Application of fluoroquinolone medicine used as polymyxin-type antibiotic sensitizer
A technology of fluoroquinolones and polymyxins, which is applied to medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., and can solve problems such as high cost and long research and development cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
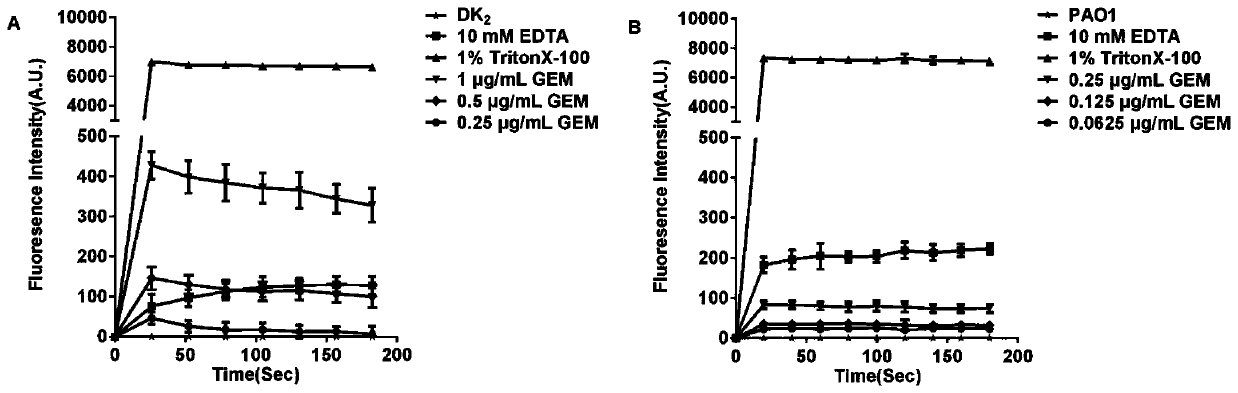
[0024] Fluoroquinolone-sensitizing polymyxin B (Polymyxin B) produces a checkerboard experiment on the growth inhibition of DK2.
[0025] (1) Experimental materials and methods
[0026] DK2 was cultured overnight and diluted with fresh LB medium to an absorbance (OD600) of about 0.01. Add 100 μL of bacterial suspension to a 96-well plate, then add corresponding concentrations of fluoroquinolones and polymyxin B, and incubate the plate at 37°C for 18 hours. Fractional inhibitory concentration (FIC) was determined by checkerboard. The minimum inhibitory concentration (Minimum Inhibitory Concentration, abbreviated as "MIC") of each drug is the concentration of the drug when the sterile growth is visible to the naked eye. The FIC for each drug was calculated as (MIC of agents when used in combination) / (MIC of agents when used alone). The FIC index (FICI) is the sum of the FICs of fluoroquinolones and polymyxin B.
[0027] (2) Experimental results
[0028] The results are show...
Embodiment 2
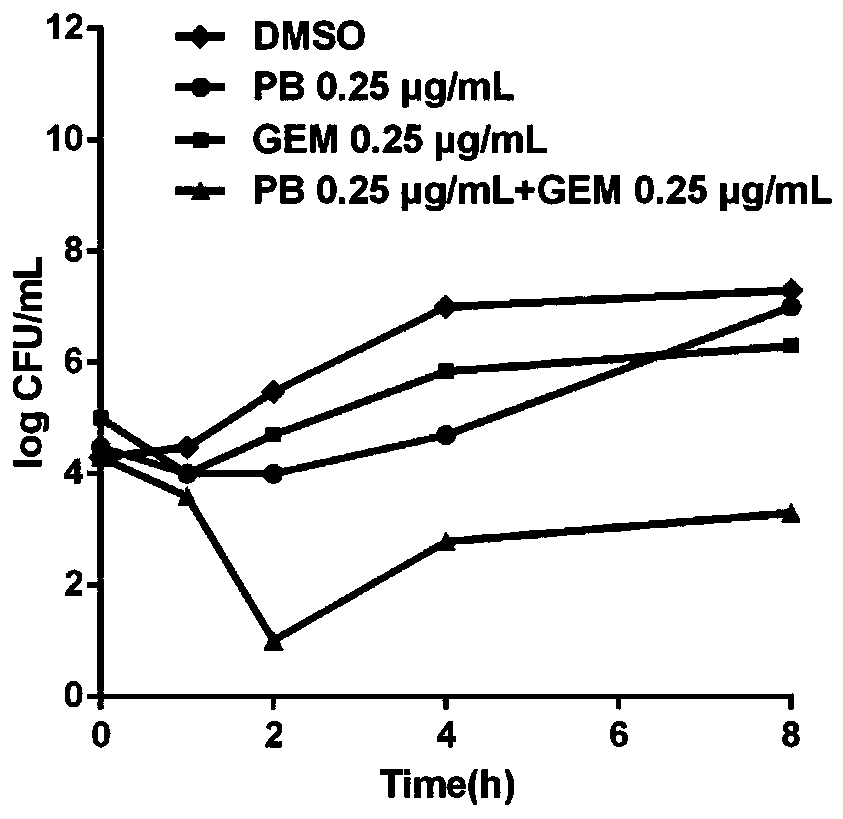
[0042] Gemifloxacin sensitized polymyxin B inhibited DK2 growth in a concentration-dependent experiment.
[0043] (1) Experimental materials and methods: the same as "Example 1".
[0044] (2) Experimental results: See Table 3 for the results.
[0045] table 3
[0046]
[0047] a Refers to the MIC of polymyxin B for DK2 when combined with the corresponding concentration of gemifloxacin.
[0048] It can be seen from Table 3 that gemifloxacin can sensitize polymyxin B to DK2 in a concentration-dependent growth inhibitory effect, and the sensitization effect of gemifloxacin is 1 μg / mL is the best.
Embodiment 3
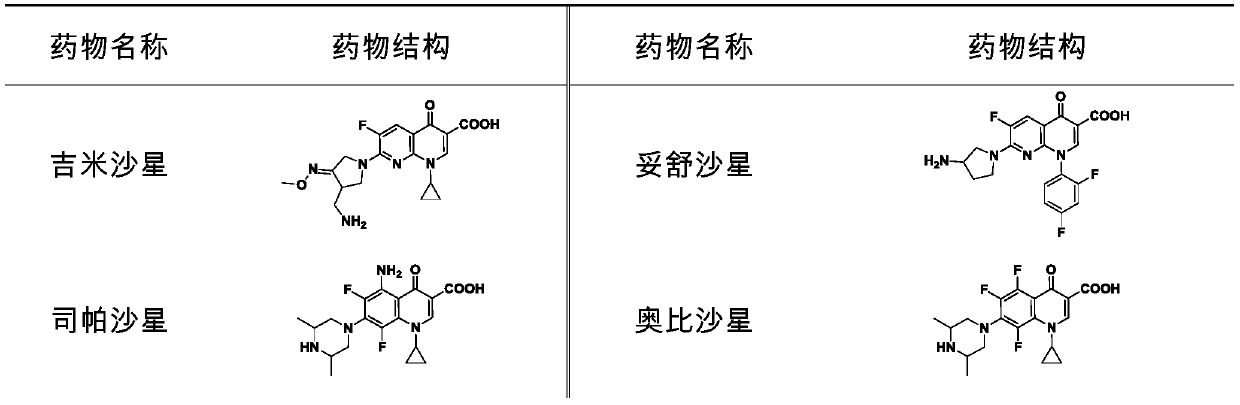
[0050] Gemifloxacin, enrofloxacin and sparfloxacin-sensitized colistin (Colistin) have growth inhibitory effects on DK2.
[0051] (1) Experimental materials and methods: the same as "Example 1". For DK2, when colistin was used alone, the MIC was >1024 μg / mL (indicating that DK2 was also severely resistant to colistin).
[0052] (2) Experimental results: the results are shown in Table 4.
[0053] Table 4
[0054]
[0055]
[0056] a Refers to the MIC of fluoroquinolones alone for DK2.
[0057] b Refers to the MIC of colistin for DK2 when combined with 1 μg / mL fluoroquinolone.
[0058] c The MIC sensitive point of colistin to aeruginosa was ≤2 μg / mL, and the drug-resistant point was ≥4 μg / mL.
[0059] It can be seen from Table 4 that gemifloxacin, enrofloxacin and sparfloxacin can sensitize the growth inhibitory effect of colistin on DK2. Among them, gemifloxacin and enrofloxacin can reduce the MIC of colistin to the range of clinical use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com