High selectivity method for synthesizing moxifloxacin
A technology of moxifloxacin and high selectivity, which is applied in the field of compound synthesis technology, can solve problems affecting product purity and yield, unsuitable for industrial production, and unfavorable for industrial production, so as to avoid difficult-to-separate impurities and high selectivity , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
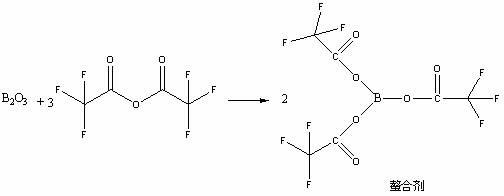
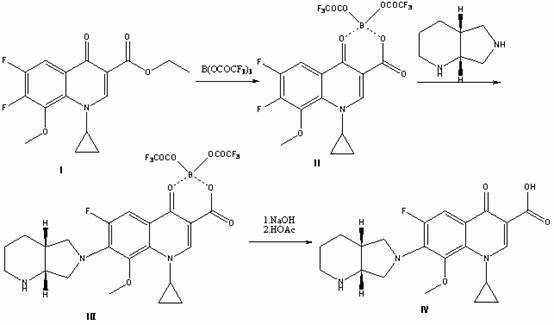
[0015] Add 69.0 g of boric anhydride and 630.0 g of trifluoroacetic anhydride in a 2000 ml three-necked flask, react at 35°C for 10 hours to obtain a thick slurry, directly add 161.1 g of 1-cyclopropyl -Ethyl 6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylate (I), react at 110°C for 2 hours, cool to room temperature, add Ice water, precipitated solid, filtered with suction, washed the filter cake with water until neutral, dried to obtain white powder 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3 - Methyl carboxylate trifluoroacetic anhydride boride chelate (Ⅱ) 231.0g, yield 87.0%;
[0016] Add 500ml of acetone to a 2000ml three-necked flask, add 231.0g of 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl trifluoroacetic anhydride Boronated chelate (Ⅱ); add 184.1 g (S,S)-2,8-diazabicyclo[4.3.0]nonane; react at 0°C for 10 hours to obtain 1-cyclopropyl-6 -Fluoro-7-([S,S]-2,8-diazabicyclo[4.3.0]nonan-8-methoxy-4-oxo-1,4-dihydro-...
Embodiment 2
[0022] Add 69.0 g of boric anhydride and 840.0 g of trifluoroacetic anhydride in a 2000 ml three-necked flask, react at 40°C for 5 hours to obtain a thick slurry, directly add 323.1 g of 1-cyclopropyl -Ethyl 6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylate (I), reacted at 80°C for 3 hours, cooled to room temperature, added Ice water, precipitated solid, filtered with suction, washed the filter cake with water until neutral, dried to obtain white powder 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3 - Methyl carboxylate trifluoroacetic anhydride boride chelate (II) 500.5g, yield 94.3%;
[0023] In a 2000 ml three-necked flask, add 500ml of acetonitrile, add 500.5 g of 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl trifluoroacetic anhydride Boronated chelate (Ⅱ); add 132.5 g (S,S)-2,8-diazabicyclo[4.3.0]nonane; react at -10°C for 24 hours to obtain 1-cyclopropyl- 6-fluoro-7-([S,S]-2,8-diazabicyclo[4.3.0]nonan-8-methoxy-4-o...
Embodiment 3
[0025] Add 69.0 g of boric anhydride and 700.0 g of trifluoroacetic anhydride in a 2000 ml three-necked flask, react at 40°C for 8 hours to obtain a thick slurry, directly add 300.0 g of 1-cyclopropane to the slurry Ethyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylate (I), reacted at 90°C for 3 hours, cooled to room temperature, Add ice water, precipitate solid, filter with suction, wash the filter cake with water until neutral, and dry to obtain white powder 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline- 3-Carboxylate methyl trifluoroacetic anhydride boride chelate (Ⅱ) 473.5g, yield 96.1%;
[0026] Add 500ml N,N'-dimethylformamide to a 2000ml three-neck flask, add 473.5g1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3 -Methyl carboxylate trifluoroacetic anhydride borate chelate (Ⅱ); add 251.0 g (S,S)-2,8-diazabicyclo[4.3.0]nonane; react at -10°C for 18 hours, 1-cyclopropyl-6-fluoro-7-([S,S]-2,8-diazabicyclo[4.3.0]nonan-8-methoxy-4-oxo-1, Ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com