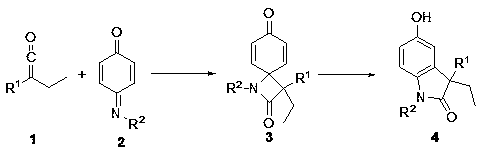
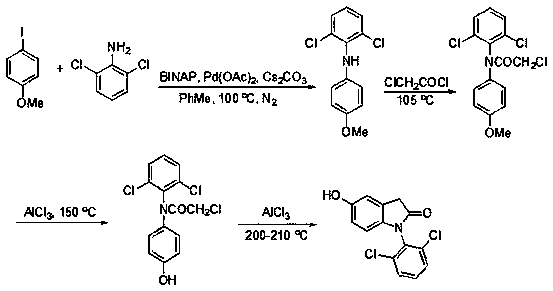
A kind of synthetic method of 3-ethyl-5-hydroxyl-1,3-diarylindolinone
A technology of aryl ethyl ketene and hydroxyl group, applied in the field of synthesizing 3-ethyl-5-hydroxyl-1, can solve the problems of long reaction time, harsh conditions and high temperature, and achieves simple method, efficient catalytic reaction, atomic High utilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of 3-ethyl-5-hydroxy-1,3-diphenylindolinone
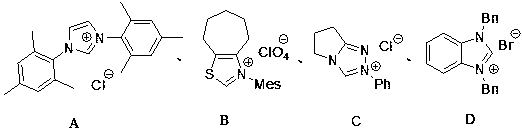
[0023] Add nitrogen heterocyclic carbene catalyst A 1,3-bis-2,4,6-trimethylphenyl-imidazole hydrochloride (13.6 mg, 0.04 mmol) and cesium carbonate (9.8 mg, 0.03 mmol) into the Schlenk bottle , replace the nitrogen three times, add 1 mL of diethyl ether (aluminum lithium hydrogen distilled to remove water), stir at room temperature for 5-10 minutes, and N -Phenyliminoquinone (36.6 mg, 0.2 mmol) and phenyl ethyl ketene (58.0 mg, 0.4 mmol) were added to the reaction system, and reacted at room temperature for 30 minutes, monitored by TLC plate, the reaction was complete, and boron trifluoride ether was added (5.0 μL, 0.04 mmol), after continuing to react for 30 minutes, remove the solvent under reduced pressure, and separate by column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain 3-ethyl-5-hydroxyl-1, 3-Diphenylindolinone (64.0 mg, 97%). 1 H NMR (400 MHz, Acetone- d 6 ) δ 8.23 (s, 1H), 7.63 ...
Embodiment 2
[0025] Preparation of 3-ethyl-5-hydroxy-1,3-diphenylindolinone
[0026] The nitrogen heterocyclic carbene catalyst C was used as the catalyst, and the rest of the operations were the same as in Example 1, and the product 4 was finally obtained with a yield of 54%.
Embodiment 3
[0028] Preparation of 3-ethyl-5-hydroxy-1,3-diphenylindolinone
[0029] The nitrogen heterocyclic carbene catalyst D was used as the catalyst, and the rest of the operations were the same as in Example 1, and the product 4 was finally obtained in a yield of 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com