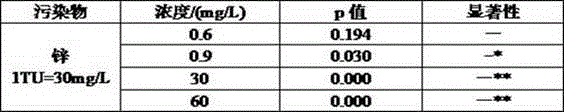
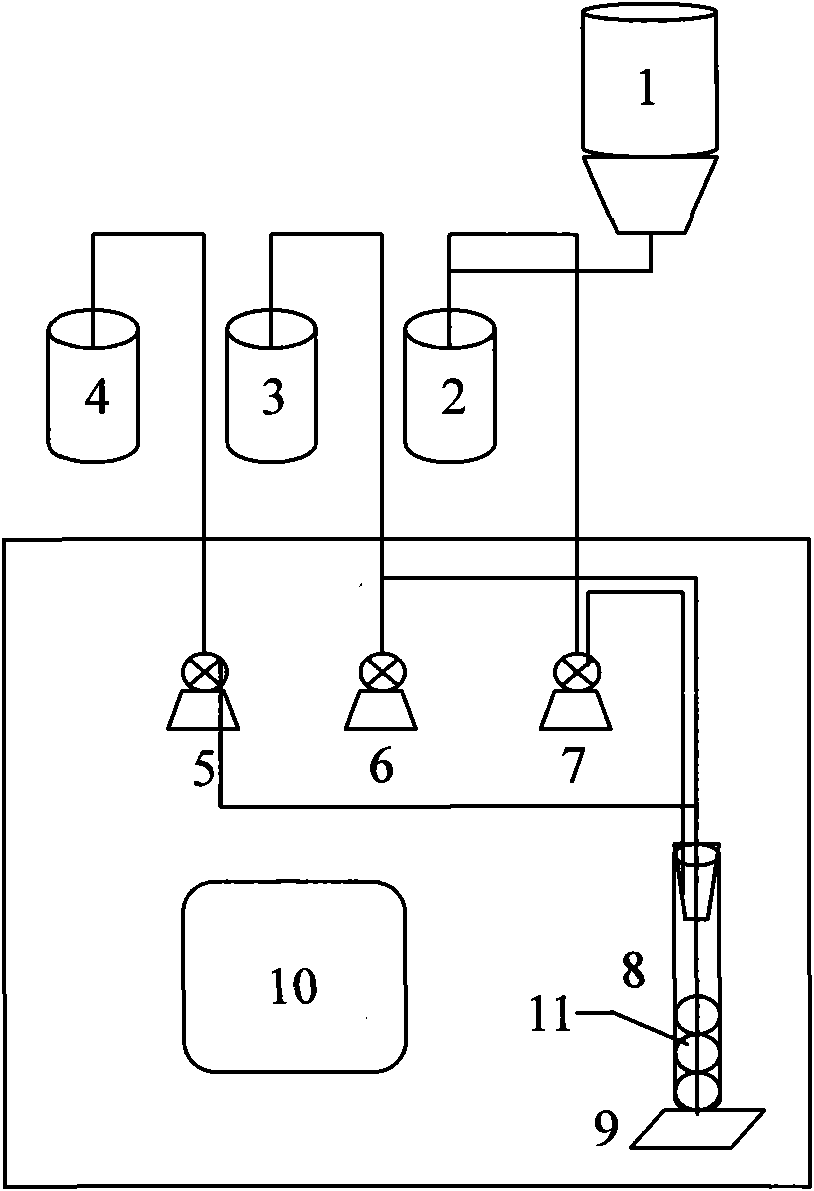
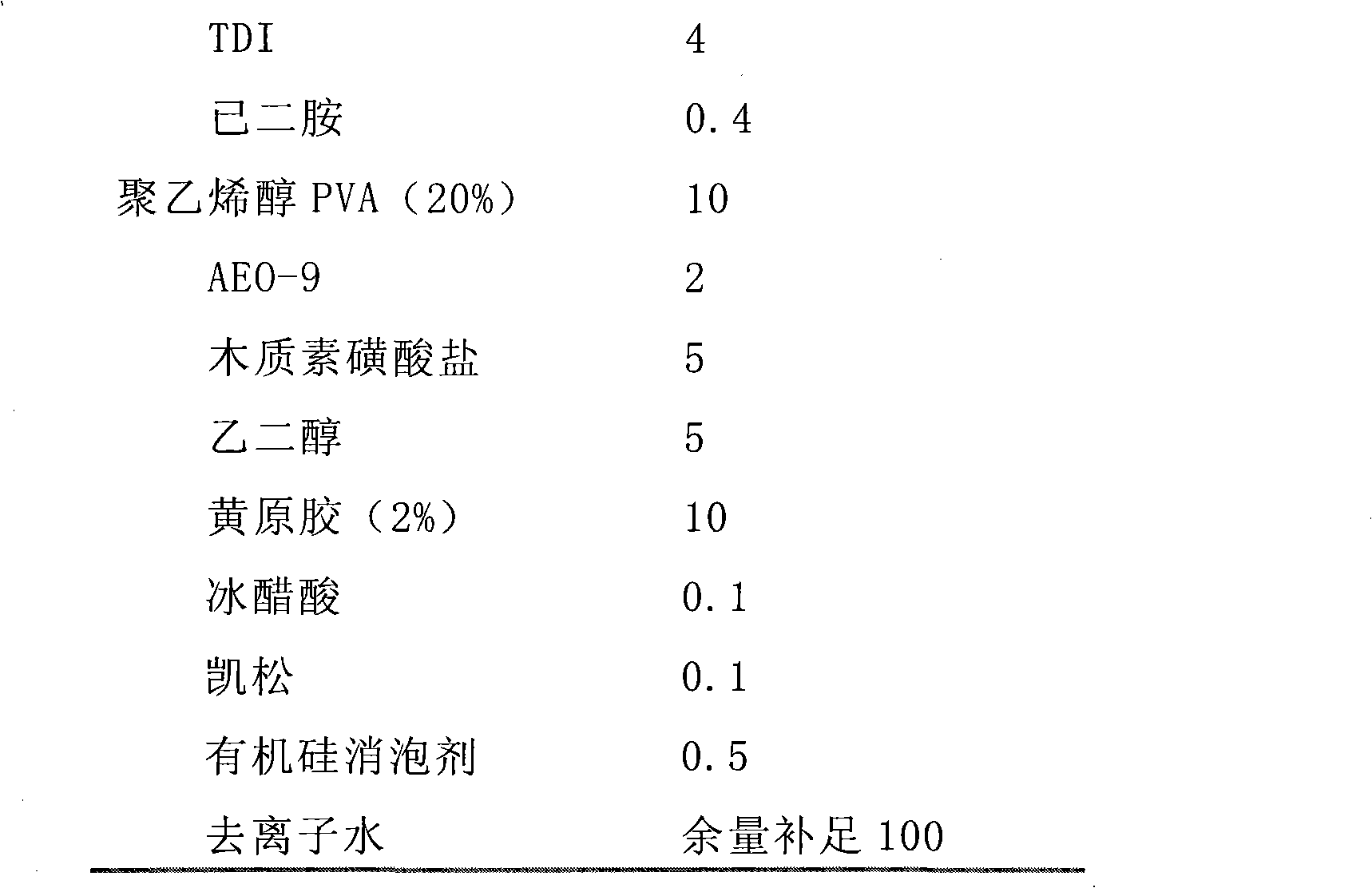
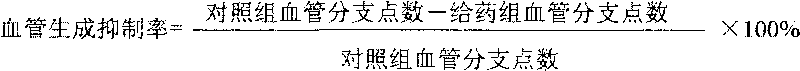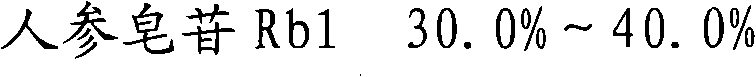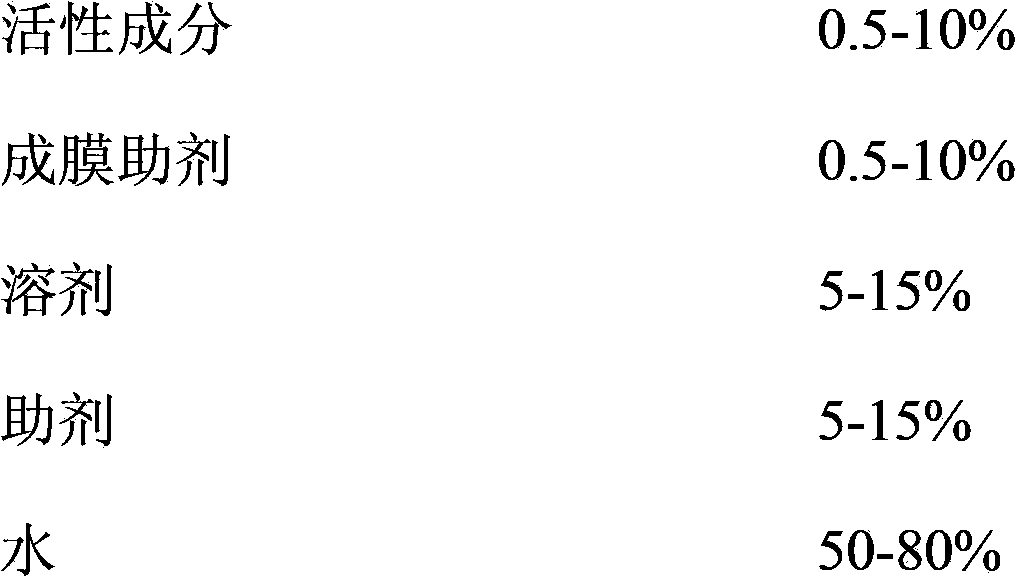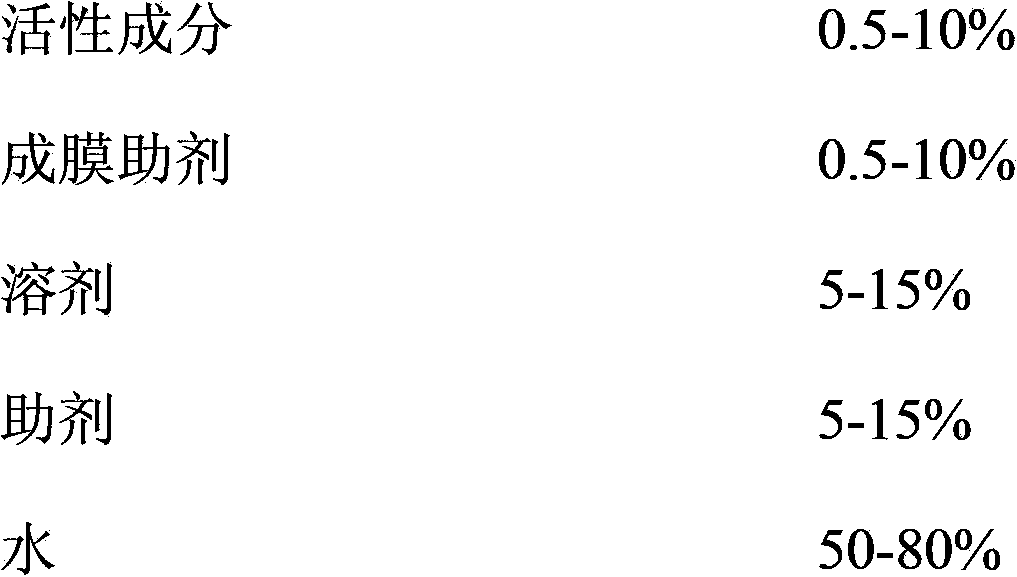Patents
Literature
484 results about "Acute toxicity testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acute toxicity is distinguished from chronic toxicity, which describes the adverse health effects from repeated exposures, often at lower levels, to a substance over a longer time period (months or years). It is widely considered unethical to use humans as test subjects for acute (or chronic) toxicity research.
Preparation of functional area of sea purse blood vessel growth inhibition factor 1 and use of the functional area of sea purse blood vessel growth inhibition factor 1 in medicaments for preventing and curing tumors
InactiveCN101724631AStrong specificityHigh expressionPeptide/protein ingredientsFermentationAbnormal tissue growthAcute toxicity testing
The invention relates to the preparation of a functional area of a sea purse blood vessel growth inhibition factor 1 and the use of the functional area of the sea purse blood vessel growth inhibition factor 1 in medicaments for preventing and curing tumors. In the invention, a gene engineering technique is used to realize the cloning, expression and recombination of the sea purse blood vessel growth inhibition factor 1; the recombinant functional fragment of the sea purse blood vessel growth inhibition factor 1 has the bioactivities for resisting the growth of blood vessels, tumor growth, tumor transplantation, acute toxicity tests and stability tests; and the functional area of the sea purse blood vessel growth inhibition factor 1 can be mixed with or dissolved in pharmaceutically acceptable carriers to prepare the medicaments for curing various tumors. The functional area of the sea purse blood vessel growth inhibition factor 1 has high action specificity, has the characteristics of easy expression, low degradation rate and the like of gene engineering medicaments and has the effects of inhibiting blood vessel growth, tumor growth and tumor transplantation; the provided gene engineering technique can realize the industrial production of the functional area of the sea purse blood vessel growth inhibition factor 1; and the functional area of the sea purse blood vessel growth inhibition factor 1 prepared by the gene engineering technique can be used in the preparation of medicaments for inhibiting blood vessel growth and preventing and curing tumors.
Owner:GUANGDONG OCEAN UNIVERSITY
Therapeutic agents for adiposity or fatty liver
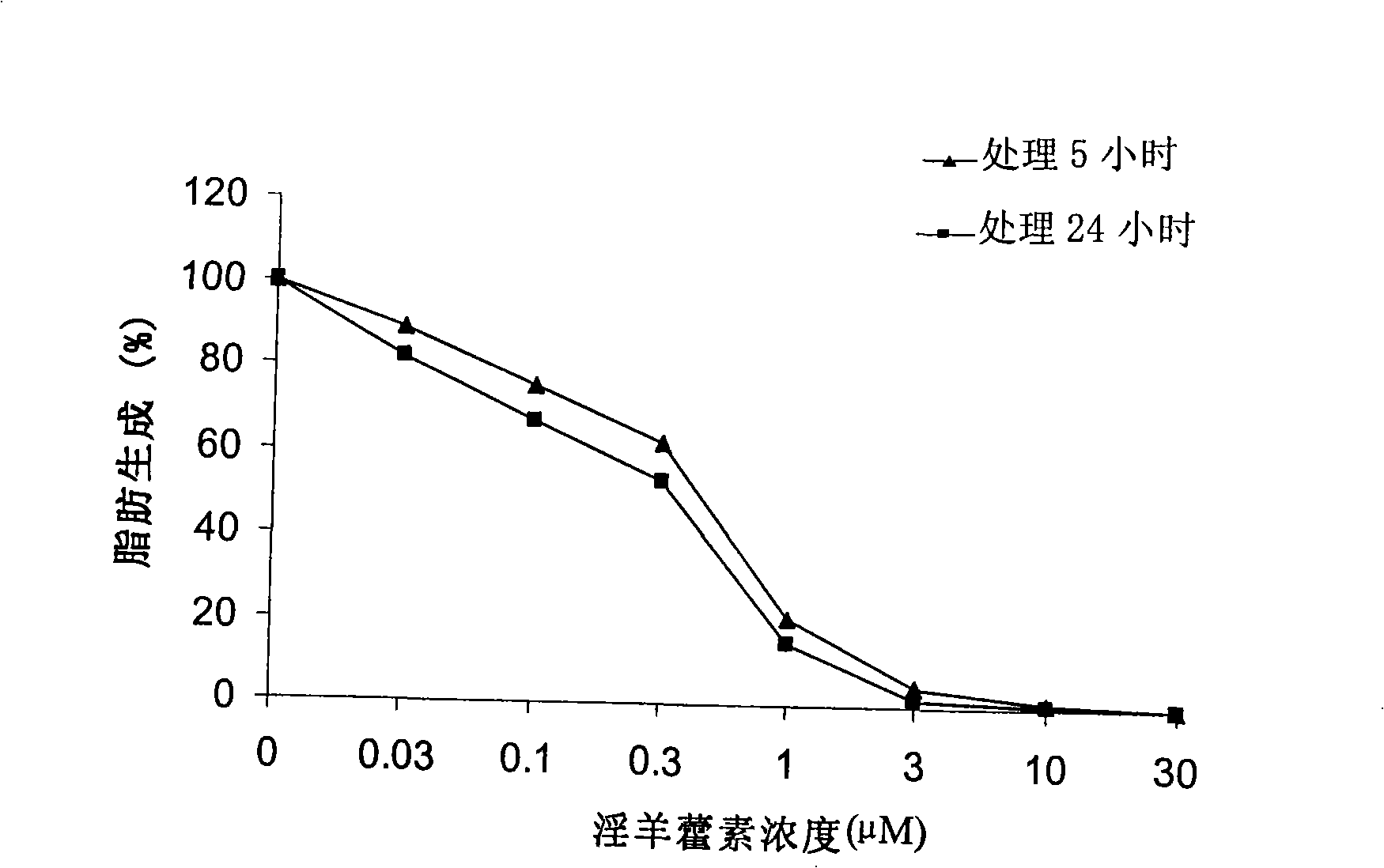
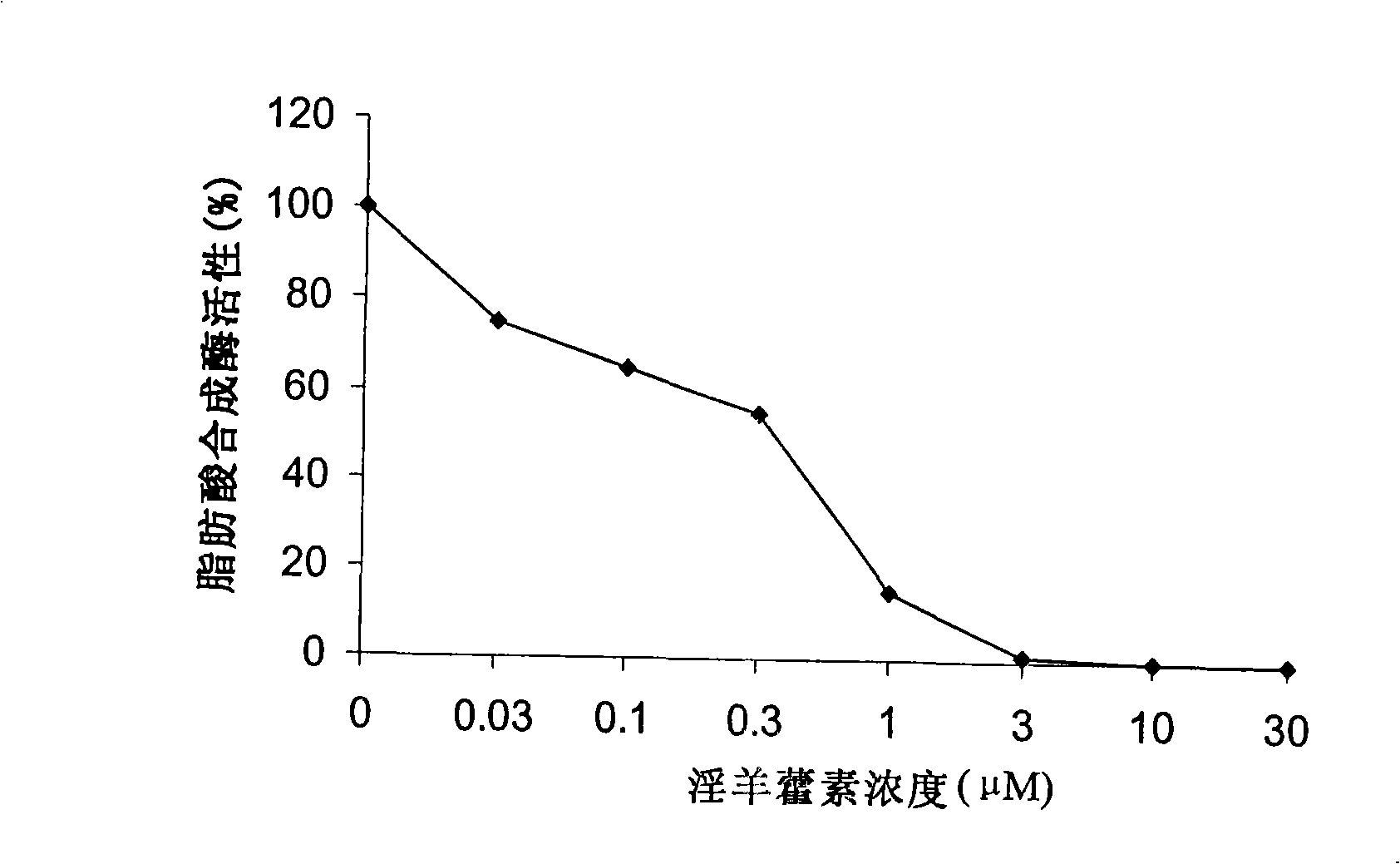
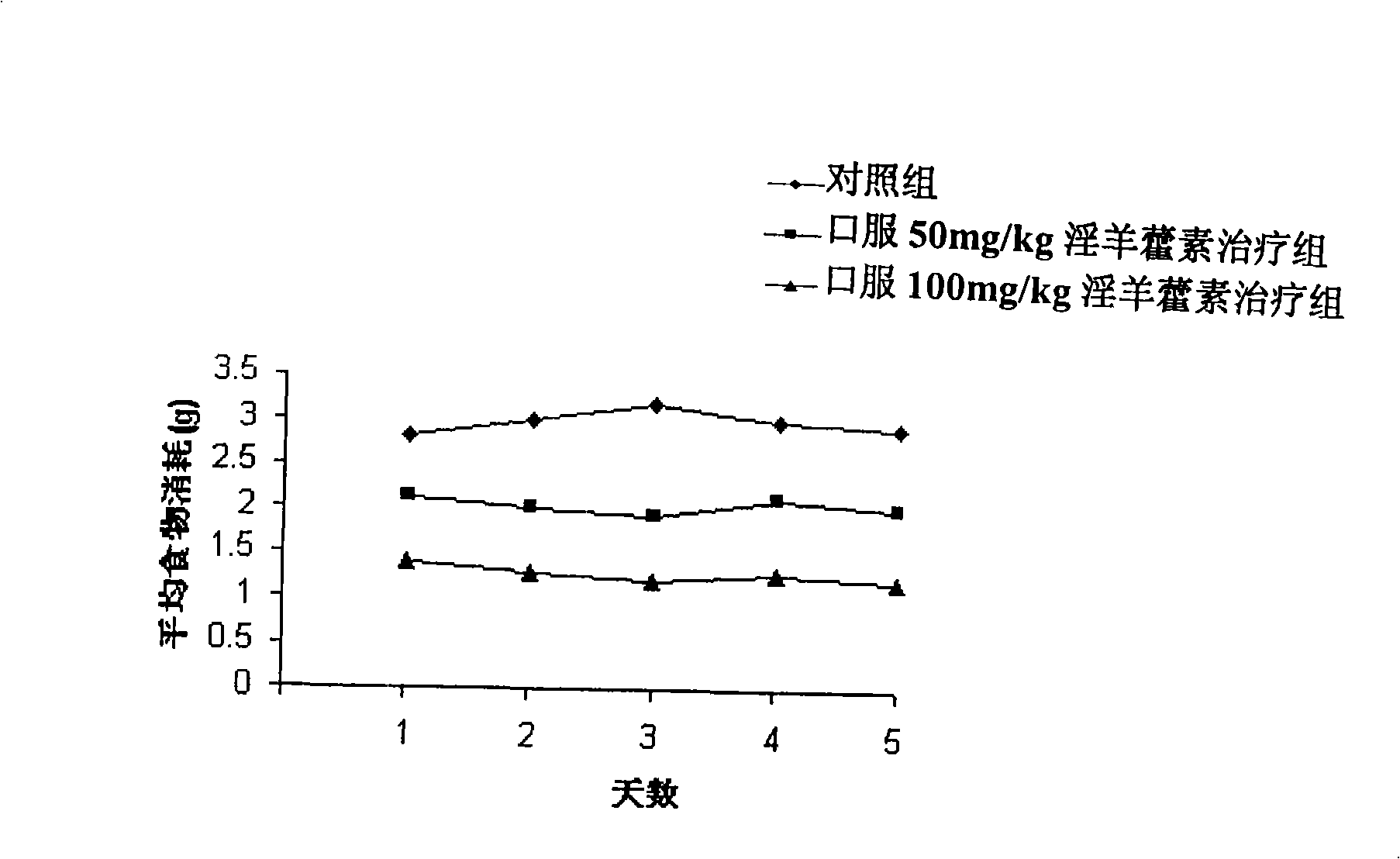
The invention relates to a medicine with icaritin as active component for treating obesity or fatty livers. The pharmacological experiment shows that the icaritin is an effective fatty acid synthetase inhibitor; can inhibit food intake and reduce weight of DIO mice; reduce adipose degeneration of the livers; and reduce the degree of ischemia / reperfusion injuries of the livers with adipose degeneration. The acute toxicity test of the icaritin on the mice shows that the icaritin has no toxicity. Therefore, the icaritin has wide development prospect, and can be used for preparing new drugs for treating obesity or fatty liver.
Owner:殷正丰 +2
Avermectin microcapsule suspending agent and preparation method thereof
InactiveCN102106345AMeet the anti-efficacy requirementsReduce decompositionBiocideNematocidesAcute toxicity testingAdjuvant
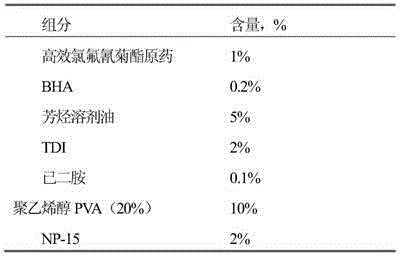
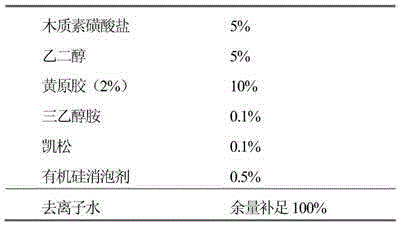
The invention discloses an avermectin microcapsule suspending agent and a preparation method thereof. The avermectin microcapsule suspending agent mainly comprises the following components in percentage by weight: 1 to 10 percent of avermectin, 5 to 40 percent of solvent, 1 to 8 percent of capsule wall material, 0.1 to 3 percent of protection glue, 2 to 5 percent of emulsifier, 3 to 5 percent of dispersant, 0 to 14 percent of adjuvant, and the balance of water. The preparation method for the avermectin microcapsule suspending agent comprises the following steps of: preparing avermectin microcapsules from an avermectin active substance serving as capsule cores and a carbamide resin serving as the capsule wall material by using an interfacial polymerization technology, and finally blending to obtain a microcapsule water suspending agent. Active ingredients of the avermectin microcapsule suspending agent are centralized in the capsule cores, and other media are almost water, so the environmental pollution is reduced and the production cost of products is also effectively reduced. The avermectin microcapsule suspending agent has the release control capacity and can prolong effective duration of pesticides by 3 to 10 times, so that the acute toxicity of technical materials is reduced and the toxicity of a preparation on non-target organisms is also reduced. The avermectin microcapsule suspending agent is safe to use and transport.
Owner:联合国南通农药剂型开发中心 +1
Composite filter tip containing biological composition
The invention relates to a composite filter tip containing a biological composition. A filament bundle filter stick of the filter tip comprises the biological composition, and after the biological composition is stuck to a carrier, when the filament bundle filter stick is formed, the carrier to which the biological composition is stuck is added into the filament bundle filter stick, wherein the biological composition comprises cobalt porphyrin and ginkgo leaf extract with the mass ratio being 1:1-80. The filter tip can not only obviously reduce the release amounts of harmful ingredients such as free radical, benzo[a]pyrene, peculiar nitrosamines of tobacco, and the like in smoke of cigarettes but also can enable the acute toxicity, subchronic toxicity, cytotoxicity and mutagenicity of the cigarettes to be lower as compared with comparison cigarettes, and at the same time has no adverse effects on the smoking quality of the cigarettes.
Owner:CHONGQING CHINA TOBACCO IND CO LTD +1
Method for culturing mycelia and sporocarps of aweto
ActiveCN101843196ASolve the problem that cannot be cultivated on a large scaleHorticultureBiotechnologyAcute toxicity testing
The invention belongs to the technical field of fungus culture, particularly to a method for culturing mycelia and sporocarps of aweto, which solves the problem that artificial aweto in the prior art can not be cultured in a large scale. The method comprises the following steps of: selecting cephalosporium acremonium and Isaria cicadae; preparing a cephalosporium acremonium liquid strain and an Isaria cicadae liquid strain; preparing a solid culture medium from corn grit, soybean flour, wheat grains, sorghum grains, silkworm chrysalis meal, whole chicken eggs, black bean coarse powder containing hulls, degreased linseed oil and a nutrient solution, wherein the nutrient solution comprises chicken blood, pine needles, carrots, black beans and cattle bone; evenly mixing, and inoculating; and culturing to obtain the sporocarps and the mycelia. The detection indicates that the sporocarps contain at least 0.02% of adenosine and at least 0.1% of cordycepin, and the mycelia contain at least 0.055% of adenosine; the finger-print test result indicates that the degree of similarity of the sporophores and the mycelia is greater than 97%; and acute toxicity experiments and long-term toxicity experiments indicate that the sporophores and the mycelia have no toxicity and can be used as substitutes of natural aweto.
Owner:杨毅
3-methoxylflavonoid compound, preparation method and application thereof
A 3-methoxy-flavone compound comprises 5,7-dihydroxy-8-(3,3-dimethyl diallyl)-3,3', 4'-trimethoxy flavone and 5, 7-2 dihydroxy-8- (3,3-dimethyl allyl)-3,4'-dimethoxy flavonoe. harmacological test results prove that: the 3-methoxy-flavone compound is an effective fatty acid synthase inhibitor, which shows the broad-spectrum anti-tumor effect in the cytotoxicity tests of various tumor cell lines and has strong tumor growth inhibiting effect on human prostate cancer cell LnCAP, human breast cancer cell ZR-75-1, human lung cancer cell NCI-H23 or human colon cancer cell HCT-116 when applied to human tumor transplant nude mice model tests. In addition, the 3-methoxy-flavone compound reveals no toxicity in mice acute toxicity tests, which then can be used as anti-tumor drug and is a new broad-spectrum anti-tumor drug with great development prospects.
Owner:殷正丰 +2
Mode creature method for medicament toxicity research
A method for studying toxicity of drugs by using model organism zebra fish comprises the following steps of: selecting 100 to 200 adult zebra fishes, respectively disposing in bottles containing a solution of 30 to 50 mL, keeping the temperature in the bottles substantially constant at 22 to 25 DEG C, randomly grouping, each group containing 10 to 20 fishes, wherein the solution in one of the groups is pure water (blank), the solutions in other groups are medicinal solutions with different concentrations, and 0.5% to 2% dimethylsulfoxide (DMSO) can be added to assist dissolving a drug that has poor water solubility, when an additional solvent comparison group prepared by adding 0.5% to 2% DMSO into the pure water is needed; recording the death number of zebra fishes in the medicinal solutions of different concentrations within 24 h, and calculating the death rate (%); calculating median lethal dose-LD50 by Bliss method according to the experiment result; and representing the acute toxicity by the value of LD50. According to the method, the LD50s of zebra fish of triptolide, matrine and emodin are respectively 5.39*10<-3>, 112.3 and 1.08*10<3> mug / mL. The method can objectively reflect the toxicity of drugs.
Owner:CHINA PHARM UNIV
Fungal fibrinolytic enzyme and cultivating method thereof
ActiveCN101503682AThrombolytic effect is goodNo obvious acute toxicityMicroorganism based processesEnzymesBiotechnologyCordyceps
The invention discloses a fungal fibrinolytic enzyme and a preparation method thereof. The fungal fibrinolytic enzyme is prepared by taking a Cordyceps militaris strain as a strain and performing liquid fermentation culture, separation and purification. The relative molecular weight of the fibrinolytic enzyme is about 21,000. The Cordyceps militaris fibrinolytic enzyme has good thrombolytic performance, is free from obvious acute toxicity, has prospects for clinical trial, development and application, and adds a new member to a rare fibrinolytic enzyme family. Particularly, a preferable fibrinolytic-enzyme Cordyceps militaris strain C.LSG-1 obtained through severe screening is rough in growth conditions, short in enzyme production cycle and capable of harvesting a large amount of Cordyceps militaris mycelium during enzyme production, and is the strain excellent in production performance. The Cordyceps militaris fibrinolytic enzyme is an extracellular enzyme which is particularly beneficial to subsequent separation and purification during preparation. The fungal fibrinolytic enzyme takes corn protein with extensive sources as a main culture medium, thereby having low cost for raw materials and providing a novel way for developing and utilizing the prior resources.
Owner:QIQIHAR UNIVERSITY
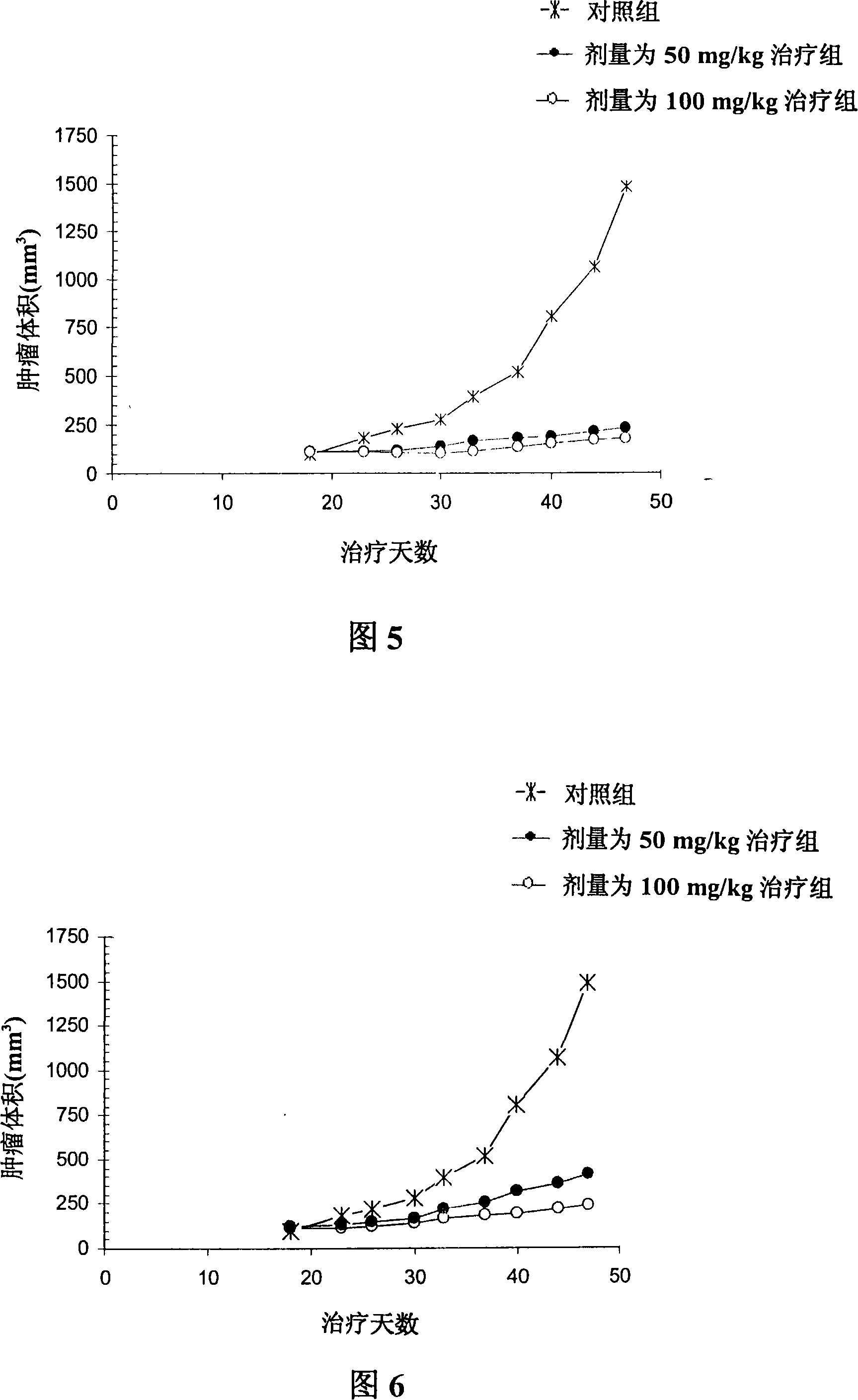
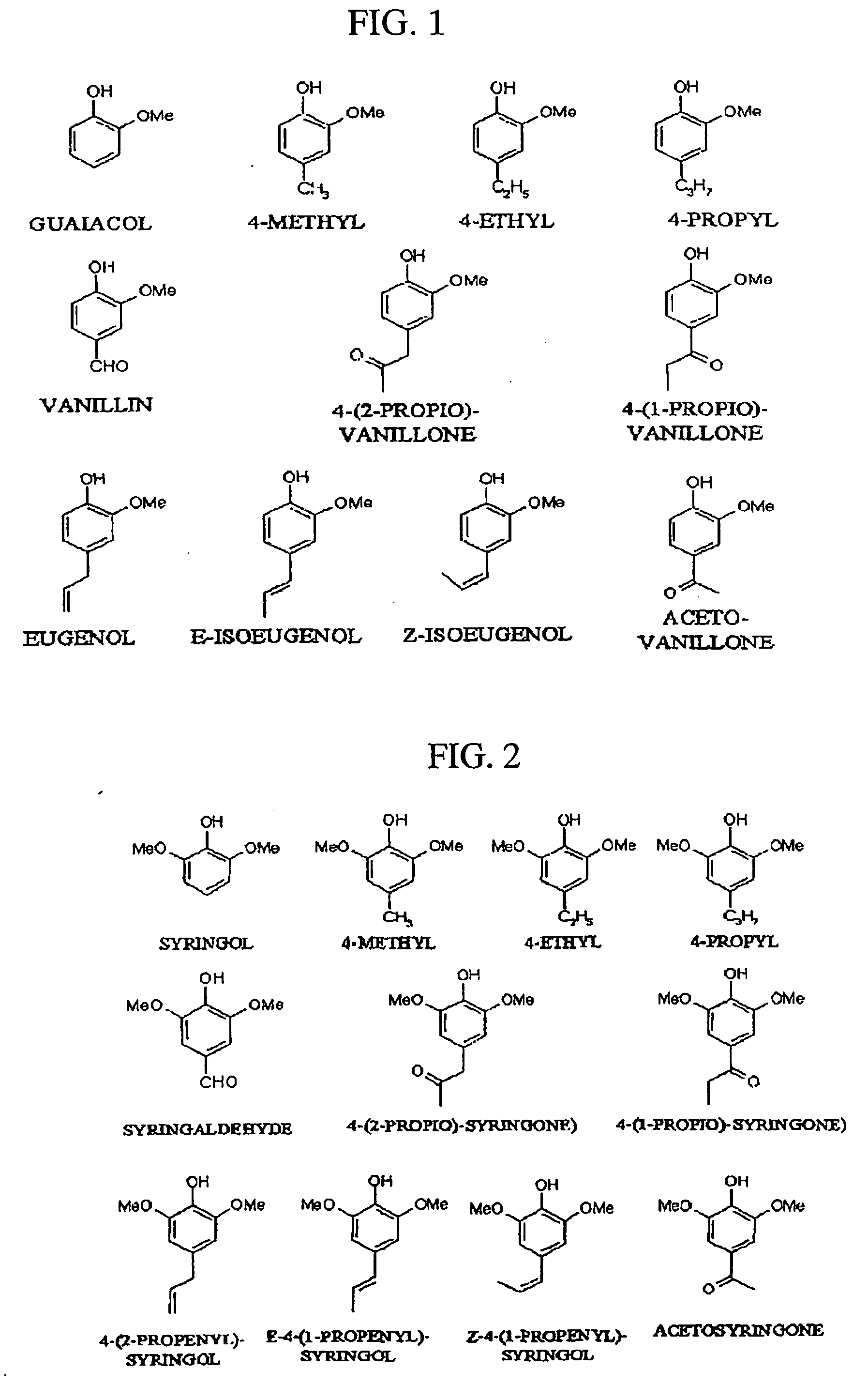
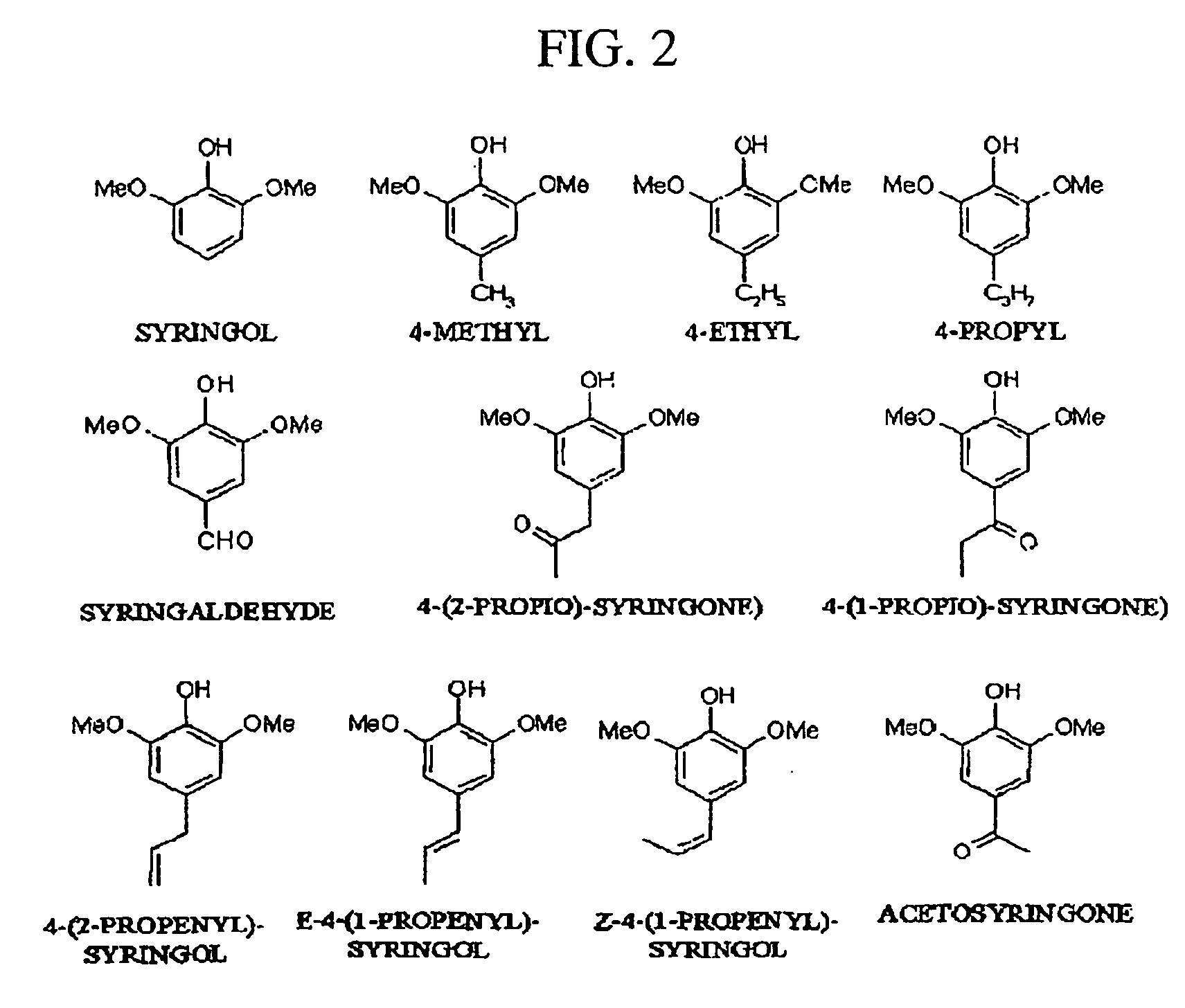
Pharmaceutical composition containing guaiacol derivatives and syringol derivatives extracted from natural plant vinegar
InactiveUS20070065524A1Increase blood flowConfirmed safety on human beingsBiocideNervous disorderAcute toxicity testingBlood flow
The present invention relates to the pharmaceutical composition comprising mainly the guaiacol family compound and the syringol family compounds, extracted from natural plant vinegar. The present invention provides that pharmaceutical compositions has effects of treating oxidative toxicity, regulating blood glucose level, improving blood flow, treating hangover and treating atopic dermatitis as well as the safe composition to be free from acute toxicity, subacute toxicity etc . . . The pharmaceutical composition of the present invention can be used as an agent or an ingredient of health functional food.
Owner:OAKY NATURAL
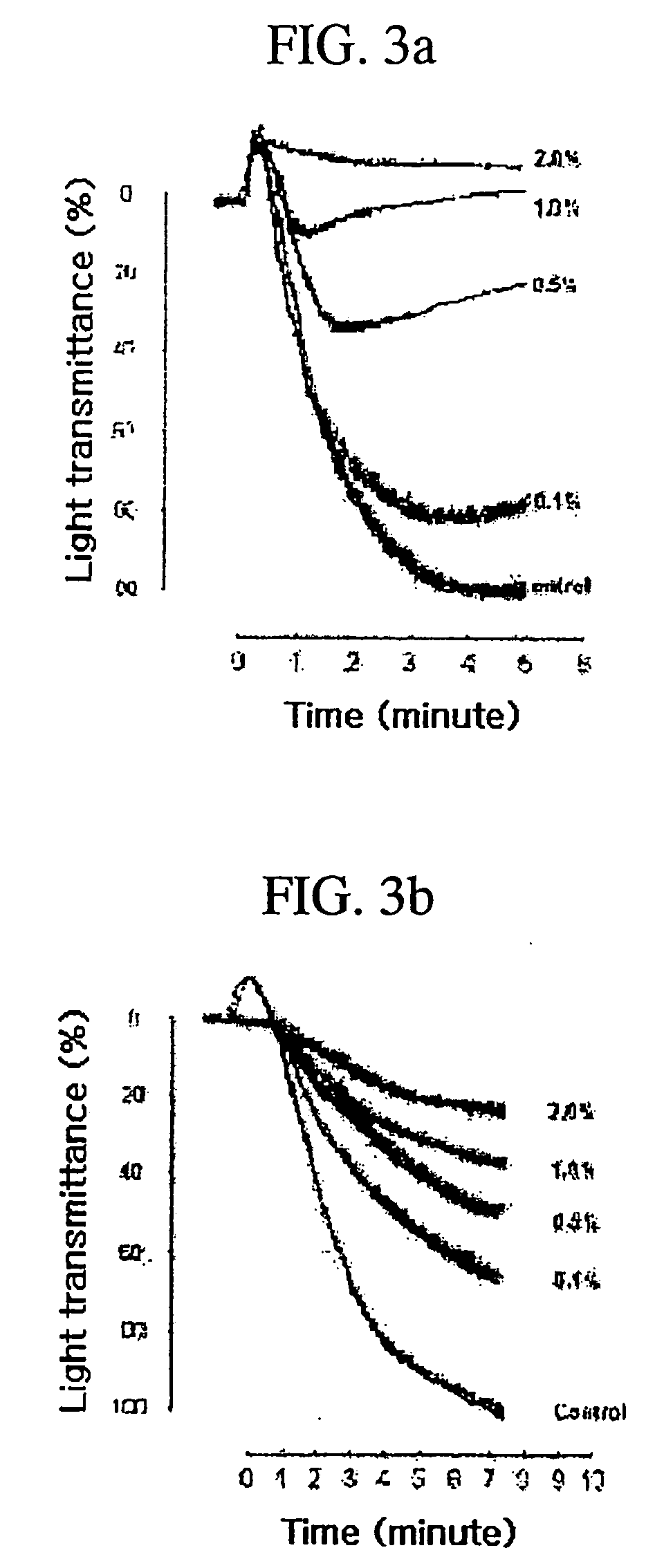
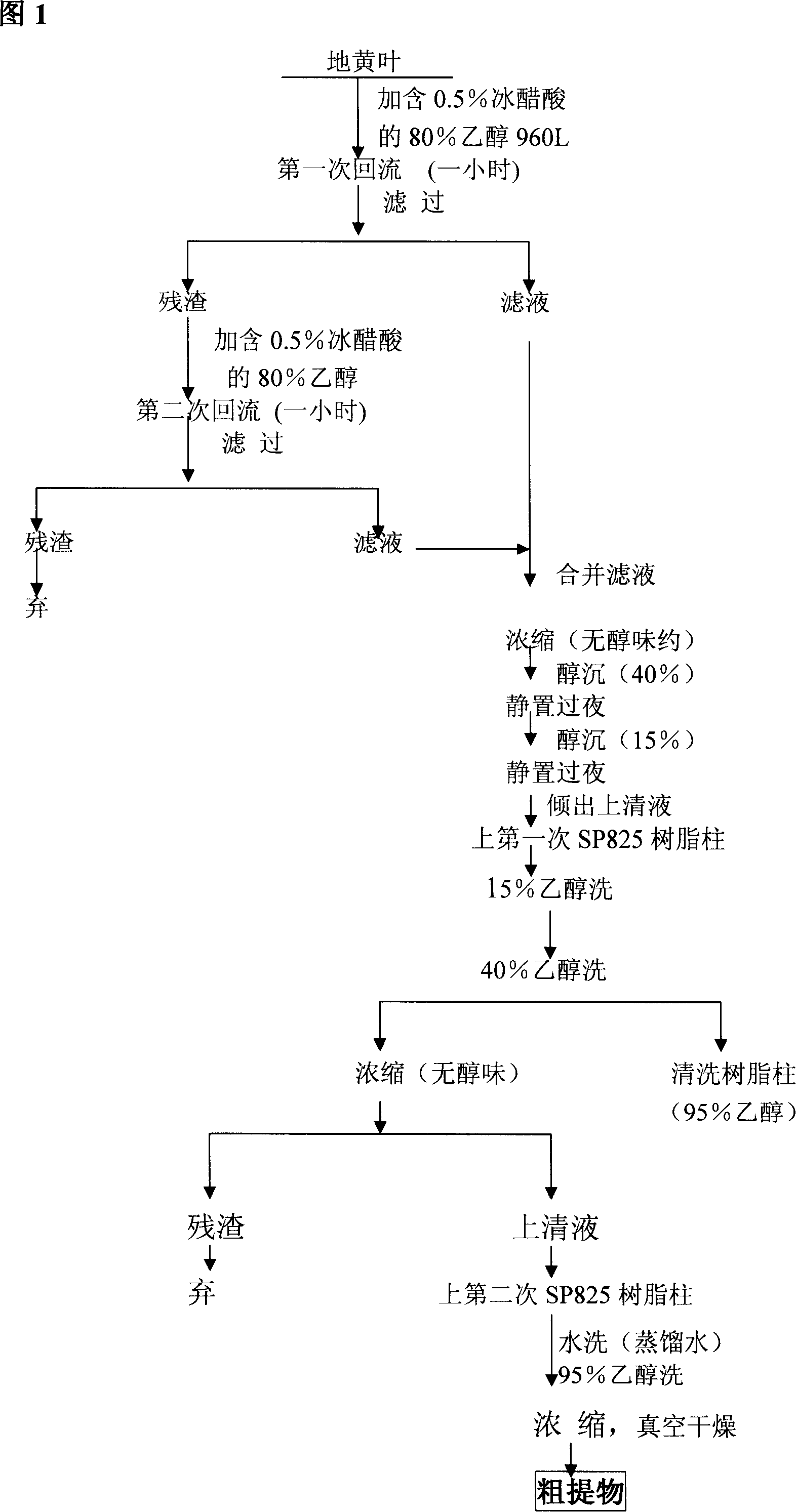
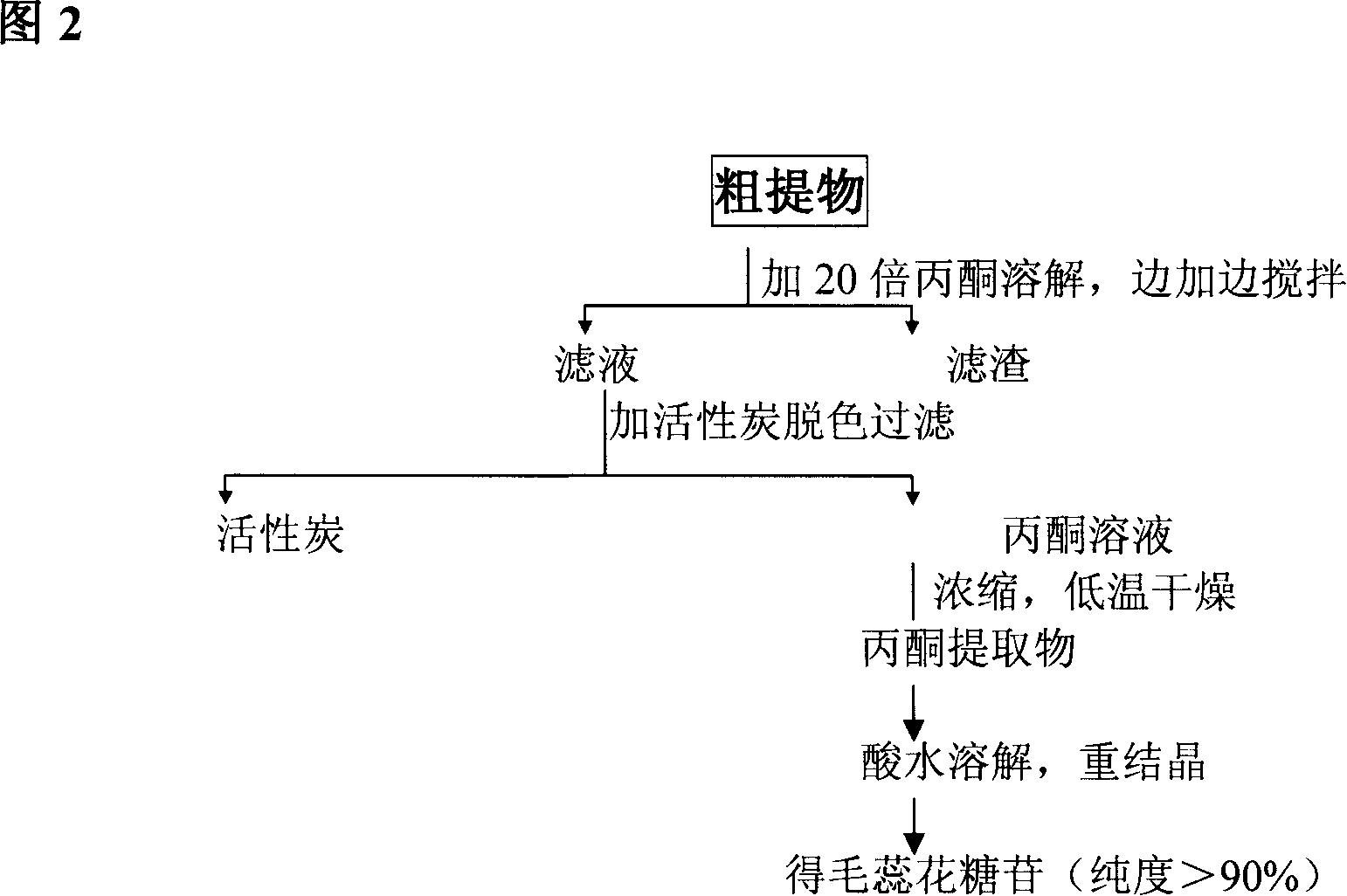
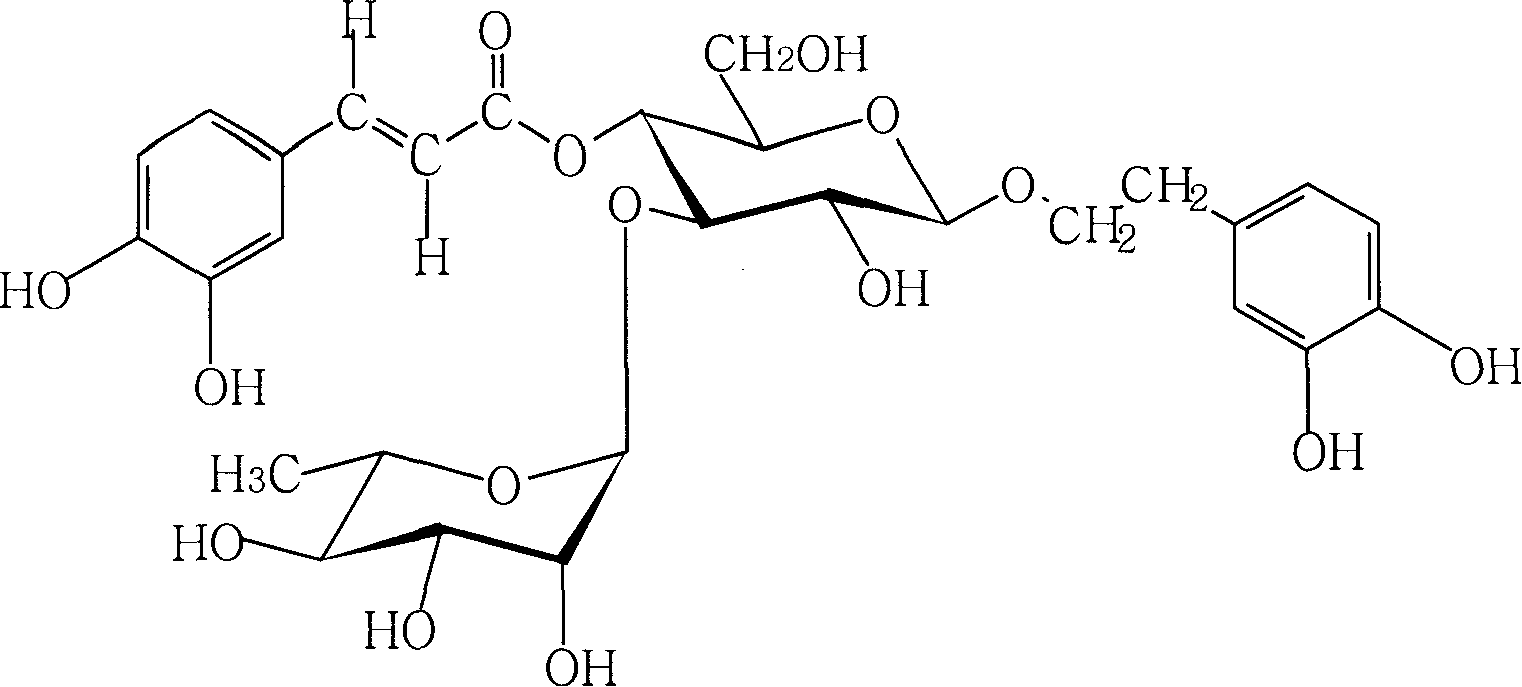
Technique for preparing verbascoside with function of curing chronic glomerulonephritis in glutinous rehmannia leaf
InactiveCN101121740ANon-toxic and safeConducive to extensive clinical applicationEsterified saccharide compoundsSugar derivativesAcute toxicity testingDigitalis
The invention belongs to the technological field of the Chinese medicine, particularly relating to an extraction and separation processes of the active ingredients with the active function of treating the chronic glomerulonephritis. The mullein indican is the active compound researched and developed for many years in our research institute; the mullein indican is extracted, separated and refined from the digitalis leaf and is an active compound with the function of treating the chronic glomerulonephritis. The invention is the research result of many years held and developed by the Chinese Medicine Research Department of the Chinese Medicine Research Institute of China and is supported by the special fund of the Central Institute of the National Science and Technology Department; now the compound has been approved by the State Food and Drug Administration Bureau as the novel drug and the mechanism research of the treatment of the chronic nephritis has been completed; the pre-clinical research work includes the main pharmacodynamic experiment, the production technology experiment (including the industrialized extraction and separation processes), the quality control standard, the experiment of the affecting factors of the quality, the experiment of the long-term stability, the acute toxicity of the rat, the experiment of the long-term toxicity, the teratogenic and mutagenic experiment, the general pharmacological experiment, the pharmacokinetics experiment, and so on. The digitalis leaf is mainly produced in the Huaiqing area of the Henan province, and is also grown wild and cultivated in the Shaanxi, Hebei and other places of China.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
High-efficiency cyhalothrin microcapsule suspension and preparation method thereof
InactiveCN105076188AMeet the anti-efficacy requirementsReduce decompositionBiocideAnimal repellantsNon target organismControlled release
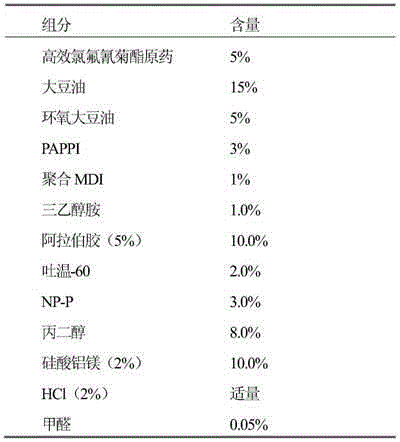
The invention relates to a microcapsule suspension, the high-efficiency cyhalothrin microcapsule suspension comprises the following components: 1-20% of high-efficiency cyhalothrin, 1-10% of a solvent, 1-8% of a capsule wall material, 0.1-3% of a protecting adhesive, 2-5% of an emulsifier, 3-5% of a dispersant, 0.01-14% of an adjuvant and 1-99% of water. The preparation method is characterized in that a high-efficiency cyhalothrin active substrate is taken as the capsule core, a polyurea resin is taken as the capsule wall material, an interfacial polymerization technology is used for preparing the high-efficiency cyhalothrin microcapsule suspension, and finally the cyhalothrin microcapsule suspension is prepared. The effective components in the cyhalothrin microcapsule suspension are concentrated in the capsule core, and other medium can be water, such that environmental pollution is reduced, and production cost is effectively reduced. The high-efficiency cyhalothrin microcapsule suspension has release controlling capability, pesticide lasting period can be prolonged by 3-10 times, acute toxicity of original medicine can be reduced, toxicity of the cyhalothrin microcapsule suspension on non-target organisms is also reduced, and the high-efficiency cyhalothrin microcapsule suspension is safe to use and transport.
Owner:南通联农佳田作物科技有限公司
Antibiotic printing ink and making method thereof
ActiveCN101084756AImprove antibacterial propertiesGood weather resistanceBiocideInksZinc compoundsProcess equipment
The invention relates to an antibiotic printing ink and its preparation method. The antibiotic printing ink comprises 5-20 wt% compound antimicrobial agents in the base material of the printing ink. The compound antimicrobial agent is a mixture of inorganic-organic compound antimicrobial agent and rosin modified phenolic resin. The inorganic-organic compound antimicrobial agent comprises inorganic antimicrobial agent and organic antimicrobial agent. The inorganic antimicrobial agent is phosphate or glassing micro powder loading silver ion, zinc ion, or silver-zinc compound ion. The inventive printing ink adopts compound antimicrobial agent, and endow the printing ink with bacteria resistance with broad spectrum, long term and high performance. The product is hard to discolor and cause no wear to the process equipment during preparing process. The inventive printing ink has no foreign odor, and no toxicity and irritation proven by rat acute toxicity test and acute rabbit skin stimulus test.
Owner:CHINA BANKNOTE INK +1
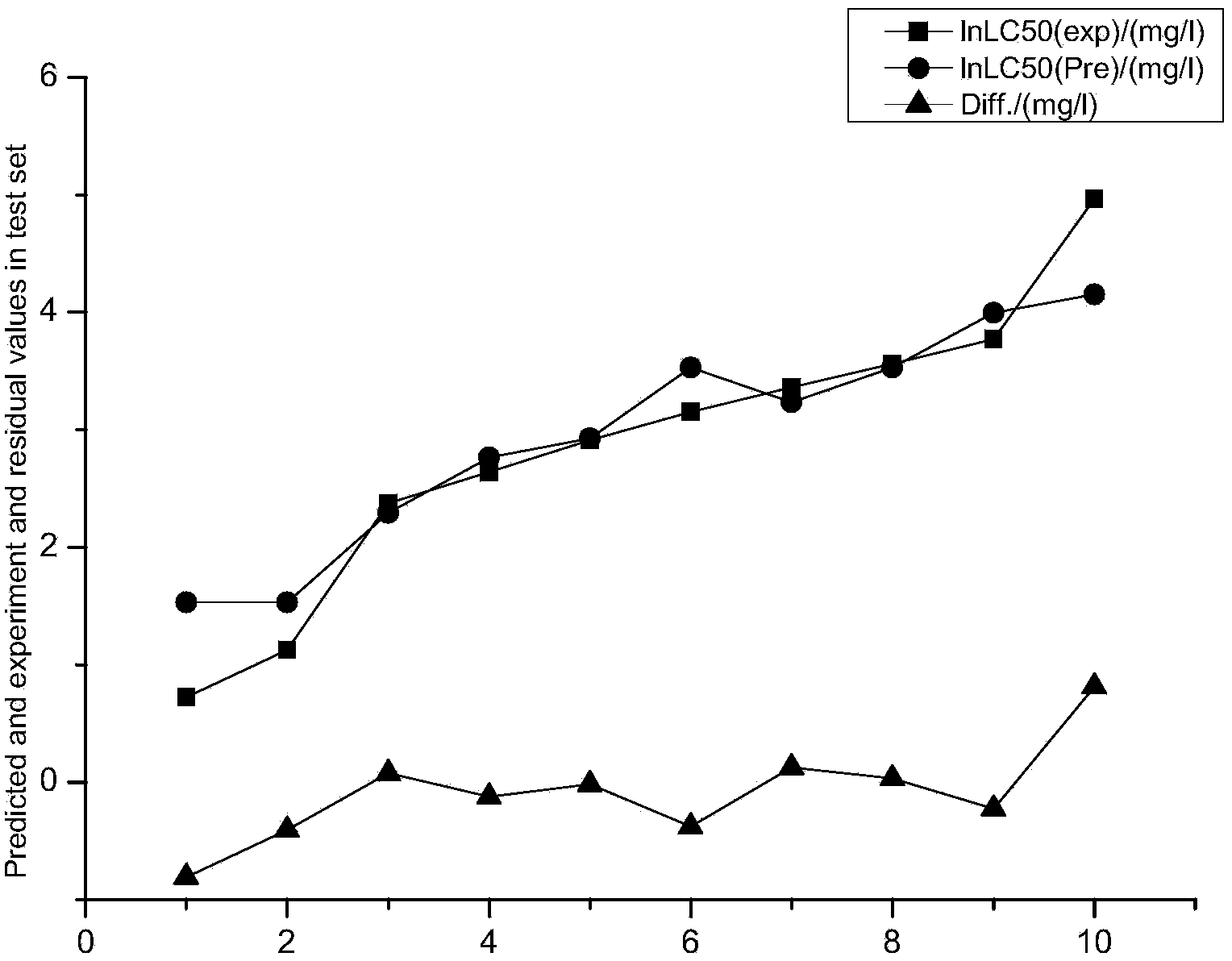
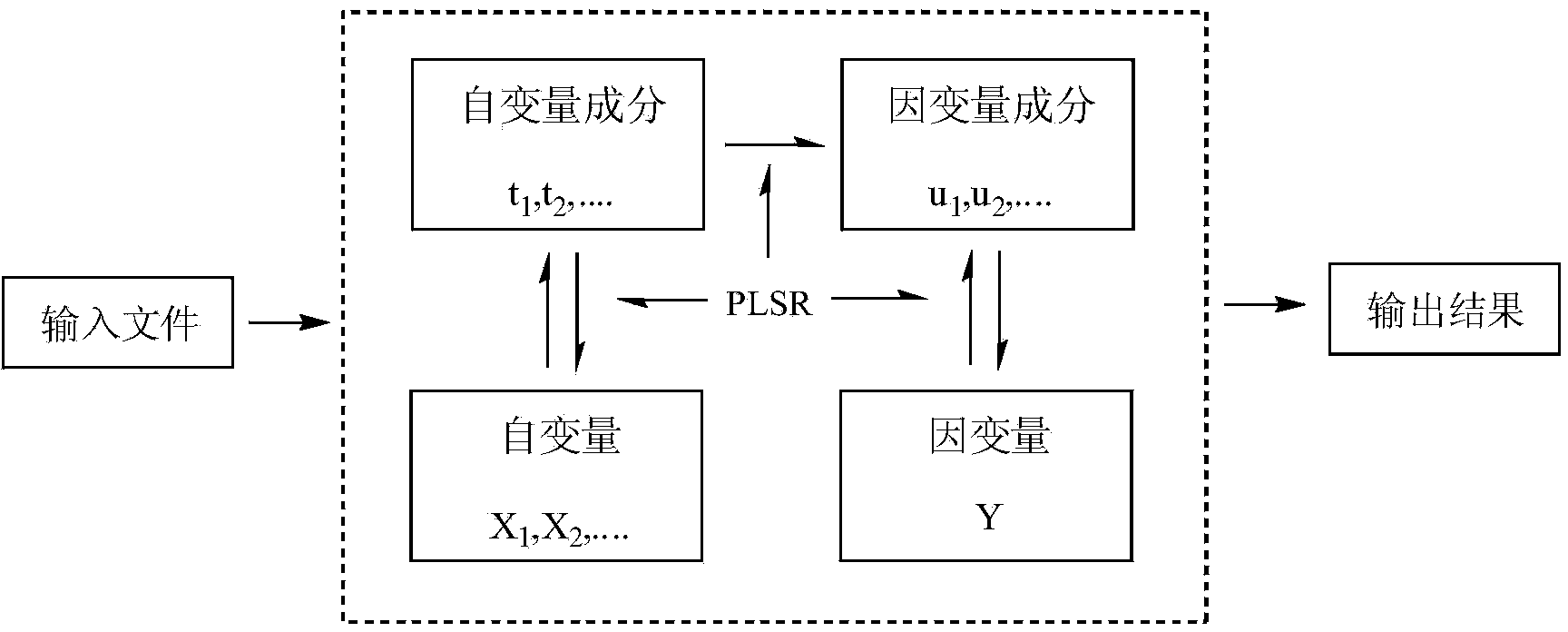
Method for forecasting acute toxicity of organic compounds by building quantitative structure-activity relationship model with quantum chemistry method
InactiveCN103646180APredict toxicityChemical property predictionSpecial data processing applicationsMolecular orbital energyAb initio quantum chemistry methods
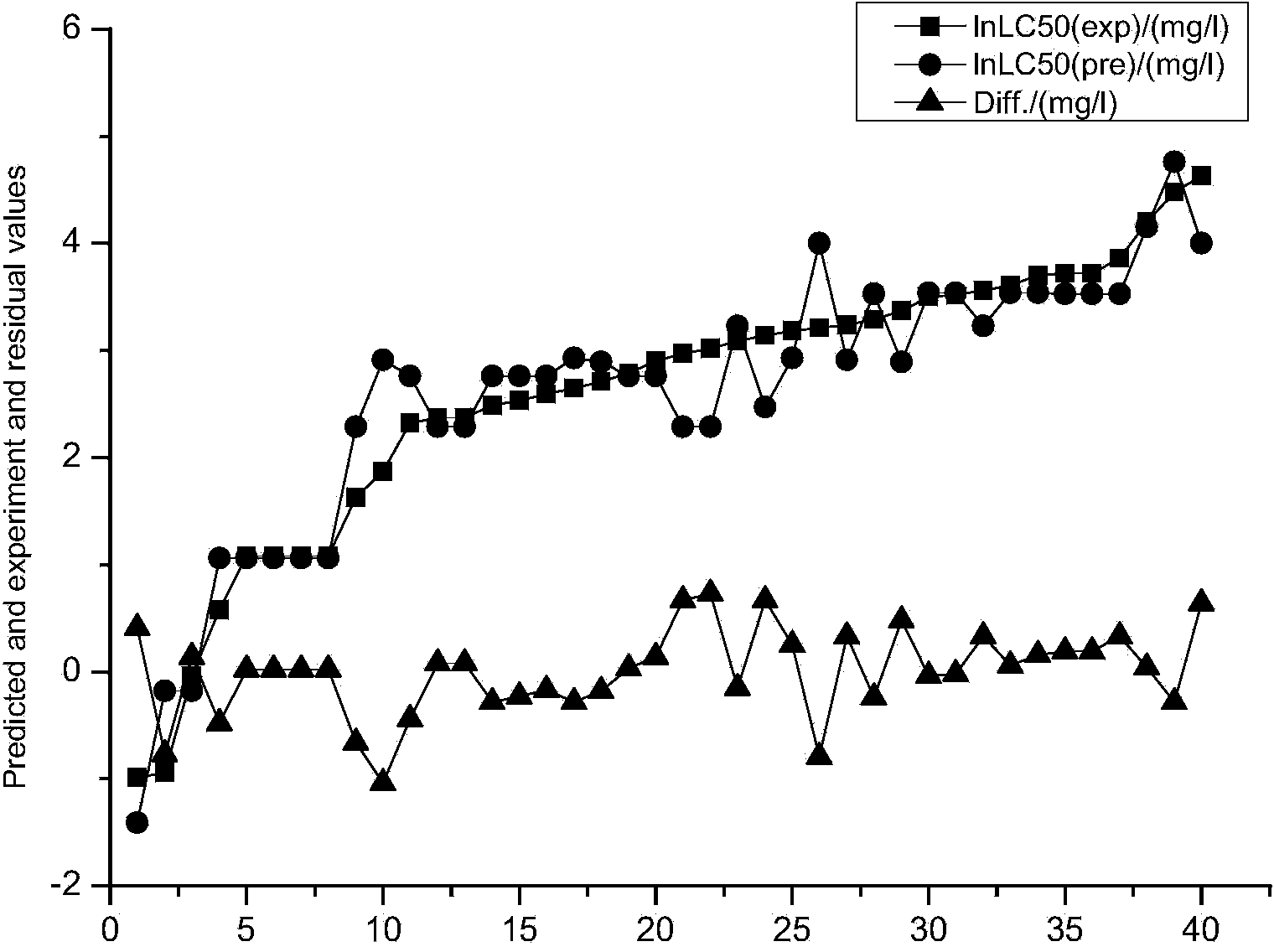
The invention discloses a method for forecasting the acute toxicity of organic compounds by building a quantitative structure-activity relationship model with a quantum chemistry method. The method fully geometrically optimizes compound structures by using a Gaussian procedure so as to obtain quantum chemistry parameters including molecular volume, relative molecular mass, highest occupied molecular orbital energy, lowest unoccupied molecular orbital energy, energy gaps of frontier molecular orbital, dipole moment, solvation energy, electron energy and the like; using the quantum chemistry parameters and a hydrophobicity parameter as structural descriptors; in combination with toxicity data, quantitative relationship equations between various structural descriptors and toxicity are established according to a written procedure based on partial least square stepwise linear regression to obtain the multiple correlation coefficient, F-test value and sum of squared residuals, and then the model is verified so as to guarantee the external predictive ability. Therefore, the method can quickly and effectively forecast the toxicity of organic compounds to be studied, and provide necessary basic data for risk assessment and supervision of chemicals.
Owner:SHANDONG UNIV
High purity cnidicin and its preparation method and medicinal composition using said compound as active component
InactiveCN1724529AOptimizing the Extraction and Separation ProcessGood reproducibilityOrganic active ingredientsOrganic chemistryStability studyAcute toxicity testing
The invention discloses a high- purity of osthol, structure proof with spectral analysis, method for preparation, stability study, acute toxicity study, the drug compositions containing osthol, and new drug utilities of the natural product. By the technique steps of extracted Chinese pharmacy conidium fruit with alcohol, decolorizing and enriching by large-hole adsorption resin, and separating and purifying, it can prepare osthol with a purity of above 98%. The study expresses that the osthol has a clear utility of anti-cancer, anti-auto lymphoma, and so on.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
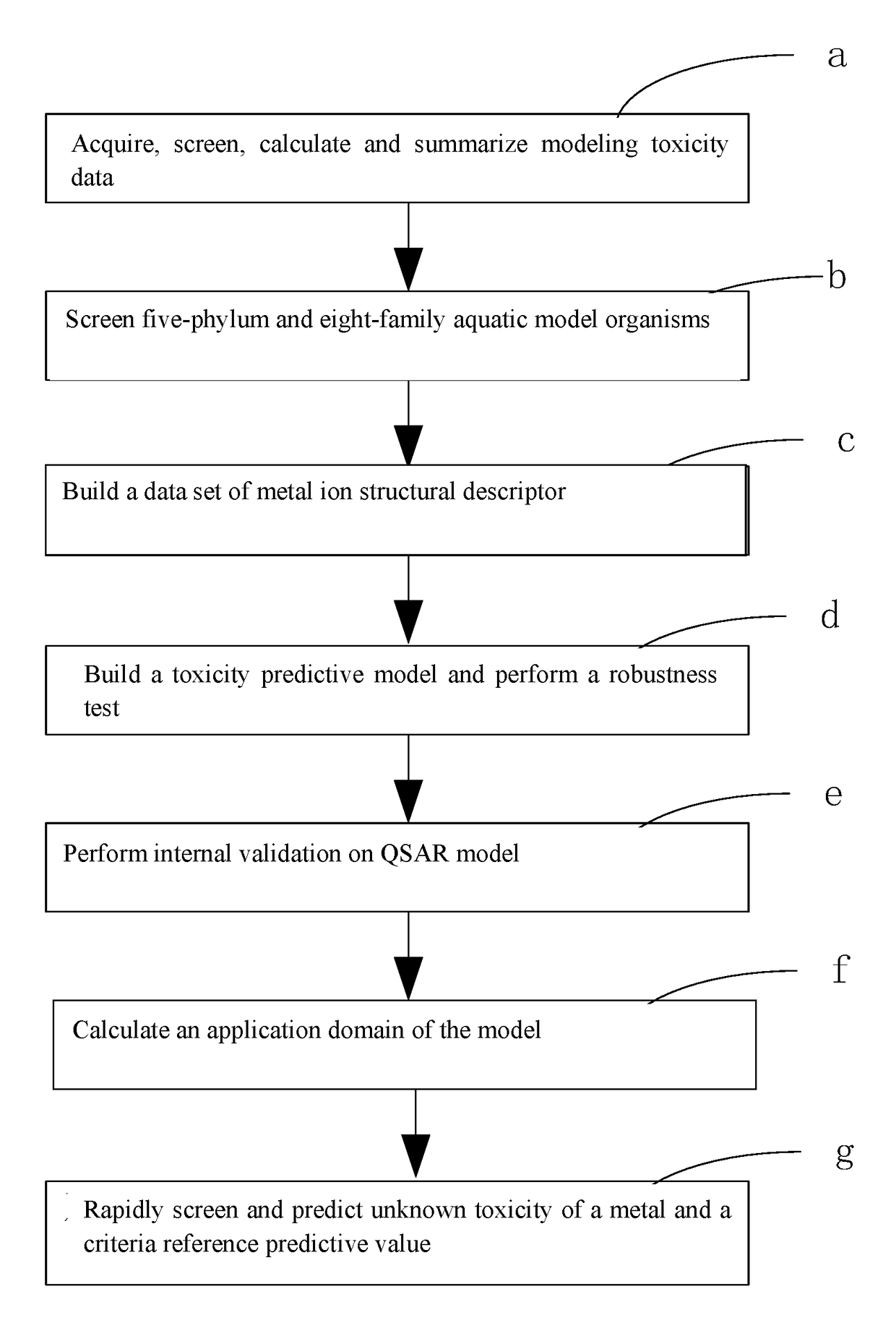
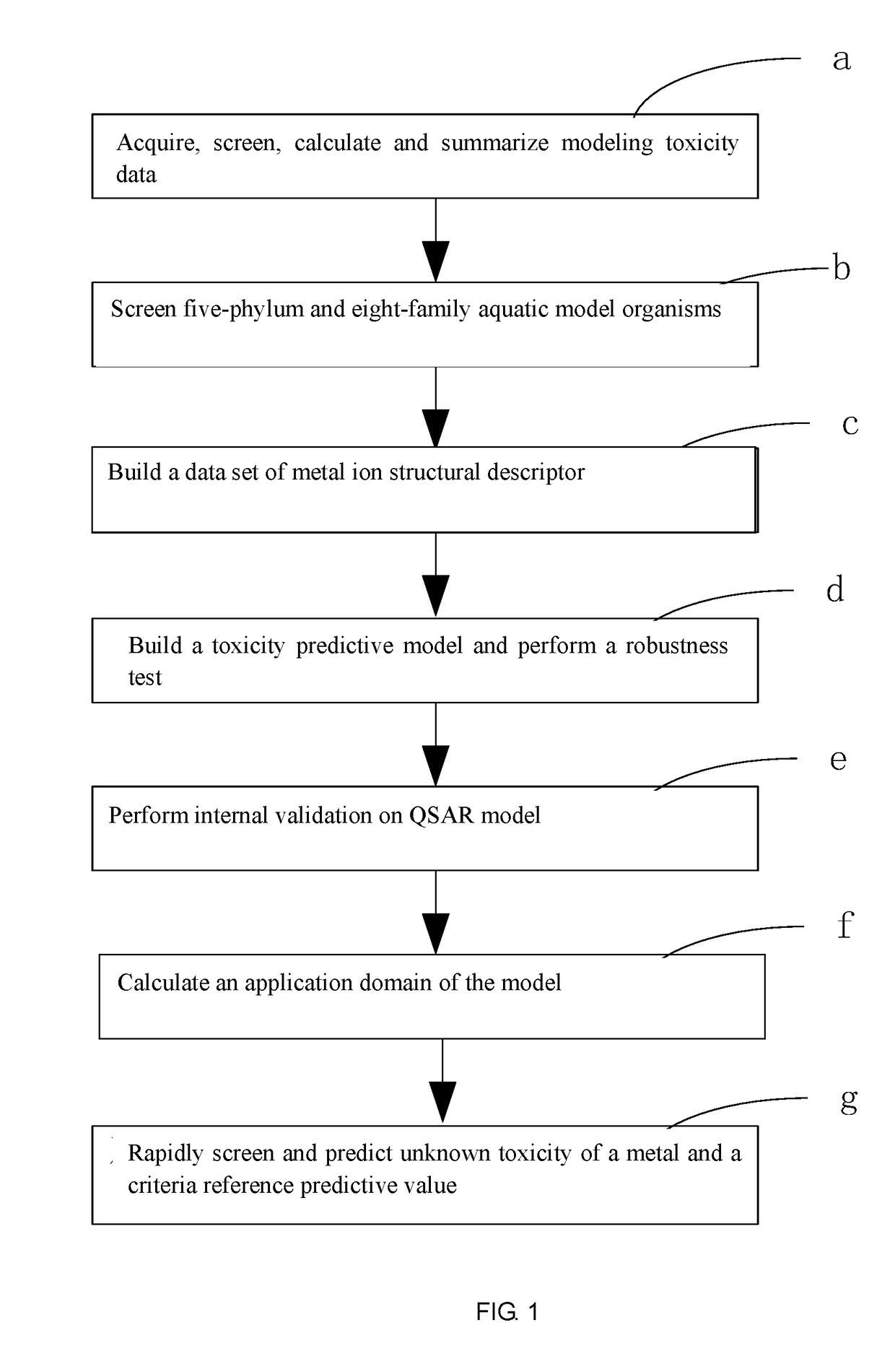
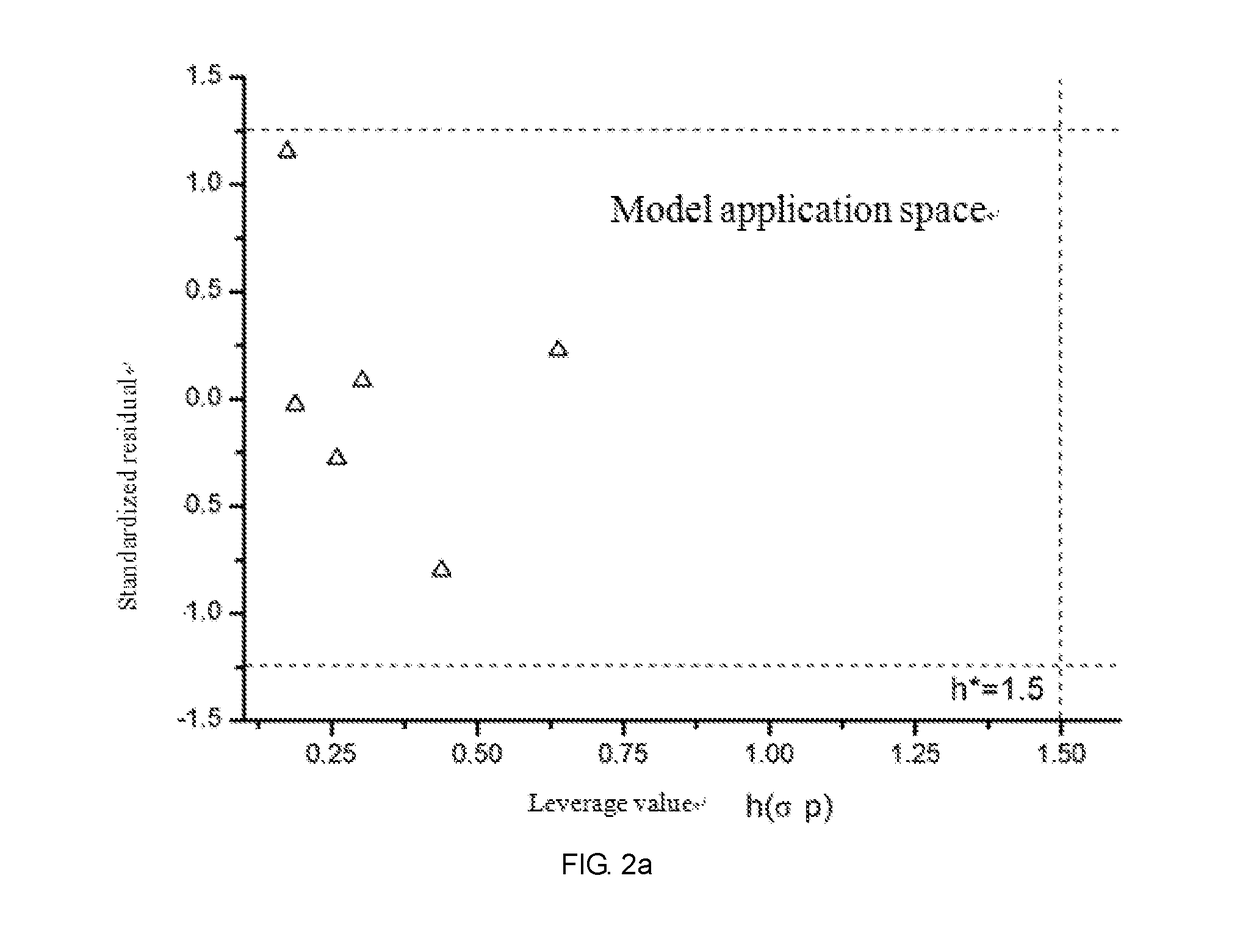
Fresh water acute criteria prediction method based on quantitative structure-activity relationship for metals
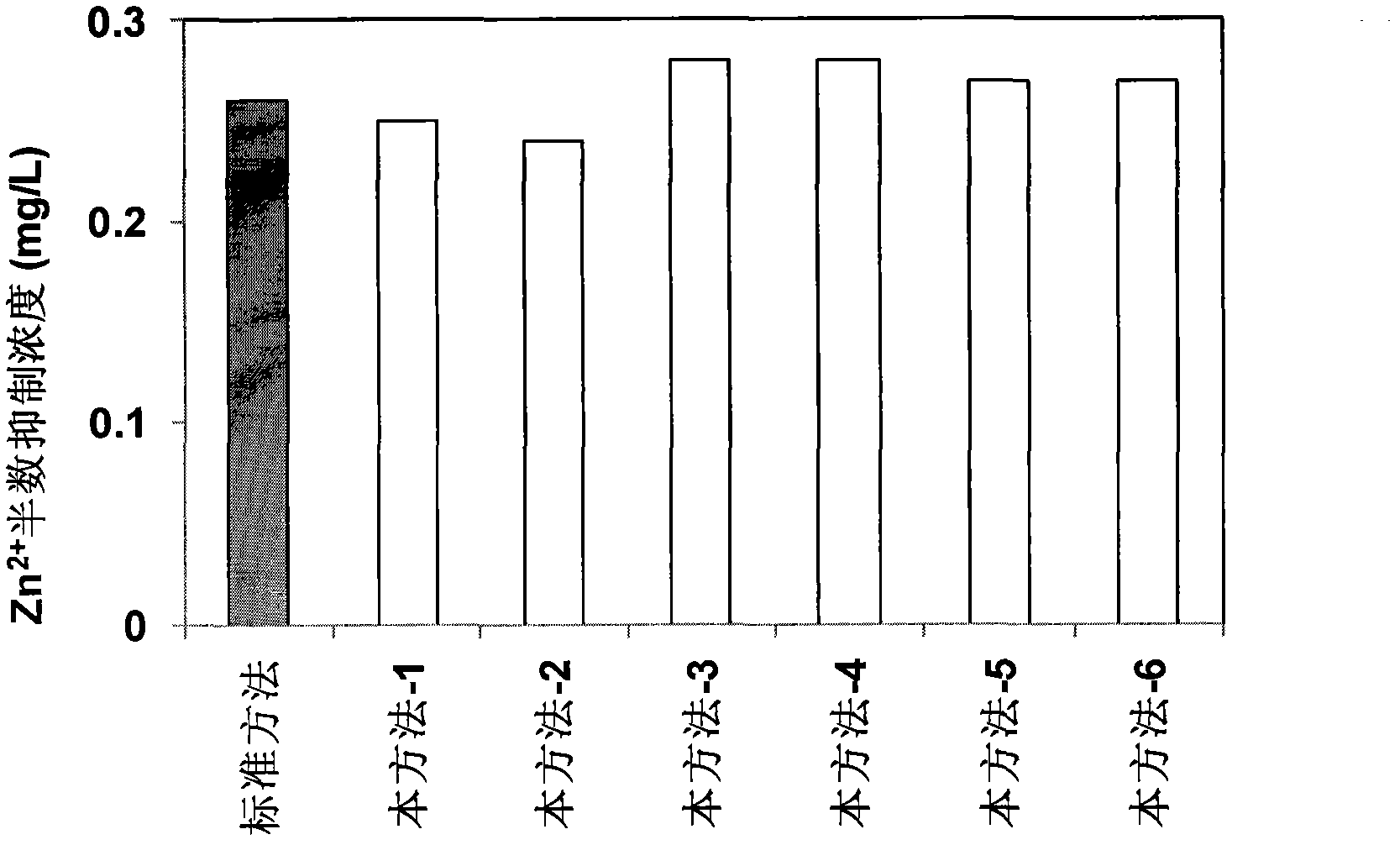
ActiveUS20170323085A1Improve precisionPredict in advanceChemical property predictionForecastingChemical structureAcute toxicity testing
The present invention relates to a fresh water acute criteria prediction method based on a quantitative structure-activity relationship for metals. An unknown toxic endpoint of a metal is predicted according to a quantitative relationship between structural characteristics of heavy metal ions and acute toxicity effects of aquatic organisms, and hazard concentrations for protecting the aquatic organisms of different proportions are derived from sensitivity distribution analysis on different species. The fresh water acute criteria prediction method is a method for establishing a metal toxicity predictive model by integrating physicochemical structural parameters of heavy metals and toxic mechanisms of different aquatic organisms and applying the metal toxicity predictive model to prediction of an unknown criteria reference value.
Owner:CHINESE RES ACAD OF ENVIRONMENTAL SCI
Medicinal food formula and medicinal edible preparation for preventing and treating hyperuricemia and gout
InactiveCN102895320ANo side effectsSignificant effectPre-extraction tea treatmentSkeletal disorderFood additiveAcute toxicity testing
The invention belongs to the field of traditional Chinese medicines, relates to traditional Chinese medicines and health care preparations, and especially relates to a medicinal food formula and a medicinal edible preparation for preventing and treating hyperuricemia and gout. The medicinal edible preparation comprises traditional Chinese medicines or active ingredients of 10 to 80 parts of plantain seed and 10 to 80 parts of semen cuscutae. The medicinal edible preparation is prepared from extract of plantain seed and semen cuscutae, and auxiliary materials, and can be processed into tablets, pills, electuary, an oral liquid, a tea bag and a health-care food additive. An animal acute toxicity test and a sub-chronic toxicity test prove that the medicinal edible preparation is safe and has no toxic or side effect. A pharmacodynamic animal test proves that the medicinal edible preparation can obviously reduce blood uric acid and improve kidney uric acid crystallization and injuries. The medicinal edible preparation as a treatment drug or a health-care product can be used for patients suffering from hyperuricemia and gout, can obviously reduce a blood uric acid level and relieve gouty arthritis symptoms, and has good safety.
Owner:张清仲
Chinese medicine extracting liquid eye plaster and preparation thereof
ActiveCN101264131ARelieve eye fatigueVisual fatigue hasSenses disorderHydroxy compound active ingredientsAcute toxicity testingIrritation
The invention relates to eye mask for healthcare product for eyes and the preparation method, which is characterized in that: the eye mask comprises non woven fabric adding medical solution; following raw material of weight are added into each 1000 ml of medical liquor: ginsenoside 2 to 3g, lucid ganoderma and spores powder 0.08 to 0.12g, pearl 2 to 3g, puerarin 0.4 to 0.6g, wild chrysanthemum flower 18 to 25g, mint 15 to 18g, musk 0.06 to 0.08g and borneol 0.2 to 0.6g. Through test of Health Supervision Test Center of Jilin Province: acute toxicity experiment of mouse skin shows that the eye mask does not have toxin; acute eye irritation experiment of rabbit shows the does not have irritation; test of 40 tested volunteers with fatigable eyesight shows that the eye mask has the function of relieving fatigue of eyesight.
Owner:力神药业(吉林)有限公司
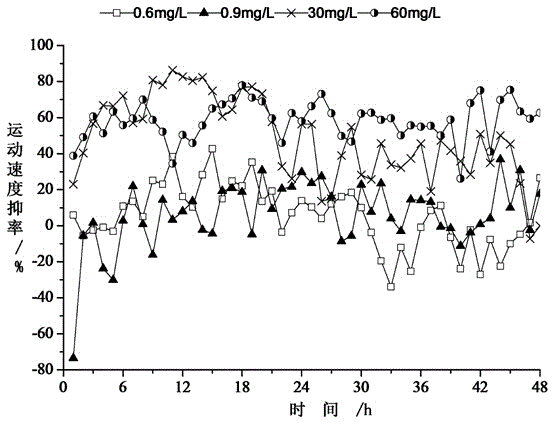
Method for determining biotoxicity of water quality based on zebrafish movement velocity change
InactiveCN104833784AImprove timelinessIncreased sensitivityGeneral water supply conservationTesting waterAcute toxicity testingWater quality
The invention discloses a method for determining biotoxicity of water quanlity based on movement velocity change of zebrafish. The method comprises the following steps: domesticating zebrafish; carrying out acute toxicity test on the domesticated zebrafish and calculating the 48 hour medial lethal concentration; and carrying out behavior test on the domesticated zebrafish, determining the movement velocity change of the zebrafish and judging the biotoxicity of water quality. With movement velocity of the zebrafish in a water environment as a biomarker, the biotoxicity of pollutants is evaluated; the movement behavior of the zebrafish in the water environment is tracked and captured; the movement track of the zebrafish is visually observed at any time; the speed index is determined in real time; quantitative evaluation for determining the biotoxicity of water quality by employing the zebrafish movement velocity change is realized; water quality fluctuation is displayed within a short period of time; the biological monitoring and early warning timeliness, sensitivity and accuracy are improved; a technical support is provided for sudden water body pollution; and the water supply quality safety is ensured.
Owner:SHANDONG JIANZHU UNIV +1
Online monitoring equipment and method for acute toxicity of water quality
InactiveCN101871927ASimple structureLow costBioreactor/fermenter combinationsBiological substance pretreatmentsData displayLuminescent bacteria
The invention discloses online monitoring equipment for acute toxicity of water quality. The equipment comprises a luminescent bacteria continuous culture system, a sample feeding system, a liquid draining system, a photoelectric detection system and a data display system, and can realize automatic operation. The invention also discloses a method for performing online monitoring on the acute toxicity of the water quality by utilizing the equipment. The online monitoring equipment for the acute toxicity of the water quality of the invention has the characteristics of simple structure, low cost and convenient operation, and can be operated and maintained by non-professionals only after a simple training. By using the method, continuous and automatic acute toxicity monitoring of the water quality can be realized completely; sensitivity is equivalent to that of an international luminescent bacteria method; and monitoring steps are simplified greatly. By using the equipment and the method, an early warning is provided for a sudden environment accident and corresponding measures are taken timely so as to reduce loss greatly.
Owner:NANJING UNIV +1
Medicinal composition and preparation method thereof
ActiveCN102028700AIncrease the maximum tolerated doseRaise the median lethal doseOrganic active ingredientsPeptide/protein ingredientsHemolysisAcute toxicity testing
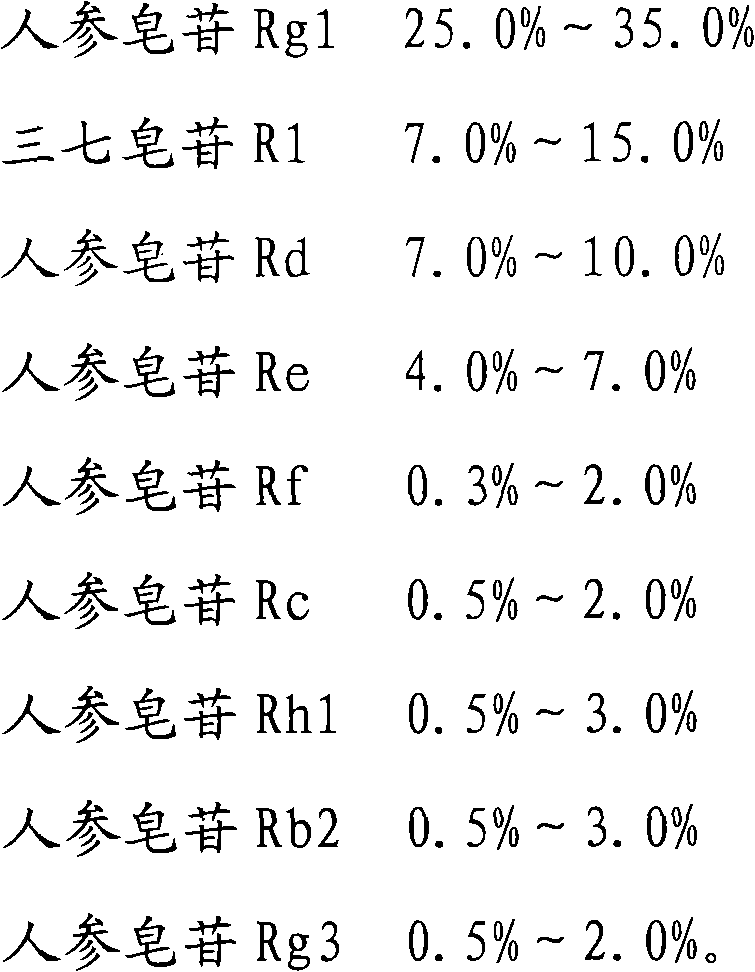
The invention provides a medicinal composition, which comprises the following components: ginsenoside Rb1, ginsenoside Rg1, notoginsenoside R1, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rc, ginsenoside Rh1, ginsenoside Rb2 and ginsenoside Rg3. The invention also provides a preparation method for the medicinal composition. The medicinal composition has definite components; compared with the total notoginsenoside in the prior art, the medicinal composition has stable quality and good controllability; and results of experiments of acute toxicity, undue toxicity, hemolysis and the like show that the medicinal composition has higher safety and wide clinical application prospect.
Owner:KPC PHARM INC
Mushroom ferment pure protein with antineoplastic activity, extracting method and formulation
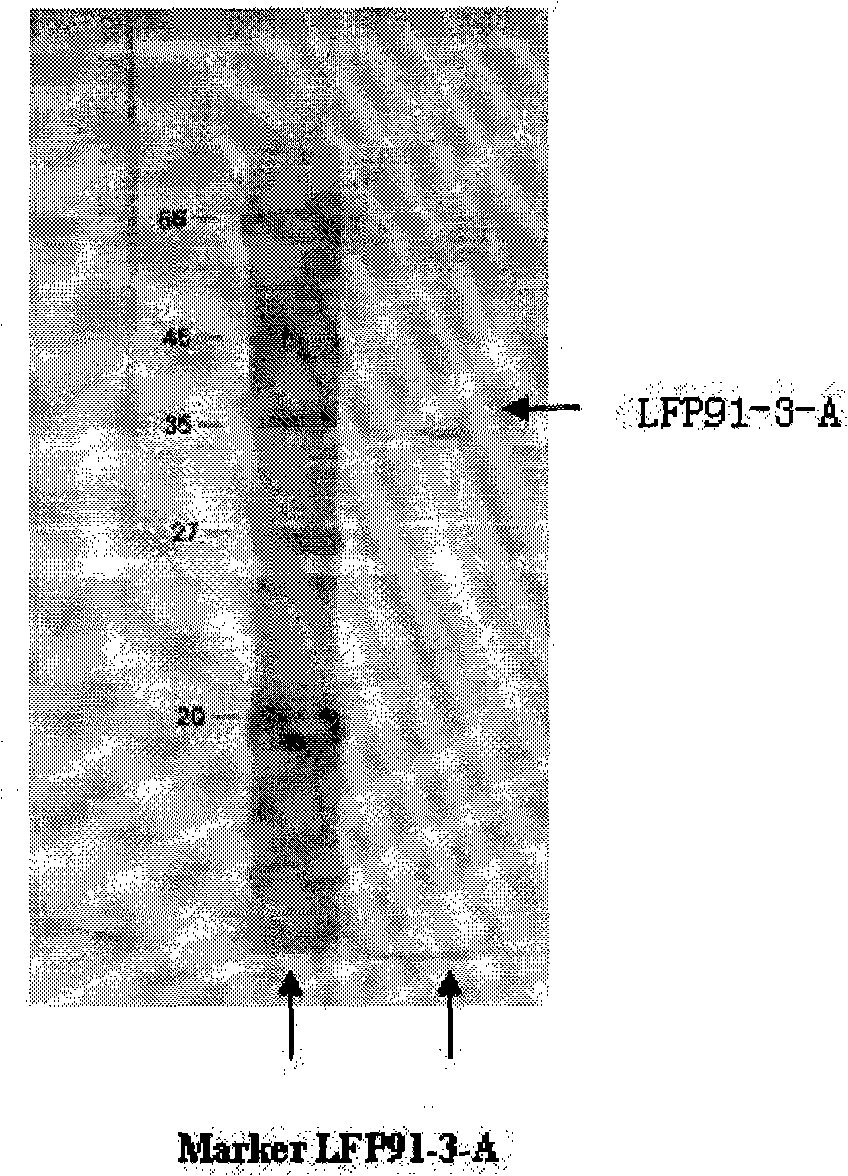
InactiveCN101343651AStrong direct killNo side effectsPowder deliveryPeptide/protein ingredientsTelomeraseSide effect
Disclosed is lentinus edodes fermented pure protein with anti-tumor activity as well as an extracting method and a preparation thereof. The protein is obtained through fermentation, salting-out, dialysis, drying and column chromatography of mycelia of lentinus edodes C91-3; the protein is light brown or earthy yellow powder which can be dissolved in water and has specific fragrance, the pH value of aqueous solution is 5.0 to 8.0, the molecular weight is 1 KD to 90 KD, and the protein contains 18 types of amino acids and various proteins. The experiment proves that in the anti-tumor experiment in vitro, the tumor restraining rate of the lentinus edodes fermented pure protein LFP91-3-A to H22 and S180 in 72 hours can achieve 59.93 percent and 80.13 percent; lentinus edodes fermented pure protein LFP91-3-A has an inducing effect on H22 cell telomerase of which the activity is obviously restrained and apoptotic. The tumor restraining rate of lentinus edodes fermented pure protein LFP91-3-B to S180 can achieve 76.7 percent; the experiment proves that the strain fermented pure protein has relatively strong effect of directly killing tumor cells. The lentinus edodes fermented pure protein has no toxic side effect through animal acute toxicity experiments and long-term toxicity experiments.
Owner:DALIAN MEDICAL UNIVERSITY
Nanofiber compound-based shale formation blocking agent for drilling fluid and preparation method
ActiveCN109810678AEffective blockingSimple methodDrilling compositionMicro nanoAcute toxicity testing
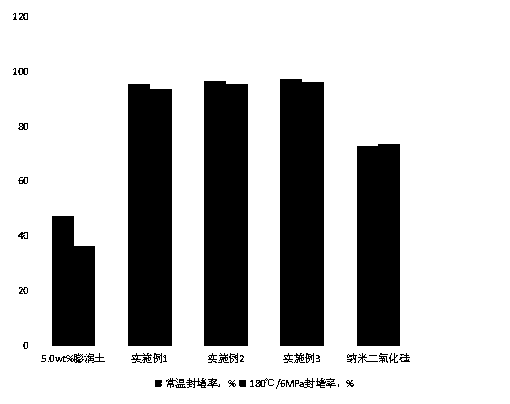
The invention relates to a nanofiber compound-based shale formation blocking agent for drilling fluid and a preparation method. The blocking agent is prepared from the following raw materials: bagasse, a dispersant, nano silica, nano graphite and deionized water. A nanofiber compound method is adopted to form the efficient blocking agent for effectively blocking nano pores and micro-nano cracks bycompounding various types of nano particles, the method is simple and reliable, and the prepared shale blocking agent has little influence on the performance of the drilling liquid, but has an obvious shale blocking effect. The blocking rate of base mud added with the amount of 3.0 percent by weight of a nanofiber compound in a shale core exceeds 96%, while under the conditions of high temperature and high pressure (180 DEG C / 6MPa), the blocking capacity remains basically unchanged (higher than 93%), and the treatment agent is non-toxic, has no adverse effects on environment, has acute toxicity EC50 which is greater than 60000, and is an efficient shale blocking agent.
Owner:CHINA PETROCHEMICAL CORP +3
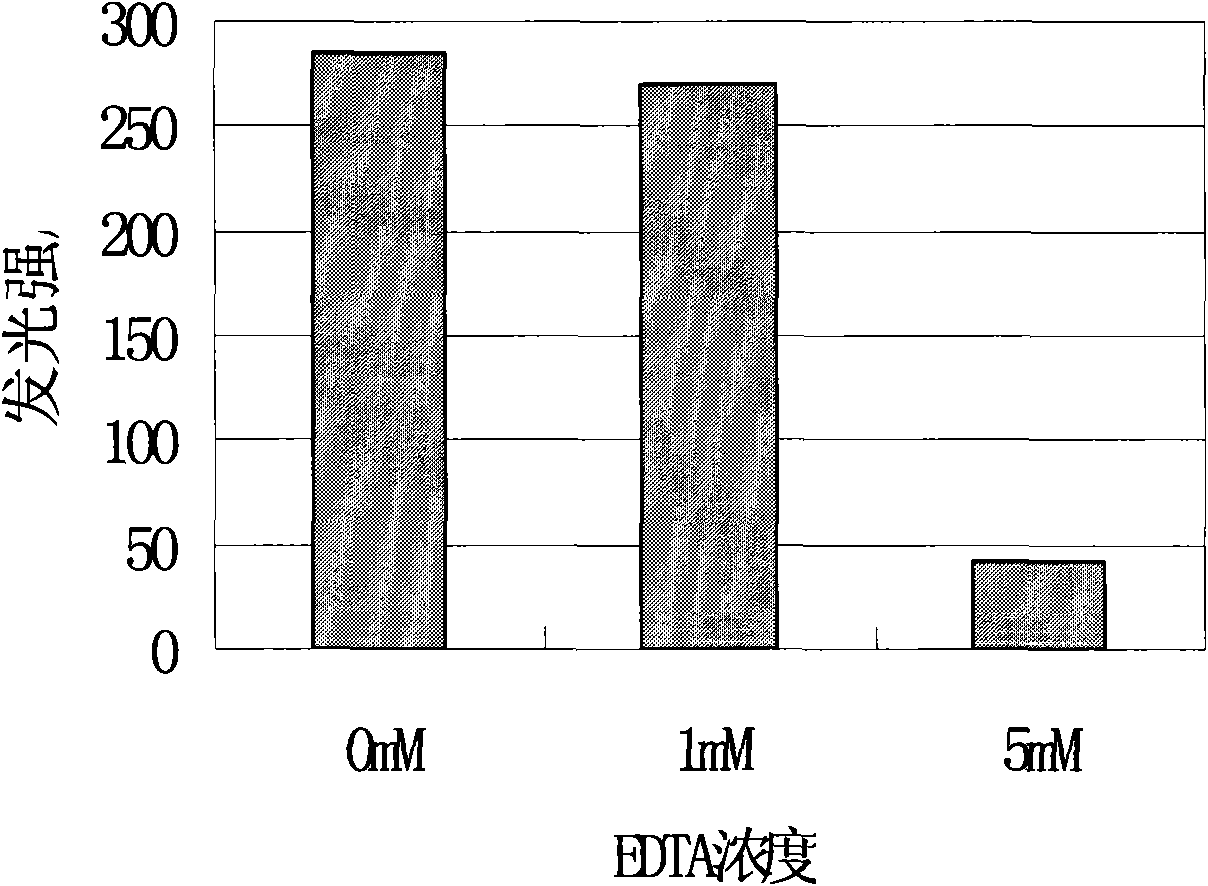
Method for detecting acute toxicity of water environment by using ATP bioluminescence
InactiveCN101865850AResponsiveImprove test performanceChemiluminescene/bioluminescencePhotometryAcute toxicity testingLuminescent bacteria
The invention discloses a method for detecting the acute toxicity of water environment by using ATP bioluminescence. The method comprises the following steps: an ATP reaction reagent, luciferase, an ATP standard sample, and a water body to be detected are sequentially added in a reaction test tube, placing the reaction test tube in an ATP fluoroscope testing instrument to detect fluorescence intensity, the detection response time is 30s and the detection is served as an experimental group; the standard sample is replaced by distilled water, the detection is served as a control group; the experimental group and the control group are repeatedly detected for three times; and the toxicity of the water body is judged by calculating relative luminance K. The method of the invention has the characteristic of stable and sensitive test effect; and the effect of a luminescent bacteria test can be reached within 3 min of the testing time at most; and the response on organic toxic substances is more sensitive than the luminescent bacteria. In the invention, a portable ATP handholding instrument with a direct current power supply is adopted, thus the ATP handholding instrument is convenient to be carried and is suitable for site test.
Owner:NANJING UNIV +1
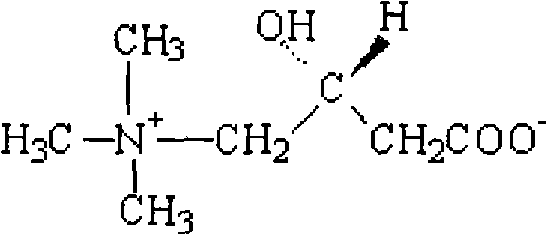
Levocarnitine liposomes injection
InactiveCN101637450AUnexpected effectImprove stabilityOrganic active ingredientsMetabolism disorderAcute toxicity testingCholesterol
The invention discloses levocarnitine liposomes injection, which is characterized by comprising the following active ingredients in parts by weight: 1 part of levocarnitine, 3-15 parts of soybean lecithin, 0.4-7.5 parts of cholesterol and 0.02-1 part of antioxidant, and a pharmaceutically acceptable carrier, wherein, the antioxidant is one or more of L-cysteine, thiourea, vitamin E and butylated hydroxyanisole and is most preferably the vitamin E. The invention also discloses a preferable preparation method of the levocarnitine liposomes injection, namely ammonium sulphate pH gradient method.The invention provides levocarnitine liposomes injection which has excellent stability, high entrapment rate and low leakage rate in the process of long-term storage. The acute toxicity tests, unusualtoxicity tests and heat source tests adopting the levocarnitine liposomes injection all conform to the specifications and are applicable to industrialized production.
Owner:HAINAN YONGTIAN PHARMA INST
Pesticide film forming agent used for controlling diseases and insect pests of forest trees, and its preparation method
InactiveCN103960228ASolve difficultySolve technical problems such as easy loss of drug effectBiocideFungicidesDiseaseAcute toxicity testing
The invention provides a pesticide film forming agent used for controlling diseases and insect pests of forest trees, and its preparation method. The pesticide film forming agent comprises 0.5-10% of an active component, 0.5-10% of a film forming aid, 5-15% of a solvent, 5-15% of an aid and 50-80% of water; and the active component is one of or a mixture comprising several of tebuconazole, difenoconazole, propiconazole and emamectin benzoate. The drug film forming agent has a good control effect on the diseases and insect pests of the forest trees, has a low price, is convenient to use, and has a good control effect on the dry rot diseases of hickory trees, the canker of various trees, stalk longhorn beetles and bark beetles, leaf aphids, red spider mites and the like especially; the drug film forming agent reduces the active component volatilization and loss possibility and changes the release performance, so the residual period is prolonged, the drug dose and the dosing frequency are reduced; and the drug film forming agent reduces the toxicity of highly toxic pesticides, reduces the acute toxicity of pesticides, reduces the drift of the pesticides, and mitigates the pollution to the environment and the hazard to crops.
Owner:ZHEJIANG FORESTRY UNIVERSITY
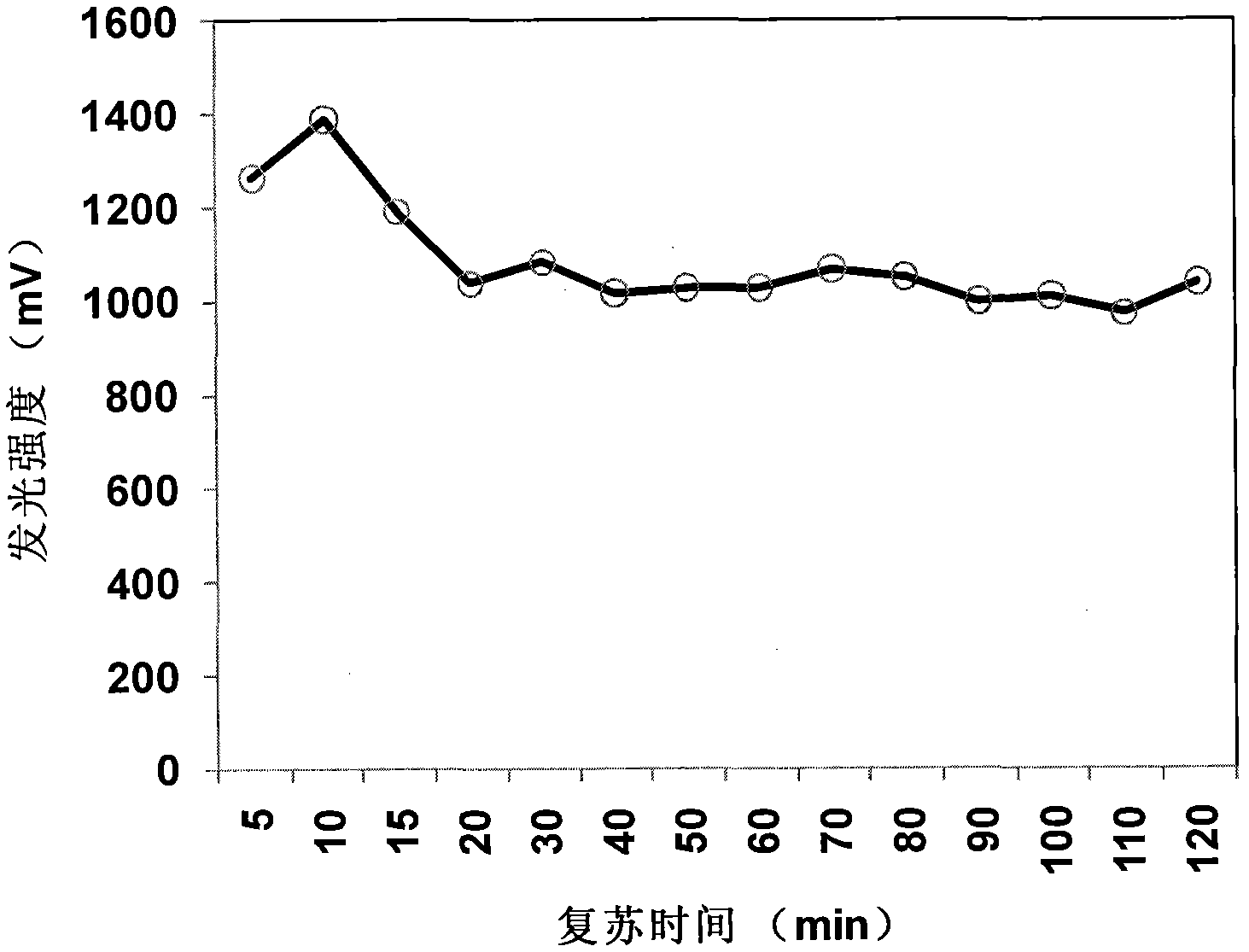
Rapid and high-flux acute toxicity test method for luminous bacteria
InactiveCN102465167AAchieve high-throughput quantizationReduce volumeMicrobiological testing/measurementAcute toxicity testingLuminous intensity
The invention relates to a rapid and high-flux acute toxicity test method for luminous bacteria, belonging to technical fields of analysis and detection. A luminous bacteria reviving solution is developed and can be used for rapidly reviving lyophilized luminous bacteria powder and cryopreserved strains within 30min, and the revived bacterium liquid can be directly used for a toxicity test as a working bacterium liquid without pre-culture after being stirred for a certain time. Meanwhile, a microporous plate is selected to serve as an exposing container and a detection tank for the toxicity test, a sample to be tested and the working bacterium liquid are added into pores and exposed for 15min, and then can be subjected to a luminous intensity test by adopting a chemiluminescence ELISA (Enzyme-Linked Immunosorbent Assay) reader. By adoption of the method, not only is a tedious and time-consuming working bacterium liquid pre-culture procedure omitted, but also the detection time is saved, the cost is reduced, the accuracy of a test result is increased, the high flux of the toxicity test is realized, and a wide popularization and application prospect is achieved.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
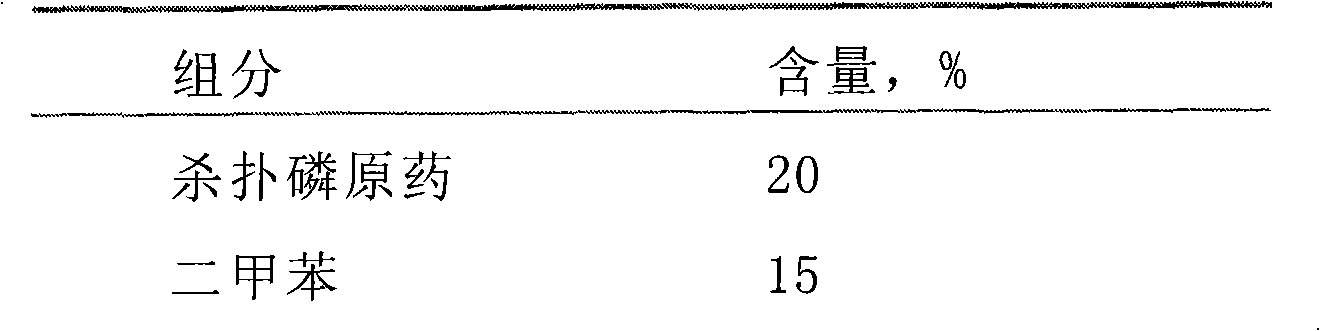
Chlorpyrifos microcapsule suspension and preparation method thereof
InactiveCN102057897ASatisfy the appropriate anti-efficacy requirementsLong durationBiocideAnimal repellantsChlorpyrifosAcute toxicity testing
The invention discloses a chlorpyrifos microcapsule suspension. The chlorpyrifos microcapsule suspension mainly comprises the following components in percentage by weight: 10 to 45 percent of chlorpyrifos, 5 to 20 percent of solvent, 1 to 10 percent of capsule wall material, 0.1 to 3 percent of lacquer, 2 to 5 percent of emulsifier, 3 to 5 percent of dispersant, 0 to 14 percent of auxiliary agentand 10 to 80 percent of water. The invention also discloses a preparation method of the chlorpyrifos microcapsule suspension. The chlorpyrifos microcapsule suspension has the advantages of reducing acute toxicity of raw medicaments, reducing environmental pollution and stimulation to people and livestock, improving prevention and control effect on insect damages such as a citrus tree scale insectand the like, greatly prolonging duration of a pesticide and reducing pesticide application frequency and agricultural cost. Since microcapsule technology is adopted and water is used as a dispersingmedium in the chlorpyrifos microcapsule suspension, the chlorpyrifos microcapsule suspension is difficult to flame and explode, and can be safely and reliably used and transported.
Owner:联合国南通农药剂型开发中心 +1
Insect antimicrobial peptide Thanatin derivant, producing method and uses of the same
InactiveCN101173005AIncrease varietyHigh antibacterial activityAntibacterial agentsAntimycoticsK pneumoniaeHemolysis
The invention relates to a group of insect Thanatin ramifications with antibacterial action and the salt which is applicable for medicinal use, as well as a preparation method and the use thereof. Approved by the results of a sterilization experiment, a hemolysis experiment and an irritable partial stimulation experiment, the insect Thanatin ramification has bigger antibacterial activity than Thanatin to colon bacillus, Candida albicans, Klebsiella pneumoniae, and staphylococcus aureus. The hemolysis reaction is not incurred when the concentration is 2.5mg / ml without acute toxicity stimulation reaction.
Owner:沈子龙
Method used for testing high throughput acute toxicity of early life stage of fish
The invention relates to a special method for testing the high throughput acute toxicity of an early life stage of fish, and belongs to the technical field of environment detection and assessment. According to the method, the sensibility of the fish at different life stages on toxic pollutants as well as the influence of conditions such as different quantities of test creatures, different exposed containers and the like on test results are discussed through developing a recombinant dilute solution for toxicity test, thus realizing high throughput on a larval fish acute toxicity test. The method is simple, convenient, economical, rapid and sensitive, has low requirements on experiment operation technical level, and is convenient for popularization and application.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
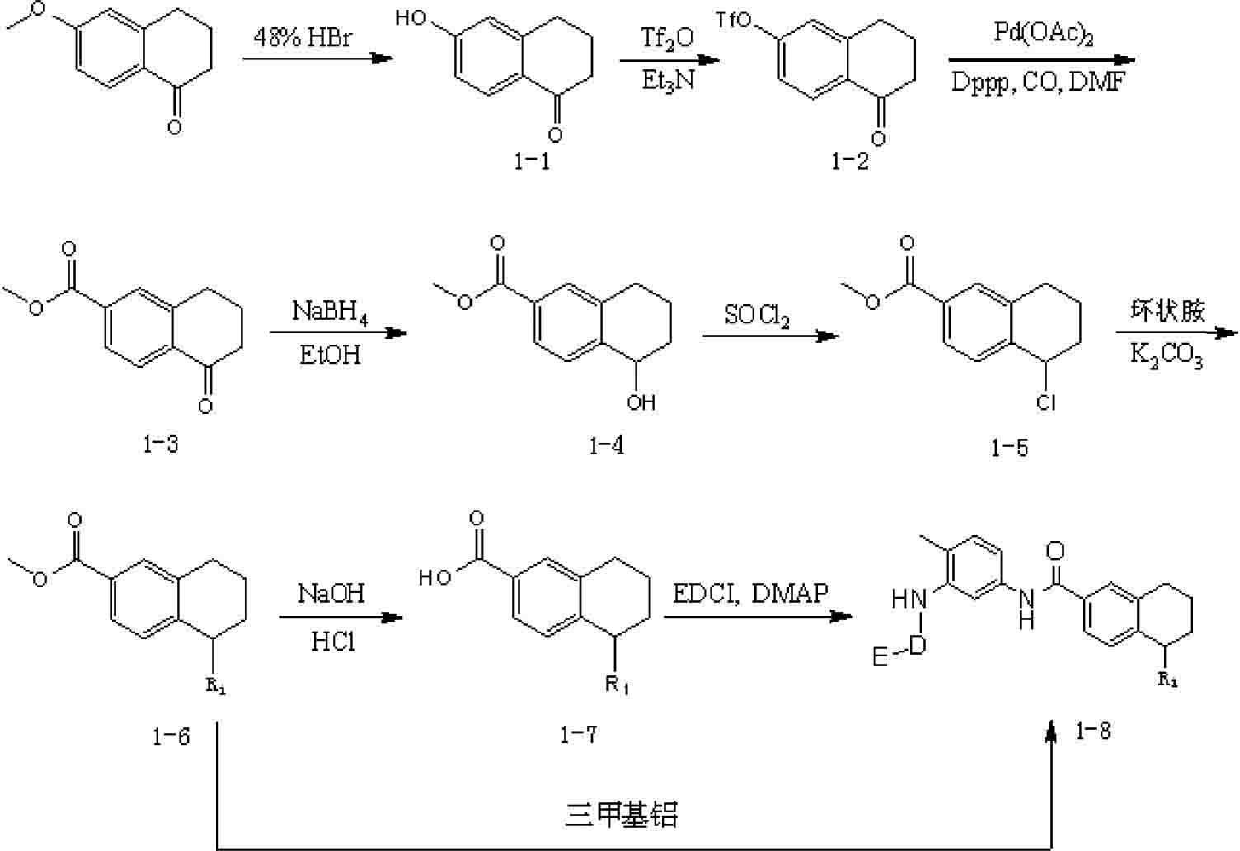
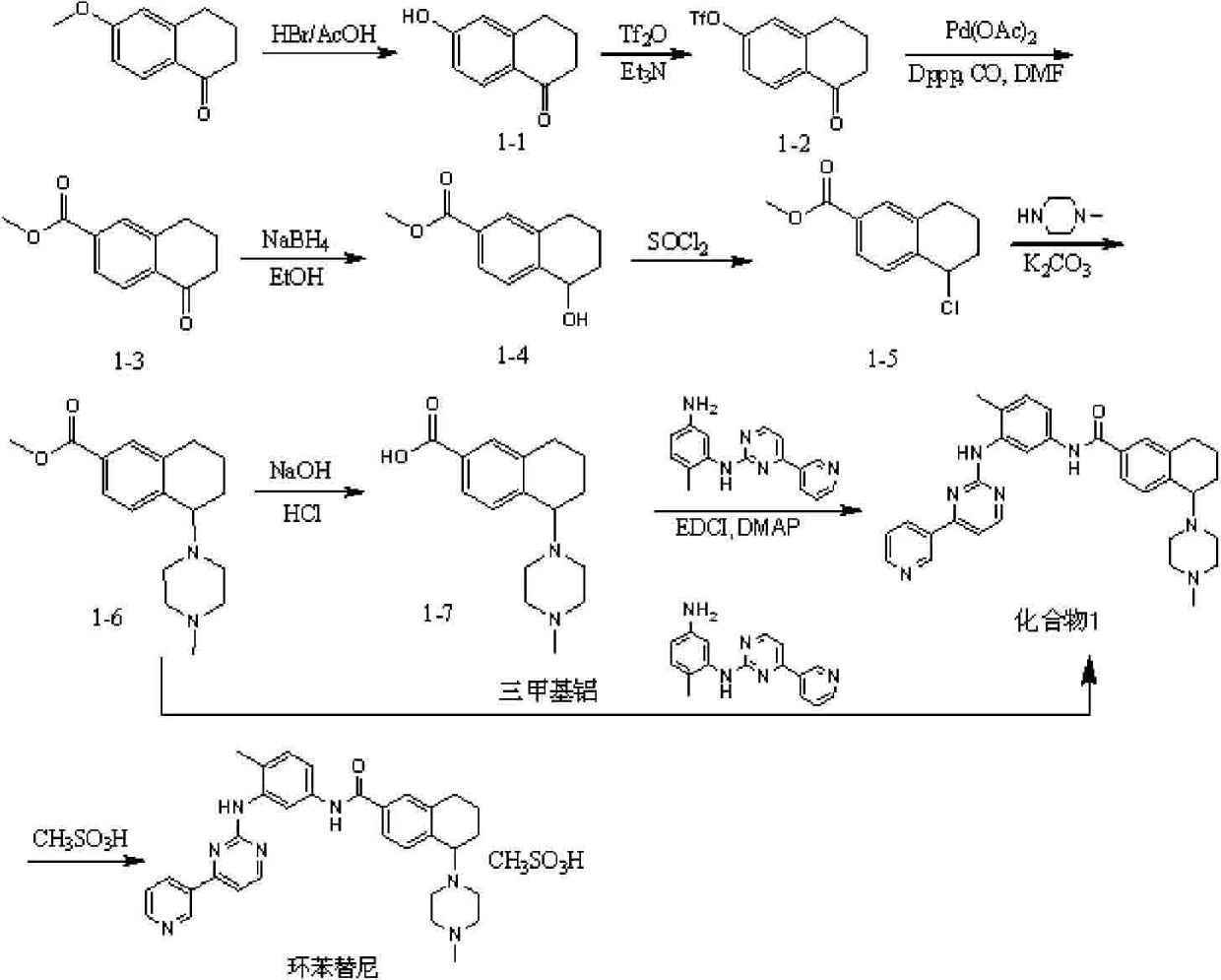
Antineoplastic drug tetrahydronaphthalene amide compound and pharmaceutically acceptable salt thereof, preparation method and application thereof
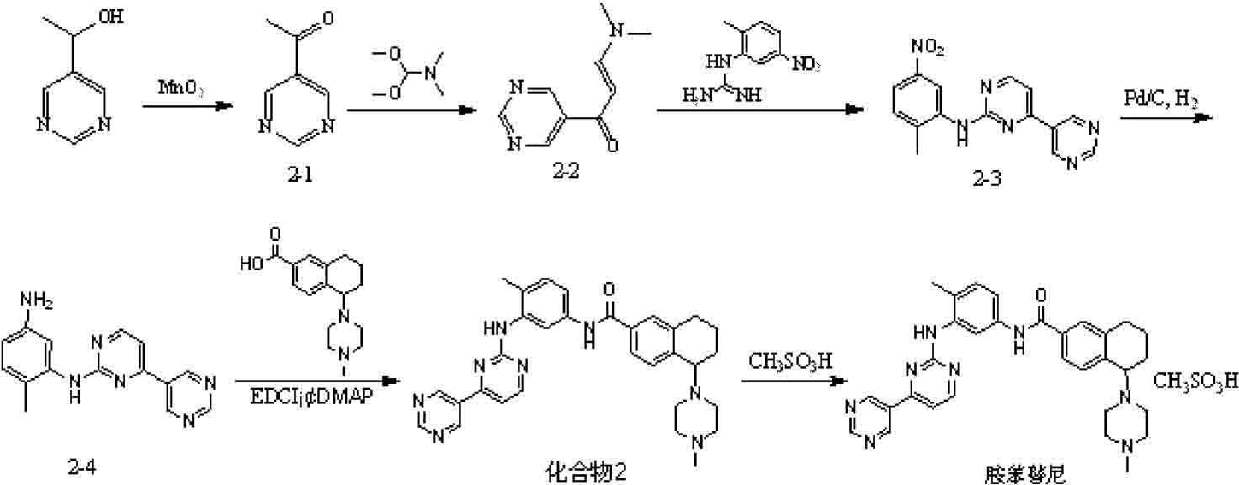
ActiveCN102295635AGood antitumor activityImprove securityOrganic active ingredientsOrganic chemistryAcute toxicity testingTetralin
The invention relates to new antitumor medicament compounds, namely tetralin amide compounds as shown in a general formula I or pharmaceutically acceptable salts thereof used as an antitumor medicament. The invention also provides a preparation method of the compounds and medicament compositions containing the compounds as well as in vitro and in vivo antitumor effect results and acute toxicity researches thereof. The antitumor medicament, namely tetralin amide compounds obtained by the preparation method, has better antitumor activity and safety, and can be applied to treatment of tumors such as leukemia, lung cancer, colon cancer, ovarian cancer, renal cancer and the like, thus the tetralin amide compounds have wide treatment ranges and also have extremely high application value in the medicine field as antitumor agents.
Owner:LIAONING UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com