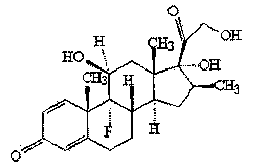
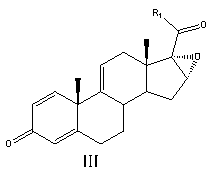
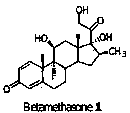
Preparation method of betamethasone intermediate
A technology of betamethasone and intermediates, which is applied in the field of preparation of steroid compounds, can solve the problems of long steps and low yield, and achieve the effects of reducing side reactions, improving yield and quality, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] sulfonation elimination reaction
[0043] Add 50 ml of acetone and 200 ml of pyridine, stir, nitrogen protection, then add 50 g of compound II (16a,17-epoxy-11a-hydroxyl-1,4-pregnadiene-3,20-dione), cool down To below -10°C, add 80 ml of methanesulfonyl chloride dropwise, and keep the temperature of the reaction solution not exceeding -10°C during the whole dropping process. After the dropwise addition, react at a constant temperature of -10°C for 5 hours. After the reaction is complete, slowly add the reaction solution dropwise to a mixed solution consisting of 1000 ml of water and 200 ml of concentrated hydrochloric acid. After the dropwise addition, keep the temperature at 0°C Stir for 4 hours, filter to obtain a off-white solid, put it into 300 ml of acetic acid, then add 60 g of potassium acetate and 3 g of magnesium chloride and heat up to 80 ° C for 2 h, after the reaction is complete, slowly add the reaction solution dropwise to 1200 ml of water, Stir for 1 h, ...
Embodiment 2
[0051] sulfonation elimination reaction
[0052] Add 80 ml n-butyl ether and 25 g imidazole, stir, nitrogen protection, then add 50 g compound II, the structural formula is
[0053]
[0054] Among them, R is β-OH; R 1 for CH 2 COOCH 2 CH 3 ;
[0055] Cool down to below 20°C, add 30 g of p-toluenesulfonyl chloride, and keep the temperature of the reaction solution not exceeding 20°C during the whole adding process. After the addition, react at a constant temperature of 10°C for 7 hours. After the reaction is complete, slowly add the reaction solution dropwise to a mixed solution composed of 1000 ml of water and 200 ml of concentrated hydrochloric acid. After the dropwise addition, stir at a constant temperature of 0°C for 4 hours. hour, filter off-white solid, put it into 300 ml of formic acid, then drop into 50 g of sodium carbonate and 3.3 g of calcium chloride and heat up to 60 ° C for 4 h, after the reaction is complete, slowly add the reaction solution dropwise to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com