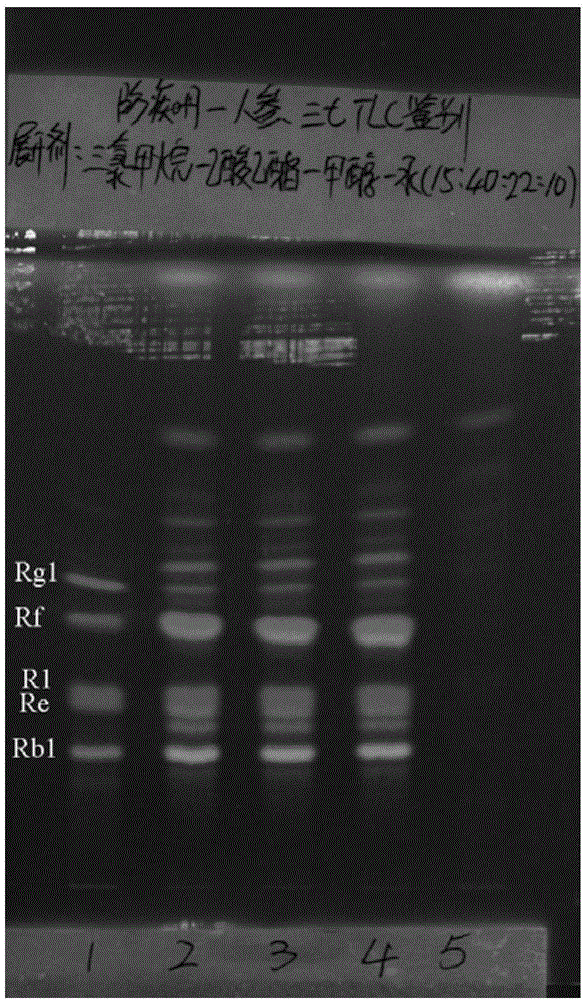
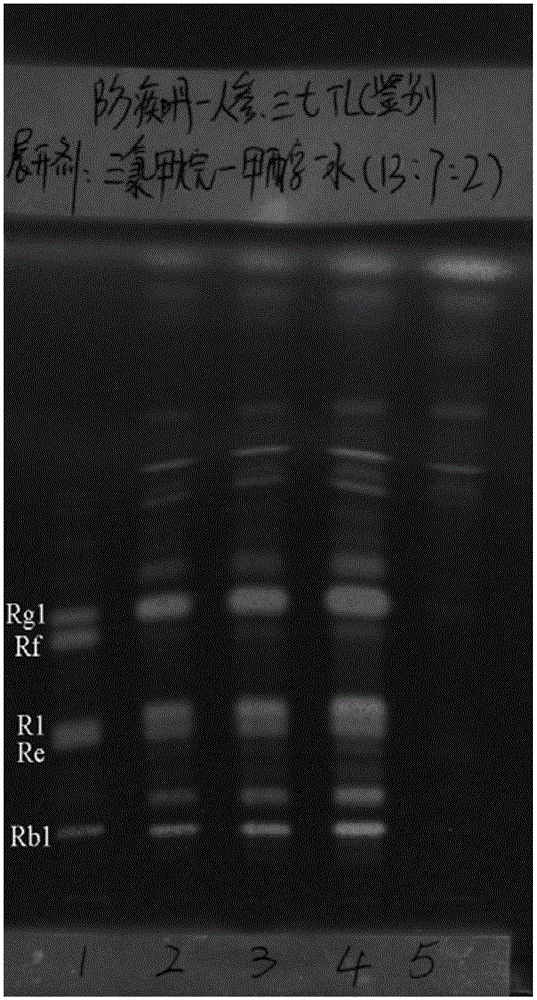
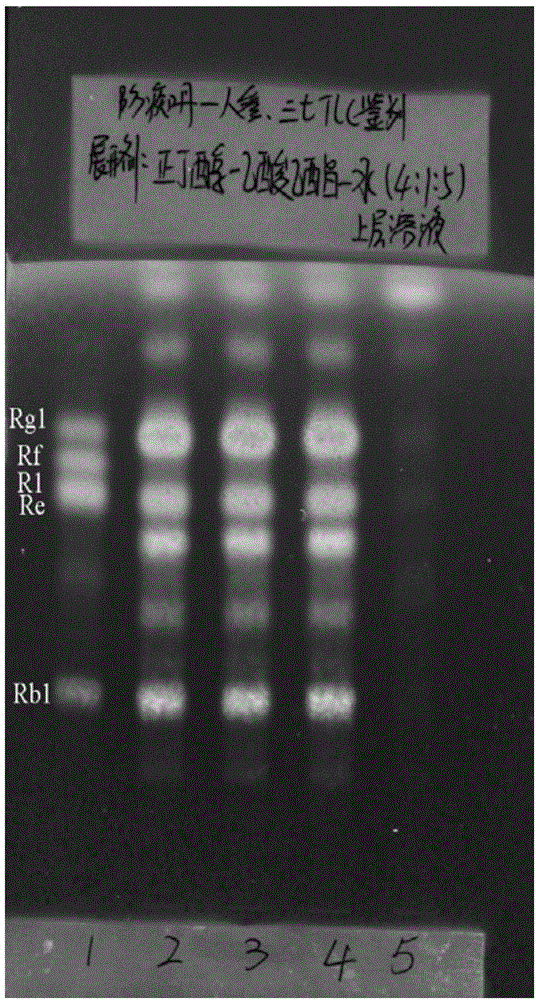
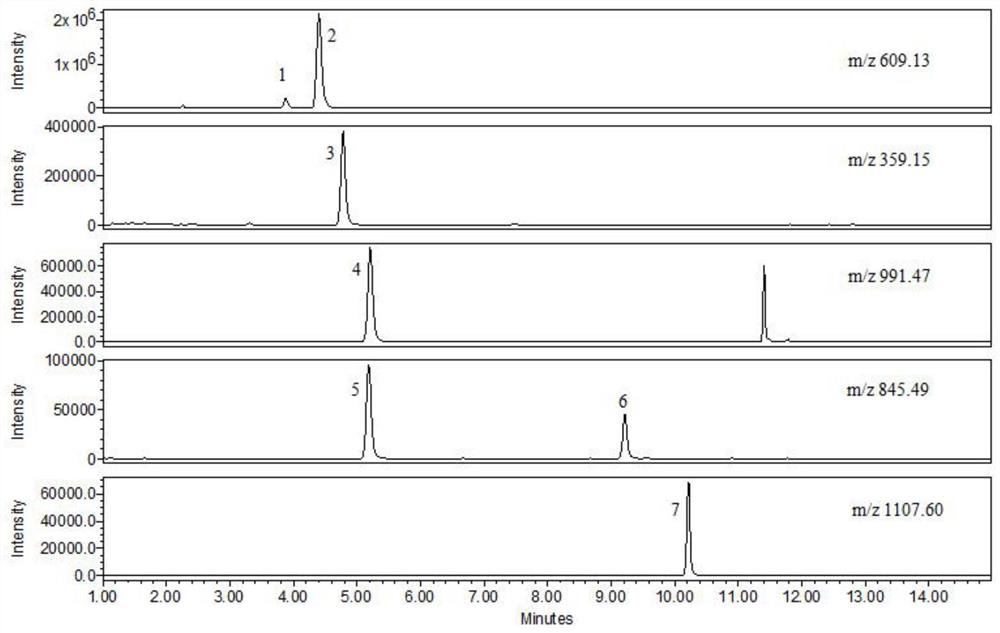
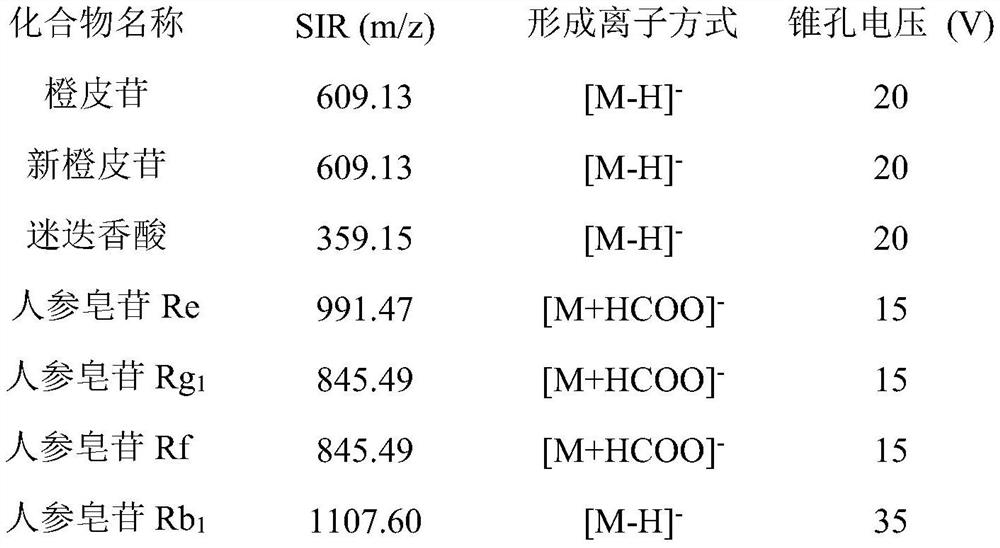
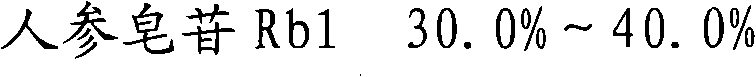Patents
Literature
32 results about "Ginsenoside Rf" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
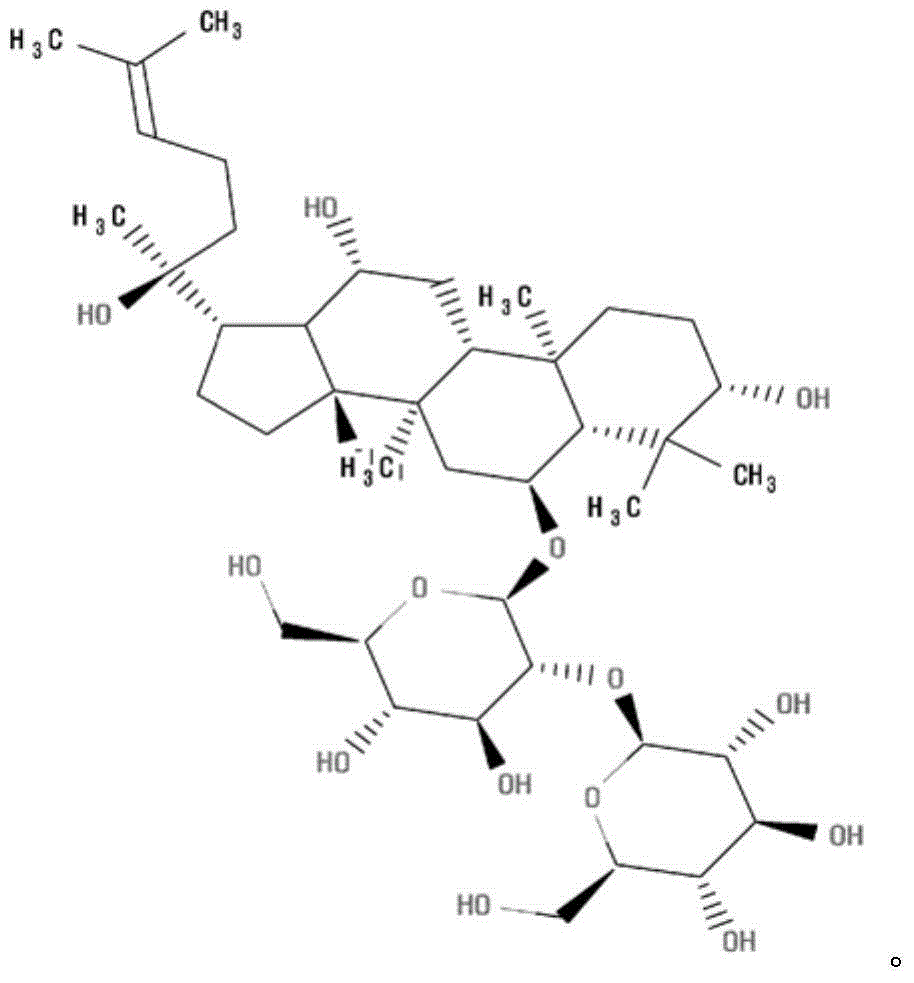
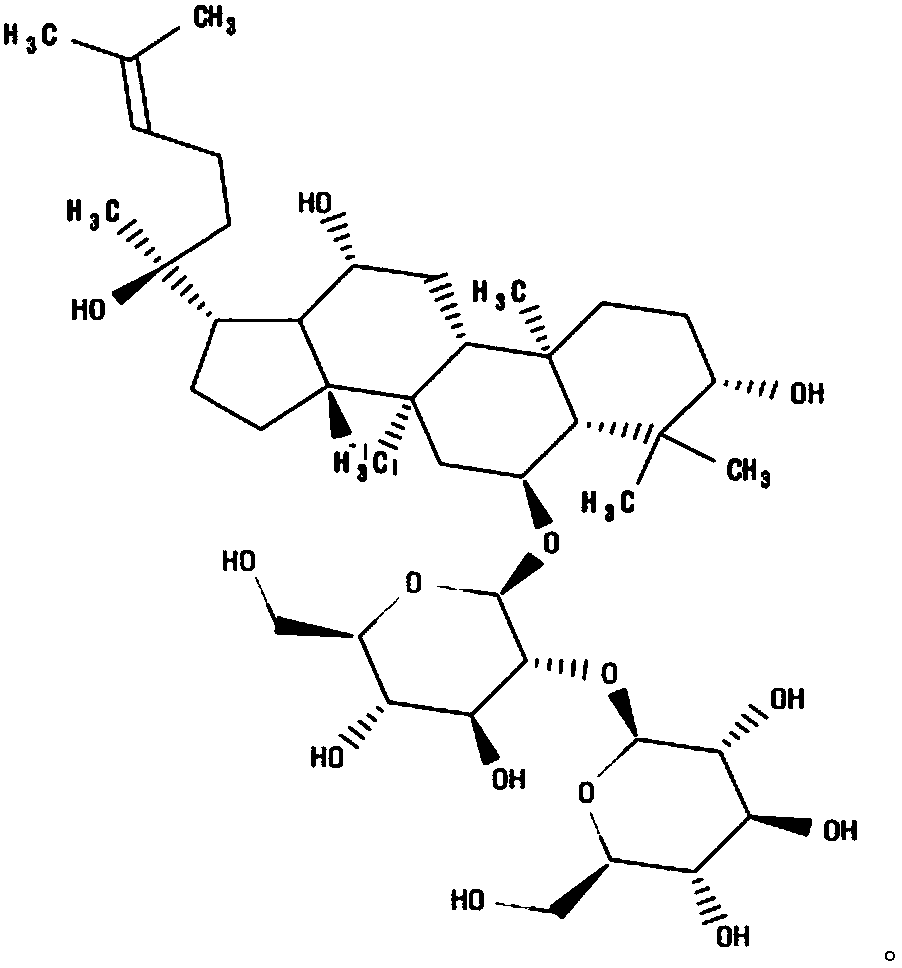
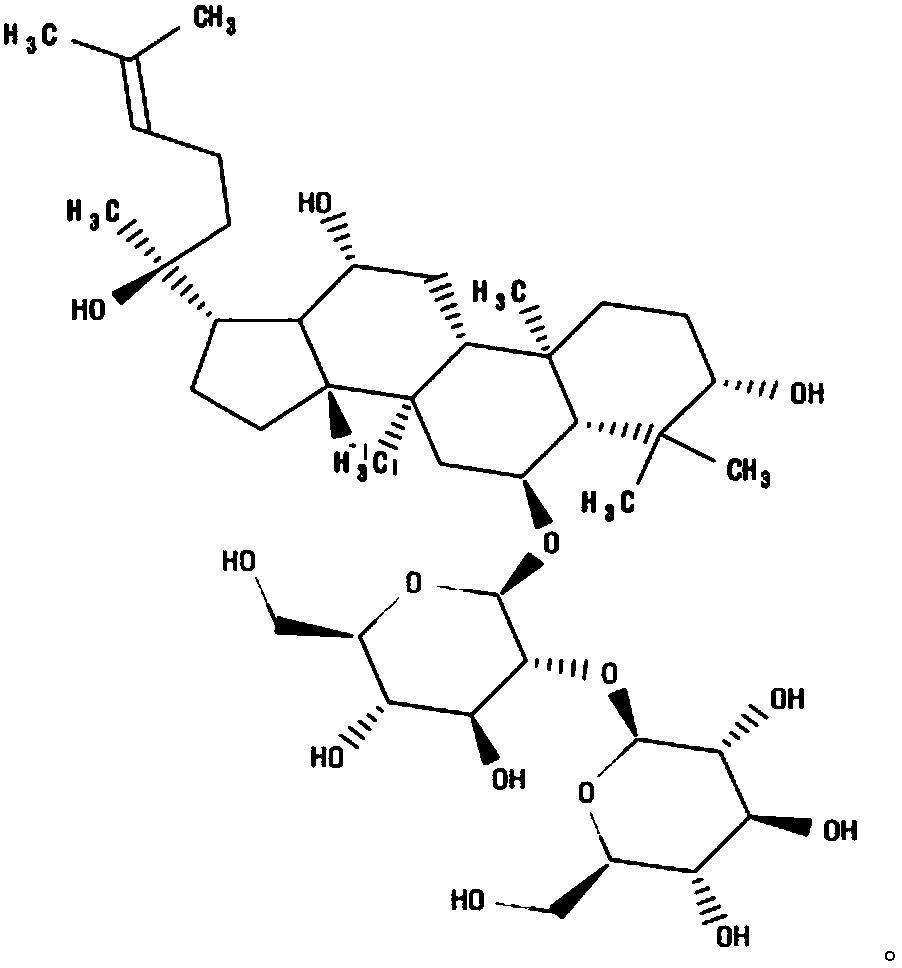
Ginsenoside Rf is a ginsenoside found in Panax ginseng and Panax japonicus var. major that is dammarane which is substituted by hydroxy groups at the 3beta, 6alpha, 12beta and 20 pro-S positions, in which the hydroxy group at position 6 has been converted to the corresponding beta-D-glucopyranosyl-(1->2)-beta-D-glucopyranoside, and in which a double bond has been introduced at the 24-25 position.
Radix notoginseng extract and preparation thereof
ActiveCN101732378AHigh purity of ingredientsIncrease concentrationCardiovascular disorderPlant ingredientsGinsenoside RcPanax notoginseng extract
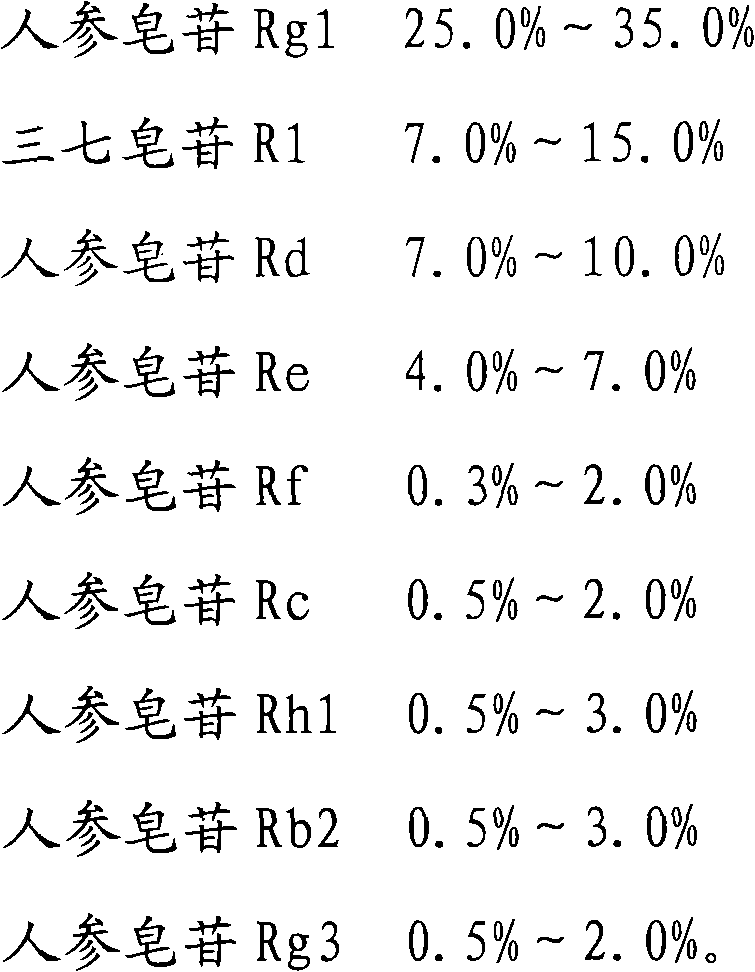
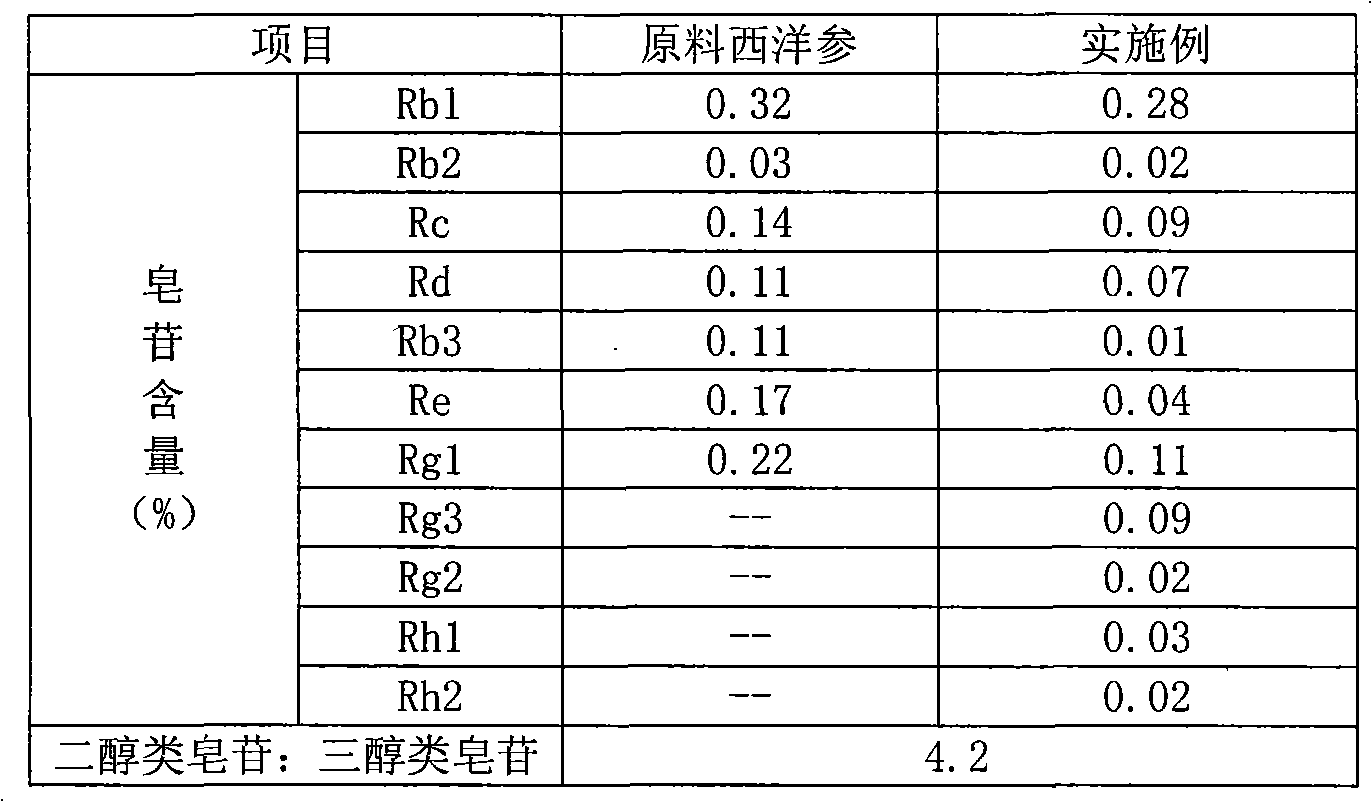
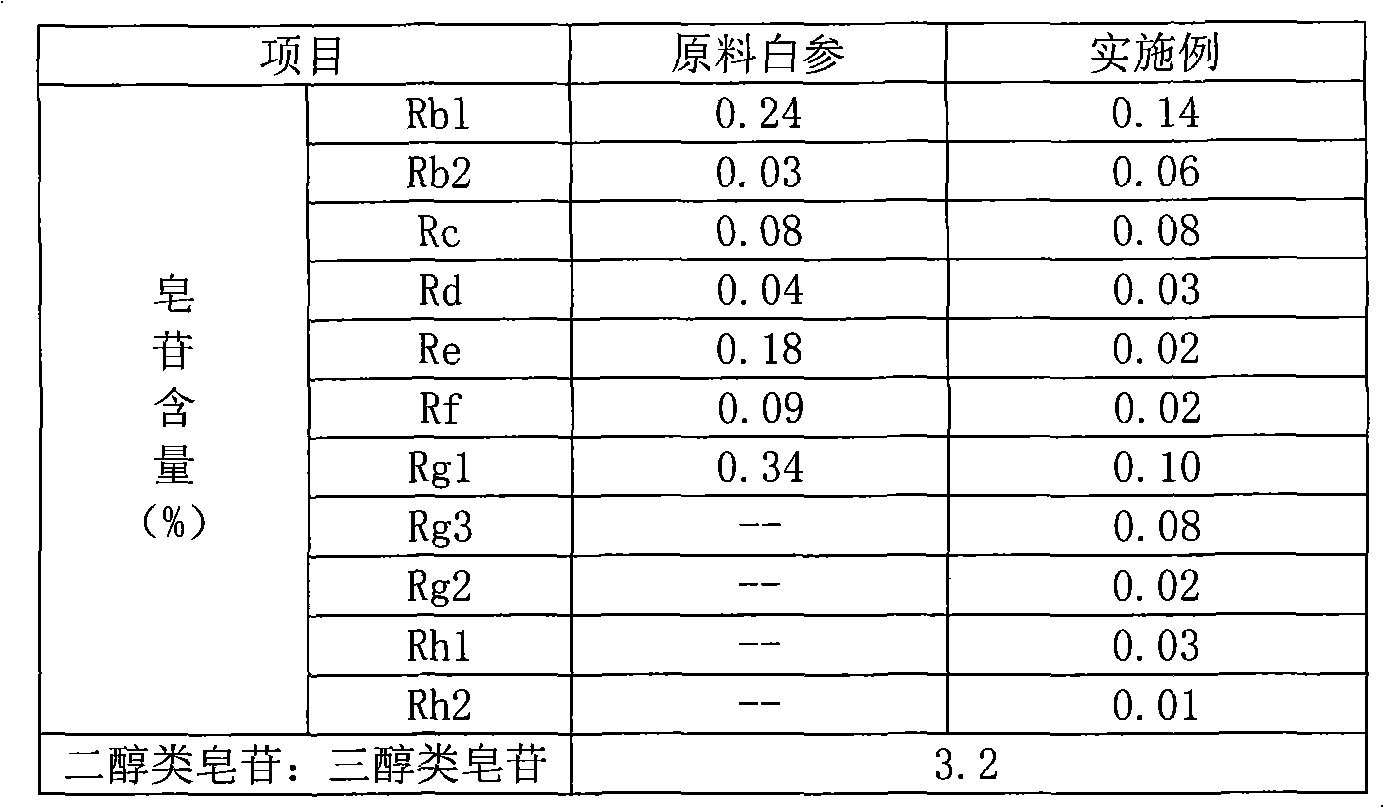
The invention provides a radix notoginseng extract which contains 5-10% of notoginsenoside R1, 25-36% of ginsenoside Rg1, 2.5-5% of ginsenoside Re, 30-39% of ginsenoside Rb1, 5-10% of ginsenoside Rd and at least 2% of ginsenoside Rf, ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2 and ginsenoside Rg3, wherein the ginsenoside R1, the ginsenoside Rg1, the ginsenoside Re, the ginsenoside Rb1 and the ginsenoside Rd account for 75-95% of the total weight. The invention also provides a preparation method of the radix notoginseng extract. The radix notoginseng extract prepared by the method has little impurities, the purity of the component of total saponin is higher, and especially, the components of the ginsenoside Rf, the ginsenoside Rh1, the ginsenoside Rc, the ginsenoside Rb2, the ginsenoside Rg3 and the like with very low content are purified. The medicinal preparation prepared by the radix notoginseng extract has better curative effect and higher safety.
Owner:HARBIN ZHENBAO PHARMA
Medicinal composition and preparation method thereof
ActiveCN102028700AIncrease the maximum tolerated doseRaise the median lethal doseOrganic active ingredientsPeptide/protein ingredientsHemolysisAcute toxicity testing
The invention provides a medicinal composition, which comprises the following components: ginsenoside Rb1, ginsenoside Rg1, notoginsenoside R1, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rc, ginsenoside Rh1, ginsenoside Rb2 and ginsenoside Rg3. The invention also provides a preparation method for the medicinal composition. The medicinal composition has definite components; compared with the total notoginsenoside in the prior art, the medicinal composition has stable quality and good controllability; and results of experiments of acute toxicity, undue toxicity, hemolysis and the like show that the medicinal composition has higher safety and wide clinical application prospect.
Owner:KPC PHARM INC
Production method of flavor panax ginseng
The invention relates to a preparation method of flavor ginseng; after ginseng is added with nature fruit juice or organic acid for reaction, the content of the precious ginsenoside in products is enhanced, the efficacies of ginseng is improved, while the specific flavor of ginseng is maintained. The main quality characteristics is that: the flavor ginseng includes at least one of the following ingredients: ginsenoside Rg3, ginsenoside Rh1 and ginsenoside 20R-Rh2; wherein, the ratio of the total amount of precious ginsenoside group, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rd and ginsenoside Rc to the total amount of ginsenoside Re, ginsenoside Rg1 and ginsenoside Rf is more than 2.5; the preparation method can convert part of the component causing internal heat, namely, panaxatriol ginsenoside, into precious ginsenoside.
Owner:JILIN HONGJIU BIO TECH
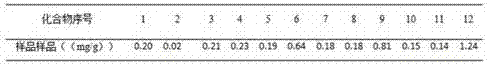
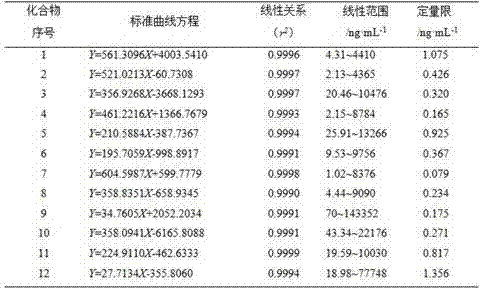
Determining method for contents of twelve components in traditional Chinese medicine composition preparation
ActiveCN104914199AQuality improvementGood repeatabilityComponent separationBiotechnologyAstragaloside
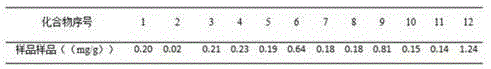
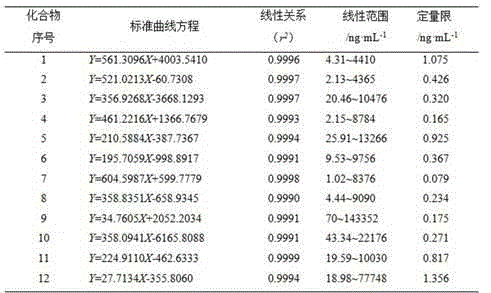
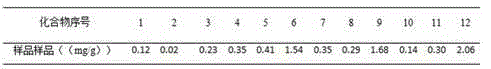
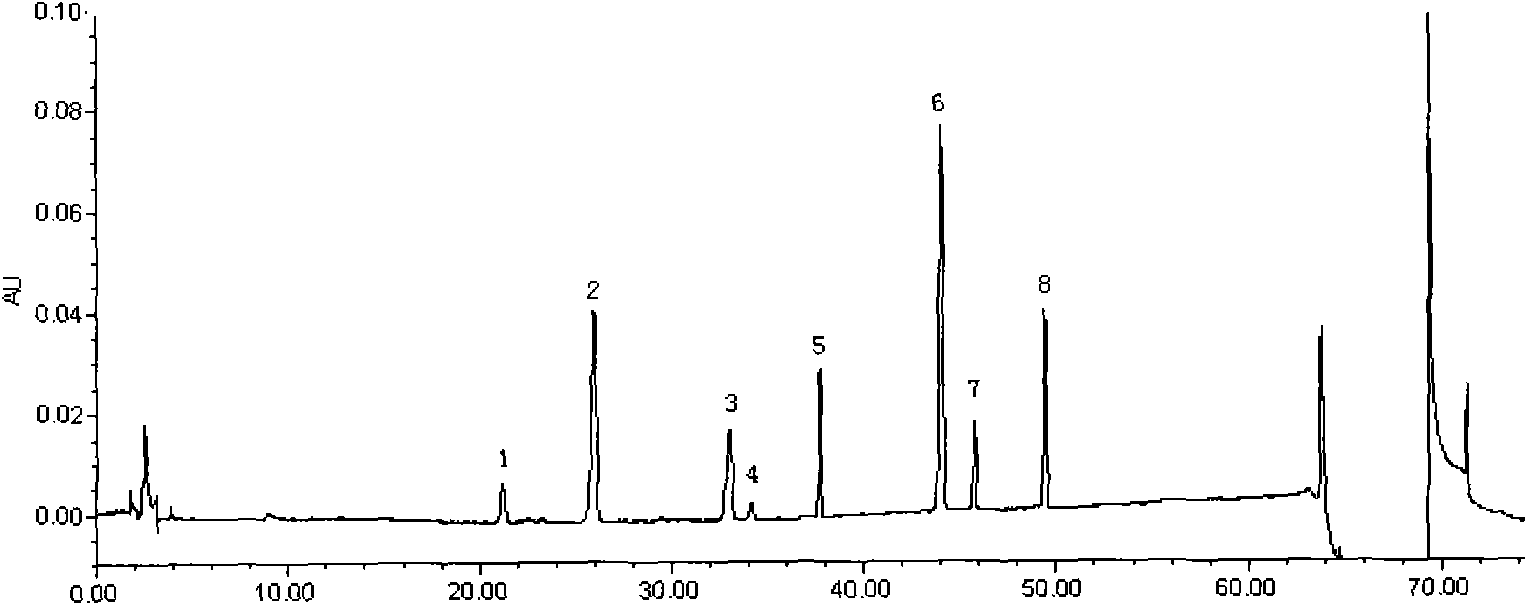
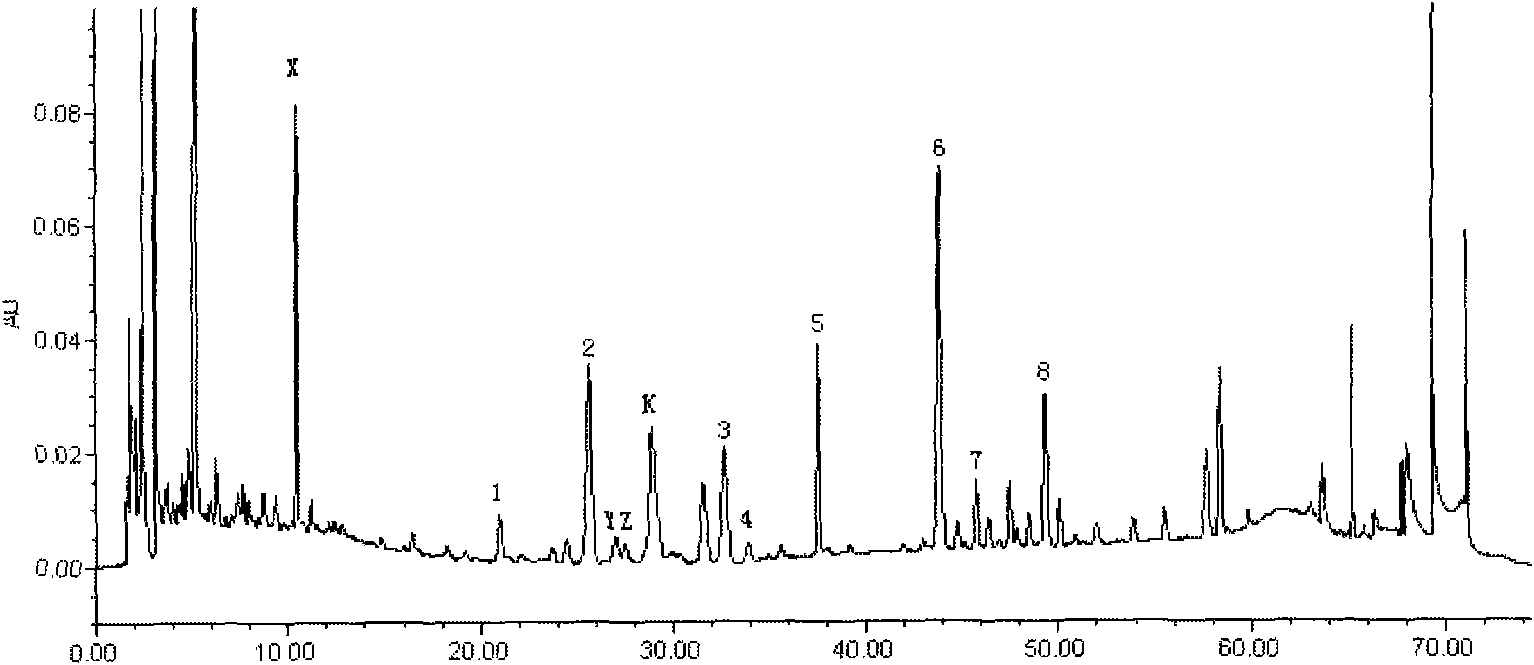
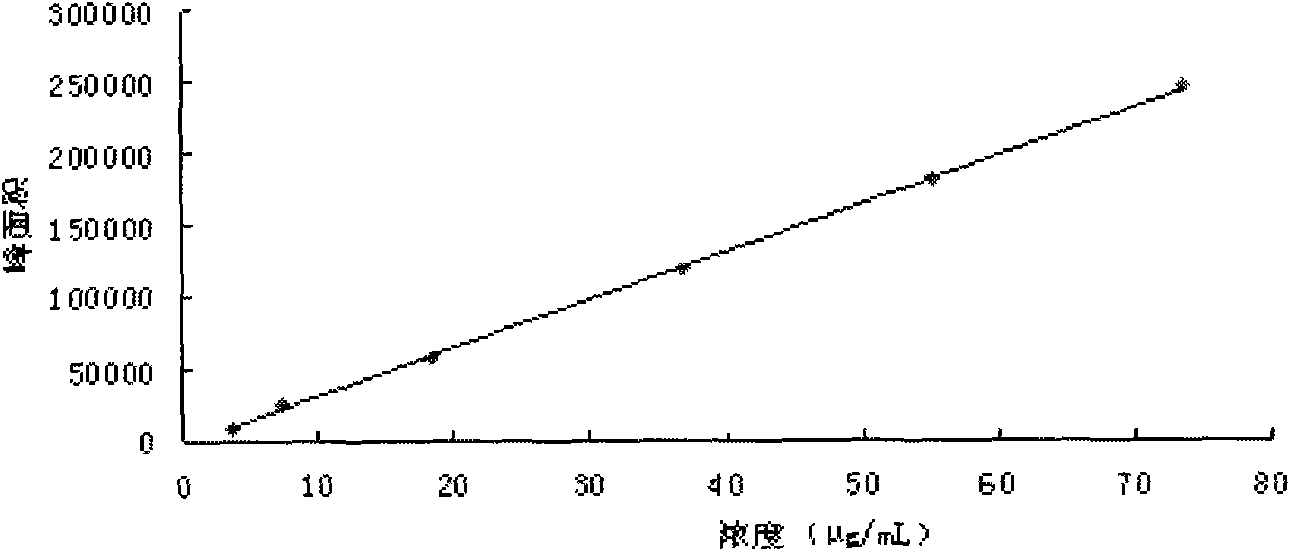
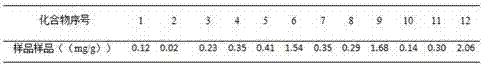
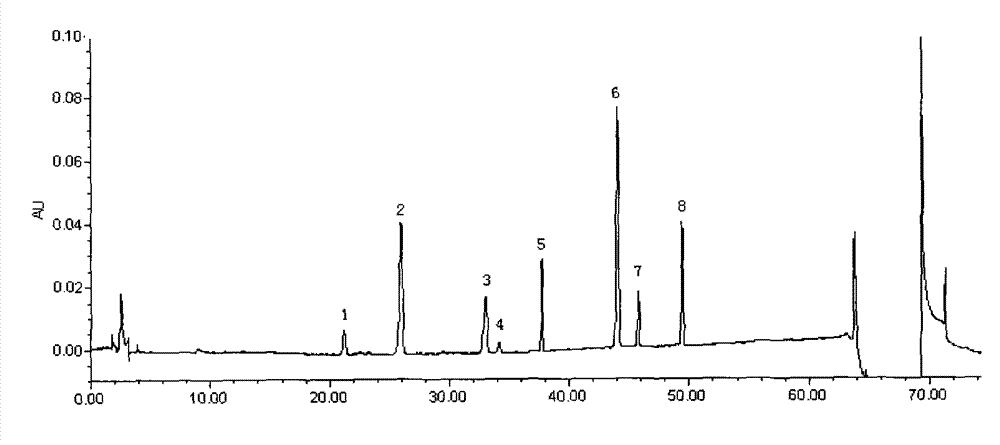
The present invention discloses a UPLC-MS quantitative method for the contents of twelve components in a traditional Chinese medicine composition preparation, specifically determination of the contents of calycosin-7-O-beta-D-glucoside (1), isoquercitrin (2), narirutin (3), hesperidin (4), ginsenoside Re (5), ginsenoside Rg1(6), periplocoside (7), ginsenoside Rf (8), ginsenoside Rb1 (9), astragaloside (10), ginsenoside Rd (11) and periplocin H1 (12) in the traditional Chinese medicine composition, and belongs to the field of traditional Chinese medicine composition preparation component detection. The determining method of the present invention has characteristics of short period, good reproducibility and high sensitivity.
Owner:HEBEI YILING MEDICINE INST
Chinese medicinal injectable powder and quality control method thereof
The invention relates to Chinese medicinal injectable powder and a quality control method thereof. The Chinese medicinal injectable powder consists of red ginseng, dwarf lilyturf tuber and Chinese magnoliavine fruit in part by weight, wherein the sum of the main active ingredient content of the injectable powder cannot be lower than 4.0mg / g; and the active ingredients comprise ginsenosides Rf, Rb1, Rb2, Rb3, Rd, Rg3 and F2, and schizandrol A. The quality control method comprises the steps of making quality standards, measuring the content, comparing the content of a sample to be measured with the quality standards, and judging whether the content reaches the preset standards or not so as to control the product quality. The method has the advantages of quickness, accuracy, sensitivity, contribution to industrial production inspection, and capability of effectively controlling the quality of a finished product.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Method for quickly quality-detecting and identifying American ginsengs, ginsengs and preparations of American ginsengs and ginsengs
InactiveCN101685089AEasy to separateSimple extraction methodComponent separationESI mass spectrometryPseudoginsenoside F11
The invention discloses a method for quickly quality-detecting and identifying American ginsengs, ginsengs and preparations of American ginsengs and ginsengs. In the method, 70-percent methanol is used as a sample ultrasonic extraction solvent, a C18 chromatographic column is adopted, a system of acetonitrile-tetrahydrofuran-formic acid-water is used as a flowing phase, an electrospray ionization(ESI) ion source is adopted, quick separation, identification and measurement of pseudoginsenoside F11 and ginsenoside Rf which are characteristics components of the American ginsengs and ginsengs respectively are performed within 2 minutes for the first time under a condition of a negative ion mode second order mass spectrometry analysis, and whether the preparation of the American ginsengs is mixed with the ginsengs is judged and the content of crude drugs mixed with the ginsengs is worked out. The method has the advantages of strong specificity, synchronous qualitative and quantitative analysis, easy and quick pre-treatment, quick detection of the contents of the American ginsengs and the ginsengs within 2 minutes, high sensitivity of detection and measurement and good repeatability, and the suitability for quick quality screening of the American ginsengs, the ginsengs and products of the American ginsengs and the ginsengs.
Owner:天津海世达检测技术有限公司 +1
Method for measuring content of five saponin components in red ginseng roots
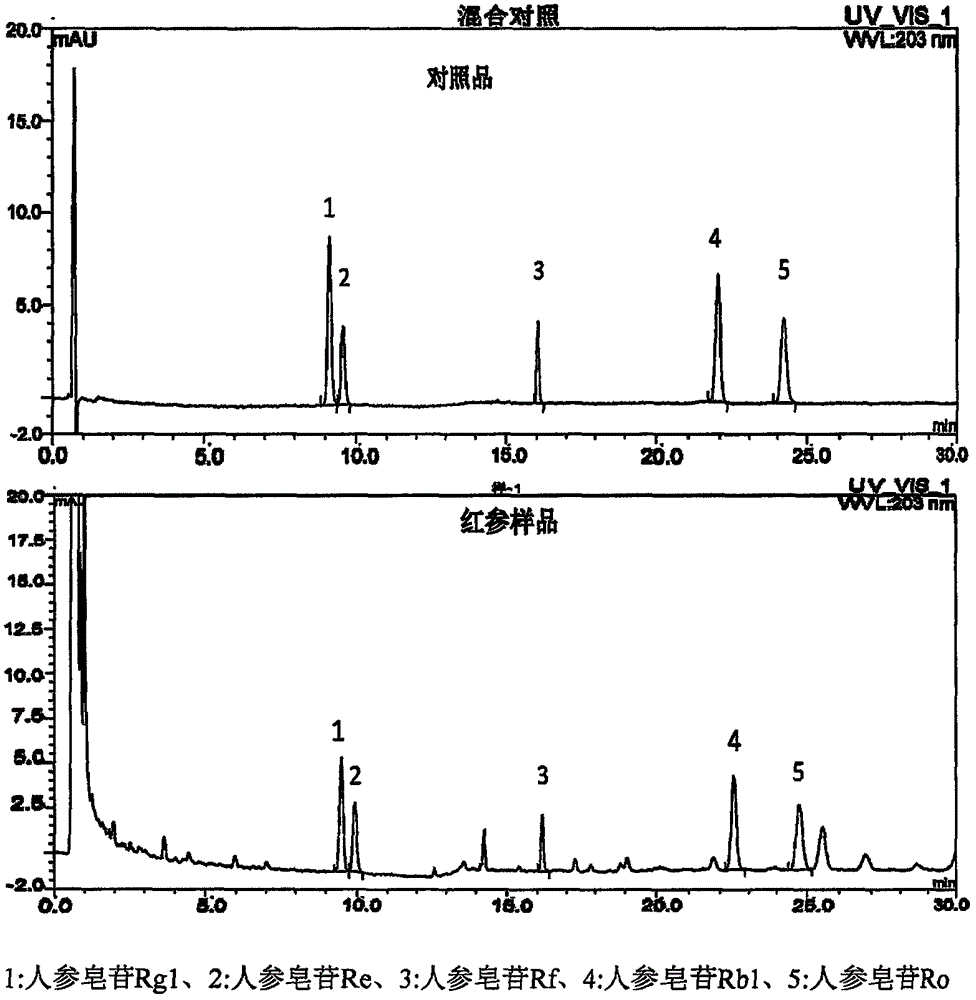
The invention provides a method for measuring content of five saponin components in red ginseng roots. Ginseng saponin components of main effective active components of the red ginseng roots and are important standards measuring advantages and disadvantages of inner quality of the red ginseng roots. According to the method, ginseng saponin Rg1, ginseng saponin Re, ginseng saponin Rf, ginseng saponin Rb1 and ginseng saponin Ro in the red ginseng roots are simultaneously measured by the aid of an ultra-high performance liquid chromatography, and the method is high in sensitivity, accurate and reliable and can be used for controlling quality of the red ginseng roots.
Owner:河北省药品检验研究院
Panax notoginseng saponin composition and preparation method and application thereof
ActiveCN105816471AClear validityClear contentOrganic active ingredientsBlood disorderGinsenoside RdLow toxicity
The invention provides a panax notoginseng saponin composition and a preparation method and application thereof.The composition is prepared from, by weight, 30-50% of ginsenoside Rg1, 25-40% of ginsenoside Rb1, 7-16% of notoginsenoside R1, 2.7-8% of ginsenoside Re, 0.5-7.0% of ginsenoside Rd, 0.5-2% of ginsenoside Rf, 0.3-2.0% of ginsenoside Rh2, 1-3% of ginsenoside Rc, 0.3-2.5% of ginsenoside Rb3 and 0.5-2.5% of ginsenoside Rg3, wherein ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 account for 70-95% of the total weight of the active components, and the sum of the contents of ginsenoside Rb1 and notoginsenoside R1 is not smaller than 8 times of the content of ginsenoside Rd.Compared with commercially available Xueshuantong injections and Xueshuantong preparations, the panax notoginseng saponin composition has a more obvious arterial and venous thrombosis resistant effect, low toxicity and high safety.
Owner:HARBIN ZHENBAO PHARMA
Detection method of ginseng/pseudo-ginseng-containing traditional Chinese medicine preparation
The invention provides a detection method of a ginseng / pseudo-ginseng-containing traditional Chinese medicine preparation. The method comprises the following steps: (1) taking a ginseng / pseudo-ginseng-containing traditional Chinese medicine composition or traditional Chinese medicine preparation, and preparing a test sample solution; (2) taking a ginseng / pseudo-ginseng-free finished product, and preparing a negative sample solution; (3) taking a ginsenoside Rb1 reference substance, a ginsenoside Re reference substance, a ginsenoside Rg1 reference substance, a ginsenoside Rf reference substance and a pseudo-ginseng saponin R1 reference substance, respectively adding methanol to prepare a mixed solution containing 0.5-2mg of respective reference substance per 1ml as a reference substance mixed solution; (4) detecting; and (5) analyzing the result. The method can simultaneously indentify ginseng and pseudo-ginseng, and simultaneously identify the index components ginsenoside Rb1, ginsenoside Re, pseudo-ginseng saponin R1, ginsenoside Rf and ginsenoside Rg1. The detection method is simple and easy to implement, and has the characteristics of high specificity and favorable repetitiveness.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Method for determining contents of seven types of ginsenosides in healthcare food
InactiveCN102183588AThe detection method is accurateConvenient and economical detection methodComponent separationRetention timeUltraviolet
The invention discloses a method for determining contents of seven types of ginsenosides in healthcare food. The seven types of ginsenosides are Rg1, Re, Rc, Rf, Rb1, Rb2 and Rd. The method comprises the steps of: (1) preprocessing a sample; (2) carrying out chromatographic analysis by using a reversed-phase high performance liquid chromatography column; (3) detecting by using an Ultraviolet (UV) detector; (4) determining the compositions of the ginsenosides in the sample according to the retention time of a chromatographic peak; and (5) determining the content of the ginsenosides in the sample by using an external standard method. By means of the method disclosed by the invention, the defects of the traditional detection technology are overcome, the determination of the ginsenoside RF is enhanced, the content of the ginsenosides in the healthcare food can be better analyzed, a basis for better judging American ginseng and ginseng can be provided, and the blank regard to the subject in relevant standards in China is made up.
Owner:PONY TESTING INT GRP SHANGHAI CO LTD
Red ginseng extract as well as preparation method and application thereof
ActiveCN103623021ASimple extraction and separation processThe chemical composition is simple and clearOrganic active ingredientsBlood disorderMethanol waterReflux extraction
The invention discloses a red ginseng extract. The extract is characterized by containing ginsenoside Rg1, ginsenoside Re, ginsenoside Rf, notoginsenoside R2 and ginsenoside Ro. A preparation method of the extract comprises the following steps: cutting red ginseng serving as a medicinal material into pieces, adding water in an amount which is 3-10 times that of the red ginseng, soaking for 0.5-2 hours, heating and performing reflux extraction for 1-2 hours, extracting for 1-3 times, combining extraction solutions, filtering and concentrating; separating and purifying a concentrated solution with a reverse phase silica gel column; eluting with 30-40 percent methanol-water by 4-10L, and discarding; and eluting with 50-70 percent methanol-water by 4-6L, collecting an eluent and concentrating to obtain active ingredients.
Owner:SHAN DONG DONG E E JIAO
Method for preparing 20-glucose-ginsenoside Rf monomers from panax japonicas
InactiveCN105348356AReduce dosageSimple processGlycoside steroidsChromatographic separationAcetic acid
The invention discloses a method for preparing 20-glucose-ginsenoside Rf monomers from panax japonicas. Components containing 20-glucose-ginsenoside Rf monomers are prepared, 20-glucose-ginsenoside Rf monomers are separated from components containing 20-glucose-ginsenoside Rf monomers by utilization of a high-speed countercurrent chromatography, wherein the high-speed countercurrent chromatography condition is as follows: n-butanol-ethyl acetate-water with a volume ratio of 4-6:1:5-7 is employed as a solvent system for high-speed countercurrent chromatography separation. In separation of 20-glucose-ginsenoside Rf column chromatography combined with high-speed countercurrent chromatography (HSCCC), the process is simple, the organic reagent dosage is reduced greatly, and the purity of the obtained monomers is more than 95%. The provided method for separation of 20-glucose-ginsenoside Rf monomers employs a high-speed countercurrent chromatography (HSCCC) for the first time. The obtained 20-glucose-ginsenoside Rf monomers can be used for preparation of medicine compositions, health care food and other products.
Owner:JILIN UNIV
Method for quickly quality-detecting and identifying American ginsengs, ginsengs and preparations of American ginsengs and ginsengs
InactiveCN101685089BEasy to separateSimple extraction methodComponent separationMass spectrometryGinsenoside Rf
The invention discloses a method for quickly quality-detecting and identifying American ginsengs, ginsengs and preparations of American ginsengs and ginsengs. In the method, 70-percent methanol is used as a sample ultrasonic extraction solvent, a C18 chromatographic column is adopted, a system of acetonitrile-tetrahydrofuran-formic acid-water is used as a flowing phase, an electrospray ionization(ESI) ion source is adopted, quick separation, identification and measurement of pseudoginsenoside F11 and ginsenoside Rf which are characteristics components of the American ginsengs and ginsengs respectively are performed within 2 minutes for the first time under a condition of a negative ion mode second order mass spectrometry analysis, and whether the preparation of the American ginsengs is mixed with the ginsengs is judged and the content of crude drugs mixed with the ginsengs is worked out. The method has the advantages of strong specificity, synchronous qualitative and quantitative analysis, easy and quick pre-treatment, quick detection of the contents of the American ginsengs and the ginsengs within 2 minutes, high sensitivity of detection and measurement and good repeatability, and the suitability for quick quality screening of the American ginsengs, the ginsengs and products of the American ginsengs and the ginsengs.
Owner:天津海世达检测技术有限公司 +1
Glycosyl transferases and method of catalyzing sugar chain extension
PendingCN112831481AHas neuroprotective propertiesActive controlTransferasesGenetic engineeringHigh activityPyran
The invention relates to a group of high-activity glycosyl transferases responsible for sugar chain extension and an application of the glycosyl transferases. Specifically, the invention provides glycosyl transferase and a derivative polypeptide thereof, which can efficiently catalyze the reaction of extending sugar chains on the first glycosyl at the C-20 site and the first glycosyl at the C-6 site of a tetracyclic triterpenoid substrate. The ginsenoside products such asginsenoside Rb1, the ginsenoside Rb3, the ginsenoside DMGG, the ginsenoside DMGX, the gynostemma pentaphyllum saponin LXXV, the gynostemma pentaphyllum saponin XVII, the gynostemma pentaphyllum saponin XIII, gynostemma pentaphyllum saponin IX, notoginsenoside U and the notoginsenoside R1, notoginsenoside R2, notoginsenoside R3, 3-O-beta-(D-xylopyranosyl)-beta-(D-glucopyranosyl)-CK, 20-O-glucosyl ginsenoside Rf, Rd-C20-O-Rha, ginsenoside Rg2, and ginsenoside Re and the like can be obtained. The glycosyl transferase disclosed by the invention can also be applied to construction of artificially synthesized ginsenoside and a plurality of new ginsenosides and derivatives thereof.
Owner:SYNBIOTECH (SUZHOU) CO LTD
New application of ginsenoside Rf
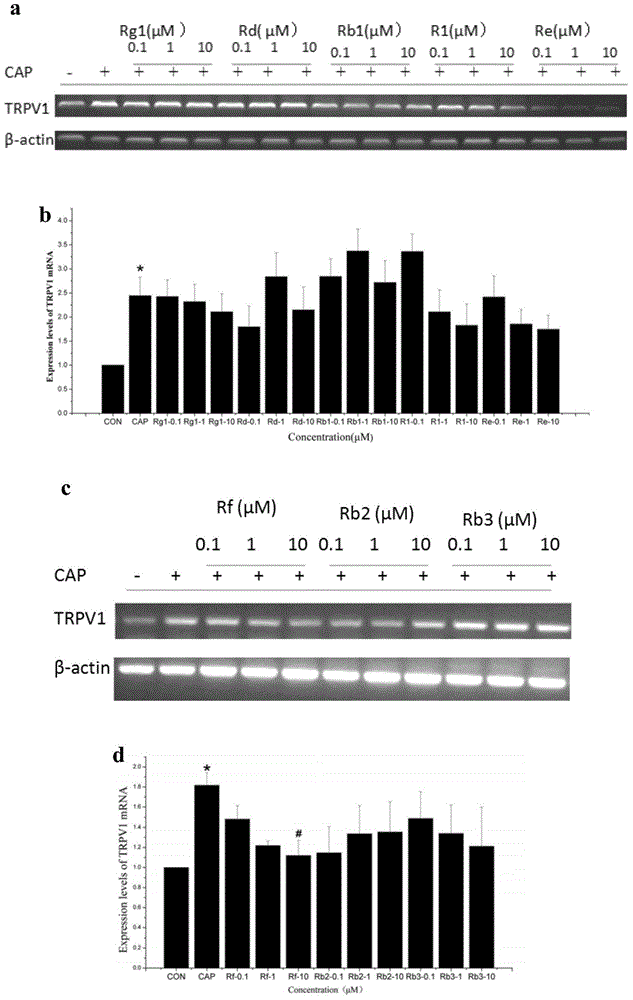
InactiveCN104382920AProlonged paw withdrawal latencyReduce pain sensitivityOrganic active ingredientsNervous disorderDiseaseTRPV1 receptor
The invention relates to the field of medicine, specifically relates to the new application of ginsenoside Rf, and in particular relates to the application of the ginsenoside Rf serving as a TRPV1 antagonist. The antagonist ginsenoside Rf is capable of remarkably inhibiting TRPV1 protein expression at the cellular level, and at the animal level, is capable of remarkably prolonging the paw withdrawal latency of a rat induced by capsaicin (CAP) and reducing the degree of sensitivity of the rat to pain. As a result, the ginsenoside Rf can be used as the TRPV1 antagonist to ease pain with TRPV1 as the target spot, and also can be used for preparing analgesics for treating diseases related to a TRPV1 receptor.
Owner:BEIJING NORMAL UNIVERSITY +1
Method for determining content of ginsenoside in panax traditional Chinese medicine
ActiveCN113433232AHigh sensitivityAccurate quality controlComponent separationBiotechnologyPseudoginsenoside F11
The invention provides a method for determining the content of ginsenoside in a panax traditional Chinese medicine, which adopts an ultra-high performance liquid chromatography electrospray detector method to simultaneously determine the content of 15 kinds of ginsenoside in the panax traditional Chinese medicine. The 15 kinds of ginsenoside comprise notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, 24 (R)-pseudoginsenoside F11, ginsenoside Rf, ginsenoside Ra2, ginsenoside Rb1, ginsenoside Rc, ginsenoside Ro, ginsenoside Rb2, ginsenoside Rb3, panax japonicus saponin IV, ginsenoside Rd, panax japonicus saponin IVa and 20 (R)-ginsenoside Rg3. By adopting the method disclosed by the invention, the contents of 15 kinds of ginsenoside in various panax traditional Chinese medicines can be simultaneously determined by reasonably selecting chromatographic conditions and detection conditions, and the method has the advantages of simplicity, convenience, accuracy, high sensitivity, strong specificity and the like, so that the method can be credibly, comprehensively and accurately used for quality control of the panax traditional Chinese medicines.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Fingerprint construction method and HPLC (High Performance Liquid Chromatography) fingerprint
InactiveCN108872417AMeet the measurement requirementsGood precisionComponent separationHplc fingerprintControl substances
Owner:亳州中药材商品交易中心有限公司
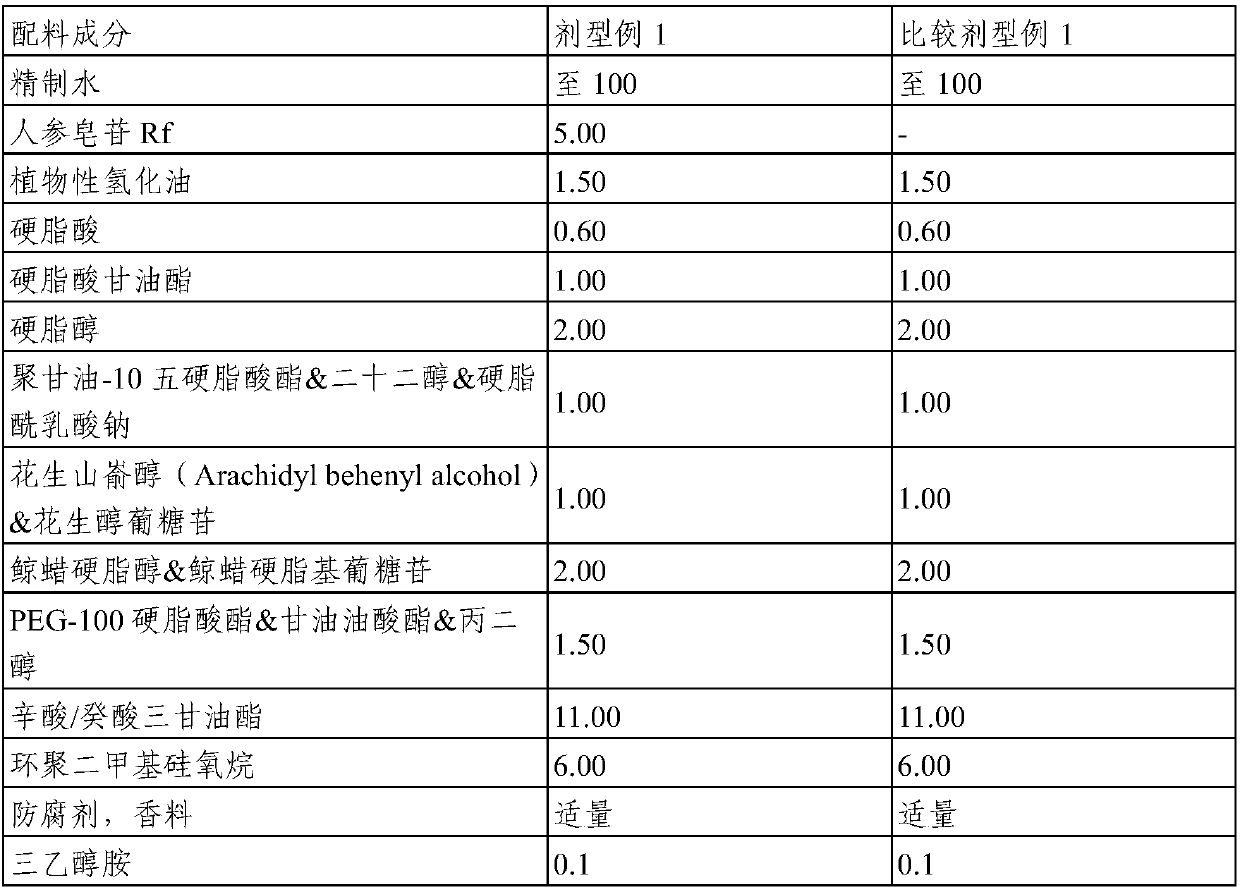
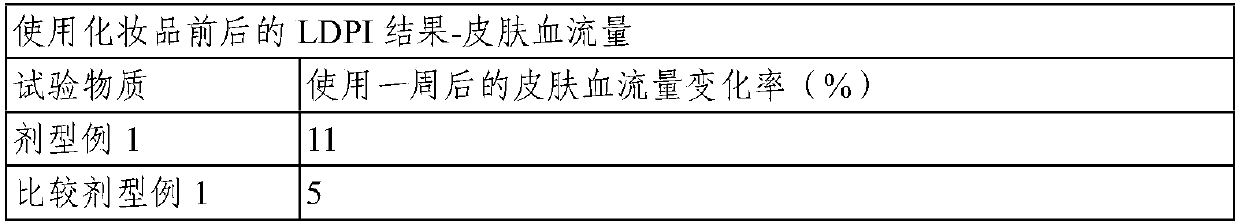
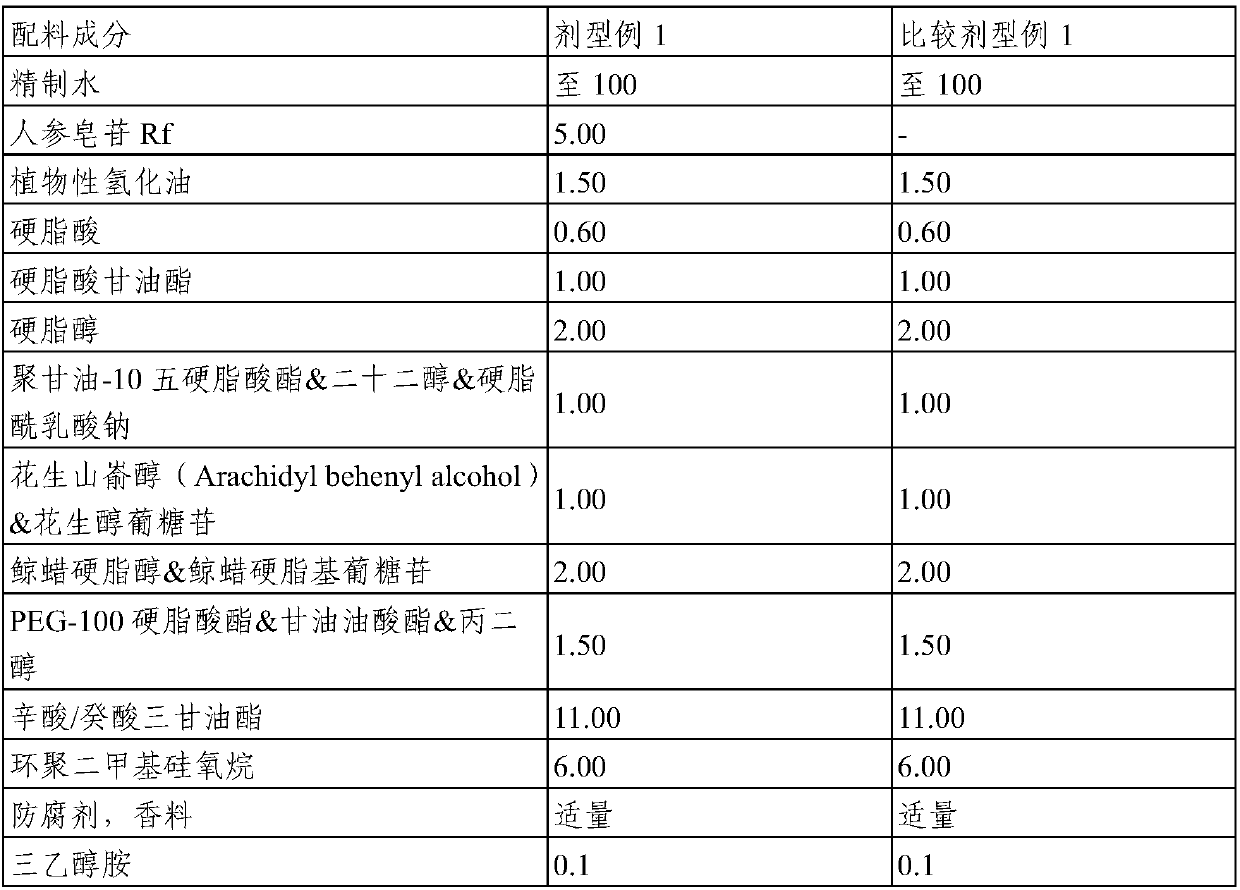
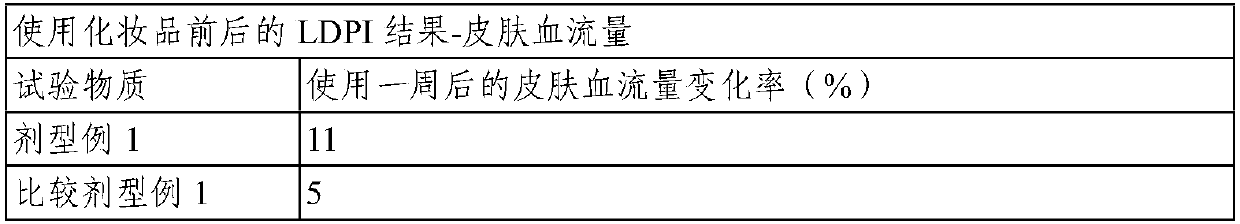
Topical composition for skin containing gincenoside RF
ActiveCN105246489AImprove blood colorPromote growthCosmetic preparationsHair cosmeticsHair growthGraying hairs
The present invention relates to a composition containing, as an active ingredient, gincenoside RF, for providing effects such as improvement of acne and skin troubles, skin clearing, pore tightening, in addition to providing effects such as skin tone improvement, hair growth promotion, improvement of graying hair, anti-dandruff, and antisepsis.
Owner:AMOREPACIFIC CORP
A kind of notoginseng total saponins composition and its preparation method and application
ActiveCN105816471BClear contentImprove effectivenessOrganic active ingredientsBlood disorderLow toxicityPANAX NOTOGINSENG ROOT
The invention provides a panax notoginseng total saponin composition and a preparation method and application thereof. The Panax notoginseng saponin composition provided by the invention contains 30-50% of ginsenoside Rg1, 25-40% of ginsenoside Rb, 7-16% of notoginsenoside R1, 2.7-8% of ginsenoside Re, Saponin Rd 0.5-7.0%, ginsenoside Rf 0.5-2%, ginsenoside Rh2 0.3-2.0, ginsenoside Rc 1-3, ginsenoside Rb3 0.3-2.5 and ginsenoside Rg 30.5-2.5%; Ginsenoside Rb1 and Panax notoginsenoside R1 account for 70-95% of the total weight of the active ingredients, and the sum of the content of ginsenoside Rb1 and notoginsenoside R1 is not less than 8 times the content of ginsenoside Rd. Compared with the commercially available Xueshuantong and Xuesaitong preparations, the Panax notoginseng saponin composition provided by the invention has more obvious anti-arteriovenous thrombosis effect and low toxicity and safety.
Owner:HARBIN ZHENBAO PHARMA
Method for determining contents of multi-index components in Weifuchun tablets
The invention discloses a method for determining the contents of multi-index components in Weifuchun tablets, which comprises the following steps: step 1, preparing a mixed reference substance solution: precisely weighing reference substances of hesperidin, neohesperidin, rosmarinic acid, ginsenoside Re, ginsenoside Rg1, ginsenoside Rf and ginsenoside Rb1, and dissolving the reference substances in methanol to prepare the mixed reference substance solution; step 2, carrying out preparation of a test solution: taking a Weifuchun tablet sample, performing grinding, precisely performing weighing, and adding the product into a methanol solution to prepare a test solution; and step 3, carrying out determination: respectively taking the mixed reference substance solution obtained in the step 1 and the test solution obtained in the step 2, performing quantitative sample introduction, and performing determination by adopting a UPLC-QDa coupling technology. The UPLC-QDa multi-index component content determination method established by the invention is used for determining 14 batches of Weifuchun tablets sold in the market. The result shows that the method can be used for evaluating the consistency between batches of the Weifuchun tablets and the controllability of the process, the quality of the Weifuchun tablets can be comprehensively controlled, and a reference is provided for improving the quality of the Weifuchun tablets.
Owner:HANGZHOU HUQINGYUTANG PHARM CO LTD
Topical composition for skin containing gincenoside RF
ActiveCN107898656AImprove blood colorPromote growthCosmetic preparationsHair cosmeticsChemical compositionHair growth
The present invention relates to a composition containing, as an active ingredient, gincenoside RF, for providing effects such as improvement of acne and skin troubles, skin clearing, pore tightening,in addition to providing effects such as skin tone improvement, hair growth promotion, improvement of graying hair, anti-dandruff, and antisepsis.
Owner:AMOREPACIFIC CORP
A method for determining the content of twelve components in a traditional Chinese medicine composition preparation
ActiveCN104914199BQuality improvementGood repeatabilityComponent separationAstragalosideGinsenoside Rd
Owner:HEBEI YILING MEDICINE INST
Serratia marcescens HGS-487 strain for converting ginsenoside Rf into ginsenoside Rg1 and application
ActiveCN113403229ALow costImprove conversion rateBacteriaMicroorganism based processesBiotechnologySerratia species
The invention provides a serratia marcescens HGS-487 strain for converting ginsenoside Rf into ginsenoside Rg1. The strain is preserved in China Center for Type Culture Collection in Wuhan University, Wuhan, China; the postcode is 430072, the preservation date is December 17, 2019, and the preservation number is CCTCC NO: M20191057; and the 16SrDNA sequence of the strain is as shown in SEQ ID NO. 1. The serratia marcescens HGS-487 strain can be used for preparing ginsenoside Rg1 through fermentation and conversion.
Owner:SHANXI UNIV OF CHINESE MEDICINE
A detection method for traditional Chinese medicine preparations containing ginseng or Panax notoginseng
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Rahnella HGS-393 strain for converting ginsenoside Rf into Rh1 and application
ActiveCN113403230ALow costImprove conversion rateBacteriaMicroorganism based processesMicrobiologyBiochemistry
The invention provides a rahnella HGS-393 strain for converting prototype ginsenoside Rf into rare ginsenoside Rh1. The strain is preserved in China Center for Type Culture Collection in Wuhan University, Wuhan, China; the postal code is 430072, the preservation date is May 7, 2021, and the preservation number is CCTCC NO: M2021498. The 16S rDNA sequence of the strain is as shown in SEQ ID NO. 1. The rahnella HGS-393 strain can be used for preparing the rare ginsenoside Rh1 through fermentation and conversion.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Topical composition for skin containing gincenoside RF
InactiveCN108042386AImprove blood colorPromote growthCosmetic preparationsHair cosmeticsChemical compositionGraying hairs
The present invention relates to a composition containing, as an active ingredient, gincenoside RF, for providing effects such as improvement of acne and skin troubles, skin clearing, pore tightening,in addition to providing effects such as skin tone improvement, hair growth promotion, improvement of graying hair, anti-dandruff, and antisepsis.
Owner:AMOREPACIFIC CORP
A kind of method that prepares 20-glucose-ginsenoside rf monomer from pearl ginseng
InactiveCN105348356BReduce dosageSimple processGlycoside steroidsChromatographic separationCountercurrent chromatography
The invention discloses a method for preparing 20-glucose-ginsenoside Rf monomers from panax japonicas. Components containing 20-glucose-ginsenoside Rf monomers are prepared, 20-glucose-ginsenoside Rf monomers are separated from components containing 20-glucose-ginsenoside Rf monomers by utilization of a high-speed countercurrent chromatography, wherein the high-speed countercurrent chromatography condition is as follows: n-butanol-ethyl acetate-water with a volume ratio of 4-6:1:5-7 is employed as a solvent system for high-speed countercurrent chromatography separation. In separation of 20-glucose-ginsenoside Rf column chromatography combined with high-speed countercurrent chromatography (HSCCC), the process is simple, the organic reagent dosage is reduced greatly, and the purity of the obtained monomers is more than 95%. The provided method for separation of 20-glucose-ginsenoside Rf monomers employs a high-speed countercurrent chromatography (HSCCC) for the first time. The obtained 20-glucose-ginsenoside Rf monomers can be used for preparation of medicine compositions, health care food and other products.
Owner:JILIN UNIV
Traditional Chinese medicine injectable powder and quality control method thereof
The invention relates to Chinese medicinal injectable powder and a quality control method thereof. The Chinese medicinal injectable powder consists of red ginseng, dwarf lilyturf tuber and Chinese magnoliavine fruit in part by weight, wherein the sum of the main active ingredient content of the injectable powder cannot be lower than 4.0mg / g; and the active ingredients comprise ginsenosides Rf, Rb1, Rb2, Rb3, Rd, Rg3 and F2, and schizandrol A. The quality control method comprises the steps of making quality standards, measuring the content, comparing the content of a sample to be measured with the quality standards, and judging whether the content reaches the preset standards or not so as to control the product quality. The method has the advantages of quickness, accuracy, sensitivity, contribution to industrial production inspection, and capability of effectively controlling the quality of a finished product.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Radix notoginseng extract and preparation thereof
ActiveCN101732378BTotal effective rate is highGood curative effectCardiovascular disorderPlant ingredientsGinsenoside RcPanax notoginseng extract
The invention provides a radix notoginseng extract which contains 5-10% of notoginsenoside R1, 25-36% of ginsenoside Rg1, 2.5-5% of ginsenoside Re, 30-39% of ginsenoside Rb1, 5-10% of ginsenoside Rd and at least 2% of ginsenoside Rf, ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2 and ginsenoside Rg3, wherein the ginsenoside R1, the ginsenoside Rg1, the ginsenoside Re, the ginsenoside Rb1 and the ginsenoside Rd account for 75-95% of the total weight. The invention also provides a preparation method of the radix notoginseng extract. The radix notoginseng extract prepared by the method has little impurities, the purity of the component of total saponin is higher, and especially, the components of the ginsenoside Rf, the ginsenoside Rh1, the ginsenoside Rc, the ginsenoside Rb2, the ginsenoside Rg3 and the like with very low content are purified. The medicinal preparation prepared by the radix notoginseng extract has better curative effect and higher safety.
Owner:HARBIN ZHENBAO PHARMA
Production method of flavor panax ginseng
The invention relates to a preparation method for flavor ginseng; after ginseng is added with nature fruit juice or organic acid for reaction, the content of the precious ginsenoside in products is enhanced, the efficacies of ginseng is improved, while the specific flavor of ginseng is maintained. The main quality characteristics is that: the flavor ginseng includes at least one of the following ingredients: ginsenoside Rg3, ginsenoside Rh1 and ginsenoside 20R-Rh2; wherein, the ratio of the total amount of precious ginsenoside group, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rd and ginsenoside Rc to the total amount of ginsenoside Re, ginsenoside Rg1 and ginsenoside Rf is more than 2.5; the preparation method can convert part of the component causing internal heat, namely, panaxatriolginsenoside, into precious ginsenoside.
Owner:JILIN HONGJIU BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com