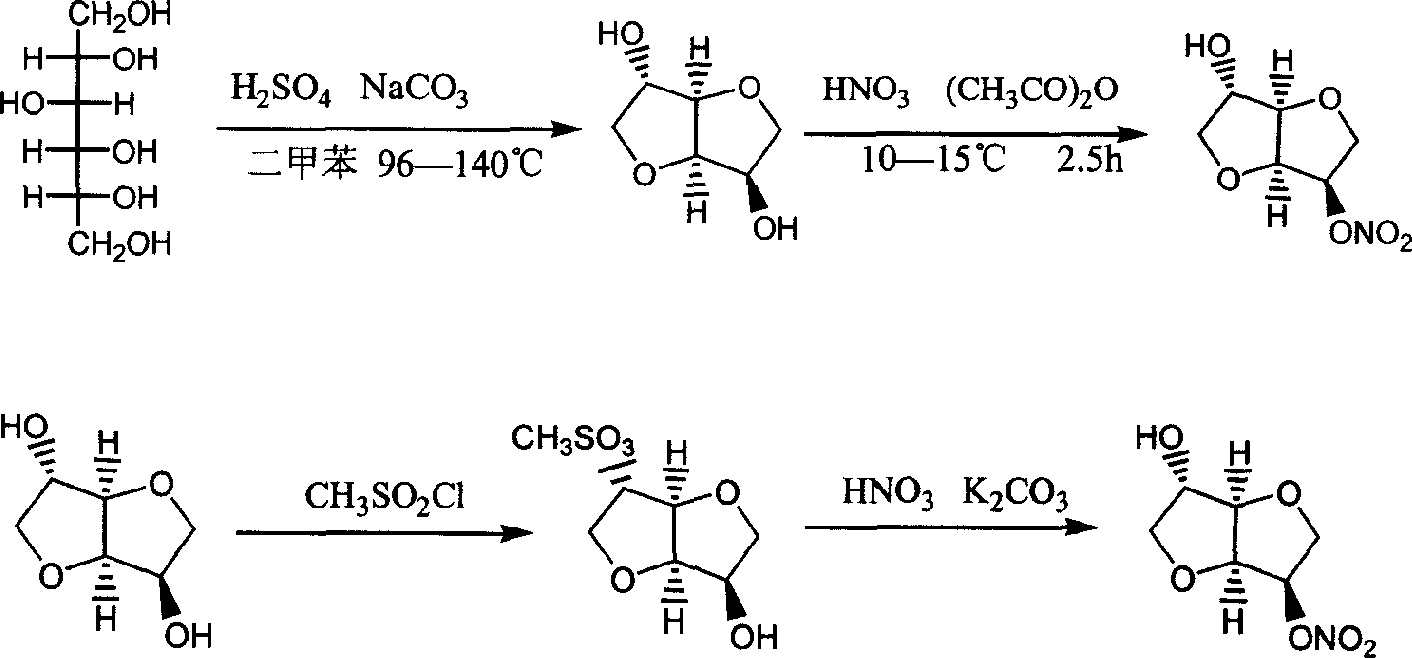
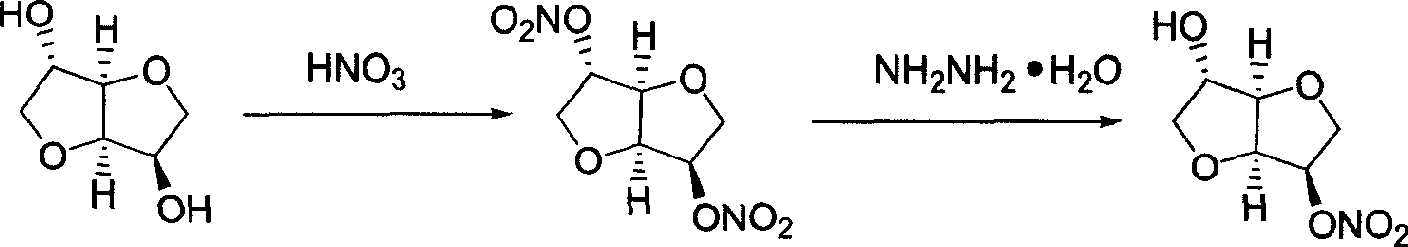
Preparation process of isosorbide mononitrate
A technology of nitric acid and sorbitol, which is applied in the fields of cardiovascular system diseases, organic chemistry, drug combination, etc., and can solve problems such as complex, difficult to control reactions, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Synthesis of intermediate 1, 4:3,6-dianhydro-D-sorbitol: 310 grams of 70% sorbitol were slowly added to 9.5 milliliters of p-toluenesulfonic acid at 40 ° C under stirring, and then the Slowly raise the temperature to evaporate the water under a certain vacuum, and the water will be basically evaporated when it rises to about 117°C. The vacuum is 0.096Mpa, and the sorbitan is collected below 220°C, with a yield of 91%.
[0019]
example 2
[0021] Preparation of compound (4): Add 100 grams of sorbitan to 180 milliliters of acetic acid, stir and heat up to dissolve, then add 5 grams of N,N-dimethylaminopyridine, stir at 30°C for 10 minutes, then add acetic anhydride dropwise at 45°C, and the addition is complete After that, continue to keep warm at 45°C for 30 minutes, drop to 20°C, stir and keep warm for 2 hours, then filter to obtain solid compound (4). Yield 95%.
[0022]
example 3
[0024] Preparation of nitrating agent: After stirring and mixing 38ml of acetic anhydride and 32ml of acetic acid, slowly add 14ml of nitric acid dropwise, keep the temperature at 15°C-20°C during the dropwise addition, and keep warm at 15°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com