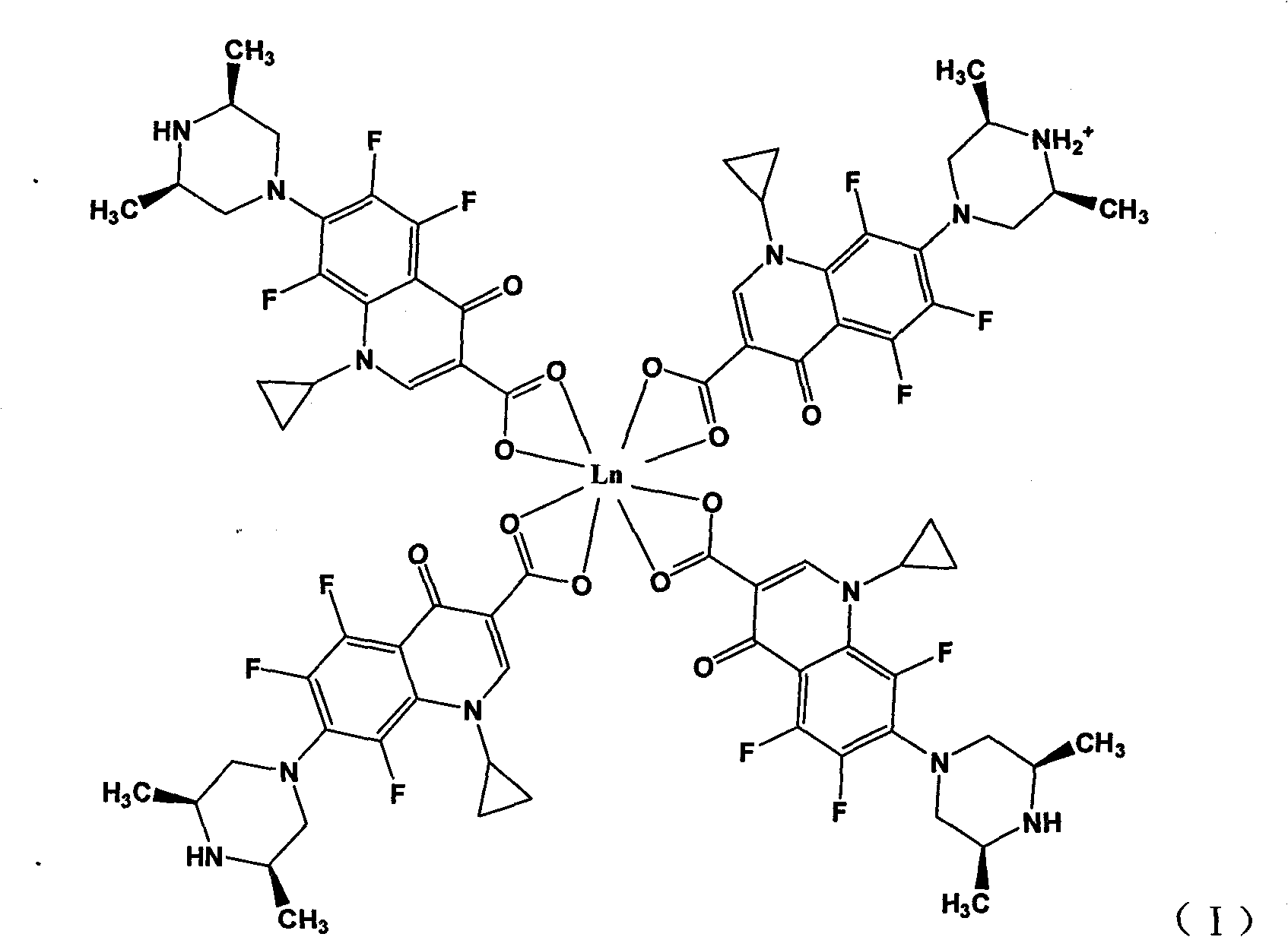
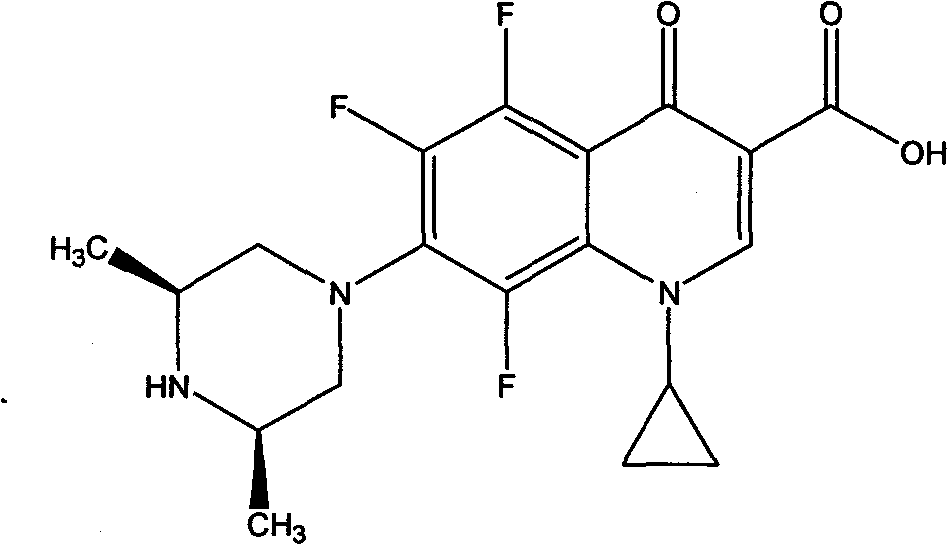
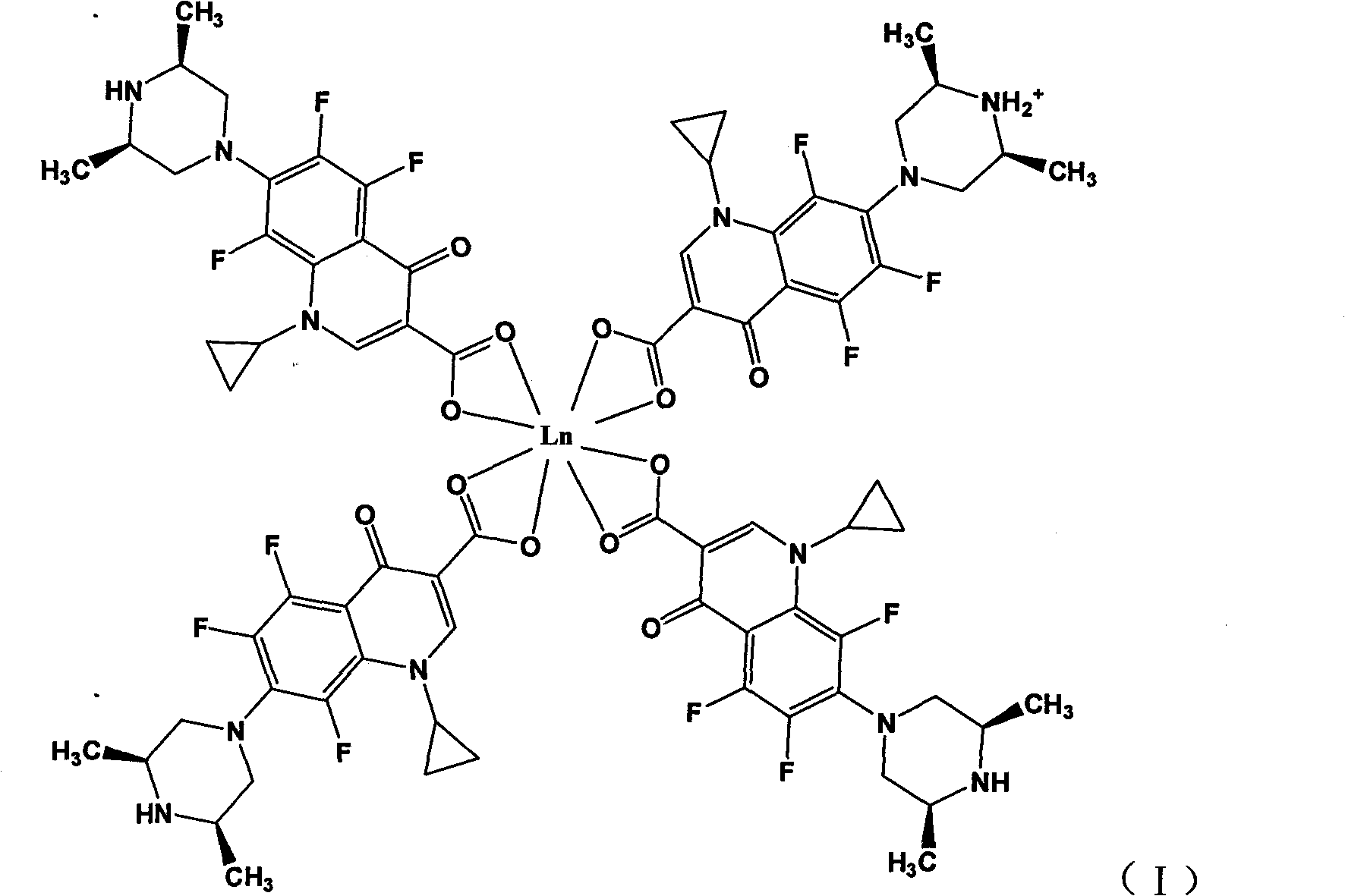
Rare earth metal complexes using orbifloxacin as ligand, method for synthesizing same and application thereof
A technology of rare earth metals and orbifloxacin, which is applied in the fields of pharmaceutical formulations, organic active ingredients, and medical preparations containing active ingredients, etc., and can solve the problems that there are no public research reports on the synthesis of metal complexes of orbifloxacin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Synthesis of Orbi-Nd (III) complex by solution method:
[0033] Dissolve 4mmol of H-Orbi in 100mL of water to form a suspension (suspension due to the low solubility of H-Orbi in water); dissolve 4mmol of NaOH in 10mL of water and add to the aqueous suspension of H-Orbi , the solution gradually became clear, indicating that Orbi had been converted to the sodium salt form. Then take 1mmol of NdCl 3 .6H 2 O was dissolved in 10 mL of water, added dropwise to the above aqueous solution, and mixed and reacted at room temperature for 6 hours. After the reaction, the aqueous solution was clear without precipitation. Under the condition of 70°C, most of the water solvent was distilled off under reduced pressure, and about 100mL of ethanol was added to the remaining mother liquor, and a large amount of precipitates formed. Filter, wash the precipitate with 30 mL of ethanol, and dry it under vacuum at 40°C to obtain a light green solid, which was determined to b...
Embodiment 2
[0034] Embodiment 2: Synthesize Orbi-Er (III) complex with solution method:
[0035] Dissolve 4mmol of H-Orbi in 100mL of water to form a suspension; dissolve 4mmol of NaOH in 10mL of water and add it to the aqueous suspension of H-Orbi. The solution gradually becomes clear, indicating that Orbi has been transformed into a sodium salt. form. Then take 1mmol of ErCl 3 .6H2 O was dissolved in 10 mL of water, added dropwise to the above aqueous solution, and mixed and reacted at room temperature for 2 hours. After the reaction, a large amount of precipitate was formed. After filtering, the precipitate was washed successively with 30 mL of water and 30 mL of ethanol, and dried under vacuum at 40°C to obtain a pale pink solid, which was determined to be an Orbi-Er(III) complex by infrared spectroscopy and elemental analysis, with the molecular formula [Er III (Orbi) 3 (Orbi-H + )].
Embodiment 3
[0036] Embodiment 3: Synthesis of Orbi-Sm (III) complex by solution method:
[0037] Dissolve 4mmol of H-Orbi in 100mL of water to form a suspension; dissolve 4mmol of NaOH in 10mL of water and add it to the aqueous suspension of H-Orbi. The solution gradually becomes clear, indicating that Orbi has been transformed into a sodium salt. form. Then take 1mmol of SmCl 3 .6H 2 O was dissolved in 10 mL of water, added dropwise to the above aqueous solution, and mixed and reacted at room temperature for 1 hour. After the reaction, a large amount of precipitate was formed. After filtering, the precipitate was washed successively with 30mL water and 30mL ethanol, and dried under vacuum at 40°C to obtain a light yellow solid, which was determined to be the product Orbi-Sm(III) complex by infrared spectroscopy and elemental analysis, with the molecular formula [Sm III (Orbi) 3 (Orbi-H + )].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com