Patents
Literature
195 results about "Moxifloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Moxifloxacin is used to treat a variety of bacterial infections.
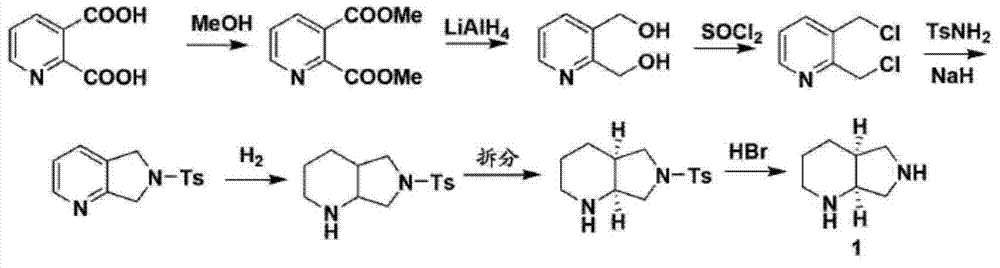
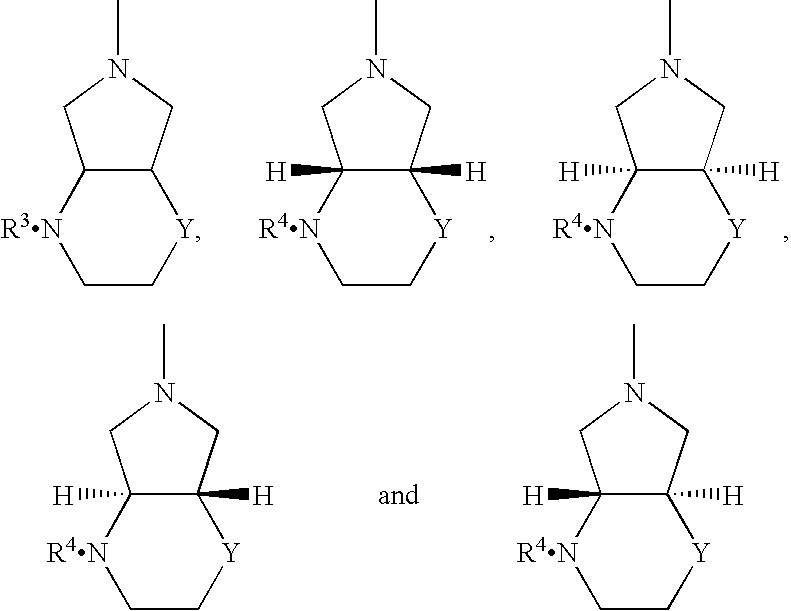
Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof
The invention discloses a method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and a chiral isomer thereof. The method comprises the steps of: taking 8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane or (1S,6R)-8-benzyl-7,9-dioxo2,8-diazabicyclo[4,3,0]nonane as a raw material, and adopting a metal borohydride / BF3 reduction system for reduction to obtain a corresponding product, namely the 8-benzyl-2,8-diazabicyclo[4,3,0]nonane or (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane. The method adopts the metal borohydride / BF3 reduction system for reduction, avoids the use of an expensive and dangerous reagent, namely lithium aluminum hydride, reduces production cost, improves the safety of the operation, and provides a safe and economical production method for industrial mass production of a moxifloxacin intermediate, namely the (S,S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
Moxifloxacin aqueous solution type injection
InactiveCN101732246AGood water solubilityImprove stabilityAntibacterial agentsOrganic active ingredientsO-Phosphoric AcidMedicine
The invention discloses a moxifloxacin aqueous solution type injection which contains moxifloxacin or pharmaceutically acceptable salt, weak acid sodium salt or phosphoric acid sodium salt and water for injection, wherein the content of the moxifloxacin is 0.8-4 percent (g / ml), and the molar concentration of the weak acid sodium salt or the phosphoric acid sodium salt is 0.0002-1mol / L. The moxifloxacin aqueous solution type injection product has strong dissolubility, human body acceptable pH value, easier control of product quality, stability in a storage period, good compatibility with clinical common isotonic solution, low production cost, small volume and convenient transportation and storage.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Freeze dry powdered injection of moxlfloxacin or its salts and preparation process thereof
InactiveCN1729978AQuality improvementImprove stabilityAntibacterial agentsOrganic active ingredientsFreeze-dryingMoxifloxacin
The invention provides a moxfloxacin for injection or freeze dried powder injection of its salts, and its preparation process, which is prepared from moxifloxacin or its salts, excipient, pH modifier and water for injection through freeze drying.
Owner:吴祥根
Oral medicinal formulation of moxifloxacin and its preparation method
ActiveCN1762357APromote dissolutionDissolution unchangedAntibacterial agentsOrganic active ingredientsCarboxymethyl cellulosePolyethylene glycol
The invention relates to an oral medicinal formulation of moxifloxacin and its preparation method, which comprises Moxifloxacin or its salt and / or its hydrate, and at least one film forming material for preparing intermediate particles of the preparation selected from hydroxy propyl methyl cellulose, hydroxy ethyl methyl cellulose, cellulose methyl, hydroxy propyl cellulose, hydroxy ethyl cellulose, sodium carboxymethyl cellulose, polyacrylic resins, polyethylene glycol, polyvinyl pyrrolidon-vinyl acetate copolymer, polyvinyl alcohol-polyethylene glycol grafted copolymer, carbopol, gelatine, poloxamer and polyvinyl pyrrolidon. The invention also discloses the process for preparing the pharmaceutical preparation.
Owner:JIANGSU TIANYISHI PHARMA
Moxifloxacin oral preparation and preparation method thereof
ActiveCN101890169ALow priceEasy to buyAntibacterial agentsOrganic active ingredientsMedicineMoxifloxacin
The invention relates to an oral pharmaceutical preparation containing moxifloxacin, salts and / or hydrates of the moxifloxacin, soluble starch and pregelatinized starch. The preparation is characterized by containing 2.9%-14.5% of soluble starch and 1.4%-6.5% of pregelatinized starch, wherein all percents are calculated on the basis of the weight of the pharmaceutical preparation. The invention also relates to a preparation method of the preparation and an application of the preparation for treating or preventing bacterial infection of people or animals.
Owner:JIANGSU YABANG QIANGSHENG PHARMA
Freeze dry powdered injection of moxfloxacin or its salts and preparation process thereof
InactiveCN1729977AQuality improvementImprove stabilityAntibacterial agentsOrganic active ingredientsFreeze-dryingMoxifloxacin
The invention provides a moxfloxacin for injection or freeze dried powder injection of its salts, and its preparation process, which is prepared from moxifloxacin or its salts, excipient, pH modifier and water for injection through freeze drying.
Owner:吴祥根
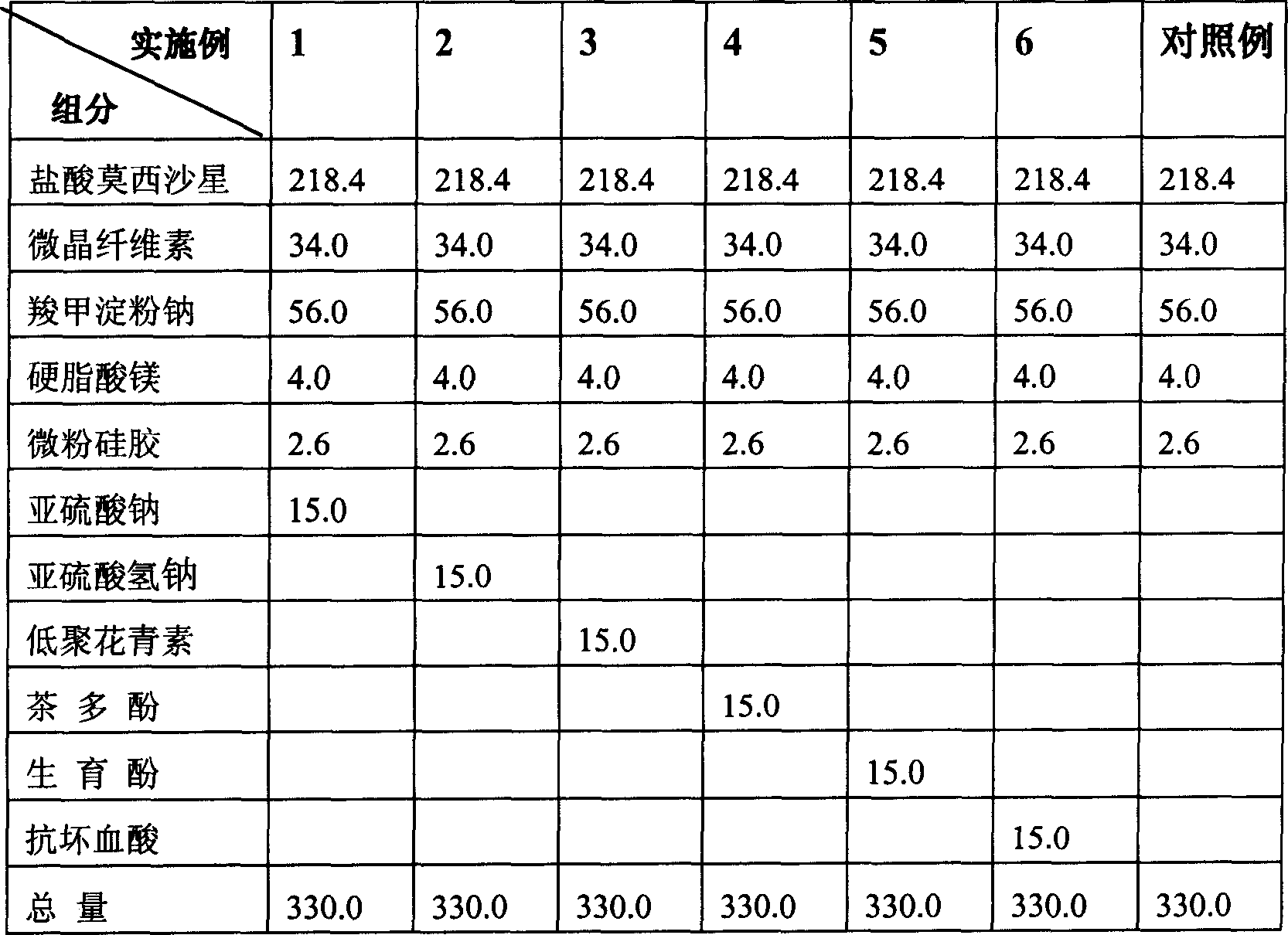
Moxifloxaci gelatin capsule and preparation process thereof
ActiveCN1736379APlay an inhibitory roleEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsBeta-CaroteneGreen Tea Polyphenols
The invention discloses a moxifloxacin gelatin capsule, whose contents comprises moxifloxacin or its salts and / or its hydrate and additive, the additive is at least one selected from the following substances: sodium sulfite, sodium acid sulfite, low-molecular proanthocyanidin, L-glutathion, sesame polyphenols, green tea polyphenols, tocopherol, ascorbic acid, isoascorbic acid, vitamin B1, vitamin B2, beta-carotene, soybean isoflavones, L-sulfo-aminolactic acid, pyrophosphoric acid, polyphosphoric acid and their medicinal salts.
Owner:JIANGSU TIANYISHI PHARMA
Compositions for treatment of ear infections
InactiveUS20070212343A1Facilitate trans-tympanic deliveryBiocideSenses disorderMoxifloxacinTreatment level
Topical otic pharmaceutical compositions comprising moxifloxacin or a pharmaceutically useful hydrate or salt thereof and a proteolytic enzyme. The compositions facilitate trans-tympanic delivery of a therapeutic level of the moxifloxacin.
Owner:ALCON INC
Pharmaceutical compositions for intraocular administration and methods for fabricating thereof
InactiveUS20160175323A1Removing issuePositive patient outcomeBiocideOrganic active ingredientsPrednisoloneMoxifloxacin
Pharmaceutical ophthalmic compositions are described, the compositions consisting essentially of a therapeutically effective quantity of an anti-bacterial agent (such as moxifloxacin), a therapeutically effective quantity of an anti-inflammatory agent (such as prednisolone), at least one pharmaceutically acceptable excipient and a pharmaceutically acceptable carrier. Methods for fabricating the compositions and using them are also described.
Owner:HARROW IP LLC
3-aminopyrrolidine compounds, and synthetic method and uses thereof
The invention relates to 3-aminopyrrolidine compounds, and a synthetic method and uses thereof. 3-pyrrolidone compounds (compounds IIIa or IIIb) are adopted as substrates and subjected to transaminase reactions under the actions of transaminase and an amino donator to obtain the (S)-3-aminopyrrolidine compounds (compounds II). Then intramolecular cyclization and removal of amino protective groups are performed to obtain (S,S)-2,8-diazabicyclo[4,3,0] nonane (a compound I). According to the 3-aminopyrrolidine compounds, the synthetic method and the uses, a synthetic technology that is low in cost, short in process steps and environmental friendly is provided for a moxifloxacin intermediate, and the synthetic technology will have great market application value and is suitable for large-scale industrial production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Sanitizer
The invention relates to the field of sanitizer manufacture, in particular to a sanitizer. The sanitizer is characterized by comprising components in parts by weight as follows: 5-6 parts of glycol stearate, 6-10 parts of citric acid, 4-8 parts of sodium dodecyl sulfate, 5-6 parts of sodium carbonate, 2-5 parts of sodium aluminate, 9-11 parts of an anionic or nonionic surfactant, 6-7 parts of moxifloxacin, 6-10 parts of trichloro hydroxydiphenyl ether, 10-20 parts of lauric acid, 100-105 parts of ethanol, 0.05-0.1 parts of iodine, 5-10 parts of acetic acid and 505-605 parts of water.
Owner:CHENGDU SHUNFA DISINFECTANT & WASHING TECH
Air disinfectant
InactiveCN102067889AReasonable formulaImprove disinfection effectBiocideDisinfectantsTriclosanAlcohol
The invention discloses an air disinfectant and aims at providing an air disinfectant with reasonable formula and better disinfecting effect. The technical scheme adopted by the invention is as follows: the air disinfectant is characterized by comprising the following components in percent by weight: 5 percent of moxifloxacin, 1 percent of triclosan, 3 percent of lauric acid, 80 percent of alcohol, 0.5 percent of natural folium artemisiae argyi essential oil and 9.5 percent of water. The invention has the advantages of reasonable formula, good disinfecting effect, environment protection and low production cost.
Owner:孟庆云
Stable moxixacin tablet and its prepn process
InactiveCN1672680AStable and no discolorationHigh hardnessAntibacterial agentsOrganic active ingredientsMedicineHypromellose
The present invention relates to stable moxixacin tablet and its preparation process. The preparation contains moxixacin or its salt and / or hydrate and tablet supplementary material. The present invention features that during preparation, hypromellose or other filming material may be added to prepare granule before tabletting so that the medicine is stable without color change during pelletizing and tabletting.
Owner:深圳市天一时科技开发有限公司
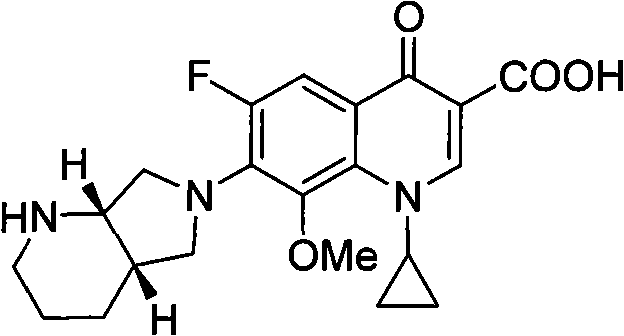
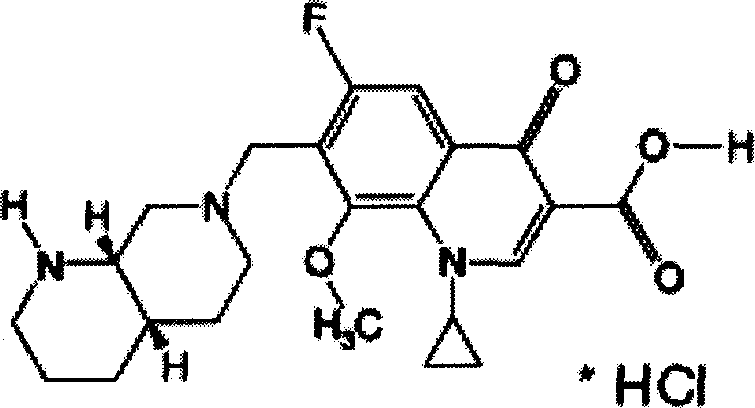
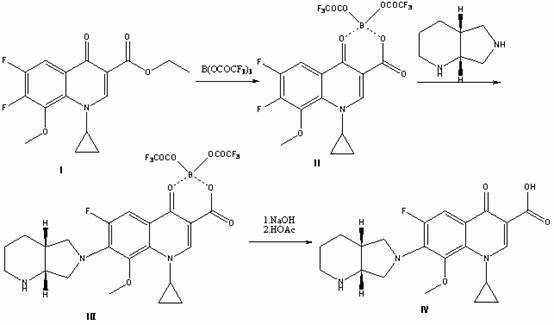
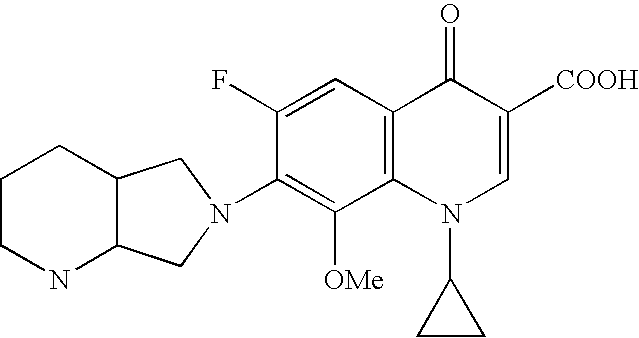
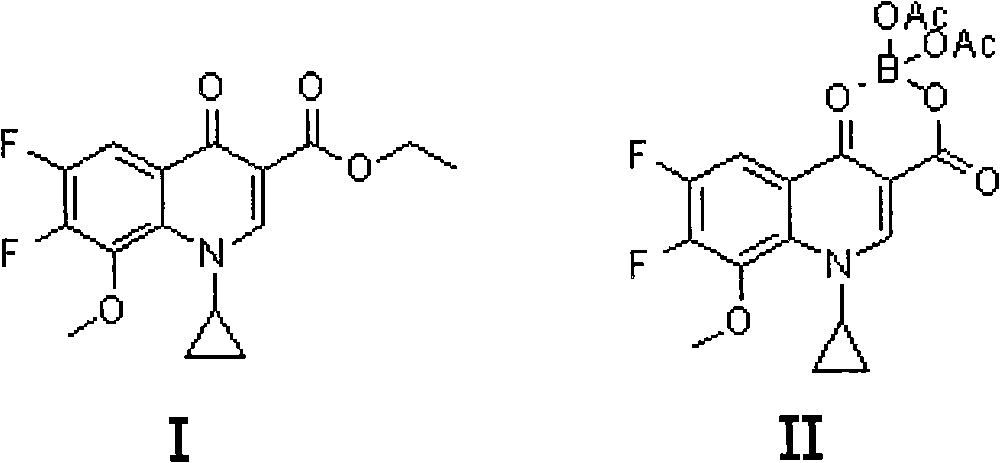
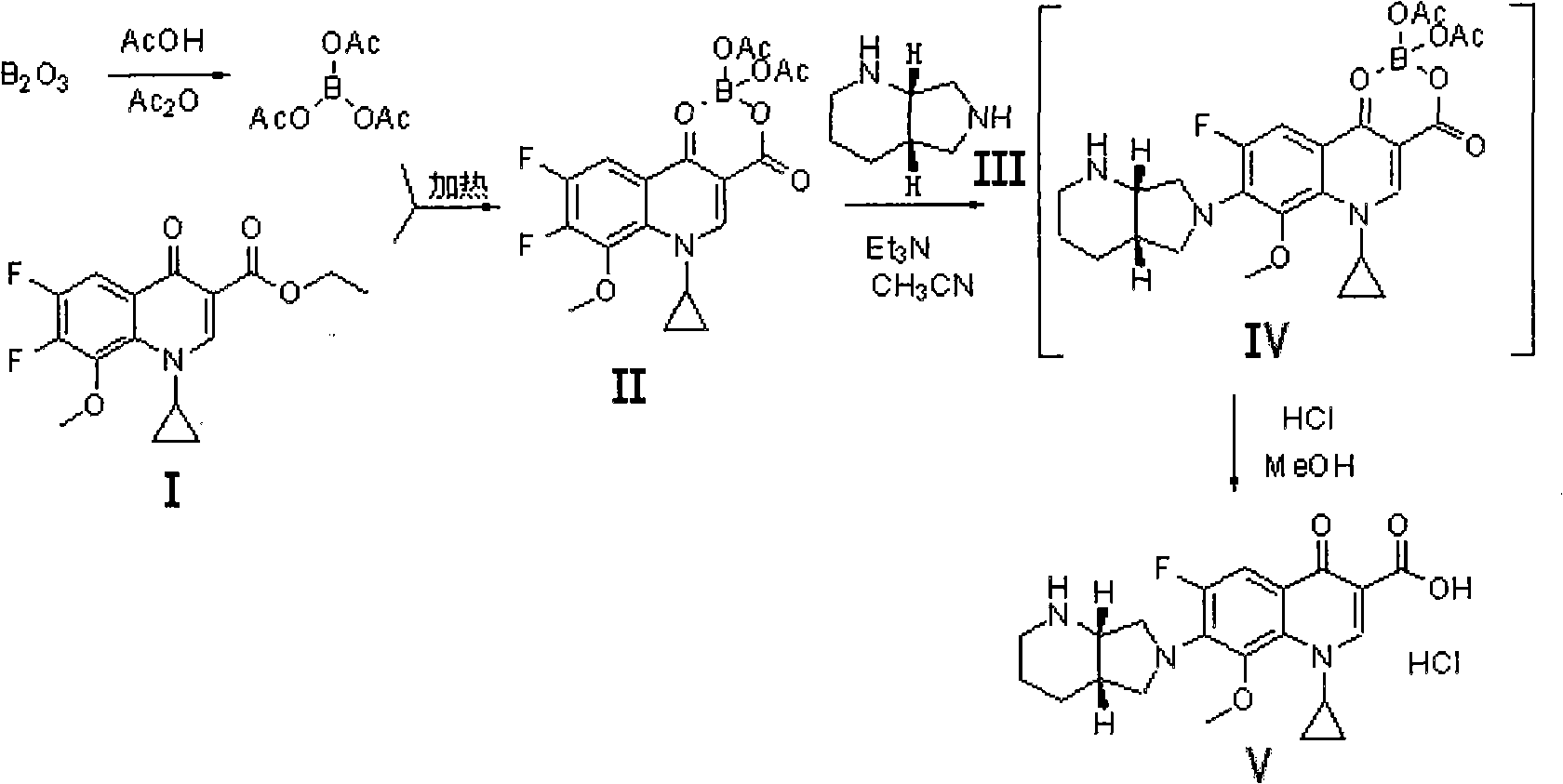
Preparation method for moxifloxacin and hydrochloride thereof
ActiveCN103012452AAvoid thermal effectsQuality assuranceGroup 3/13 element organic compoundsAcetic anhydrideIce water
The invention discloses a preparation method for moxifloxacin and hydrochloride thereof. A conventional process has the following defects that a one-pot material addition process has safety hidden troubles such as bumping materials and explosion which are caused by suddenly increased temperature, and a great amount of wastewater is produced because ice water is employed in a separation process of a borane chelate to perform crystallization process to separate the chelate. The method provided by the invention is characterized in that in a chelating reaction, solid boric acid is added in acetic anhydride in a continuous feeding manner to form a chelating agent through reaction, the chelating agent reacts with 1-cyclopropyl-6,7-difluoro-8-methoxyl-1,4-dihydro-4-oxo quinoline-3-ethyl carboxylate to prepare the borane chelate, and the borane chelate is purified through crystallization and separation by using an organic solvent. The preparation method is simple in process, mild in conditions and safe in the chelating reaction process and solves the problem of the great amount of the wastewater in a preparation process of the chelate. The method has high yield, good selectivity and high product purity and is suitable for industrialized production.
Owner:ZHEJIANG NHU CO LTD
Water-soluble salt of aspartic acid carbostyril series antibacterial drugs and injection dosage forms thereof
The invention provides an aspartate quinolone antibiotics water-soluble salt and the injection formulation thereof, including sparfloxacin, gatifloxacin, rufloxacin, pefloxacin, tosufloxacin, moxifloxacin, and so on; the invention improves the water solubility of quinolone antibiotics and enhances the anti-bacterial effect of quinolone antibiotics. Compared with the oral liquid of quinolone drugs of the prior art, the invention has the advantages that: water solubility of the drug is good, as the drug enters the blood directly, the invention can not only achieve treatment function rapidly, but can also be absorbed by the human body fully, and the invention has significant effects in the two aspects of fast onset of action and low consumption. Compared with the injection of the quinolone drugs of the prior art, the invention has the advantages that: due to the existence of the L-aspartate, the antibacterial activity of quinolone drugs can be enhanced.
Owner:SHENYANG WOSEN PHARMA INST
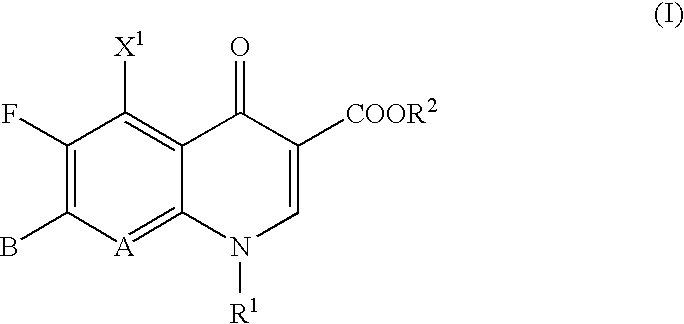
High selectivity method for synthesizing moxifloxacin
ActiveCN102351858AAvoid it happening againSimple processing methodOrganic chemistryAcetic anhydrideIce water
The invention discloses a high selectivity method for synthesizing moxifloxacin. The method comprises the following steps of: reacting boric anhydride with trifluoro acetic anhydride to obtain a chelant; reacting 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester with the chelant, cooling to room temperature, adding ice water, performing suction filtration, and washing a filter cake with water until neutrality to obtain a 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate; and reacting the 1-ethyl-7-chloro-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoroacetic anhydride boronized chelate with (S,S)-2,8-diazabicyclo[4,3,0]nonane to obtain a 1-cyclopropyl-6-fluoro-7-([S,S]-2,8-diazabicyclo[4,3,0]nonane-8-methoxy-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid ethyl ester trifluoroacetic anhydride boronized chelate, recycling a solvent under reduced pressure, adding alkali, refluxing, discoloring, filtering, freezing, performing suction filtration, and drying a filter cake. The method is simple, mild in conditions, and high in selectivity, avoids difficultly separated impurities, is high in reaction yield and product purity, and is suitable for industrial production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Air disinfectant
Owner:QINGDAO SANDING SANITARY PROD
Method of treating ophthalmic infections with moxifloxacin compositions
Ophthalmic, otic and nasal compositions containing a new class of antibiotics (e.g., moxifloxacin) are disclosed. The compositions preferably also contain one or more anti-inflammatory agents. The compositions may be utilized to treat ophthalmic, otic and nasal conditions by topically applying the compositions to the affected tissues.
Owner:NOVARTIS AG
Method for preparing moxifloxacin or its medicinal salt and its intermediate
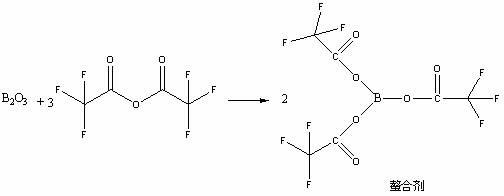
ActiveCN102617622AReduce harmLight colorGroup 3/13 element organic compoundsAcetic acidAcetic anhydride
The invention provides a novel method for preparing moxifloxacin or its medicinal salt and its intermediate, which comprises the following steps: dissolving boron trioxide in a certain amount of acetic anhydride and an acetate mixing solution, reacting under the temperature of 90-120 DEG C, cooling a reaction solution; adding cyclized quinolinecarboxylic ester in the reaction solution and reacting at the reaction temperature of 50-80 DEG C, cooling, adding a certain amount of an ether solvent, stirring and then filtering, washing by the ether solvent, drying to obtain the product; reacting with (4aR, 7aR)-octahydropyrrolo[3,4-b]pyridine to obtain the moxifloxacin or its medicinal salt. The reaction method of the invention is mild and controllable, the common equipments enable production, the obtained product has the advantages of high purity and good color, boron oxide substitutes boric acid for chelating to reduce the damage of equipments and human body caused by acid mist in the reaction.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
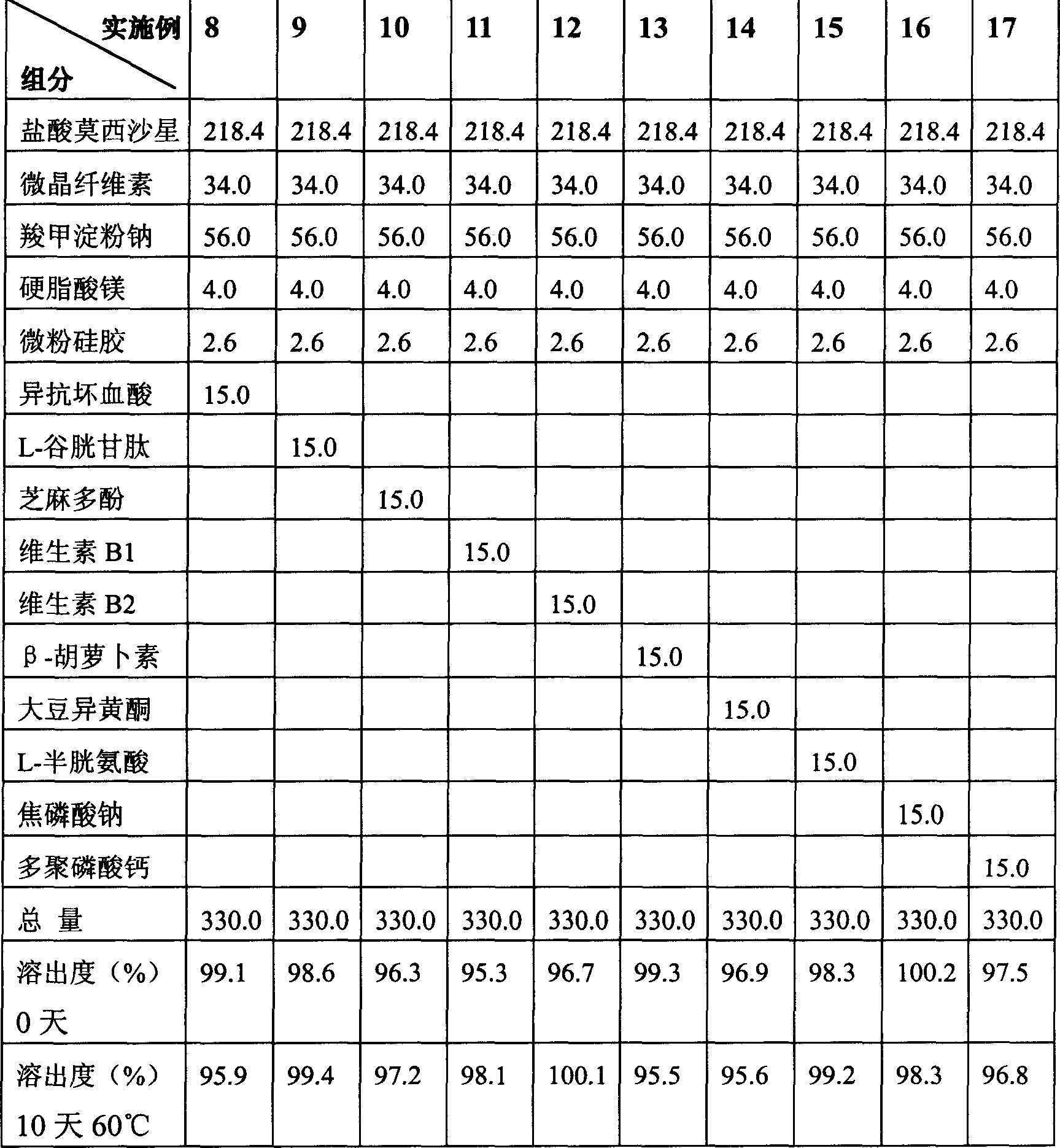
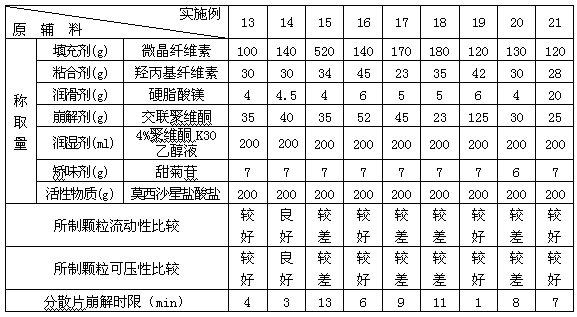
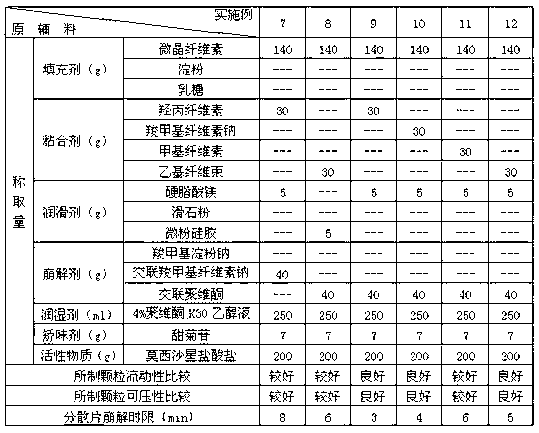
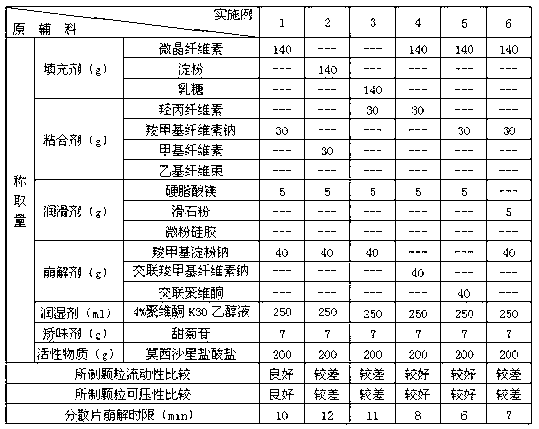
Moxifloxacin dispersible tablet and preparation method thereof
ActiveCN103284962AImprove liquidityGood compressibilityAntibacterial agentsOrganic active ingredientsCross-linkAlcohol
The invention discloses a dispersible tablet of moxifloxacin or a salt thereof or / and hydrate thereof and a preparation method of the dispersible tablet. The prescription of dispersible tablet comprises the following components in percentage by weight: 15%-65% of filler, 5%-10% of adhesive, 5%-25% of disintegrating agent, 1%-5% of lubricating agent, and a proper amount of wetting agent and other auxiliary materials. The preparation method of the moxifloxacin dispersible tablet comprises the following steps of: respectively screening the main material and the auxiliary materials; uniformly mixing the main material and the auxiliary materials according to a prescription ratio; using a proper amount of povidone K30 alcohol solution to prepare a soft material; sieving and pelletizing, drying, and sieving the dry particles; adding a disintegrating agent cross-linked povidone and a lubricating agent magnesium stearate, uniformly mixing and tabletting to obtain the dispersible tablet. The dispersible tablet of moxifloxacin or the salt thereof or / and hydrate thereof provided by the invention is short in disintegrating time, good in dispersing status, quick in dissolving out of medicines, convenient and flexible to take, and can be swallowed, also can be taken after being dispersed in water.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
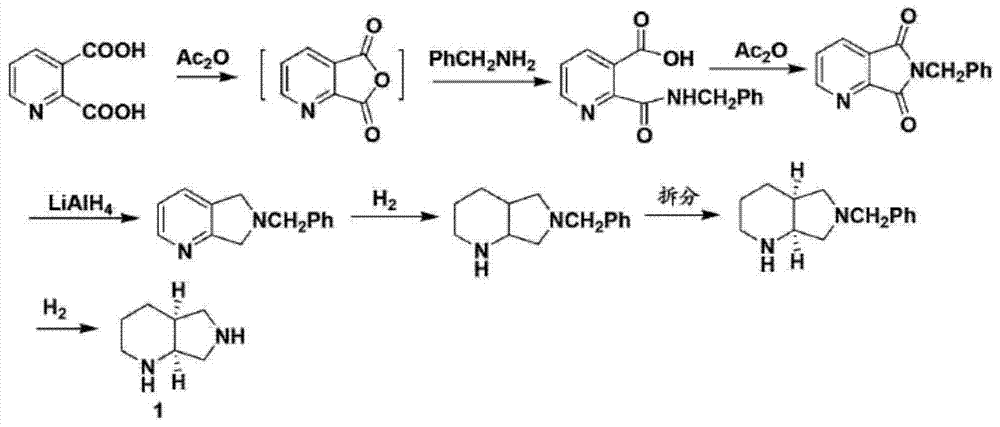
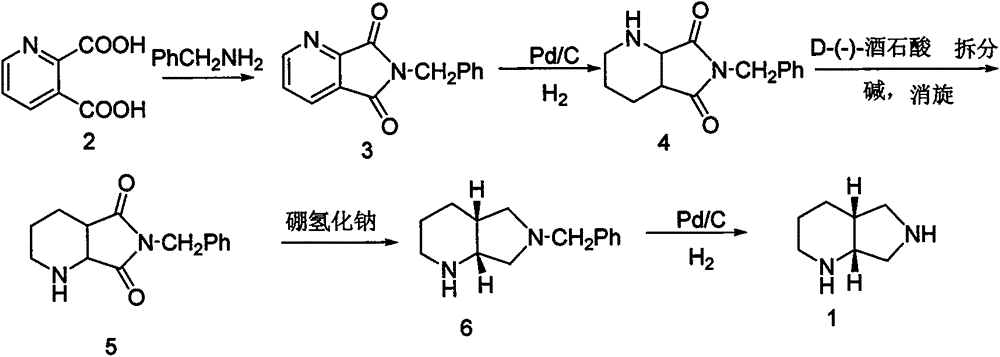
Synthesis method for moxifloxacin side chain
InactiveCN105777750AReduce manufacturing costImprove productivityOrganic chemistrySynthesis methodsSide chain
The invention discloses a synthesis method for a moxifloxacin side chain.With 2,3-dipicolinic acid being a raw material, cyclization, catalytic hydrogenation, resolution and racemization are performed, and then chemical reduction and debenzylation are performed to obtain moxifloxacin side chain.Resolution, racemization and chemical reduction are performed in sequence, sodium borohydride is used for replacing high-risk lithium aluminum hydride to synthesize the moxifloxacin side chain, and the synthesis method has the advantages that industrial waste materials are reduced, the production cost is lowered, and productivity is increased.
Owner:江西中德诚信科技有限公司 +1
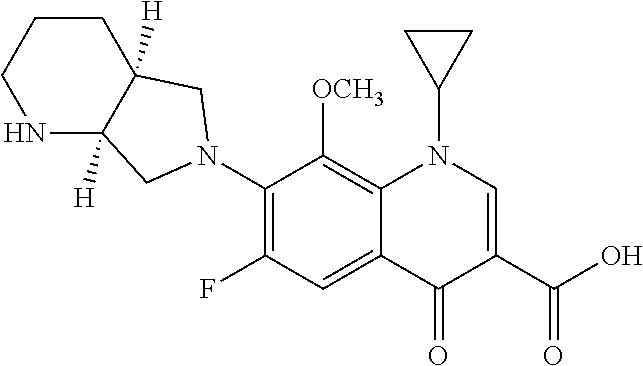
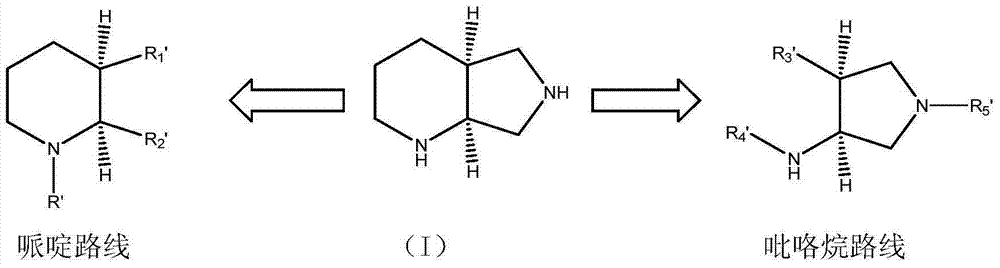
Novel and economical process for preparing (S, S)-2, 8-diazabicyclo[4.3.0]nonane and its enantiomer
ActiveUS20080221329A1High efficient resolutionSafe reaction conditionsOrganic chemistryQuinoloneMoxifloxacin
The present invention relates to a novel and economical process for preparing (S,S)-2, 8-diazabicyclo[4.3.0]nonane, a valuable intermediate used for constructing quinolone and naphthyridine derivatives having antibacterial effectiveness, e.g. moxifloxacin and its enantiomer.
Owner:LIANYUNGANG JINKANG PHARMA TECH
Method for industrial production of moxifloxacin side chain
InactiveCN103275078AReduce manufacturing costSuitable for industrial productionOrganic chemistryNonaneSide chain
The invention discloses a method for industrial production of moxifloxacin side chain. The method comprises the steps of A, dissolving 3-aldehyde pyridine-2-carboxylic acid and benzylamine in a mixed solvent of distilled water and an organic solvent, putting the above solution into an autoclave to carry out catalytic hydrogenation, and thus 6-benzyl-hexahydro-pyrrolo[3,4-b] pyridine-7-one is obtained; B. dissolving 6-benzyl-hexahydro-pyrrolo[3,4-b] pyridine-7-one in an organic solvent, adding a reducing agent in the solution to carry out a reduction reaction, and thus 6-benzyl-octahydro pyrrolo[3,4-b]pyridine is obtained; C. dissolving 6-benzyl-octahydro pyrrolo[3,4-b]pyridine in an organic solvent, adding D-(-)-tartaric acid to carry out resolution, and thus (s, s)-6-benzyl-octahydro-pyrrolo[3,4-b]pyridine is obtained; and D. dissolving (s, s)-6-benzyl-octahydro-pyrrolo[3,4-b]pyridine in an organic solvent, putting the solution in the autoclave to carry out catalytic hydrogenation for debenzylation, and thus (S,S)-2,8-diazabicyclo[4.3.0]nonane is obtained. The technical solution provided by the invention is simple and practical, low in cost and high in production efficiency, and is suitable for industrialized production.
Owner:SUZHOU MICRODIAG BIOLOGICALS
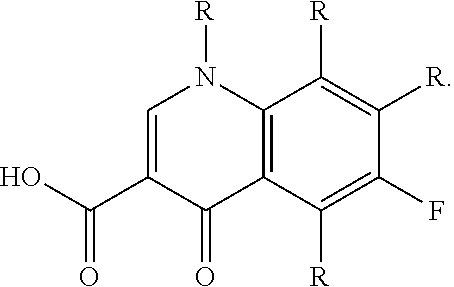
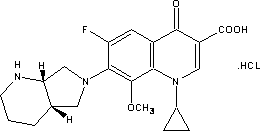
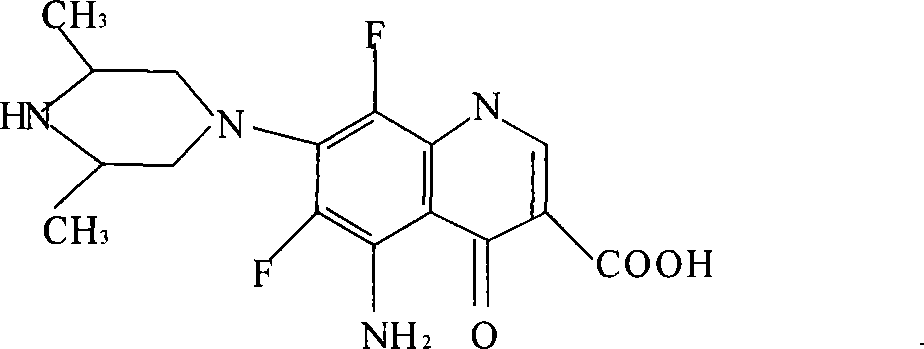
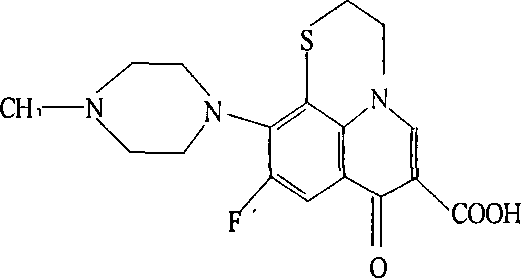
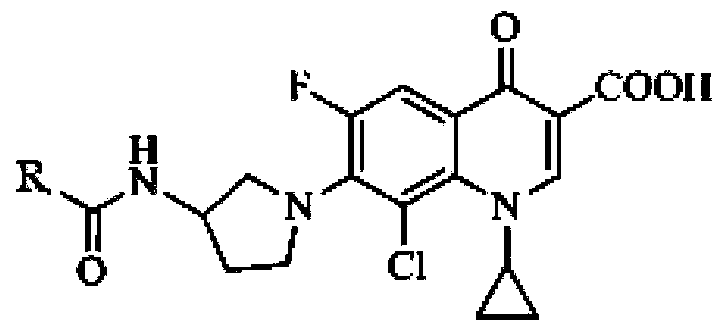
Application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments
ActiveCN103405435AHas inhibitory effectStrong anti-tuberculosis activityAntibacterial agentsOrganic active ingredientsThioureaMoxifloxacin
The invention discloses an application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments. In the structural general formula of the clinafloxacin amino derivatives, R is -(CH2)nNR<1>R<2>, -CH(CH2CH2XCH3)NH2, 2-pyrrolidyl or -OR3; n is 0 or 1; R1 and R2 are separately hydrogen, methyl, 2-hydroxyethyl, (R)-1-ethyl-2-hydroxyethyl, 2-aminoethyl, 3-(dimethylamino)propyl, hydroxyl, amino, methylamino, methoxyl or thiourea group; X is S or SO2; R3 is methyl or isobutyl; the compounds have certain inhibitory effect on standard sensitive strains, clinically isolated sensitive strains and clinically isolated drug-resistant strains of mycobacterium tuberculosis, the antitubercular activity of a part of compounds is stronger than that of ofloxacin and clinafloxacin and weaker than that of moxifloxacin, thus providing a new research direction for the antitubercular medicaments, and being beneficial to clinical treatment of tuberculosis.
Owner:SOUTHWEST UNIVERSITY
Method for preparing moxifloxacin side chain through biological method
The present invention provides a method for preparing a moxifloxacin side chain through a biological method, particularly to a method for preparing a compound represented by a formula 2. The method comprises: catalyzing a compound represented by a formula 4 with transaminase to form a compound represented by formula 3, and carrying out spontaneous ring closure on the compound represented by the formula 3 to obtain the compound represented by the formula 2, wherein the transaminase is selected from the omega-transaminase in Arthrobacter, Aspergillus terreus, Vibrio fluvialis, Bacillus megaterium, Sphingomonas paucimobilis, Hyphomonasneptunium, and Chromobacterium violaceum, and in the formulas 2, 3 and 4, R is selected from benzyloxycarbonyl, benzyl and ethoxycarbonyl. The formulas 2, 3 and 4 are defined in the specification.
Owner:ENZYMEWORKS
Medicinal composition for treating ophthalmic inflammation and application thereof
The invention discloses a Chinese medicinal composition for treating ophthalmic inflammation, which consists of collagen and Chinese medicaments for resisting bacterium and diminishing inflammation, and belongs to the field of pharmacy. Raw material medicaments of effective components of the medicinal composition comprise collagen and medicaments for resisting bacterium and diminishing inflammation such as erythrocin, ciprofloxacin, moxifloxacin, levofloxacin and the like which are prepared into various eye preparations by combining the prior art. The technical scheme creatively combines and applies the collagen and the effective components of the medicaments in the eye preparations so as to enlarge the application range of the collagen and achieve remarkable effect in the aspect of treating various ophthalmic inflammations.
Owner:BEIJING HERUN INNOVATION PHARMA TECH DEV
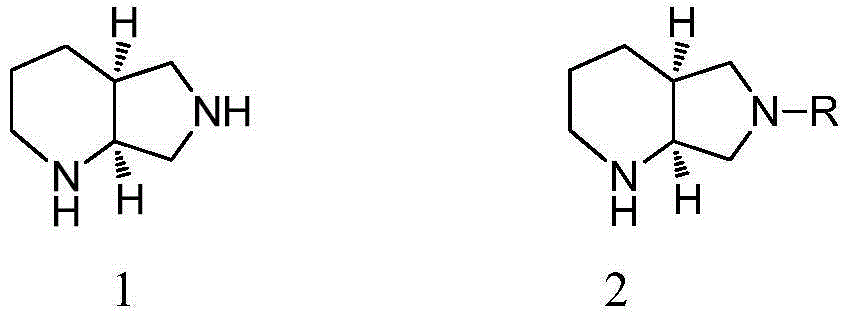
SYNTHESIS OF (4aS,7aS)-OCTAHYDRO-1H-PYRROLO[3,4-b]PYRIDINE
InactiveUS20110137036A1Avoid recyclingSimple methodOrganic chemistryFermentationEnzymatic hydrolysisKetone
The present invention relates to the stereoselective synthesis of (4aS,7aS)-octahydro-1H-pyrrolo[3,4-b]pyridine, as well as the conversion thereof, to give Moxifloxacin. Particularly, the present invention relates to a method for the synthesis of (4aS,7aS)-octahydro-1H-pyrrolo[3,4-b]pyridine of formula (I) comprising: (a) the optical resolution by enzymatic hydrolysis of the intermediate dialkyl-1-alkylcarbonylpiperidine-2,3-dicarboxylate racemate of formula (II) to give, following isolation, the intermediate dialkyl-(2S,3R)-1-alkylcarbonyl-piperidine-2,3-dicarboxylate of formula (III) in which AIk is a straight or branched C1-C5 alkyl group; (b) the conversion of the intermediate (III) to (4aR,7aS)-1-alkylcarbonylhexahydrofuro[3,4-b]pyridine-5,7-dione of formula (IV) in which AIk has the meanings set forth above; (c) the conversion of the intermediate (IV) to (4aS,7as)-octahydro-1H-pyrrolo[3,4-b]pyridine of formula (I) with an optical purity above 99%.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Medicinal composition for resisting helicobacter pylori and preparation method as well as application thereof
ActiveCN104814964AAct quicklyReduce adverse reactionsAntibacterial agentsDigestive systemMedicineVonoprazan
The invention relates to a medicinal composition for resisting helicobacter pylori. The medicinal composition comprises the following components in parts by weight: 50-500 parts of component A, 100-500 parts of component B and 5-50 parts of component C, wherein the component A is amoxicillin; the component B is selected from levofloxacin and pharmaceutically acceptable salts thereof, moxifloxacin and pharmaceutically acceptable salts thereof; the component C is selected from Vonoprazan and pharmaceutically acceptable salts thereof. The invention also provides a preparation method and application of the medicinal composition. The medicinal composition for resisting the helicobacter pylori has a strong effect of resisting the helicobacter pylori and has less adverse reaction and wide application, and the preparation method is simple and is easy to operate.
Owner:山东豪瑞恩制药有限公司
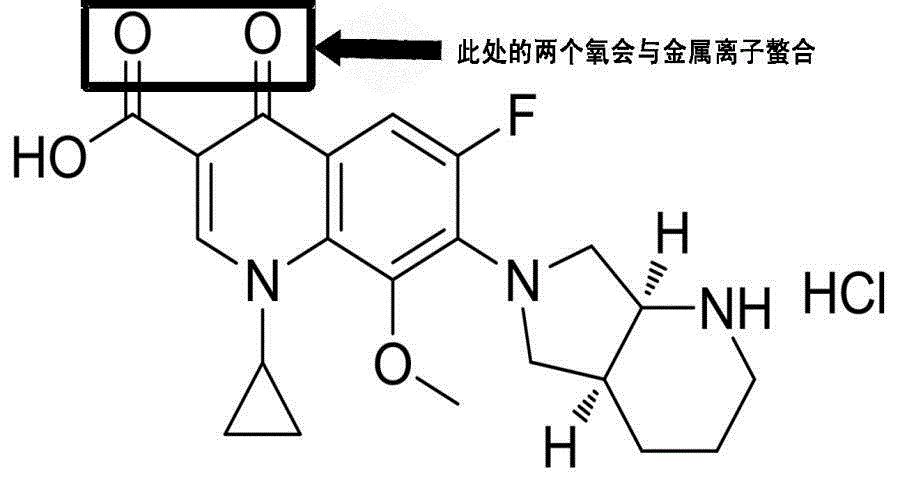
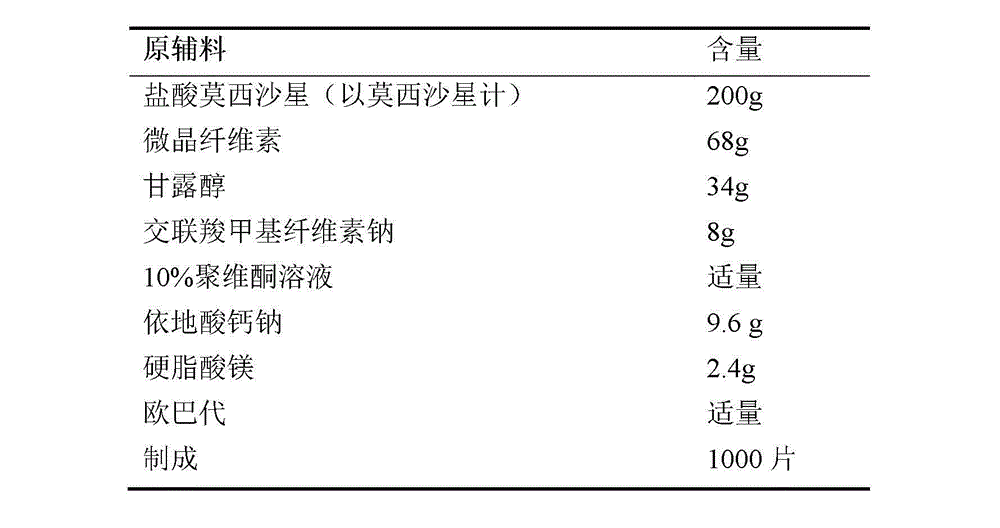
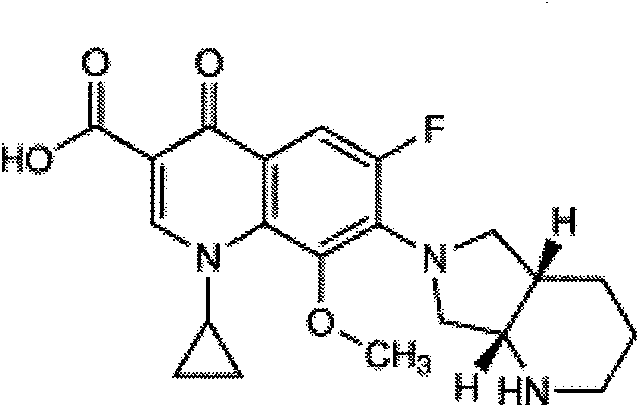
Moxifloxacin-containing pharmaceutical composition
ActiveCN102908323AGood dissolution propertiesHigh hardnessAntibacterial agentsOrganic active ingredientsProcess qualityAdditive ingredient
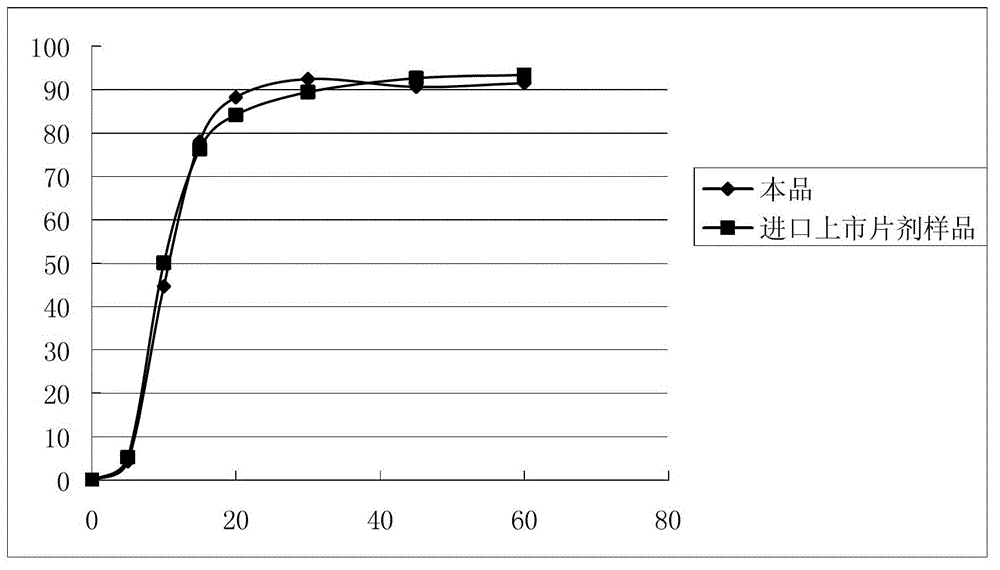
The invention relates to a moxifloxacin-containing pharmaceutical composition which is a solid pharmaceutical composition prepared from moxifloxacin and minor ingredients acceptable pharmaceutically. The composition can be prepared into orally-taken solid preparations according to the technical scheme, and metal ion chelating agents added into a formula avoid generation of red moxifloxacin metal ion chelates during production of the solid preparations, so that stability of moxifloxacin tablets is improved. Besides, the solid preparations have the dissolution behavior and hardness similar to those of imported tablets in the market. The preparation formula and process quality are stable and controllable.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Triple compound microsphere vascular targeted embolization sustained-release preparation containing antituberculous drug as well as preparation method and application of preparation
ActiveCN104324032AExcellent anti-tuberculosis effect in vitro and in vivoReduce concentrationAntibacterial agentsOrganic active ingredientsAntituberculous drugHemoptyses
The invention relates to a triple compound microsphere vascular targeted embolization sustained-release preparation containing an antituberculous drug as well as a preparation method and application of the preparation. The sustained-release agent comprises a carrier and drugs, wherein the drugs are coated with the carrier; the carrier is sodium alginate or chitosan, and the drugs are triple antituberculous compound drugs including rifampicin, isoniazid and pyrazinamide or moxifloxacin. The three antituberculous drugs are matrix drug solutions, the sodium alginate or chitosan is a carrier solution, the matrix drug solutions and the carrier solutiona are mixed to prepare a solution, the polymer solution containing drugs is dispersed into fogdrops with a certain diameter by adopting a high-voltage electrostatic droplet mode, and the fogdrops are sprayed into a solidifying liquid to prepare antituberculous drug microspheres under the action of calcium ions. The embolization sustained-release preparation can be used for treating tuberculosis, massive hemoptysis of pulmonary tuberculosis, tuberculosis cavity, renal tuberculosis, osteoarticular tuberculosis, genital tuberculosis, tuberculosis of thyroid gland, tuberculosis of cervical lymph nodes, tuberculosis of pericardium, tuberculosis of chest wall, pleural tuberculosis and other kinds of tuberculosis in a body.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A2009101002270002C1.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700051.PNG)
![Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof Method for reducing 8-benzyl-2,8-diazabicyclo[4,3,0]nonane and chiral isomer thereof](https://images-eureka.patsnap.com/patent_img/d773f92e-9397-45a1-b63c-9d0abefeecf0/A20091010022700052.PNG)

![Novel and economical process for preparing (S, S)-2, 8-diazabicyclo[4.3.0]nonane and its enantiomer Novel and economical process for preparing (S, S)-2, 8-diazabicyclo[4.3.0]nonane and its enantiomer](https://images-eureka.patsnap.com/patent_img/918a2ba4-be39-482e-a73f-b2c9b747c9de/US20080221329A1-20080911-C00001.png)
![Novel and economical process for preparing (S, S)-2, 8-diazabicyclo[4.3.0]nonane and its enantiomer Novel and economical process for preparing (S, S)-2, 8-diazabicyclo[4.3.0]nonane and its enantiomer](https://images-eureka.patsnap.com/patent_img/918a2ba4-be39-482e-a73f-b2c9b747c9de/US20080221329A1-20080911-C00002.png)
![Novel and economical process for preparing (S, S)-2, 8-diazabicyclo[4.3.0]nonane and its enantiomer Novel and economical process for preparing (S, S)-2, 8-diazabicyclo[4.3.0]nonane and its enantiomer](https://images-eureka.patsnap.com/patent_img/918a2ba4-be39-482e-a73f-b2c9b747c9de/US20080221329A1-20080911-C00003.png)
![SYNTHESIS OF (4aS,7aS)-OCTAHYDRO-1H-PYRROLO[3,4-b]PYRIDINE SYNTHESIS OF (4aS,7aS)-OCTAHYDRO-1H-PYRROLO[3,4-b]PYRIDINE](https://images-eureka.patsnap.com/patent_img/1b74e857-b50d-49ee-8e5d-05143bddf94f/US20110137036A1-20110609-D00001.png)
![SYNTHESIS OF (4aS,7aS)-OCTAHYDRO-1H-PYRROLO[3,4-b]PYRIDINE SYNTHESIS OF (4aS,7aS)-OCTAHYDRO-1H-PYRROLO[3,4-b]PYRIDINE](https://images-eureka.patsnap.com/patent_img/1b74e857-b50d-49ee-8e5d-05143bddf94f/US20110137036A1-20110609-D00002.png)
![SYNTHESIS OF (4aS,7aS)-OCTAHYDRO-1H-PYRROLO[3,4-b]PYRIDINE SYNTHESIS OF (4aS,7aS)-OCTAHYDRO-1H-PYRROLO[3,4-b]PYRIDINE](https://images-eureka.patsnap.com/patent_img/1b74e857-b50d-49ee-8e5d-05143bddf94f/US20110137036A1-20110609-D00003.png)