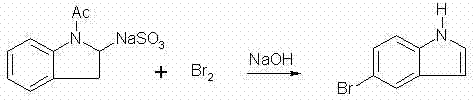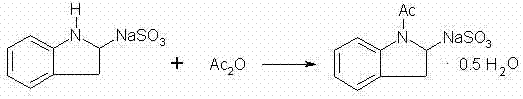Method for preparing 5-bromoindole
A technology of bromoindole and indole, which is applied in the field of preparing 5-bromoindole, can solve the problems of high cost, large solvent consumption, and low atom economy, and achieve the effects of convenient operation, high product quality, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
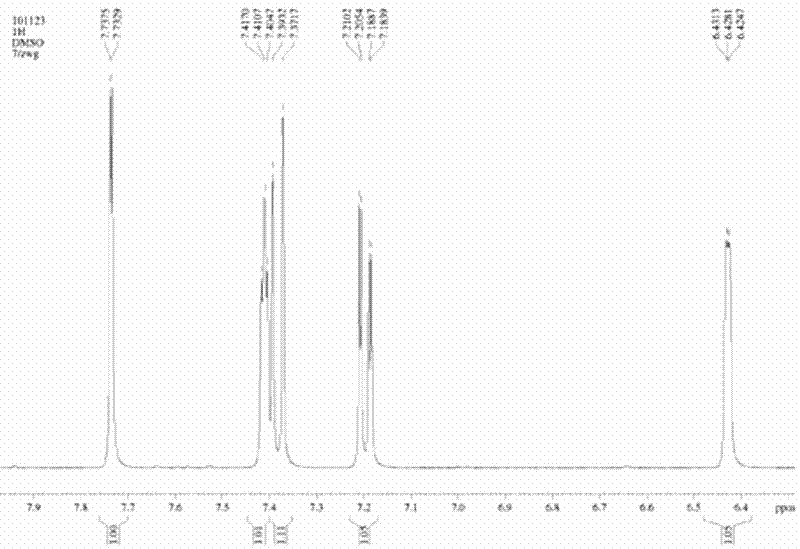
[0024] A method for preparing 5-bromoindole, comprising the steps of:
[0025] ①Synthesis of 2-sodium sulfonate-indole: Dissolve 7.2g of indole in 80ml of ethanol, then add 52g of sodium bisulfite aqueous solution with a mass concentration of 27% (it is advisable to complete the dropwise addition within 1.5h), at 28°C After 18 hours of reaction, the reaction solution was filtered, washed, and dried to obtain 13.2 g of light blue intermediate I (the intermediate I was 2-sodium sulfonate-indole);
[0026] ②Synthesis of 2-sodium sulfonate-1-acetylindole: take 6g of intermediate I and 23g of acetic anhydride and stir and mix, then raise the temperature to 70°C for 2.5h, then add 8g of ethyl acetate to continue the reaction for 30min, cool down after the reaction To room temperature, the reaction solution was filtered, washed, and dried to obtain 5.5 g of white powder intermediate II (the intermediate II is 2-sodium sulfonate-1-acetylindole);
[0027] ③Synthesis of 5-bromoindole: ...
Embodiment 2
[0029] A method for preparing 5-bromoindole, comprising the steps of:
[0030] ① Dissolve 7.2g of indole in 86ml of isopropanol, then add dropwise 64g of potassium bisulfite aqueous solution with a mass concentration of 22%, and react at 25°C for 20h. After the reaction, the reaction solution is filtered, washed, and dried to obtain 12.4g of shallow Blue Intermediate I;
[0031] ② Stir and mix 6g of intermediate I and 27g of acetic anhydride, then raise the temperature to 73°C for 3 hours, then add 9g of ethyl propionate to continue the reaction for 1 hour, after the reaction is completed, cool to room temperature, and the reaction solution is filtered, washed and dried to obtain 5.4g White powder intermediate II;
[0032] ③Dissolve 4.8g of intermediate II in 40g of water, add 8.6g of bromine dropwise at 0°C, and keep the reaction for 1.5h, then rise to room temperature, and continue the reaction for 1h, then add 22g of 5.4% potassium bisulfite aqueous solution After reactin...
Embodiment 3
[0034] A method for preparing 5-bromoindole, comprising the steps of:
[0035] ①Dissolve 7.2g of indole in a mixture of 40ml of ethanol and 35ml of methanol, then add dropwise 64g of potassium bisulfite aqueous solution with a mass concentration of 22%, and react at 25°C for 20h. After the reaction, the reaction solution is filtered, washed, and dried 13.4g light blue intermediate I was obtained;
[0036] ② Stir and mix 6g of intermediate I and 28g of acetic anhydride, then raise the temperature to 68°C and react for 3h, then add 9g of benzene to continue the reaction for 1h, after the reaction is completed, cool to room temperature, and the reaction solution is filtered, washed, and dried to obtain 5.5g of white powder Intermediate II;
[0037] ③ Dissolve 4.8g of intermediate II in 42g of water, add 7.7g of bromine dropwise at 0°C, and keep the reaction for 1.5h, then rise to room temperature, continue the reaction for 1h, and then add 20g of 7% potassium bisulfite aqueous s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com