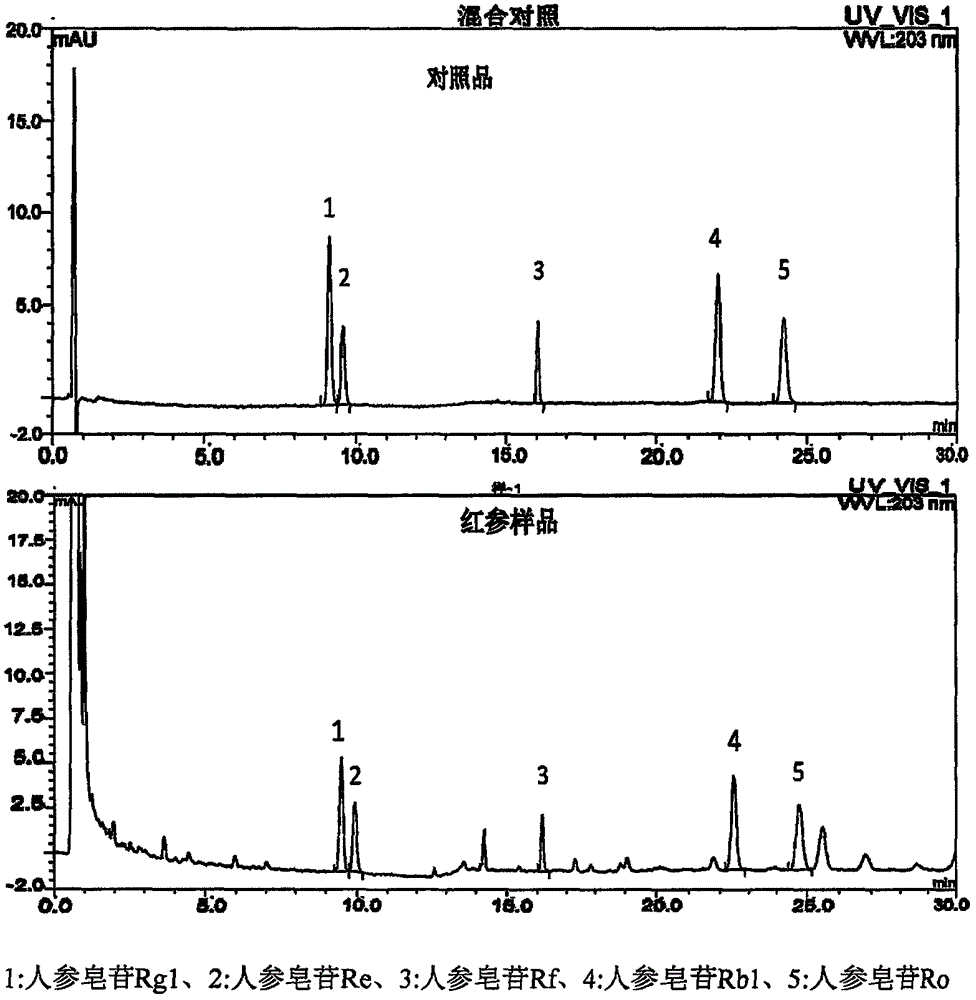
Method for measuring content of five saponin components in red ginseng roots
A method of determination, technology of ginsenosides, applied in measuring devices, material separation, analysis of materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
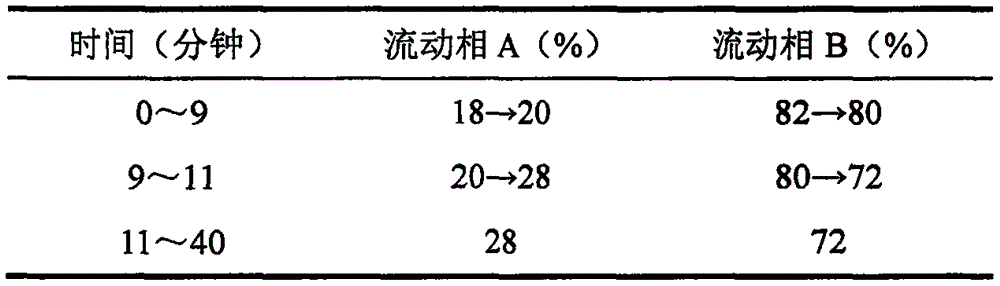
[0081] Chromatographic conditions and system suitability test column adopts BEH C 18 Chromatographic column; use acetonitrile as mobile phase A, 0.05% phosphoric acid as mobile phase B, and perform gradient elution. The elution gradient is: 0-9 minutes, mobile phase A: 18%→20%; 9-11 minutes, mobile phase A: 20%→28%; 11-40 minutes, the mobile phase A maintains 28%; the detection wavelength is 203nm; the flow rate is 0.4ml / min;
[0082] Preparation of mixed reference solution
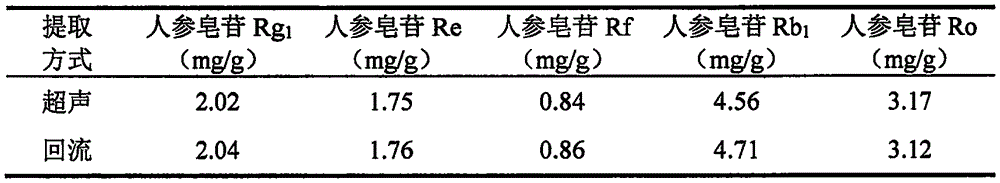
[0083] Take an appropriate amount of ginsenoside Rg1 reference substance, ginsenoside Re reference substance, ginsenoside Rf reference substance, ginsenoside Rb1 reference substance, ginsenoside Ro reference substance, weigh them accurately, add methanol to make each 1ml contain 80 μg of ginsenoside Rg1 reference substance, A mixed solution of 60 μg of ginsenoside Re reference substance, 50 μg of ginsenoside Rf reference substance, 150 μg of ginsenoside Rb1 reference substance, and 100 μg of ginsenoside ...
Embodiment 2
[0087] Chromatographic conditions and system suitability test column adopts BEH C 18 Chromatographic column; use acetonitrile as mobile phase A, 0.10% phosphoric acid as mobile phase B, carry out gradient elution, the elution gradient is: 0-9 minutes, mobile phase A: 18%→20%; 9-11 minutes, mobile phase A: 20%→28%; 11-40 minutes, the mobile phase A maintains 28%; the detection wavelength is 203nm; the flow rate is 0.4ml / min;
[0088] Preparation of mixed reference solution
[0089] Take an appropriate amount of ginsenoside Rg1 reference substance, ginsenoside Re reference substance, ginsenoside Rf reference substance, ginsenoside Rb1 reference substance, ginsenoside Ro reference substance, weigh them accurately, add methanol to make each 1ml contain 80 μg of ginsenoside Rg1 reference substance, A mixed solution of 60 μg of ginsenoside Re reference substance, 50 μg of ginsenoside Rf reference substance, 150 μg of ginsenoside Rb1 reference substance, and 100 μg of ginsenoside Ro...
Embodiment 3
[0093] Chromatographic conditions and system suitability test column adopts BEH C 18 Chromatographic column; use acetonitrile as mobile phase A, 0.05% phosphoric acid as mobile phase B, and perform gradient elution. The elution gradient is: 0-9 minutes, mobile phase A: 18%→20%; 9-11 minutes, mobile phase A: 20%→28%; 11-40 minutes, the mobile phase A maintains 28%; the detection wavelength is 203nm; the flow rate is 0.4ml / min;
[0094] Preparation of mixed reference solution
[0095] Take an appropriate amount of ginsenoside Rg1 reference substance, ginsenoside Re reference substance, ginsenoside Rf reference substance, ginsenoside Rb1 reference substance, ginsenoside Ro reference substance, weigh them accurately, add methanol to make each 1ml contain 80 μg of ginsenoside Rg1 reference substance, A mixed solution of 60 μg of ginsenoside Re reference substance, 50 μg of ginsenoside Rf reference substance, 150 μg of ginsenoside Rb1 reference substance, and 100 μg of ginsenoside ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com