Moxifloxacin dispersible tablet and preparation method thereof
A technology of dispersible tablet and weight percentage, which is applied in the field of dispersible tablet of moxifloxacin or its salt and/or its hydrate and its preparation, preparation of medicine and pharmaceutical preparation, which can solve the problems of less adverse reactions and inconvenience for patients to take , achieve the effects of short disintegration time, rapid drug dissolution and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
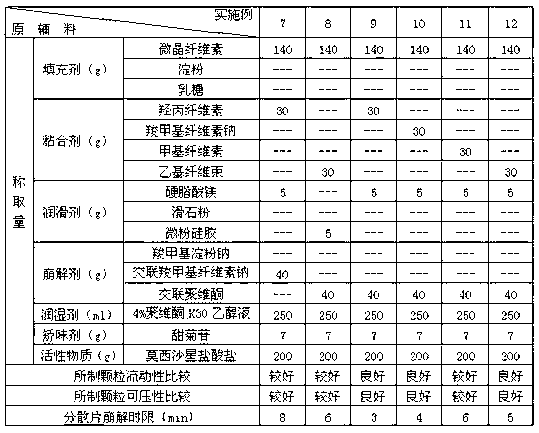
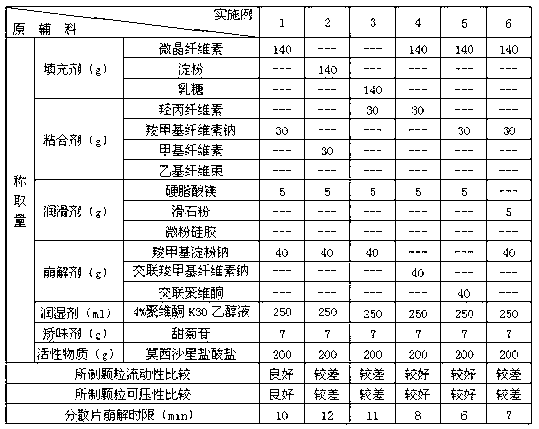
Examples
Embodiment 1-12
[0031] Test the selection of various excipients in the prescription, take each component according to the amount in Table 1 and 2, pass moxifloxacin hydrochloride through an 80 mesh sieve, filler, disintegrant (accounting for 65% of the total disintegration dose), Adhesives, flavoring agents, etc. are passed through a 60-mesh sieve, and the above components are mixed evenly. Subsequently, with an appropriate amount of 4% povidone K 30 The ethanol solution is prepared into a soft material, granulated through a 30-mesh sieve, dried at 55°C, and then granulated through a 24-mesh sieve. Then add a lubricant and a disintegrant (accounting for 35% of the total disintegrating dose), mix evenly and press into tablets to prepare dispersible tablets each containing 200 mg of moxifloxacin hydrochloride.
[0032] Table 1 The composition and investigation results of each component in Examples 1-6
[0033]
[0034] Table 2 The composition and investigation results of each component in ...
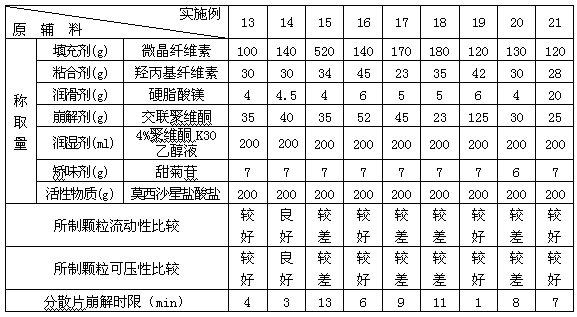
Embodiment 13-21
[0038] According to the preferred auxiliary materials in Example 9 above, the proportions of the various components of the auxiliary materials were studied in Examples 13-21, and each component was measured according to Table 3. Pass moxifloxacin hydrochloride through a 80-mesh sieve, microcrystalline cellulose, crospovidone (accounting for 65% of the total), hydroxypropyl cellulose, stevioside, etc. through a 60-mesh sieve, and mix the above components evenly . Subsequently, with an appropriate amount of 4% povidone K 30 The ethanol solution is prepared into a soft material, granulated through a 30-mesh sieve, dried at 55°C, and then granulated through a 24-mesh sieve. Magnesium stearate and crospovidone (accounting for 35% of the total amount) were then added, mixed uniformly and pressed into tablets to prepare dispersible tablets each containing 200 mg of moxifloxacin hydrochloride.
[0039] Table 3 Embodiment 13-21 each component weight composition and investigation resu...
Embodiment 22
[0043] Prepare each dispersible tablet containing 50mg of moxifloxacin hydrochloride active substance, each active substance content is about 33%, take each component according to the following weight:
[0044] Moxifloxacin hydrochloride 50g
[0045] Crospovidone 20g
[0046] Microcrystalline Cellulose 50g
[0047] Hypromellose 23g
[0049] Stevioside 4g
[0050] 4% povidone K 30 Proper amount of ethanol solution
[0051] Pass 50 g of the above-mentioned moxifloxacin hydrochloride through a 80-mesh sieve, 13 g of crospovidone, 50 g of microcrystalline cellulose, 23 g of hydroxypropyl cellulose, and 4 g of stevioside through a 60-mesh sieve, and mix the above components evenly. Subsequently, with an appropriate amount of 4% povidone K 30 The ethanol solution is prepared into a soft material, granulated through a 30-mesh sieve, dried at 55°C, and then granulated through a 24-mesh sieve. Then 7 g of crospovidone and 3 g of magnesium stearate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com