Synthesis method of medroxyprogesterone acetate
The technology of a methadone and a synthetic method, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of high cost and low yield, and achieve the effects of reducing processing cost, good production safety, and easy recycling and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
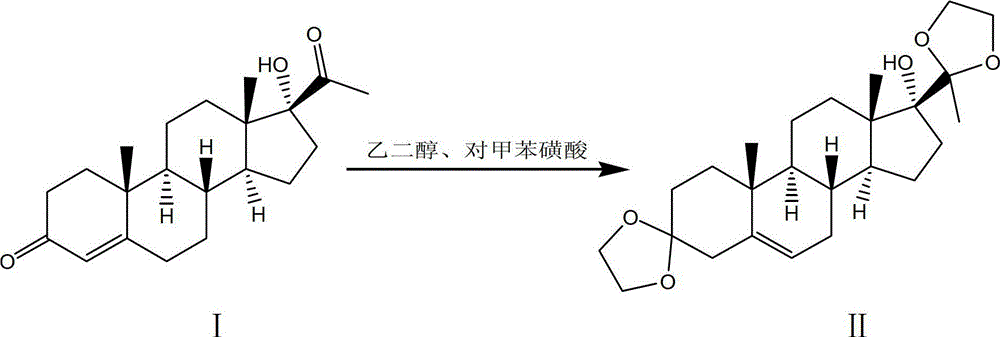
[0052] Step 1. Using 17α-hydroxyprogesterone as a raw material, react with ethylene glycol under the catalysis of p-toluenesulfonic acid to obtain a ketal product through ketal reaction:
[0053] 101. In a clean 2000L enamel reaction kettle, pump in 1150kg of fresh benzene, 440kg of ethylene glycol, put in 50kg of 17α-hydroxy progesterone, stir, azeotropically distill off water for 1.5h, then add 2.2kg of toluenesulfonic acid into the reaction kettle , continue azeotropic distillation to remove water for 1 hour, and reflux at 100°C for 9 hours; then add 7.5kg of pyridine to the reactor to terminate the reaction, let it stand after cooling, discard the upper solution, and use 500kg of carbonic acid with a mass percentage concentration of 5% for the lower solution After washing with sodium hydrogen solution, wash with water until neutral;
[0054] 102. Concentrate under reduced pressure the lower layer solution washed to neutral in 101 to obtain a concentrated solution, add 400k...
Embodiment 2
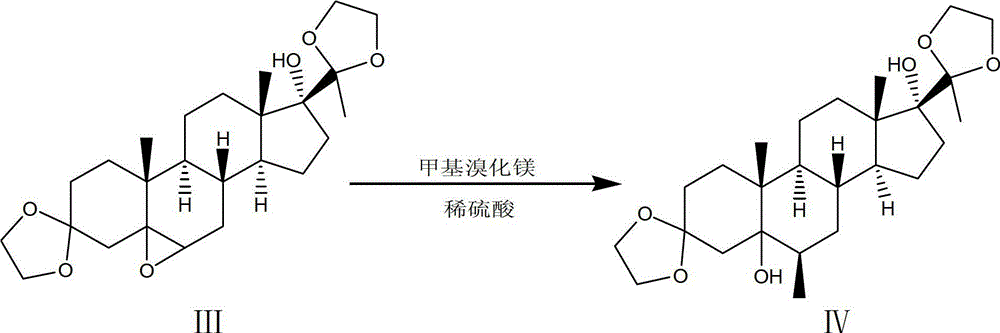
[0073] Step 1. Using 17α-hydroxyprogesterone as a raw material, react with ethylene glycol under the catalysis of p-toluenesulfonic acid to obtain a ketal product through ketal reaction:
[0074] 101. In a clean 2000L enamel reaction kettle, pump in 1150kg of fresh benzene, 400kg of ethylene glycol, put in 50kg of 17α-hydroxyprogesterone, stir, azeotropically distill off water for 1 hour, add 2kg of toluenesulfonic acid into the reaction kettle, continue Azeotropic distillation to remove water for 1.5h, reflux reaction at 110°C for 8h; then add 5kg of pyridine to the reaction kettle to terminate the reaction, let it stand after cooling, discard the upper layer solution, and use 400kg mass percentage concentration of 7% sodium bicarbonate for the lower layer solution After washing with the solution, wash with water until neutral;
[0075] 102. Concentrate under reduced pressure the lower layer solution washed to neutral in 101 to obtain a concentrated solution, add 300kg of met...
Embodiment 3
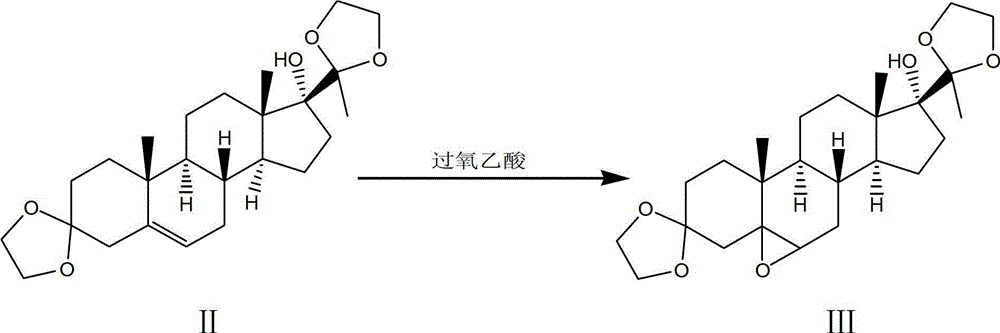
[0094] Step 1, using 17α-hydroxyprogesterone as a raw material, reacting with ethylene glycol through ketal reaction under the catalysis of p-toluenesulfonic acid to obtain a ketal product:
[0095] 101. In a clean 2000L enamel reaction kettle, pump in 1150kg of fresh benzene, 500kg of ethylene glycol, put in 50kg of 17α-hydroxyprogesterone, stir, azeotropically distill off water for 2 hours, then add 2.5kg of toluenesulfonic acid into the reaction kettle, Continue azeotropic distillation to remove water for 0.5h, and reflux reaction at 110°C for 8h; then add 10kg of pyridine to the reaction kettle to terminate the reaction, let it stand after cooling, discard the upper layer solution, and use 600kg mass percentage concentration of 3% hydrogen carbonate for the lower layer solution After washing with sodium solution, wash with water until neutral;
[0096] 102. Concentrate under reduced pressure the lower layer solution washed in 101 to neutrality to obtain a concentrated solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com