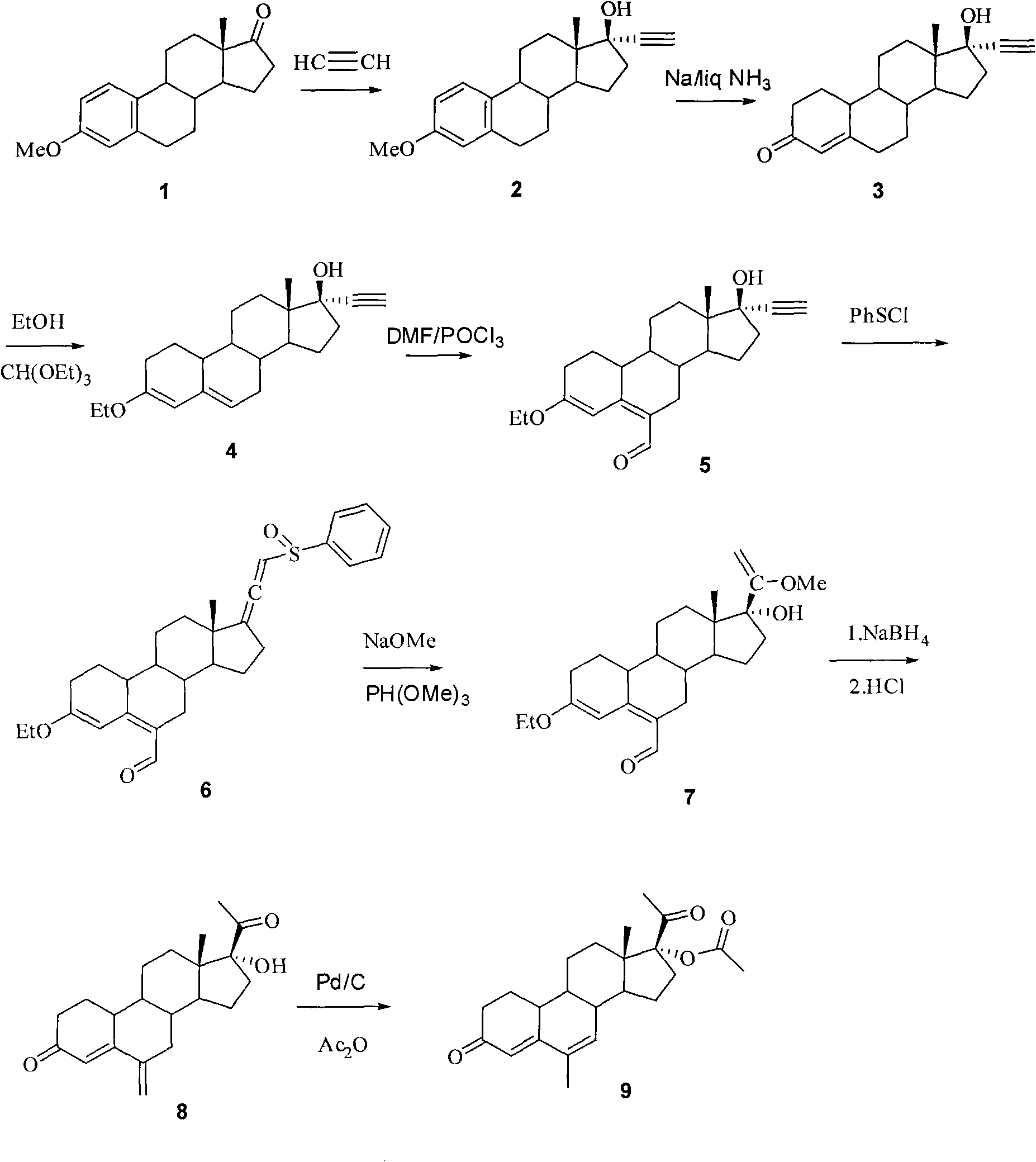
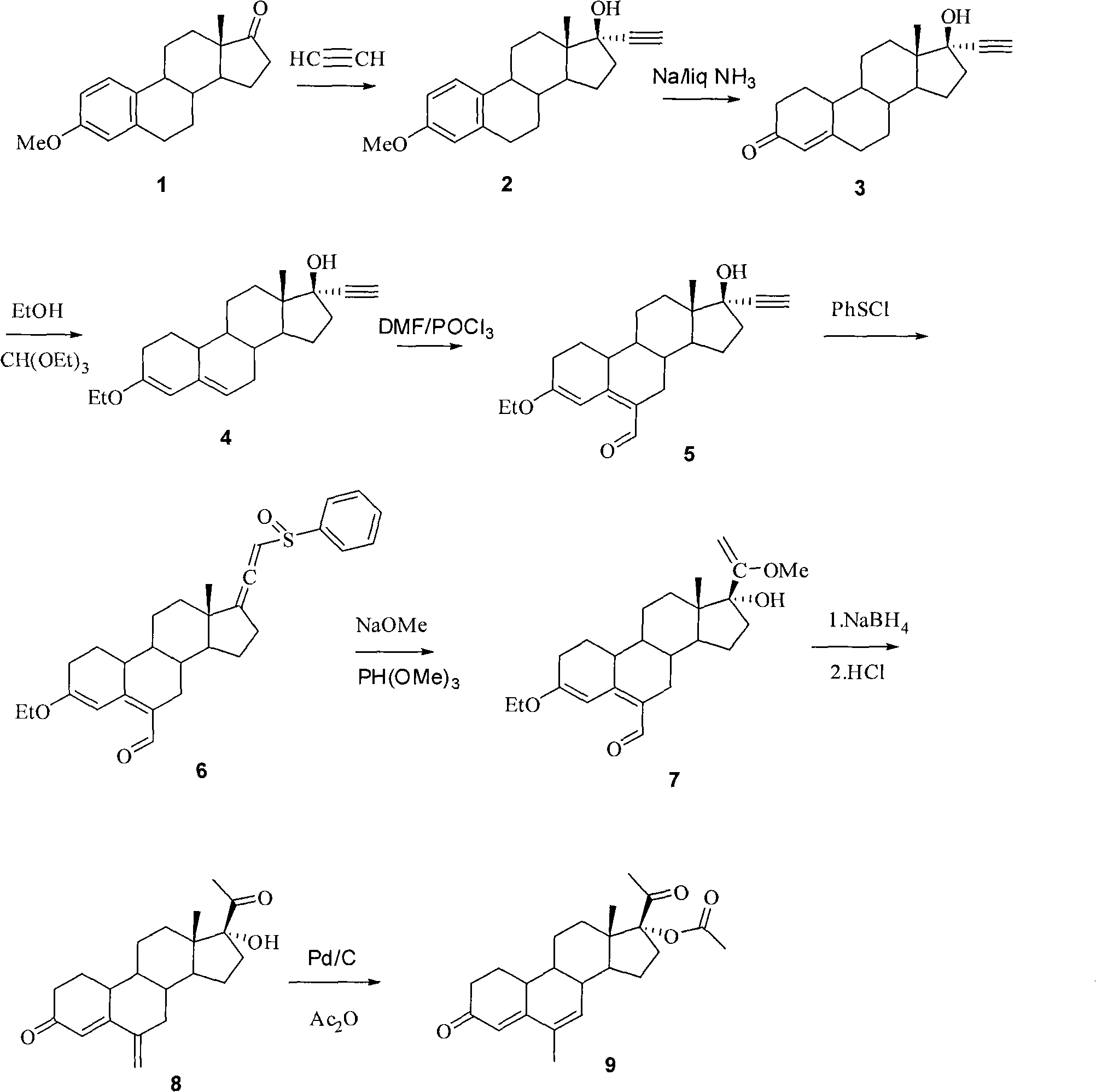
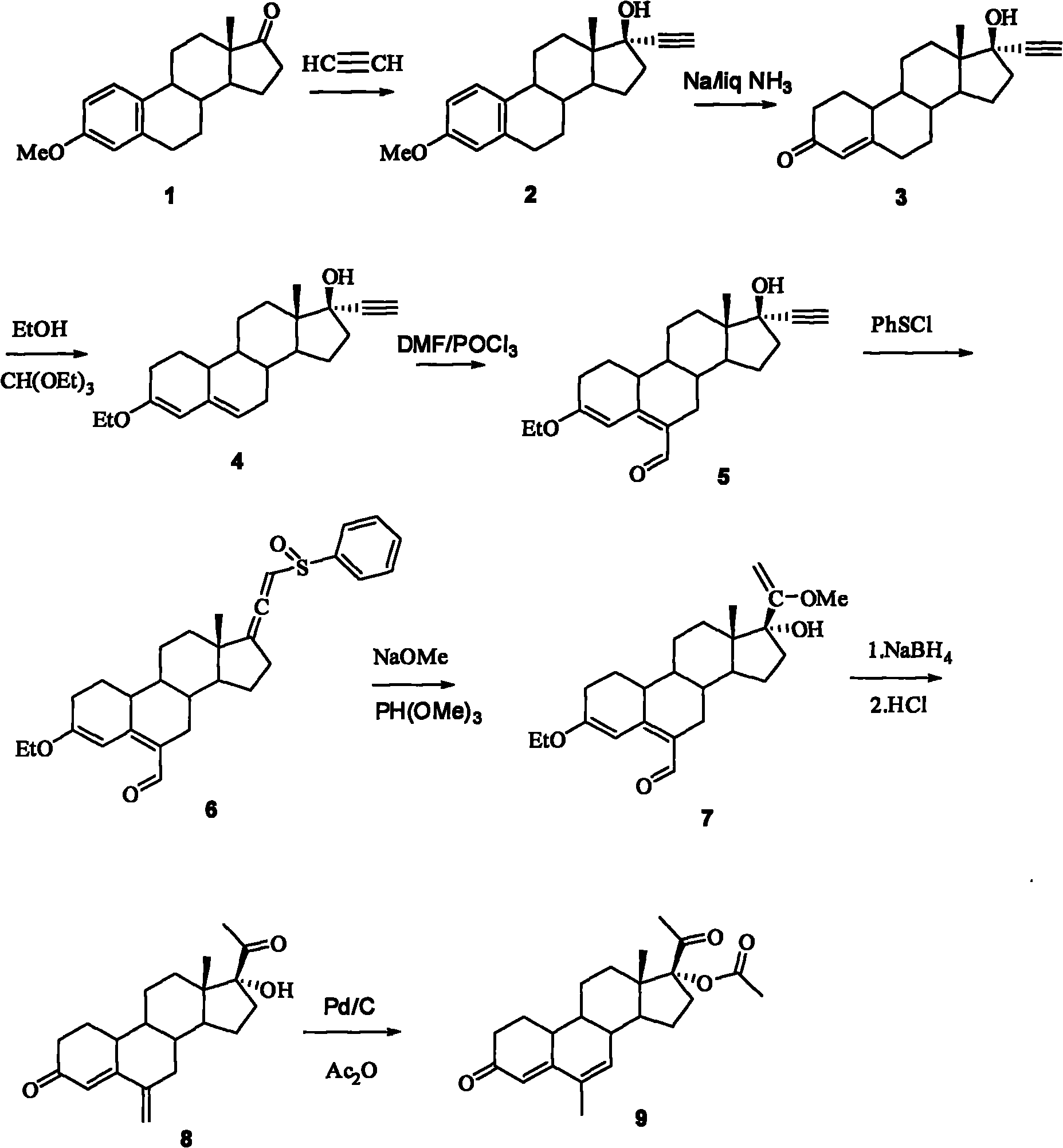
Method for synthesizing 6-methyl-17alpha-acetoxyl-19-norpregnane-4,6-diene-3,20-diketone
A technology of acetoxyl and norpregnant, applied in the field of drug synthesis, can solve problems such as difficulty in obtaining, and achieve the effects of simplified operation, reduced production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. 17α-ethynylestradiol-3-methyl ether (2)
[0022] Put 60 grams of potassium tert-butoxide powder into a 3-liter reaction flask, add THF (1.4 L), pass acetylene gas into the reaction flask until saturated, cool to 0° C., and pass acetylene gas for 30 minutes. Add estrone alcohol-3-methyl ether (100g, 0.35mol), and stir the reaction solution at room temperature for 1 hour, add saturated aqueous ammonium chloride solution, separate the organic layer, extract the aqueous layer with tetrahydrofuran, combine the organic phases, and wash with saturated chlorine Wash with sodium chloride water, concentrate part of the organic solvent, pour it into ice-water, filter the precipitated solid, and obtain 105 g of 17α-ethynylestradiol-3-methyl ether after drying, the yield is 97%, and TLC shows one point, It can be directly used in the next reaction.
[0023] 2. 17α-ethynyl-17β-hydroxy-19-desmethylandrost-4-en-3-one (3)
[0024] Under the cooling of dry ice-acetone, 2kg of liquid...
Embodiment 2
[0036] 1. 17α-ethynylestradiol-3-methyl ether (2)
[0037] Put 60 grams of potassium tert-butoxide powder into a 3-liter reaction flask, add THF (1.4 L), pass acetylene gas into the reaction flask until saturated, cool to -10°C, and pass acetylene gas for 30 minutes , add estrone alcohol-3-methyl ether (100g, 0.35mol), the reaction solution was stirred at room temperature for 1 hour, added saturated ammonium chloride aqueous solution, the organic layer was separated, the aqueous layer was extracted with tetrahydrofuran, the combined organic phase was washed with saturated Wash with sodium chloride water, concentrate part of the organic solvent, pour into ice-water, filter the precipitated solid, and obtain 105 g of 17α-ethynylestradiol-3-methyl ether after drying, the yield is 96%, and TLC shows one point , which can be directly used in the next reaction.
[0038] 2. 17α-ethynyl-17β-hydroxy-19-desmethylandrost-4-en-3-one (3)
Embodiment 3
[0051] 1. 17α-ethynylestradiol-3-methyl ether (2)
[0052] Put 60 grams of potassium tert-butoxide powder into a 3-liter reaction flask, add THF (1.4 L), pass acetylene gas into the reaction flask until saturated, cool to -5°C, and then pass acetylene gas for 30 minutes , adding estrone alcohol-3-methyl ether (100g, 0.35mol), the reaction solution was stirred at room temperature for 1 hour, adding saturated aqueous ammonium chloride solution, the organic layer was separated, the aqueous layer was extracted with tetrahydrofuran, the combined organic phase was washed with saturated After washing with sodium chloride water, after concentrating part of the organic solvent, pour it into ice-water, filter the precipitated solid, and obtain 105 grams of 17α-ethynylestradiol-3-methyl ether after drying, the yield is 98%, and TLC shows one point , which can be directly used in the next reaction.
[0053] 2. 17α-ethynyl-17β-hydroxy-19-desmethylandrost-4-en-3-one (3)
[0054] Under the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com