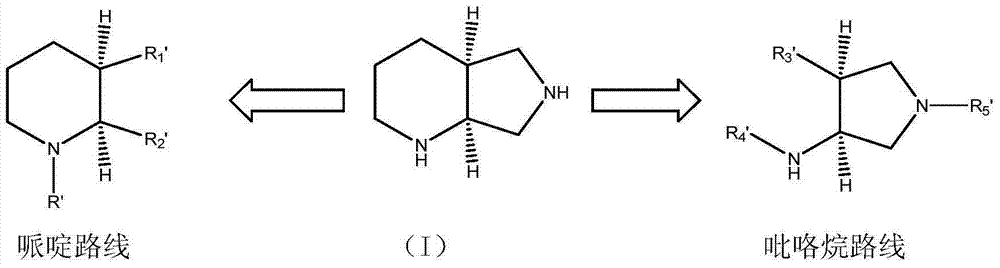
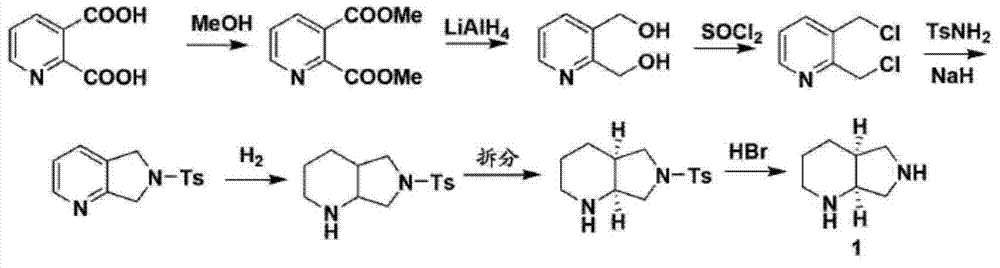
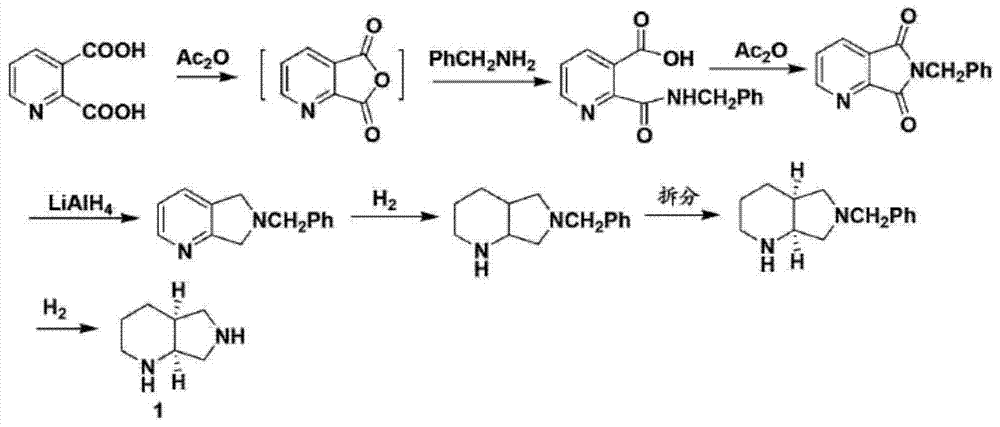
3-aminopyrrolidine compounds, and synthetic method and uses thereof
A technology of aminopyrrolidine and compounds, applied in the field of biomedicine, can solve the problems of high process cost, unfriendly environment, expensive reducing agent, etc., and achieve the effect of short process flow, huge market application value and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of (3S)-1-benzyl-3-amino-4-(3-ethoxycarbonylpropyl)-pyrrolidine
[0051]
[0052]Dissolve 100 g of isopropylamine in 100 ml of water, adjust the pH value to 8.0 with hydrochloric acid aqueous solution under ice-water bath cooling, and add 20 ml of tetrahydrofuran, then dilute to 700 ml with 0.1 M Tris-HCl buffer solution and preheat to 30 ° C, then Add 200ml of tetrahydrofuran solution containing 50g of 1-benzyl-4-(3-ethoxycarbonylpropyl)-3-pyrrolidone (3-1), and finally add 1g of ω-transaminase freeze-dried powder and 0.8g of PLP (pyridoxal phosphate) , the reaction is controlled by 20% isopropylamine aqueous solution to pH = 7-10, the temperature is converted at 10-50° C. for more than 24 hours, and the reaction is completed by TLC monitoring. The solid was removed by filtration, the mother liquor was extracted three times with ethyl acetate, the combined organic phases were dried over anhydrous sodium sulfate, and concentrated to obtain 45 g ...
Embodiment 2
[0053] Example 2 Preparation of (1S,6S)-8-benzyl-2,8-diazabicyclo[4,3,0]nonane
[0054]
[0055] (3S)-1-benzyl-3-amino-4-(3-ethoxycarbonylpropyl)-pyrrolidine (compound 2-1 in Example 1, 27.4g, 100mmol), 270ml toluene, 35ml acetic acid, The temperature was raised to 70°C for 16h. After the reaction was carried out completely, it was washed with aqueous sodium bicarbonate to a pH of about 8, the combined water phase was extracted with 100ml toluene, the combined toluene phase was washed with water, the toluene phase was dried over anhydrous sodium sulfate, and the solvent was concentrated under reduced pressure to obtain an oily product (1 -2) (21.2 g, yield 92%). 1 H-NMR (400MHz, CDCl 3 ):δ7.14(2H),7.07(1H),7.06(2H),3.68(1H),3.62(2H),2.60(2H),2.34(2H),2.23(2H),2.20(1H),1.65 (2H). MS-ESI: m / z: 229 (M + +1).
[0056] Add 35.0 g of lithium aluminum hydride into 50 ml of anhydrous tetrahydrofuran, and slowly add a solution of the product (compound 1-2, 21.2 g, 92 mmol) dis...
Embodiment 3
[0057] Example 3 Preparation of (S, S)-2,8-diazabicyclo[4,3,0]nonane
[0058]
[0059] The product from the previous step (compound 1-1, 10.8g, 50mmol) was dissolved in 250ml of methanol, 0.5g of 10% palladium carbon was added to the autoclave, and hydrogenation reaction was carried out at room temperature under a pressure of 1.0MPa for 24 hours. After the reaction was complete, the palladium carbon was removed by filtration , the filtrate was adjusted to PH=10.0 with sodium methoxide / methanol solution, filtered, the filtrate was concentrated to dryness and then distilled under reduced pressure to obtain 5.3 g of the product (S,S)-2,8-diazabicyclo[4,3,0]nonane, The yield is about 84%, and the ee value is 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com