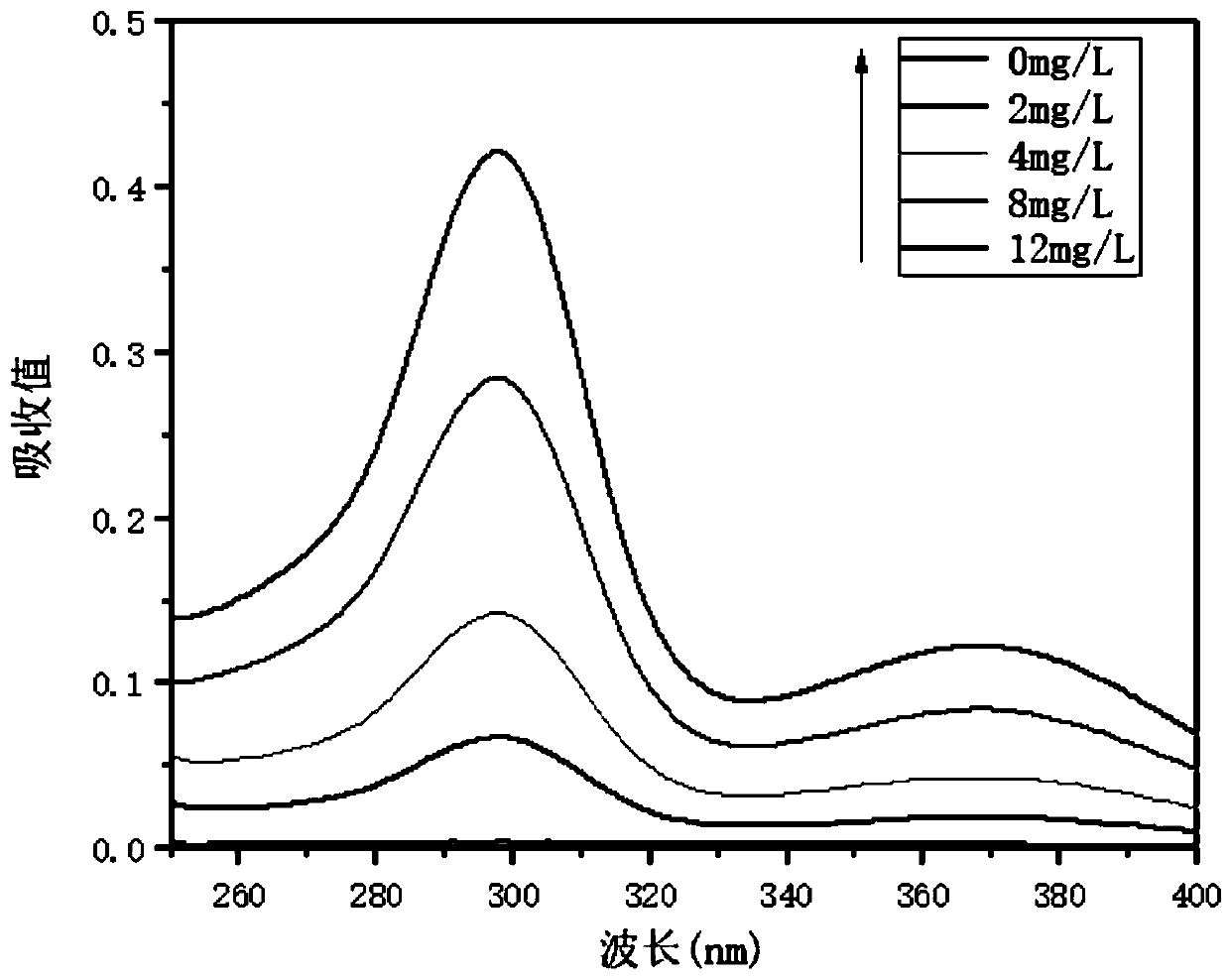
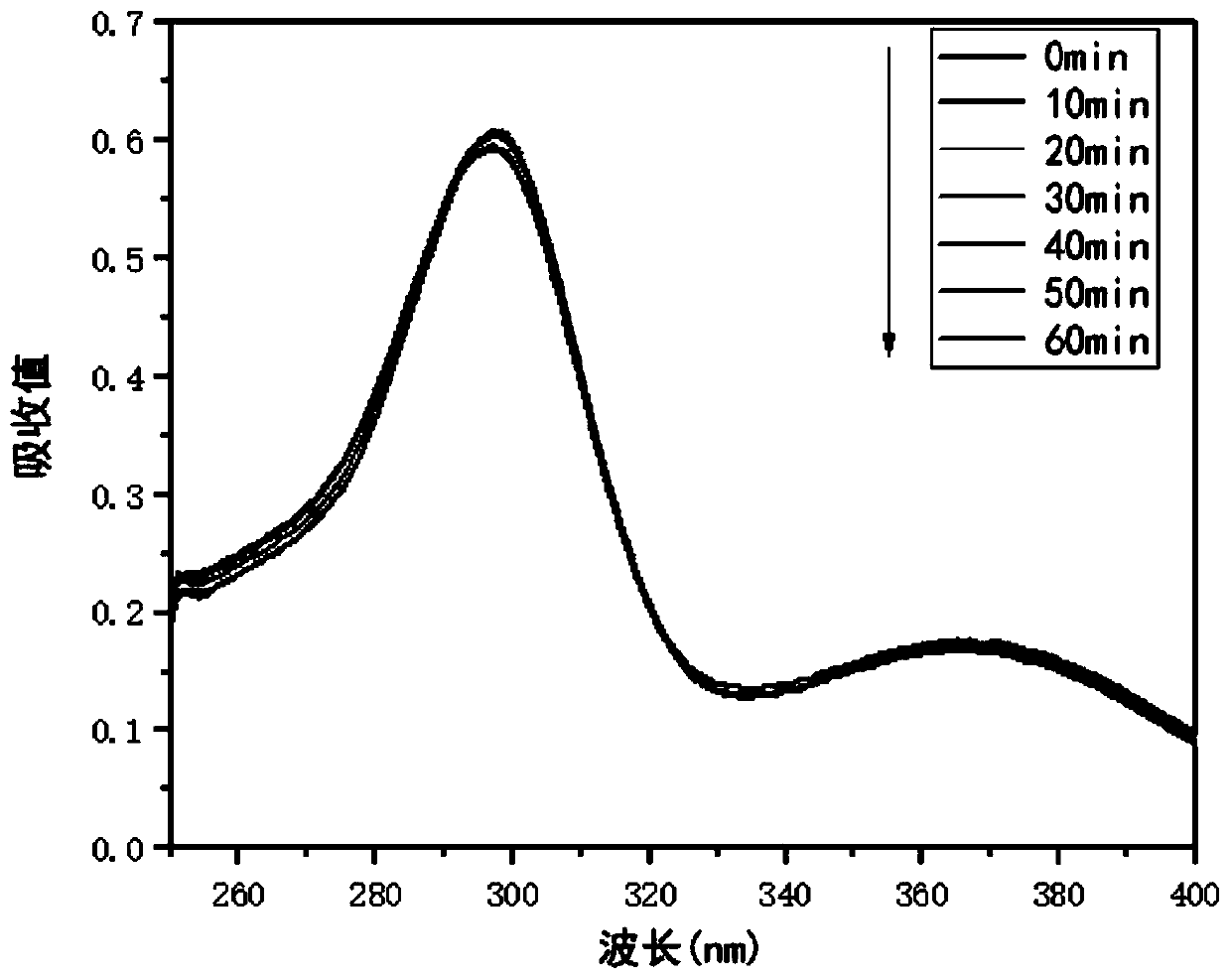
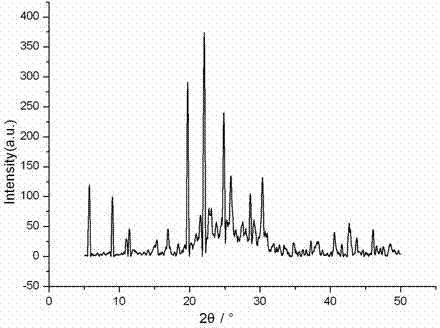
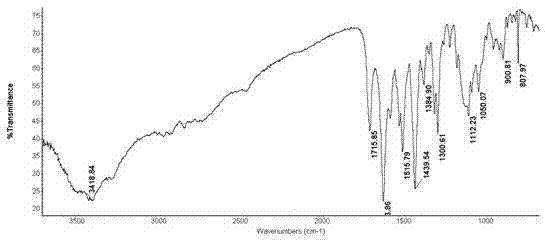
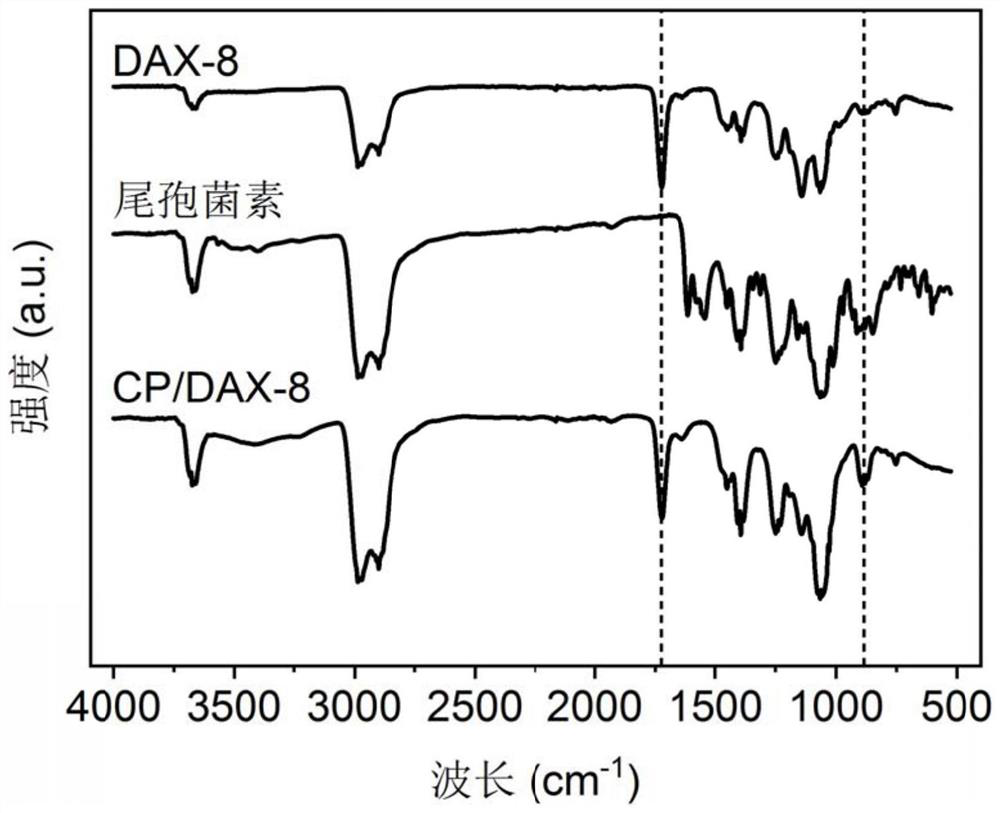
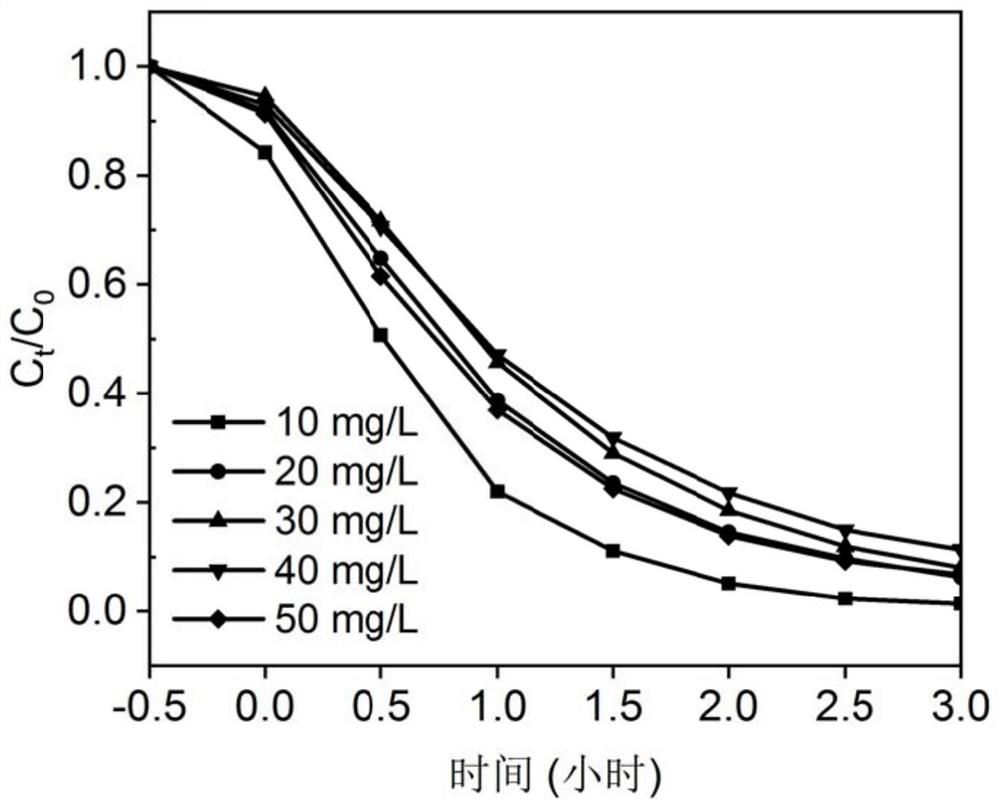
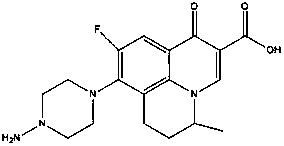
Patents
Literature
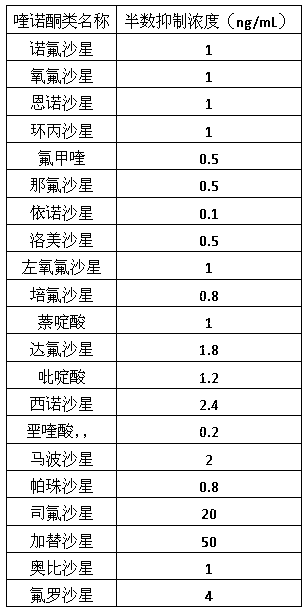
30 results about "Sparfloxacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
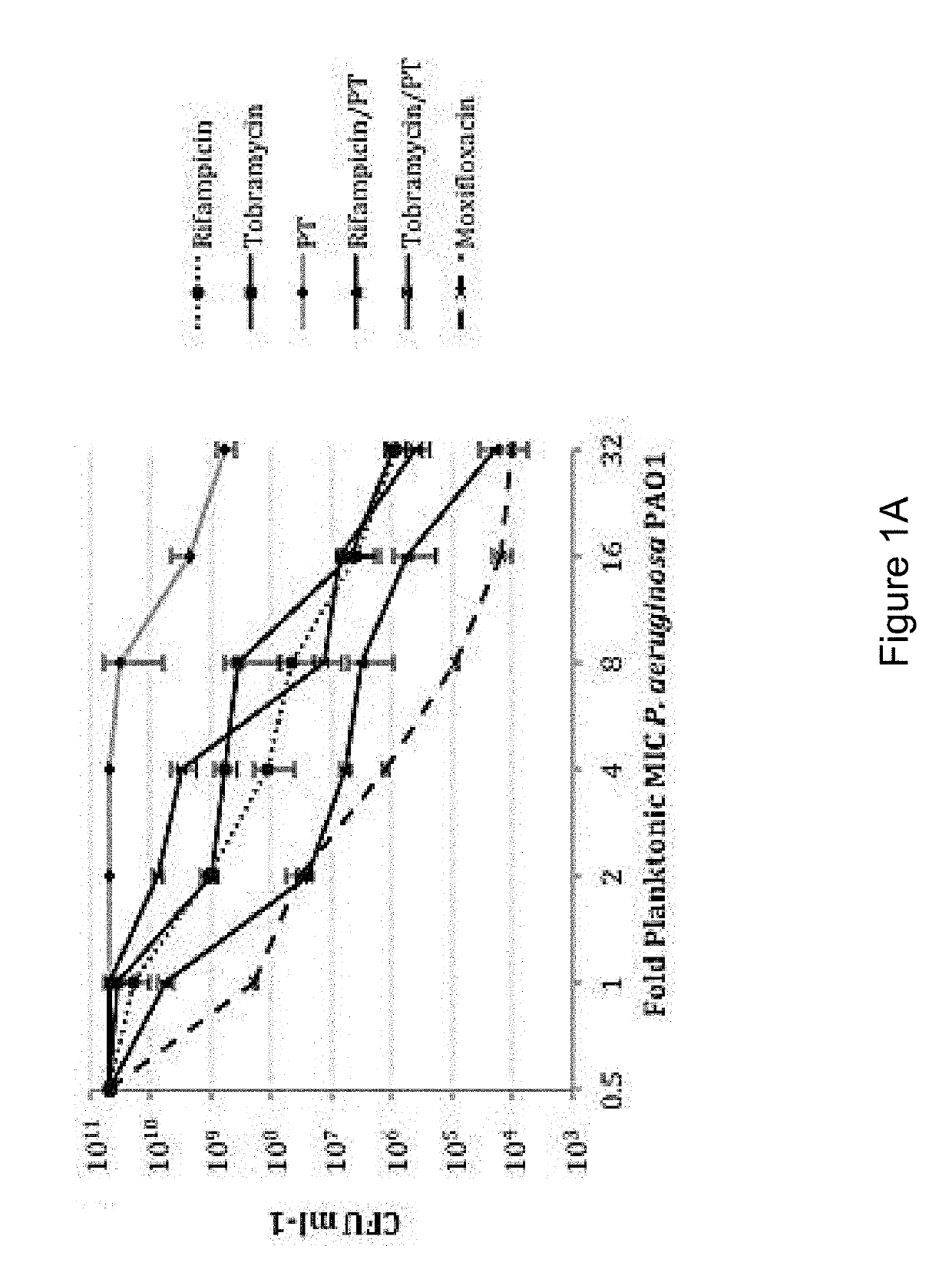
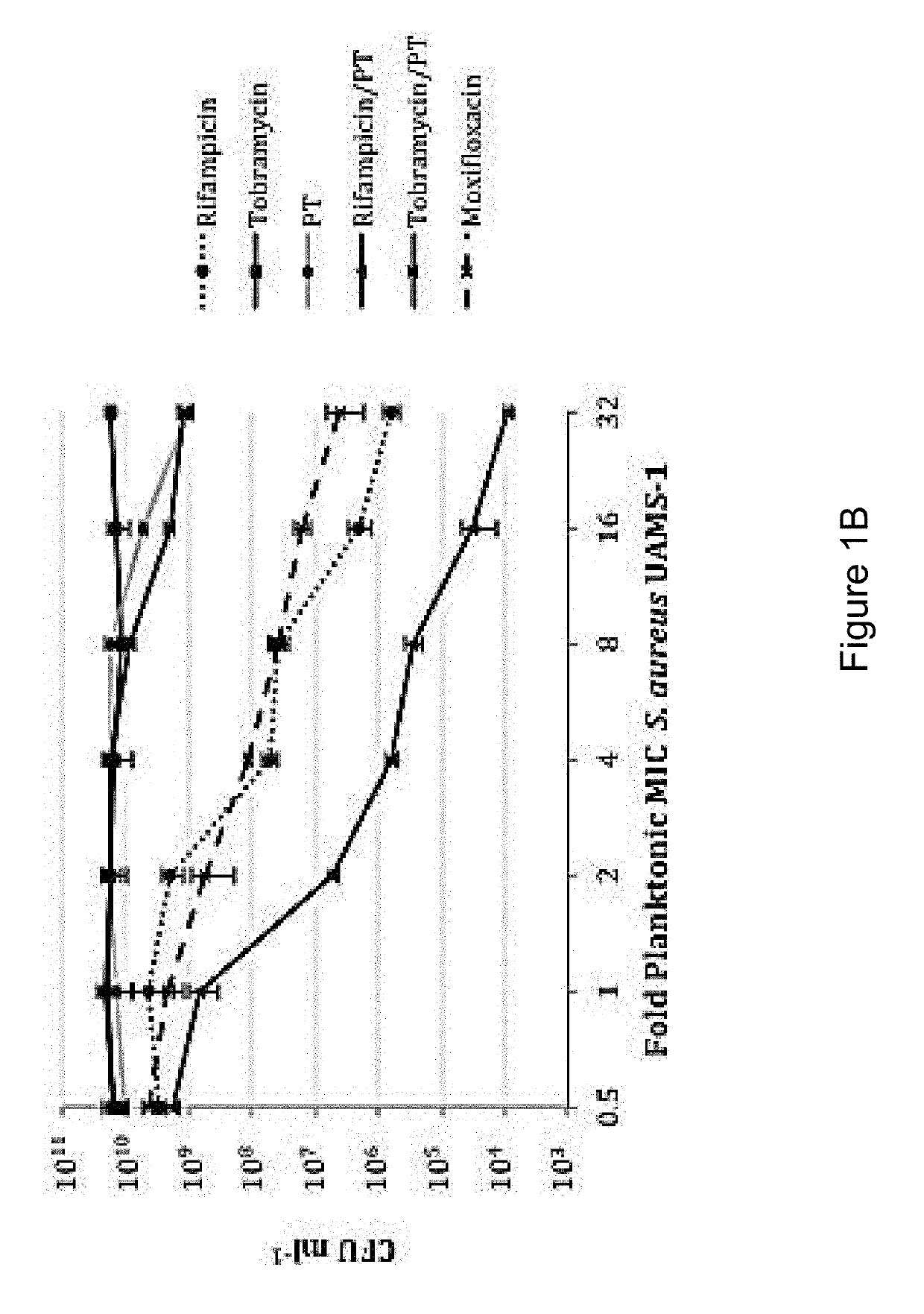
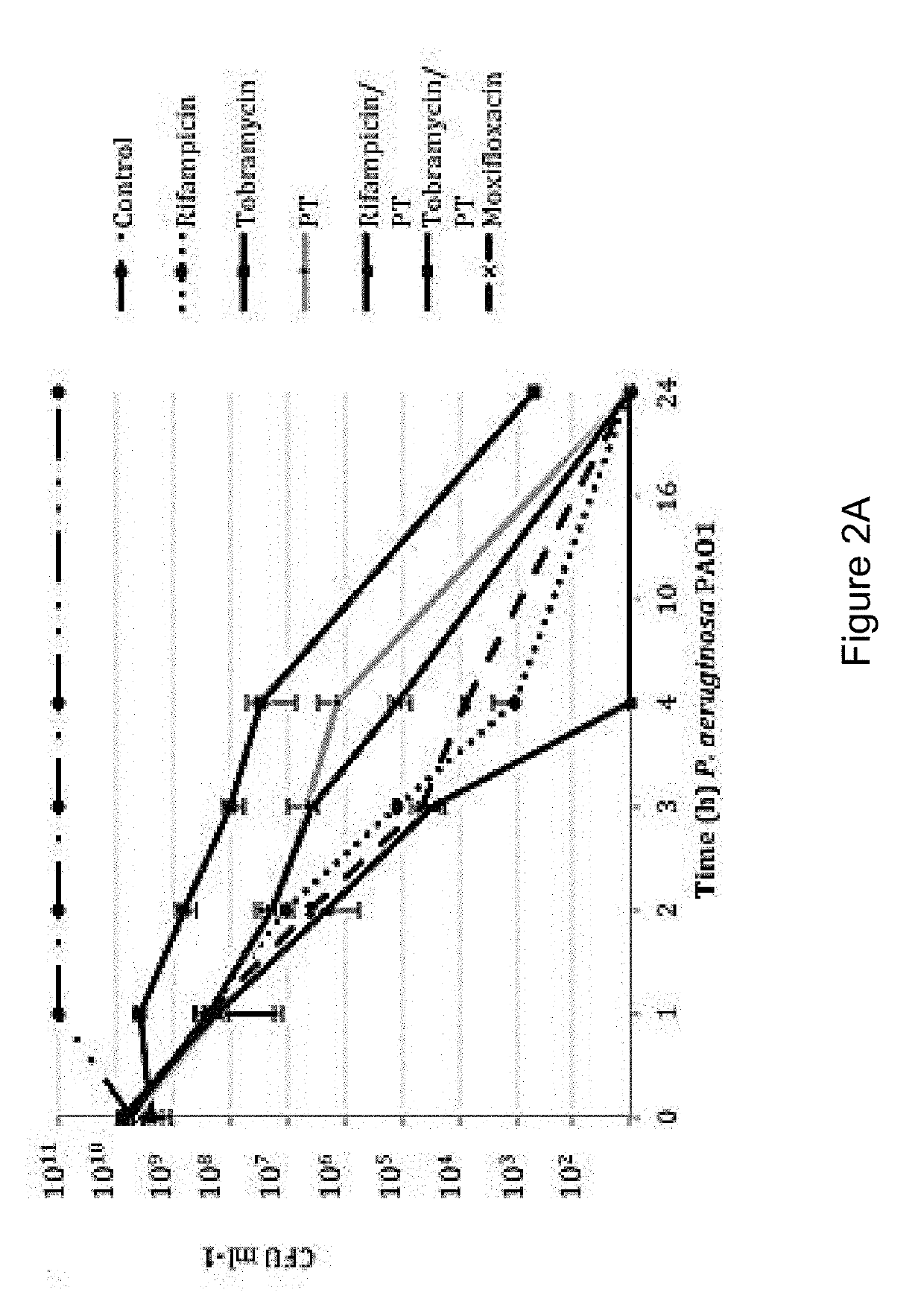
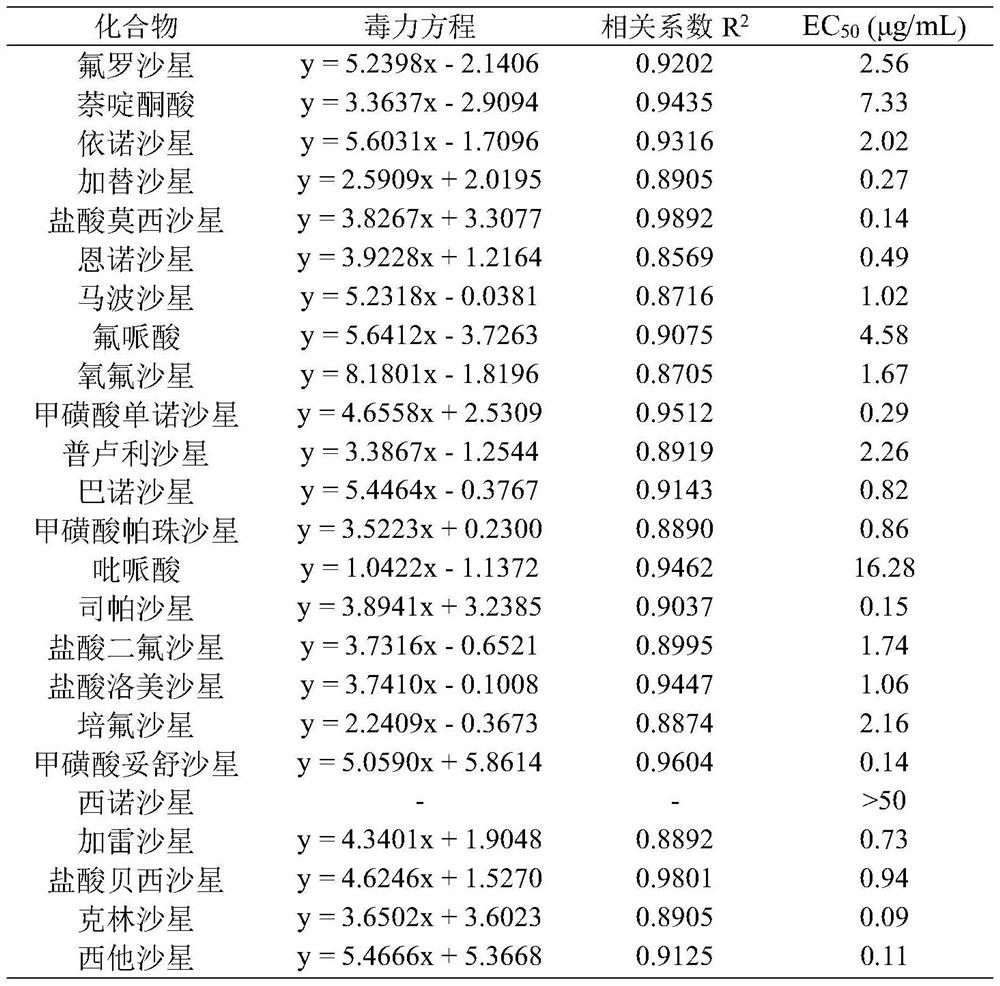
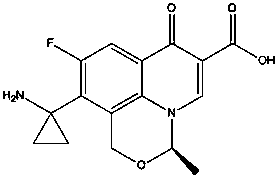
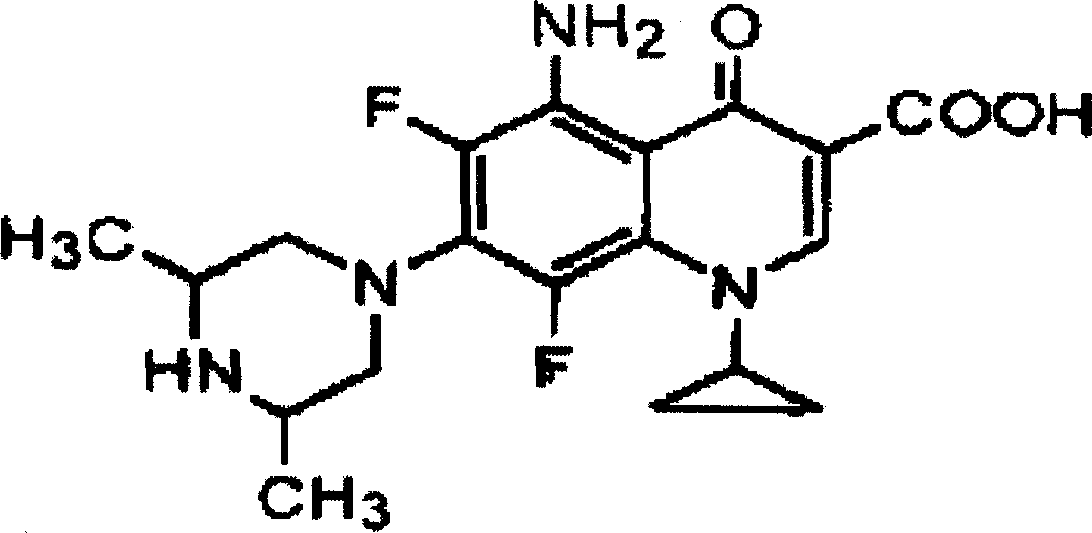
Sparfloxacin is a fluoroquinolone antibiotic used in the treatment of bacterial infections. It has a controversial safety profile. It was patented in 1985 and approved for medical use in 1993. Zagam is no longer available in the United States.
Water-soluble salt of aspartic acid carbostyril series antibacterial drugs and injection dosage forms thereof
The invention provides an aspartate quinolone antibiotics water-soluble salt and the injection formulation thereof, including sparfloxacin, gatifloxacin, rufloxacin, pefloxacin, tosufloxacin, moxifloxacin, and so on; the invention improves the water solubility of quinolone antibiotics and enhances the anti-bacterial effect of quinolone antibiotics. Compared with the oral liquid of quinolone drugs of the prior art, the invention has the advantages that: water solubility of the drug is good, as the drug enters the blood directly, the invention can not only achieve treatment function rapidly, but can also be absorbed by the human body fully, and the invention has significant effects in the two aspects of fast onset of action and low consumption. Compared with the injection of the quinolone drugs of the prior art, the invention has the advantages that: due to the existence of the L-aspartate, the antibacterial activity of quinolone drugs can be enhanced.
Owner:SHENYANG WOSEN PHARMA INST
Method for determining 10 quinolone antibiotics in bean sprouts by ultra-high performance liquid chromatography-tandem mass spectrometry
InactiveCN109406680AReduce distractionsEliminate the effects ofComponent separationFleroxacinAntibiotic Y
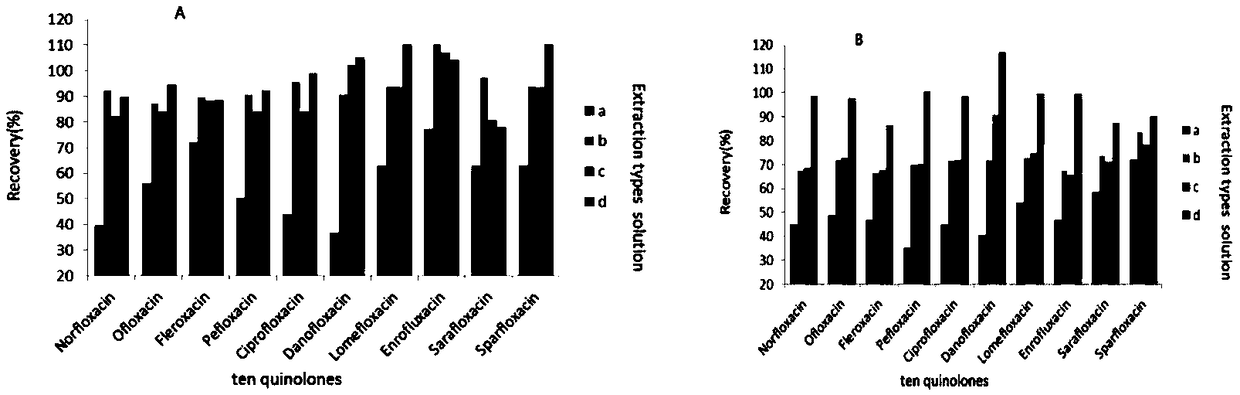
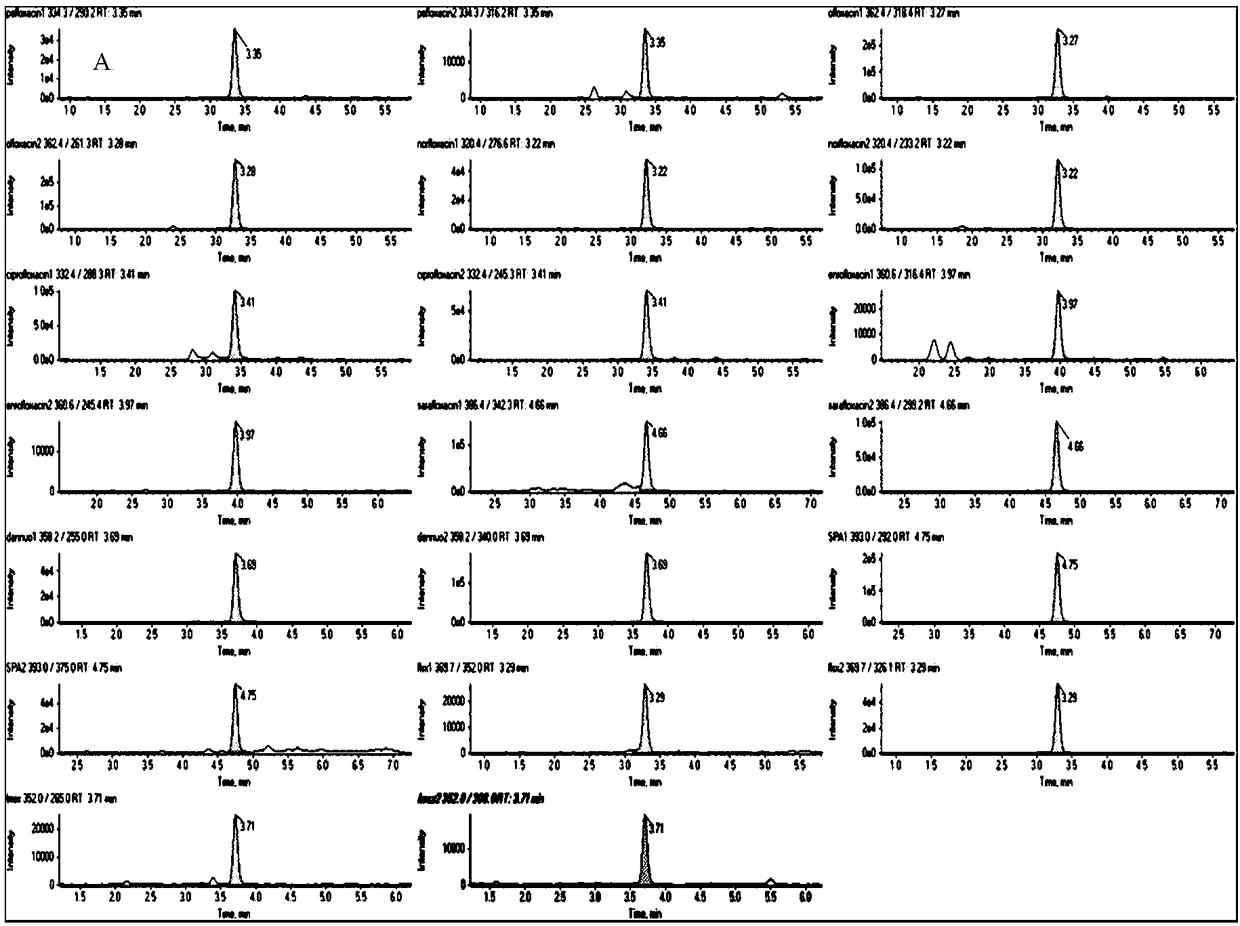
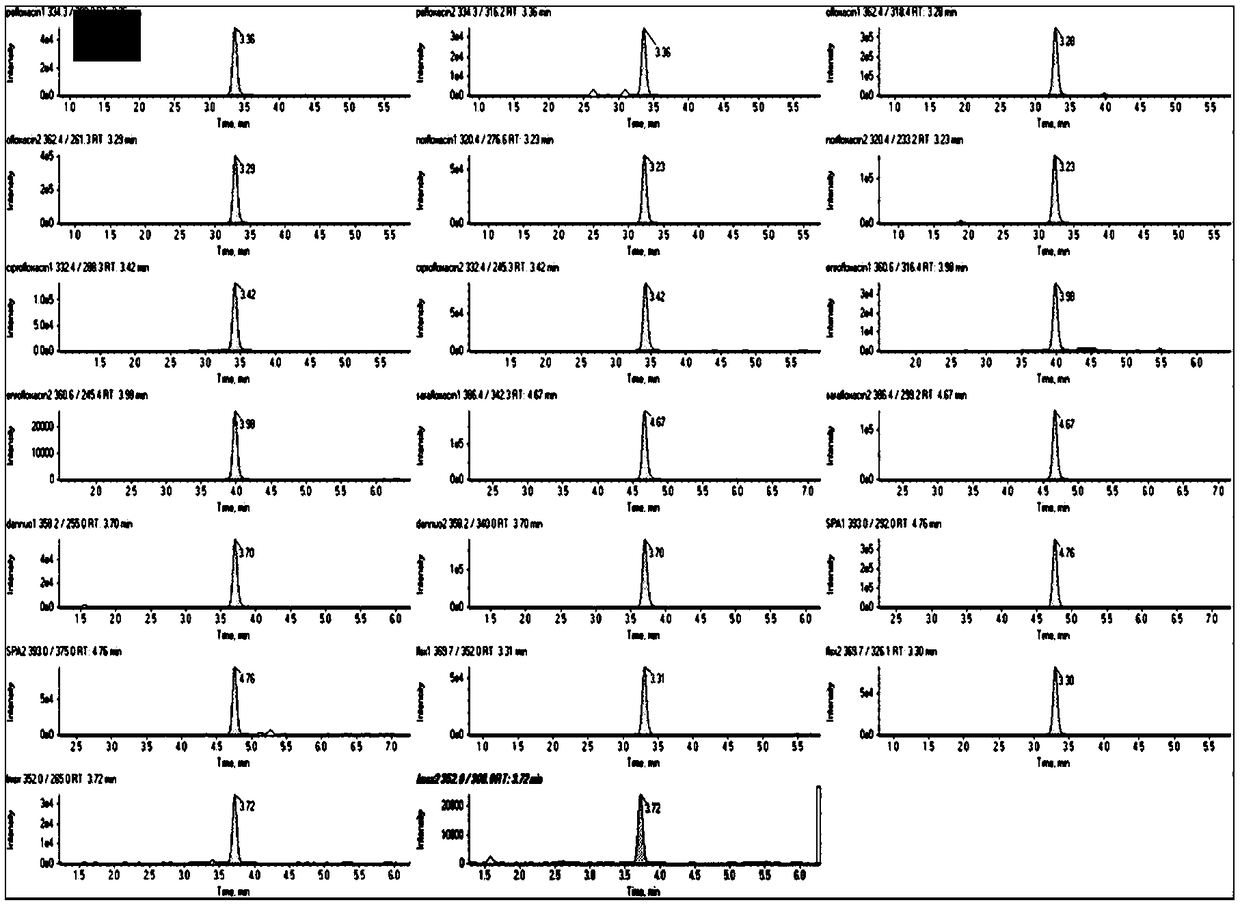
The invention discloses a method for determining 10 quinolone antibiotics in bean sprouts by an ultra-high performance liquid chromatography-tandem mass spectrometry, which is characterized by comprising the following steps of: preprocessing; preparing a standard solution; obtaining a liquid chromatography mass spectrogram and a regression equation; and analyzing and detecting samples by the ultra-high performance liquid chromatography-tandem mass spectrometry. A ultra-high performance liquid chromatography-electrospray tandem mass spectrometry is adopted in this study to carry out an analysisand determination of enrofloxacin, ciprofloxacin, norfloxacin, pefloxacin, ofloxacin, sarafloxacin, danofloxacin, sparfloxacin, fleroxacin and lomefloxacin in the bean sprouts. Sample extraction conditions, purification means, liquid chromatography-mass spectrum parameters and the like are optimized and the matrix effect of the method is evaluated. The result shows that an establishment of the method solves the problem of a simultaneous determination of various quinolone antibiotics in the bean sprouts and provides a technical reference for risk assessment and government supervision.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
Slow released medicine containing antituberculotic
InactiveCN1857218AAntibacterial agentsOrganic active ingredientsCarboxymethyl celluloseTreatment effect
The slow released implanting agent and injection containing antituberculotic are set or injected to local tuberculosis focus for maintaining the local effective medicine concentration while lowing the systemic toxicity. The slow released injection consists of slow released microsphere and solvent. The slow released microsphere includes antituberculotic selected from Cycloserine, Ofloxacin, Ciprofloxacin and Sparfloxacin and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The suspending agent is carboxymethyl cellulose sodium, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released implanting agent may be prepared with slow released microsphere. The present invention has obvious and unique treating effect on various kinds of intractable tuberculosis.
Owner:JINAN KANGQUAN PHARMA TECH
Anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and application thereof
ActiveCN106520704AHigh sensitivityBiological material analysisMicroorganism based processesOrbifloxacinEnzyme linked immunoassay
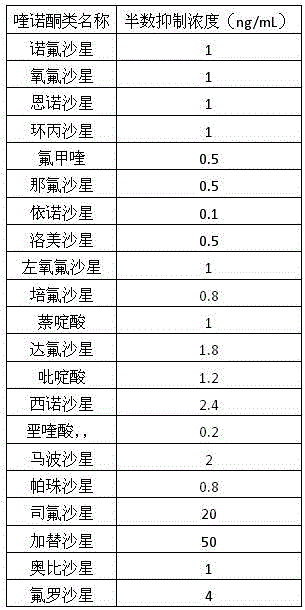
An anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and an application thereof belong to the technical field of immunochemistry. The monoclonal cell strain YH6 is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC No.12024. A monoclonal antibody secreted by the YH6 is detected by indirect competitive enzyme-linked immunosorbent assay, and has cross reaction with the following 21 pyrethroids: norfloxacin, ofloxacin, enrofloxacin, ciprofloxacin, flumequine, nafloxacin, enoxacin, lomefloxacin, levofloxacin, pefloxacin, nalidixic acid, danofloxacin, pyridine acid, cinoxacin, oxolinic acid, marbofloxacin, pazufloxacin, sparfloxacin, gatifloxacin, orbifloxacin and fleroxacin, and the IC50 value of the monoclonal antibody is 0.1-50 ng / mL. The class specific monoclonal antibody can be used for developing colloidal gold immunochromatographic test strips and immunosensors, provides a raw material for the immunodetection of quinolone antibiotic residues in foods, and has practical application values.
Owner:JIANGNAN UNIV
Slow released compound antituberculotic preparation containing synergist
The slow released compound antituberculotic preparation containing synergist is implanting agent or slow released injection comprising slow released microsphere and solvent. The slow released microsphere consists of slow releasing supplementary material, at least one antituberculotic selected from rifampicin, isoniazid and pyrazinamide and at least one antituberculotic synergist selected from cycloserine, ofloxacin, ciprofloxacin, sparfloxacin and capreomycin. The solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released compound antituberculotic preparation can release antituberculotic in the local tubercolosis part for 30-40 days so as to maintain the local effective medicine concentration while lowering systemic toxicity. The present invention has obvious unique treating effect on various kinds of intractable tuberulosis.
Owner:JINAN SHUAIHUA PHARMA TECH
Pharmaceutical Composition Containing Polymyxin B/Trimethoprim based Therapeutics
ActiveUS20190175550A1Increase virulenceAntibacterial agentsOrganic active ingredientsRifabutinTrimethoprim
The present invention features an antibacterial composition comprising 1) a composition A comprising polymyxin B and trimethoprim; and 2) an antibiotic agent selected from the group consisting of rifampicin, rifabutin, rifapentine, rifaximin, pefloxacin mesylate, sparfloxacin, sarafloxacin HCl, tobramycin, lomefloxacin, besifloxacin, danofloxacin mesylate, enrofloxacin, nadifloxacin and clinafloxacin, a topical pharmaceutical thereof, and a method of treating bacterial infections using mixtures of 1 and 2.
Owner:UNIVERSITY OF ROCHESTER
Ureaplasma urealyticum/mycoplasma hominis combined rapid culture and drug sensitivity detection kit
InactiveCN104988206AResolve detectionResolve accuracyMicrobiological testing/measurementMicroorganism based processesPenicillinArginine
Owner:姜洪波 +1
Sparfloxacin salt and synthesis method and use thereof
InactiveCN1117085CGuaranteed to be free from interferenceOrganic active ingredientsOrganic chemistrySynthesis methodsSparfloxacin
Owner:孙卫东
New application of quinolone compounds in prevention and treatment of plant bacterial diseases such as citrus canker
PendingCN111771895AStrong antibacterial activityHigh antibacterial activityBiocideDisinfectantsPipemidic acidFleroxacin
The invention discloses a new application of quinolone compounds as bactericides in prevention and treatment of bacterial diseases and citrus canker of crops. The quinolone compounds comprise floroxacin, enofloxacin, gatifloxacin, moxifloxacin hydrochloride, enrofloxacin, marbofloxacin, floxacin, mononorfloxacin mesylate, prulifloxacin, Balofloxacin, pazufloxacin mesylate, pipemidic acid, sparfloxacin, difloxacin hydrochloride, lomefloxacin hydrochloride, pefloxacin, tosufloxacin mesylate, Cinoxacin, galafloxacin, besifloxacin hydrochloride, ofloxacin, nalidixic acid, Clinafloxacin and Sitafloxacin. The quinolone compounds can be used for preventing and treating bacterial diseases caused by citrus canker pathogens, especially gatifloxacin, moxifloxacin hydrochloride, mononorfloxacin mesylate, sparfloxacin, tosufloxacin mesylate, clinafloxacin and sitafloxacin, has excellent bacteriostatic activity on citrus canker pathogens, and can be used for preventing and treating bacterial diseases of crops.
Owner:LANZHOU UNIVERSITY
Method for detecting sparfloxacin enantiomer in aquatic product
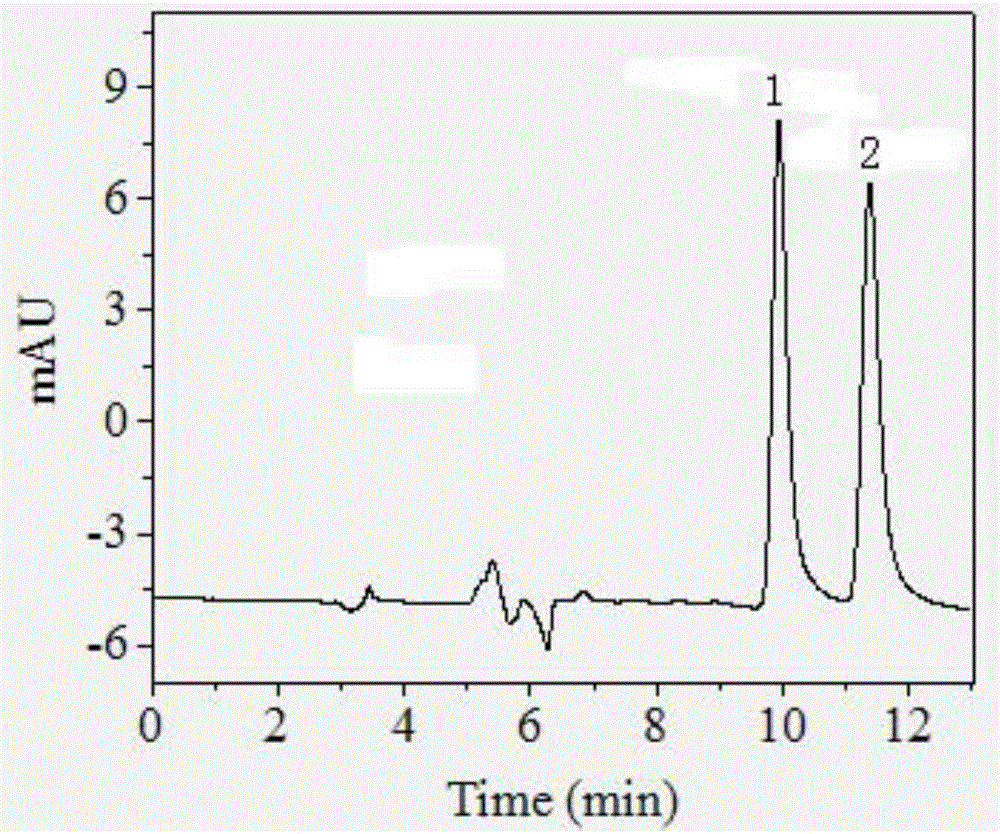
The invention relates to a method for detecting sparfloxacin enantiomer in an aquatic product. The solid-phase extraction conditions are optimized, so that the solid-phase extraction time is shortened, and the efficiency is high; besides, by means of the high performance liquid chromatography method, the concentration accuracy of sparfloxacin enantiomer is high, the speed is high, the operation is simple and the cost is lower; the detection method has good linear relation for a target compound, the recovery rate is high, relatively small deviation is caused, a used reagent is low-toxicity and small in dosage, and the method is environment-friendly.
Owner:ZHEJIANG OCEAN UNIV
Slow released antituberculotic preparation
InactiveCN1857714AIncrease concentrationReduce concentrationAntibacterial agentsOrganic active ingredientsMicrosphereWhole body
The slow released antituberculotic preparation contains the composition of ofloxacin, ciprofloxacin or sparxacin, capreomycin, sodium taurocholate and / or deoxysodium taurocholate. The slow released antituberculotic preparation is slow released injection or slow released implanting agent. The injection consists of slow released microsphere and solvent. The slow released microsphere contains slow releasing supplementary material and antituberculotic and the solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released implanting agent is prepared through a certain process. The present invention has obvious unique treating effect on various kinds of intractable tuberulosis.
Owner:JINAN SHUAIHUA PHARMA TECH
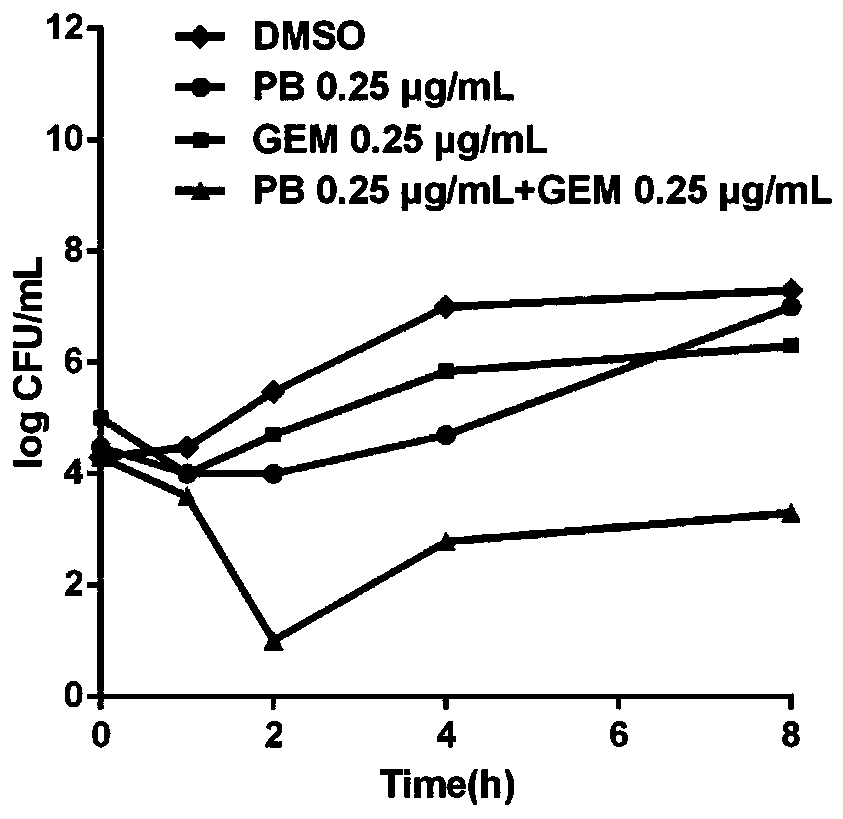
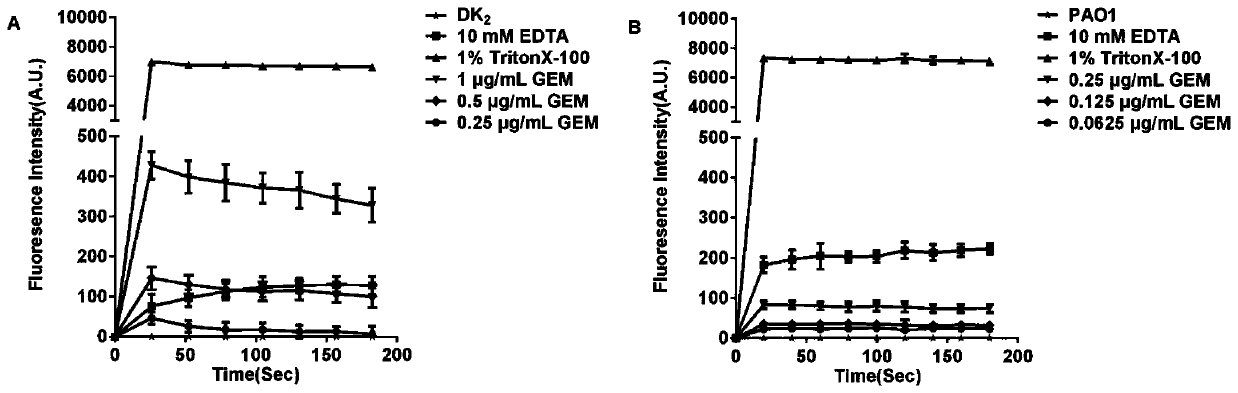
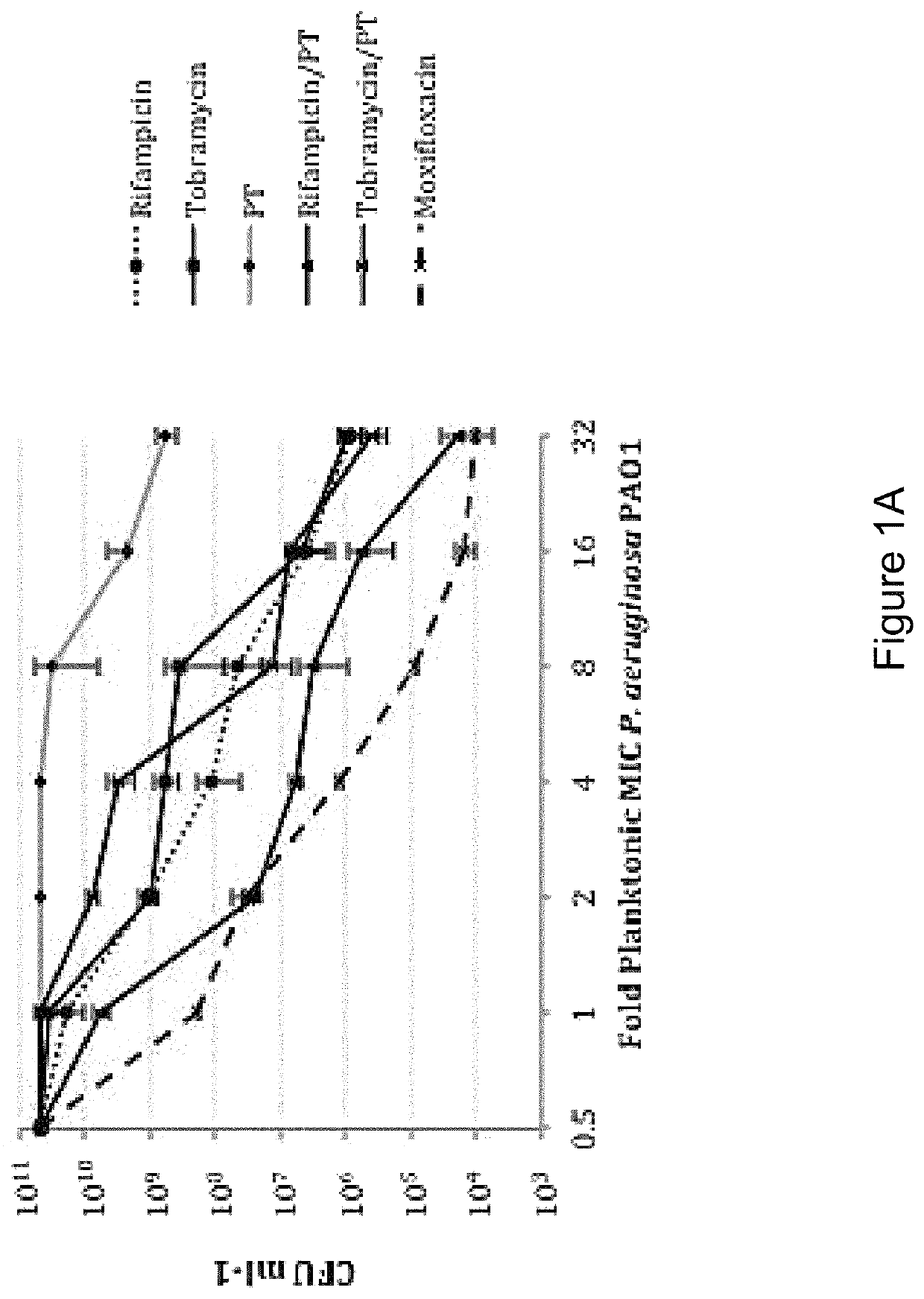
Application of fluoroquinolone medicine used as polymyxin-type antibiotic sensitizer
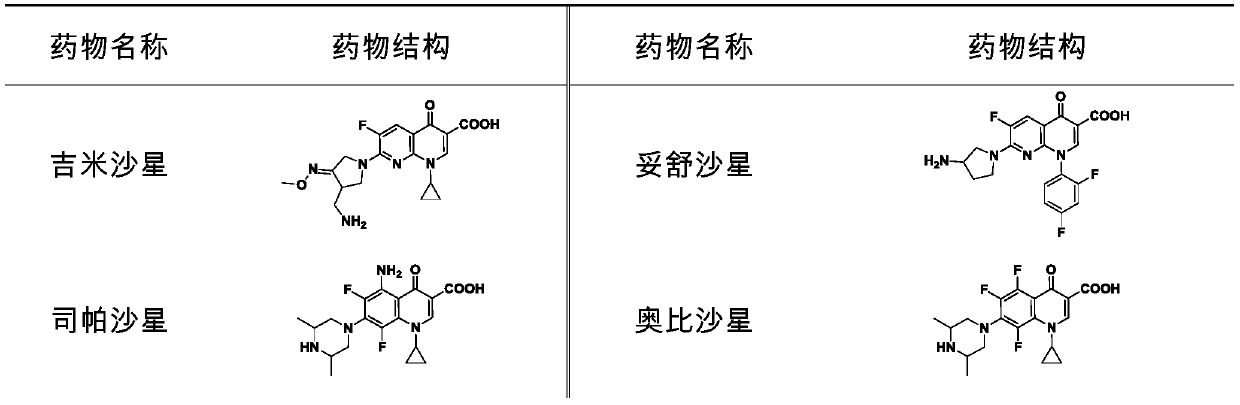
The invention discloses novel application of a fluoroquinolone medicine and application of the fluoroquinolone medicine in preparation of a sensitizer of a pseudomonas aeruginosa (P.aeruginosa) inhibitor. The fluoroquinolone medicine is prepared from gemifloxacin, sparfloxacin, enrofloxacin, ciprofloxacin, sarafloxacin, moxifloxacin, pefloxacin, tosufloxacin, orbifloxacin, prulifloxacin, marbofloxacin, levofloxacin, flumequine or / and pazufloxacin; the pseudomonas aeruginosa is pseudomonas aeruginosa DK2 or PAO1; the pseudomonas aeruginosa inhibitor is polymyxin B or colistin.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Method for preparing Sparfloxacin
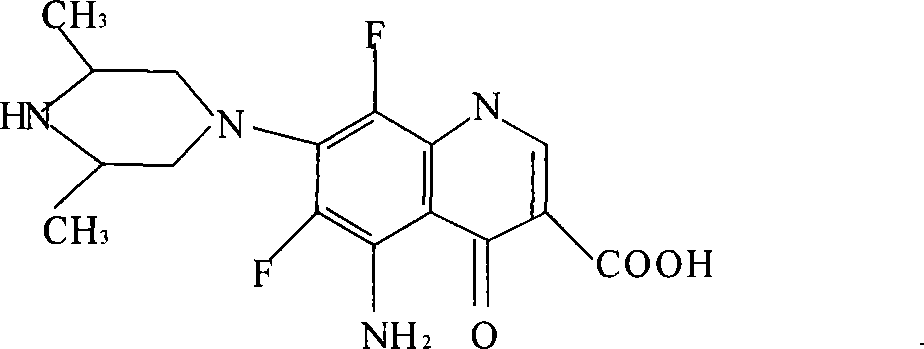
The invention discloses a method for preparing Sparfloxacin. The method comprises the steps of sequentially adding cis-2,6-dimethylpiperazine, 5-amino-1-cyclopropyl-6,7,8-trifluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid and an aprotic polar solvent into a reaction tank according to a feeding ratio, performing a condensation reaction so as to obtain a wet crude product of Sparfloxacin, carrying out baking on the wet crude product of Sparfloxacin so as to control the water content of the wet crude product of Sparfloxacin, adding the dried Sparfloxacin crude product, an alkaline aqueous solution, water, hydrochloric acid, EDTA and medicinal charcoal into a reaction tank according to a feeding ratio, carrying out heating and stirring, carrying out cooling after dissolving is completed, carrying out discharging, centrifuging and spin-drying so as to obtain a wet Sparfloxacin product, subjecting the wet Sparfloxacin product to drying and performing crushing, thereby obtaining a finished product. As a whole, the method has the advantages that the product is good in stability and easy to treat in medicine processing, and the preparation process is simple.
Owner:河南精康制药有限公司
Fructose injection of antibiotic medicine
InactiveCN108721625AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention relates to fructose injection of an antibiotic medicine. The fructose injection of the antibiotic medicine consists of antibiotics, fructose and water and also comprises proper additives, wherein the antibiotics comprise gatifloxacin, levofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin as well as medicinal acid addition salt, esterification compounds, derivatives and the like; the fructose injection is prepared from the antibiotics; the advantages that the injection is convenient to use and takes effect rapidly are achieved; and compared with the glucose injection, the fructose injection is easier to absorb and utilize, more suitable for antisepsis and anti-inflammation, energy supply and body liquid supplementation of patientssuffering from diabetes, heart diseases and liver diseases, and enlarges the use range.
Owner:WEIHAI HAOTONG MEDICAL SCI & TECH
Medicinal preparation for resisting intracellular mycoplasma infection and application thereof
PendingCN113980889AGood broad-spectrum antibacterial effectImprove the bactericidal effectCulture processArtificial cell constructsBiotechnologyCell culture media
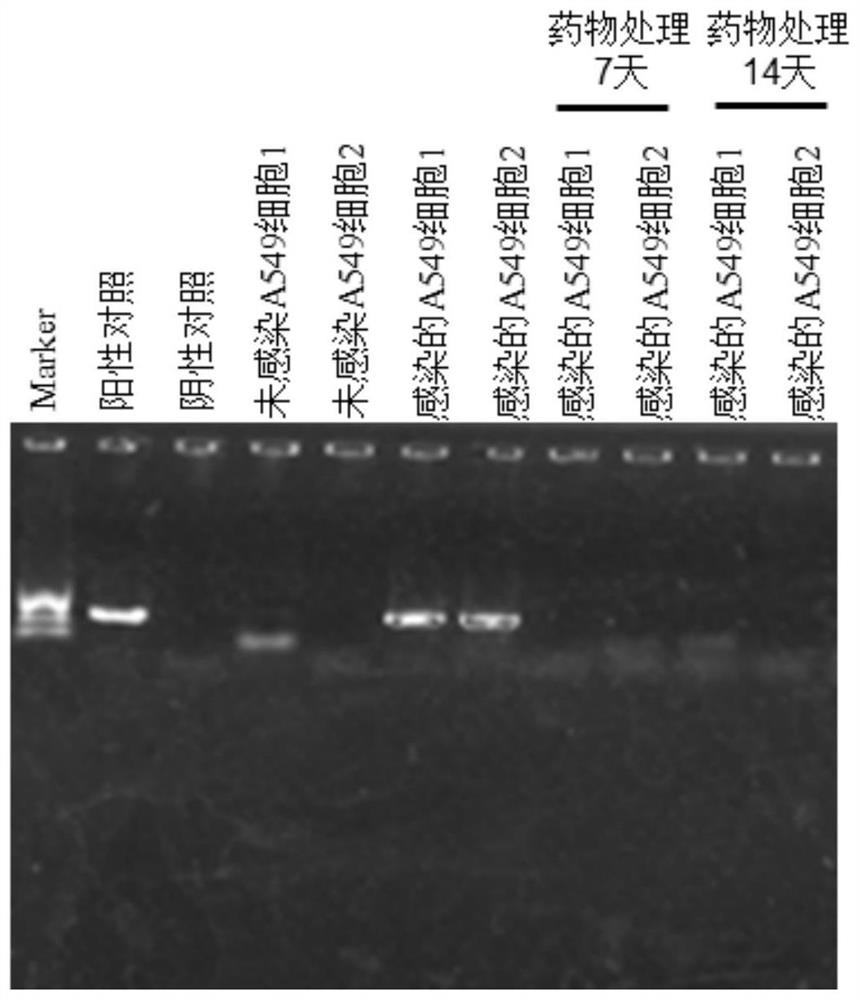
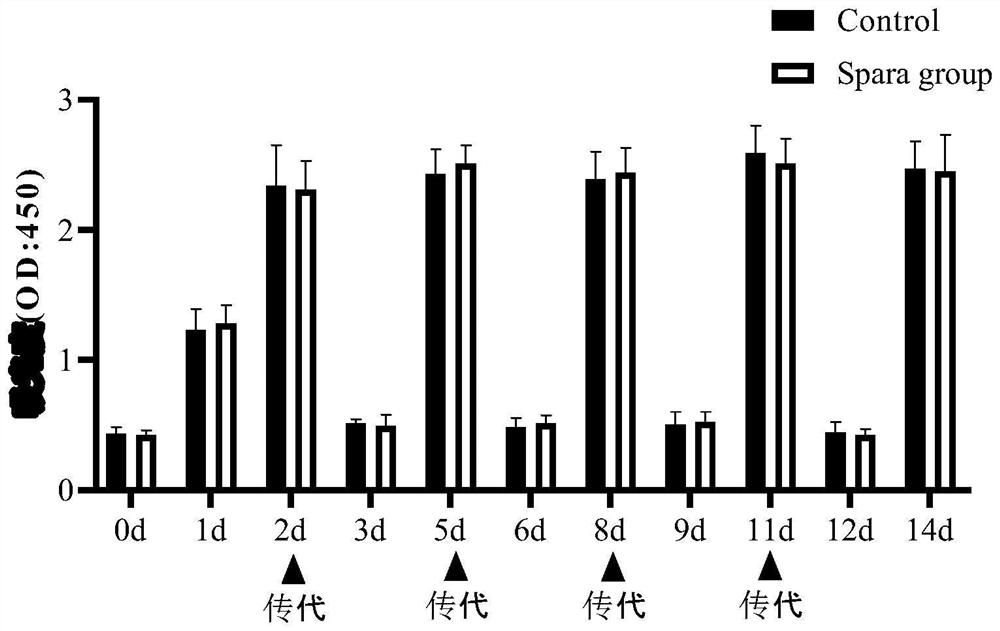
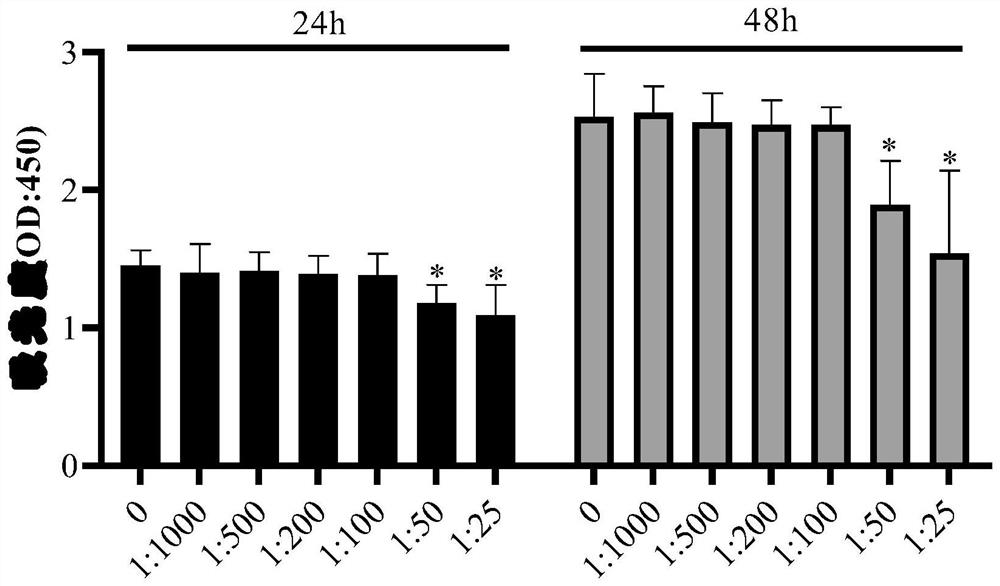
The invention discloses a medicinal preparation for resisting intracellular mycoplasma infection and an application thereof, and belongs to the technical field of medicines. The pharmaceutical preparation is a sparfloxacin solution with the final concentration of 10 mg / ml, is obtained by dissolving sparfloxacin in a 0.1 mol / l NaOH solution and sequentially filtering through a 0.45 [mu]m filter and a 0.22 mu m filter, is used for preventing cells from infecting mycoplasma and removing the mycoplasma infecting the cells during cell culture, and is continuously used for 7 days every 1-2 days according to the effective use dosage of 0.01-0.1 mg sparfloxacin / ml cell culture medium. The sparfloxacin active compound is dissolved in the NaOH solution to prepare the solution preparation by adopting an alkali solution dissolution method, the solution preparation can replace mycillin to be used for preventing cells from infecting mycoplasmas and removing the mycoplasmas infecting the cells during cell culture, the antibacterial effect is good, toxicity and drug resistance are low, damage to the cells is small while the mycoplasmas are effectively killed, and the mycoplasma can be prevented from polluting the cells again.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Polypyrrole/cadmium sulfide imprinted composite photocatalyst as well as preparation method and application thereof
ActiveCN111495432AStrong photocatalytic degradation functionEfficient degradationWater/sewage treatment by irradiationWater treatment compoundsSparfloxacinPolypyrrole
The invention discloses a polypyrrole / cadmium sulfide imprinted composite photocatalyst which comprises a crystalline cadmium sulfide photocatalyst serving as a carrier and a polypyrrole imprinted layer formed on the surface of the carrier. The polypyrrole imprinted layer is internally provided with holes formed by taking sparfloxacin as template molecules. The invention also discloses a preparation method and application of the photocatalyst, and a method for degrading sparfloxacin. The polypyrrole / cadmium sulfide imprinted composite photocatalyst provided by the invention has strong stability and can selectively and effectively degrade sparfloxacin.
Owner:SHENZHEN POLYTECHNIC
Sparfloxacin hemisulphate, its preparation method and application
InactiveCN102367248BEasy to handleImprove stabilityAntibacterial agentsOrganic active ingredientsSocial benefitsSparfloxacin
The invention discloses Sparfloxacin hemisulphate, its preparation method and application. Characterized by clear crystal form, good stability, easy treatment during medicament processing and simple preparation technology, the Sparfloxacin hemisulphate provided in the invention has substantial economic and social benefits.
Owner:FUJIAN AGRI & FORESTRY UNIV
Fructose injection of antibiotic drug
InactiveCN106668862AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention provides a fructose injection of an antibiotic drug. The injection is composed of antibiotics, fructose and water. The injection also can contain proper additives. The antibiotic comprise gatifloxacin, levofloxacin, ofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin and medicinal acid additive salts, esterified compounds, derivatives and the like. The antibiotics are prepared into the fructose injection. Besides the advantages of convenience in use and quickness to take effect of the injection, compared with a gluconic infection, the injection provided by the invention is more easily absorbed and utilized, is more suitable for preventing bacteria and diminishing inflammation, supplying energy and supplementing body fluids for patients with diabetes, heart disease and liver disease, so that the application range of the injection is expanded.
Owner:威海恒基伟业信息科技发展有限公司
Sparfloxacin compound combination preparation and preparation method thereof
InactiveCN102188430BPromote dissolutionImprove stabilityAntibacterial agentsOrganic active ingredientsSparfloxacinArginine
The invention provides a Sparfloxacin compound combination preparation comprising Sparfloxacin, arginine and citruline, and the weight of the citruline is half that of the arginine. The Sparfloxacin compound combination preparation is good in quality stability and dissolving effect and suitable for wide crowds. The invention further provides a preparation method of Sparfloxacin capsules and Sparfloxacin tablets, which is simple in process and easy to implement.
Owner:广东好药多医药有限公司
Orbifloxacin synthesis method
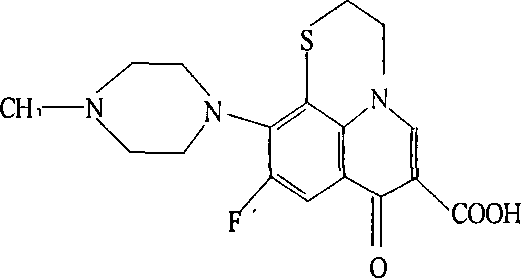
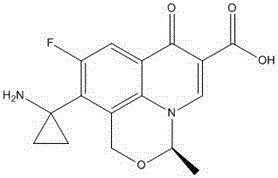
The present invention discloses an orbifloxacin synthesis method, which comprises: adopting sparfloxacin as a raw material, carrying out a diazotization reaction under the effect of hydrochloric acid and sodium nitrite to obtain a diazonium salt solution, adding tetrafluoroboric acid to the diazonium salt solution in a dropwise manner, carrying out a fluorization reaction at a temperature of -10 DEG C, filtering the reaction solution after completing the reaction, taking the filter cake, washing with ethyl ether, drying, heating the dried product until no gas is generated to obtain an orbifloxacin crude product, and purifying the orbifloxacin crude product to obtain the orbifloxacin. According to the present invention, the sparfloxacin is utilized as the starting raw material and the diazotization reaction and the fluorization reaction are used to prepare the orbifloxacin, such that advantages of new process route, high product yield, low reaction cost and short reaction time are provided, and the method is the new economical and practical technology.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Organic composite photocatalyst for degrading drugs and pathogenic bacteria and preparation method thereof
ActiveCN113058648BPromote absorptionImprove adsorption capacityWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsSuperoxide radicalTrimethoprim
The invention discloses an organic composite photocatalyst for degrading medicines and pathogenic bacteria and a preparation method thereof, and belongs to the technical field of water pollutant treatment. In the method of the present invention, a simple green method is used to load cercosporin on the macroporous adsorption resin to obtain a supported photocatalyst. It can generate singlet oxygen, superoxide radicals, etc. under the irradiation of fluorescent lamps and even sunlight. These oxygen free radicals can react with drugs and pathogenic bacteria, so as to achieve the purpose of degrading drugs and pathogenic bacteria. The method can completely degrade drugs within 3 hours of reaction under light, these drugs include: antibacterial drugs: quinolones ciprofloxacin, gatifloxacin, moxifloxacin, ofloxacin, enrofloxacin , sparfloxacin, trimethoprim sulfonamides, and antiviral drugs: chloroquine phosphate. In addition, the photocatalyst can also effectively inhibit the pathogenic bacteria Staphylococcus aureus, realizing the simultaneous removal of drugs and pathogenic bacteria in water pollution.
Owner:JIANGNAN UNIV
A group-selective monoclonal antibody hybridoma cell line yh6 against quinolone antibiotics and its application
ActiveCN106520704BHigh sensitivityBiological material analysisMicroorganism based processesOrbifloxacinNorfloxacin
An anti-quinolone antibiotic class specific monoclonal antibody hybridoma cell strain YH6 and an application thereof belong to the technical field of immunochemistry. The monoclonal cell strain YH6 is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC No.12024. A monoclonal antibody secreted by the YH6 is detected by indirect competitive enzyme-linked immunosorbent assay, and has cross reaction with the following 21 pyrethroids: norfloxacin, ofloxacin, enrofloxacin, ciprofloxacin, flumequine, nafloxacin, enoxacin, lomefloxacin, levofloxacin, pefloxacin, nalidixic acid, danofloxacin, pyridine acid, cinoxacin, oxolinic acid, marbofloxacin, pazufloxacin, sparfloxacin, gatifloxacin, orbifloxacin and fleroxacin, and the IC50 value of the monoclonal antibody is 0.1-50 ng / mL. The class specific monoclonal antibody can be used for developing colloidal gold immunochromatographic test strips and immunosensors, provides a raw material for the immunodetection of quinolone antibiotic residues in foods, and has practical application values.
Owner:JIANGNAN UNIV
Slow released capsule of sparfloxacin and bletilla tuber glue for animal and birds
InactiveCN1985957AExtended stayGood treatment effectAntibacterial agentsOrganic active ingredientsDiseaseBletilla striata
The present invention belongs to the field of veterinary medicine technology, and is especially slow released veterinary medicine capsule prepared with sparfloxacin and bletilla tuber as material. The preparation process includes the following steps: water extracting and alcohol precipitation to extract bletilla tuber glue; mixing sparfloxacin and bletilla tuber in the ratio of 1 to 3 and 10 % concentration alcohol solution of PVP as adhesive; making pellet of 20 meshes and encapsulating. The slow released veterinary medicine capsule has long medicine retaining time in the disease focus, high local medicine concentration, excellent bioadhesion and obvious medicine slow releasing characteristic.
Owner:TIANJIN RINGPU BIO TECH
Preparation method of slow-release sparfloxacin injection
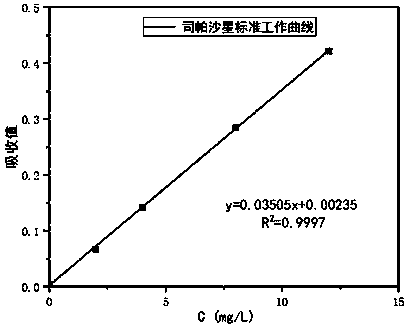
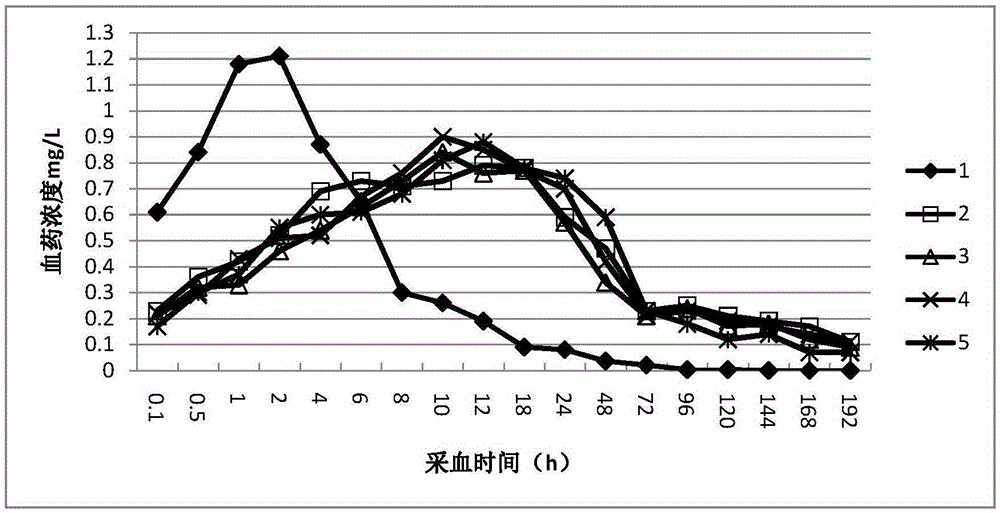
InactiveCN105362219AExtended release timeReduce emergency responseAntibacterial agentsOrganic active ingredientsBlood concentrationOrganic solvent
The invention provides a preparation method of a slow-release sparfloxacin injection and relates to a slow-release injection for animals. The sparfloxacin injection comprises components in percentage by weight as follows: 5%-20% of sparfloxacin, 1%-4% of fatty acid, 5%-35% of a stabilizer and the balance of an organic solvent. The sparfloxacin and the organic solvent are taken and stirred to be dissolved, the fatty acid is added to have a complex reaction with a dissolution product, the stabilizer is added and fully dissolved, the organic solvent is added until the mixture reaches a certain volume and is subjected to membrane filtration, and the slow-release sparfloxacin injection is prepared. New composition is formed after sparfloxacin, salt of sparfloxacin or hydrate of sparfloxacin has a reaction with the fatty acid, the release time of sparfloxacin can be prolonged, the effective blood concentration of the medicine in the animals is maintained for a long time, and accordingly, the purposes of reducing administration frequency and stress due to the medicine are achieved.
Owner:TIANJIN RINGPU BIO TECH
Fructose injection of antibiotic drug
InactiveCN109953943AEasy to useSuitable for useAntibacterial agentsOrganic active ingredientsFluconazoleSupplement use
A fructose injection of an antibiotic drug comprises antibiotics, fructose and water and can contain proper additives. The antibiotics comprise gatifloxacin, levofloxacin, ofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, cadrofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin as well as pharmaceutical acid added salts, esters and derivatives thereof. The antibiotics are prepared into the fructose injection, beside injection advantages of being convenient to use and taking effect quickly, compared with glucose injections, the injection is easier to absorb and utilize and more suitable for antisepsis andanti-inflammation, energy supply and body fluid supplement use for patients suffering from diabetes, heart disease and hepatopathy, and the use range of the injection is enlarged.
Owner:李芳凯
Pharmaceutical composition containing polymyxin B/trimethoprim based therapeutics
ActiveUS11096923B2Increase virulenceAntibacterial agentsOrganic active ingredientsRifabutinTrimethoprim
Owner:UNIVERSITY OF ROCHESTER
Sparfloxacin hemisulphate, its preparation method and application
InactiveCN102367248AEasy to handleImprove stabilityAntibacterial agentsOrganic active ingredientsMedicineCrystal
The invention discloses Sparfloxacin hemisulphate, its preparation method and application. Characterized by clear crystal form, good stability, easy treatment during medicament processing and simple preparation technology, the Sparfloxacin hemisulphate provided in the invention has substantial economic and social benefits.
Owner:FUJIAN AGRI & FORESTRY UNIV
Organic composite photocatalyst for degrading drugs and pathogenic bacteria and preparation method thereof
ActiveCN113058648APromote absorptionImprove adsorption capacityWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsSuperoxide radicalSinglet oxygen
The invention discloses an organic composite photocatalyst for degrading drugs and pathogenic bacteria and a preparation method of the organic composite photocatalyst, and belongs to the technical field of treatment of pollutants in water. According to the method disclosed by the invention, the loaded photocatalyst is obtained by loading the cephalosporin onto the macroporous adsorption resin through a simple green method. The photocatalyst can generate singlet oxygen, superoxide free radicals and the like even under the irradiation of sunlight and a fluorescent lamp. The oxygen free radicals can react with drugs and pathogenic bacteria, so that the purpose of degrading the drugs and the pathogenic bacteria is achieved. According to the method, the drugs can be completely degraded within 3 hours under illumination, and the drugs comprise antibacterial drugs such as quinolone ciprofloxacin, gatifloxacin, moxifloxacin, ofloxacin, enrofloxacin and sparfloxacin, sulfonamide trimethoprim and antiviral drugs such as chloroquine phosphate. In addition, the photocatalyst can effectively inhibit pathogenic bacteria staphylococcus aureus, and simultaneous removal of drugs and pathogenic bacteria in water pollution is achieved.
Owner:JIANGNAN UNIV
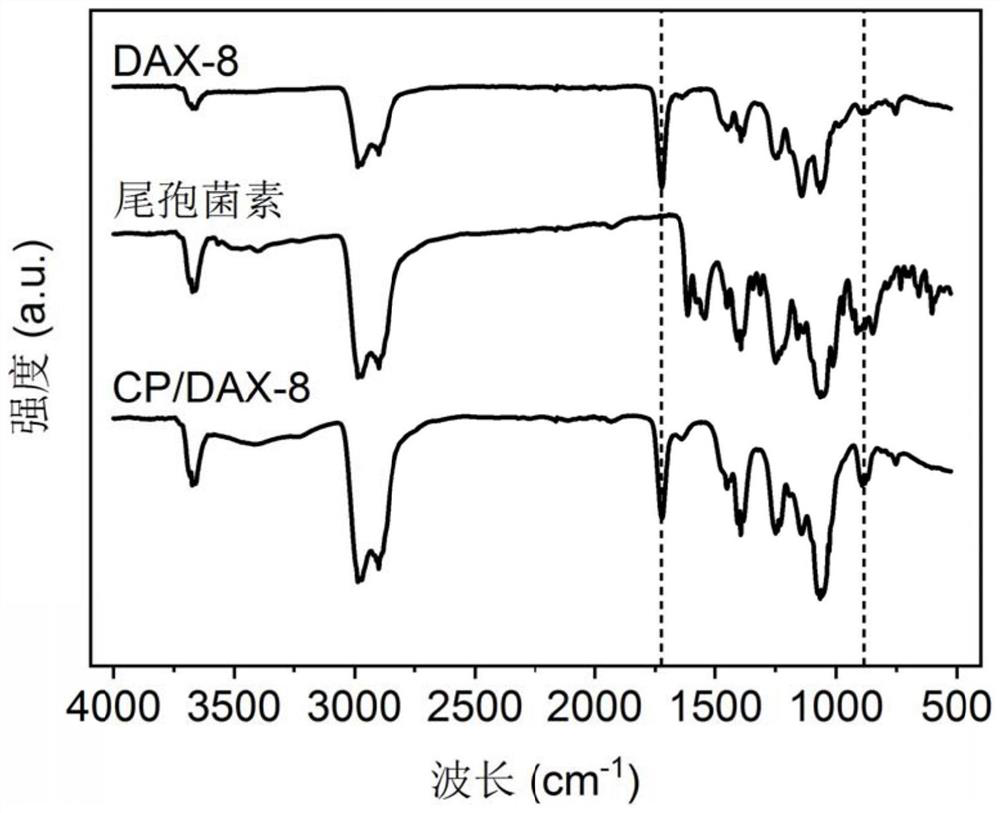
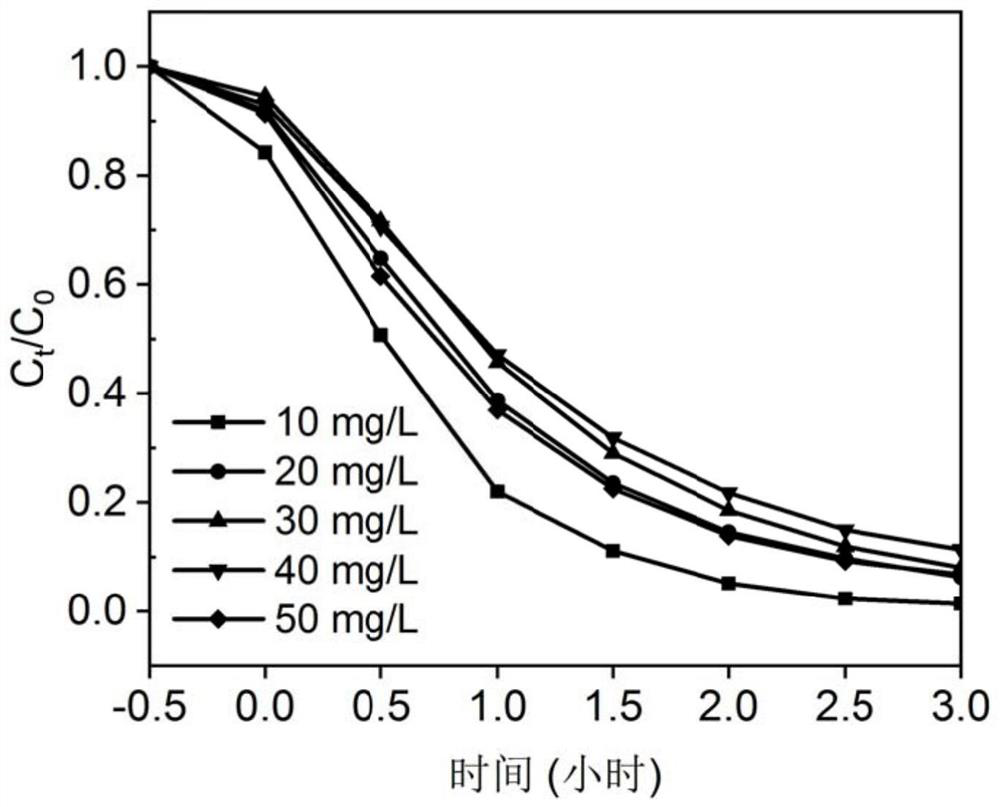
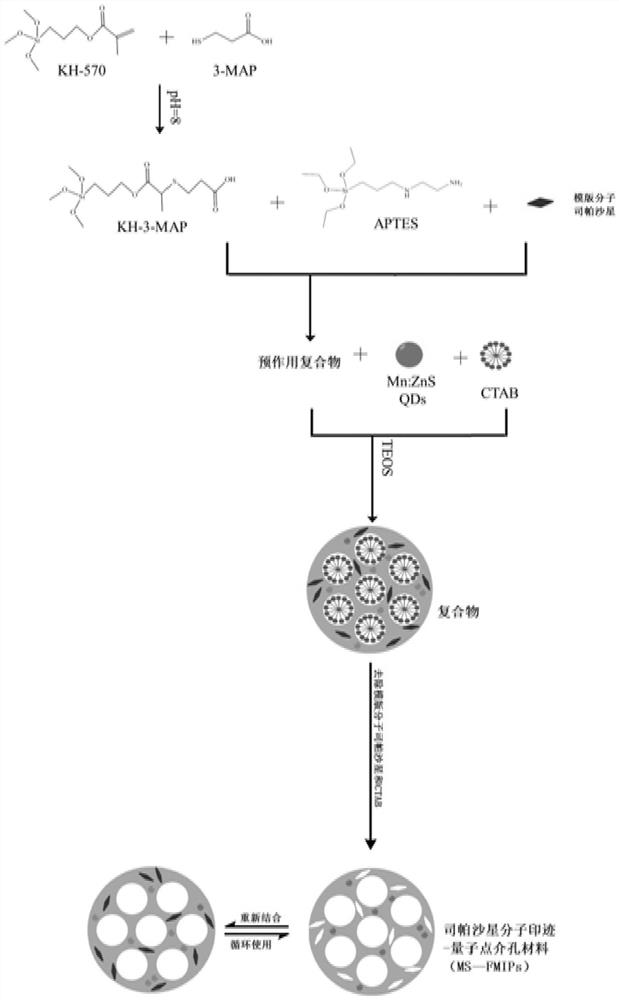
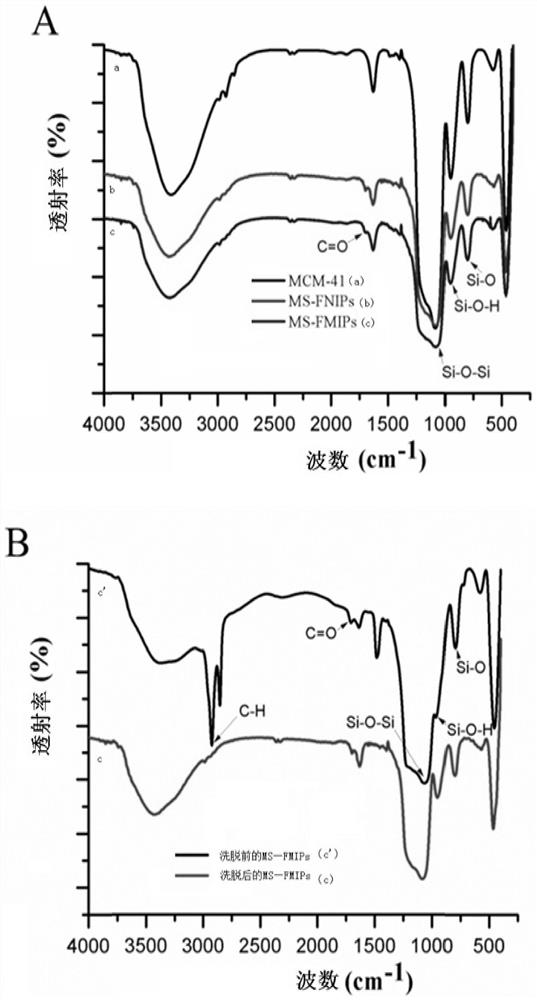
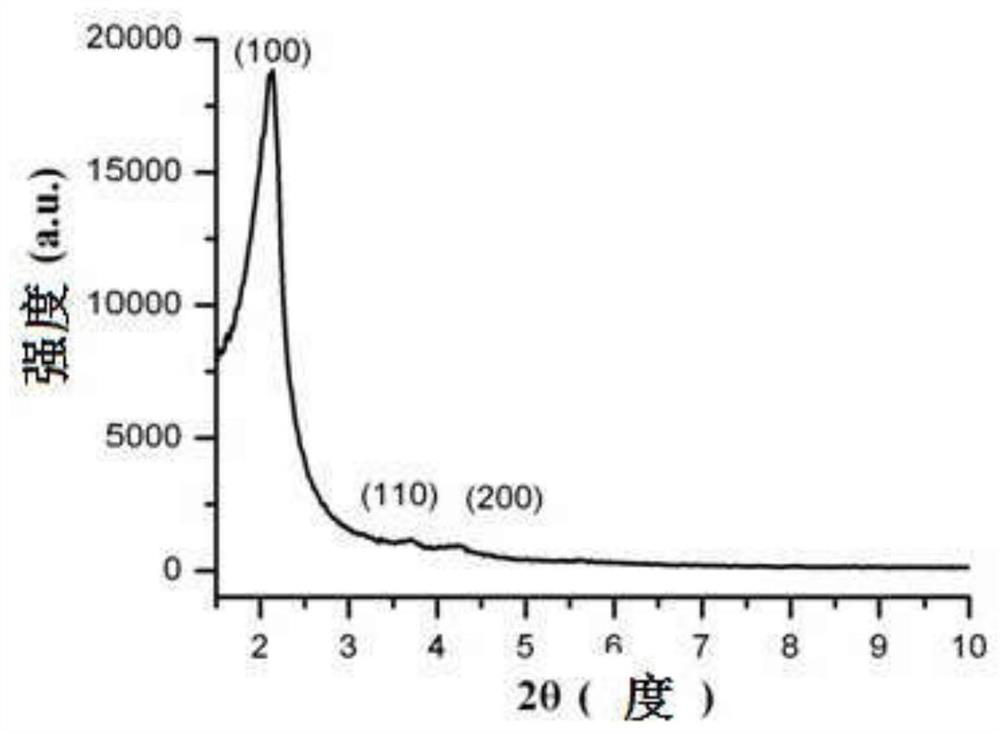
A kind of sparfloxacin molecularly imprinted-quantum dot mesoporous material and its preparation method and application
ActiveCN108318461BEasy to eluteMild reaction conditionsFluorescence/phosphorescencePropanoic acidActive agent
The invention provides a preparation method of a sparfloxacin molecular imprinting-quantum dot mesoporous material. The preparation method comprises the following steps: firstly, preparing a preactioncompound from a template molecule sparfloxacin, 3-aminopropyl triethoxy silane and propionic acid sulfenyl methyl acetoxyl propyl trimethoxy silane; secondly, preparing a compound from a surfactant,a cross-linking agent, the preaction compound and L-cysteine modified Mn doped ZnS quantum dots; finally, deluting the template molecule sparfloxacin and the surfactant in the compound, thereby obtaining the sparfloxacin molecular imprinting-quantum dot mesoporous material. The invention further provides the sparfloxacin molecular imprinting-quantum dot mesoporous material prepared by using the preparation method, application of the material in detecting the content of sparfloxacin in a sample, and particularly application of the material in detecting the content of sparfloxacin in serum. Thepreparation method provided by the invention is simple in step, and by adopting the molecular imprinting-quantum dot mesoporous material, sparfloxacin in a sample can be rapidly, sensitively and specifically detected.
Owner:SOUTH CHINA NORMAL UNIVERSITY +1
Lung-targeting sparfloxacin microsphere for animal and birds and its preparing method
InactiveCN1985824AReduce releaseGood curative effectAntibacterial agentsOrganic active ingredientsSide effectMicrosphere
The present invention belongs to the field of veterinary medicine technology, and is especially lung targeting sparfloxacin microsphere for animal and its preparation process. The sparfloxacin microsphere is prepared with sparfloxacin as medicine component and gelatin as carrier in the weight ratio of 1 to 2, and through dissolving sparfloxacin in gelatin solution and adding Span-80 and liquid paraffin through stirring to obtain emulsion; cooling in icy bath to below 5deg.c and adding glutaraldehyde through stirring for cross-linking and curing; dewatering with isopropyl alcohol and suction filtering; washing with isopropyl alcohol and ethyl ether to eliminate glutaraldehyd, washing with petroleum ether to eliminate liquid paraffin in the surface of microsphere and vacuum drying at room temperature to obtain sparfloxacin microsphere. The medicine has raised tissue selectivity, delayed release, raised curative effect and lowered toxic side effect.
Owner:TIANJIN RINGPU BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com