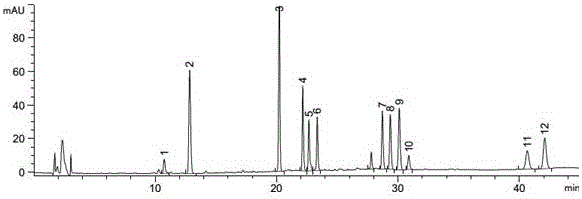
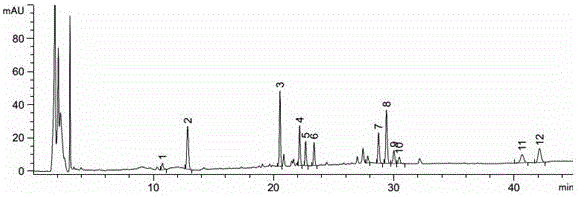
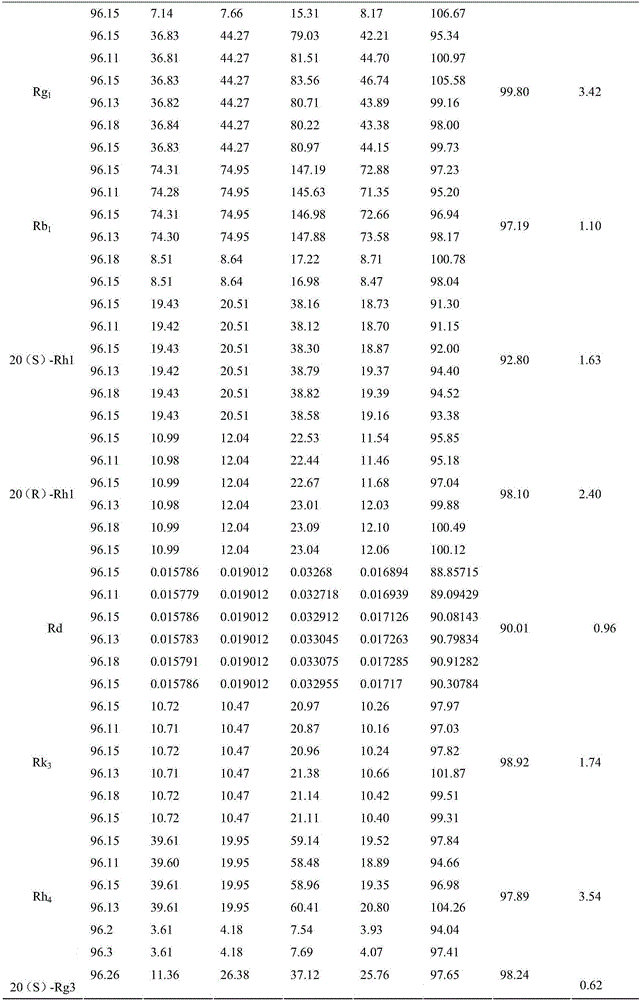
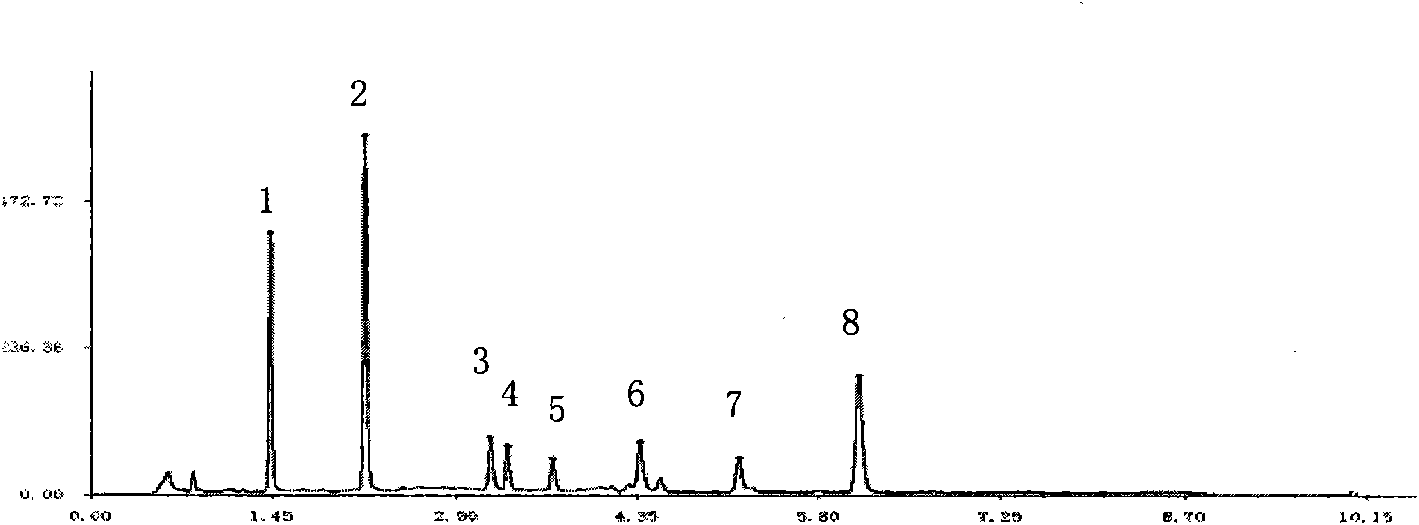
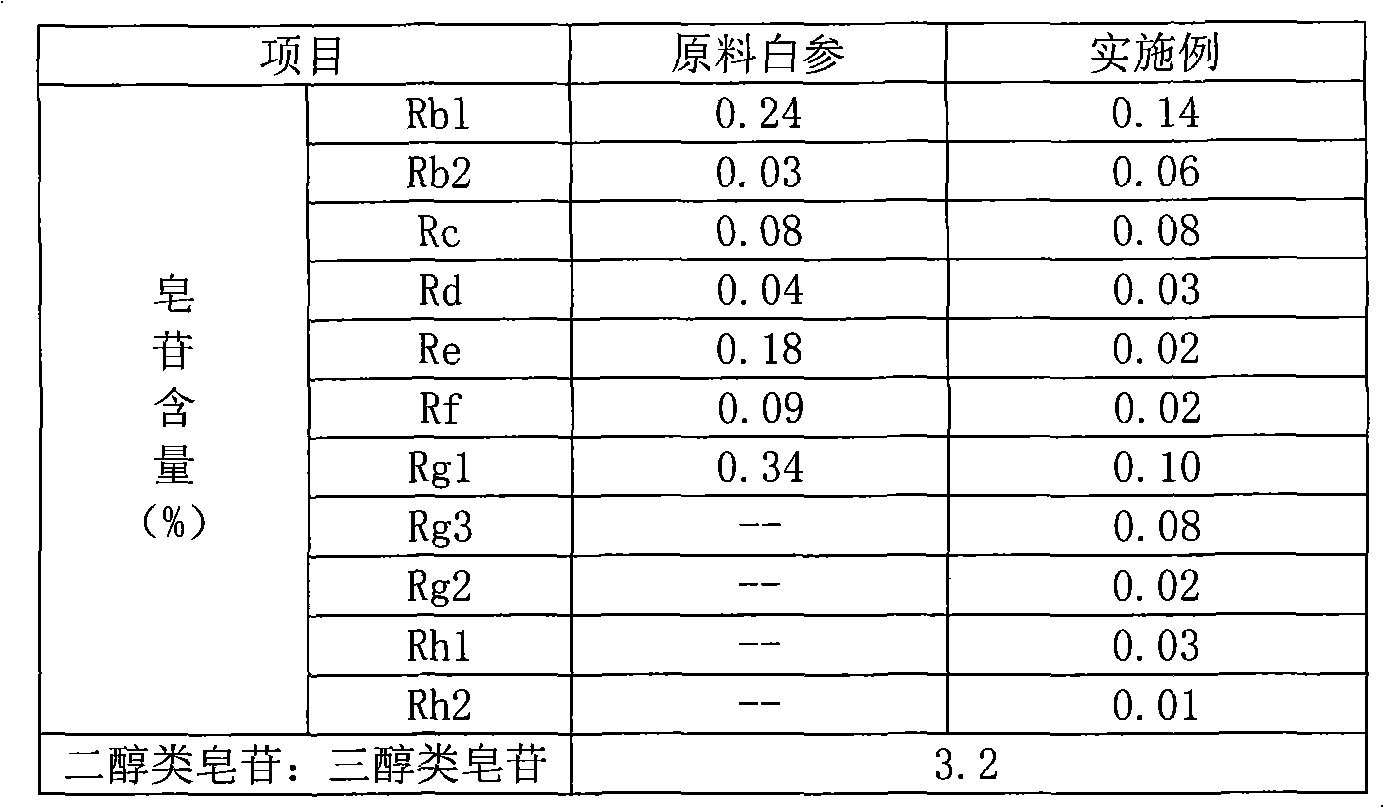
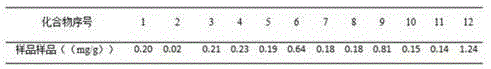
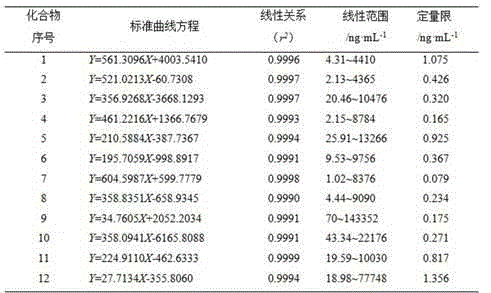
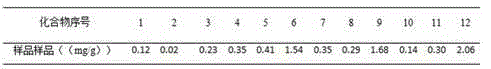
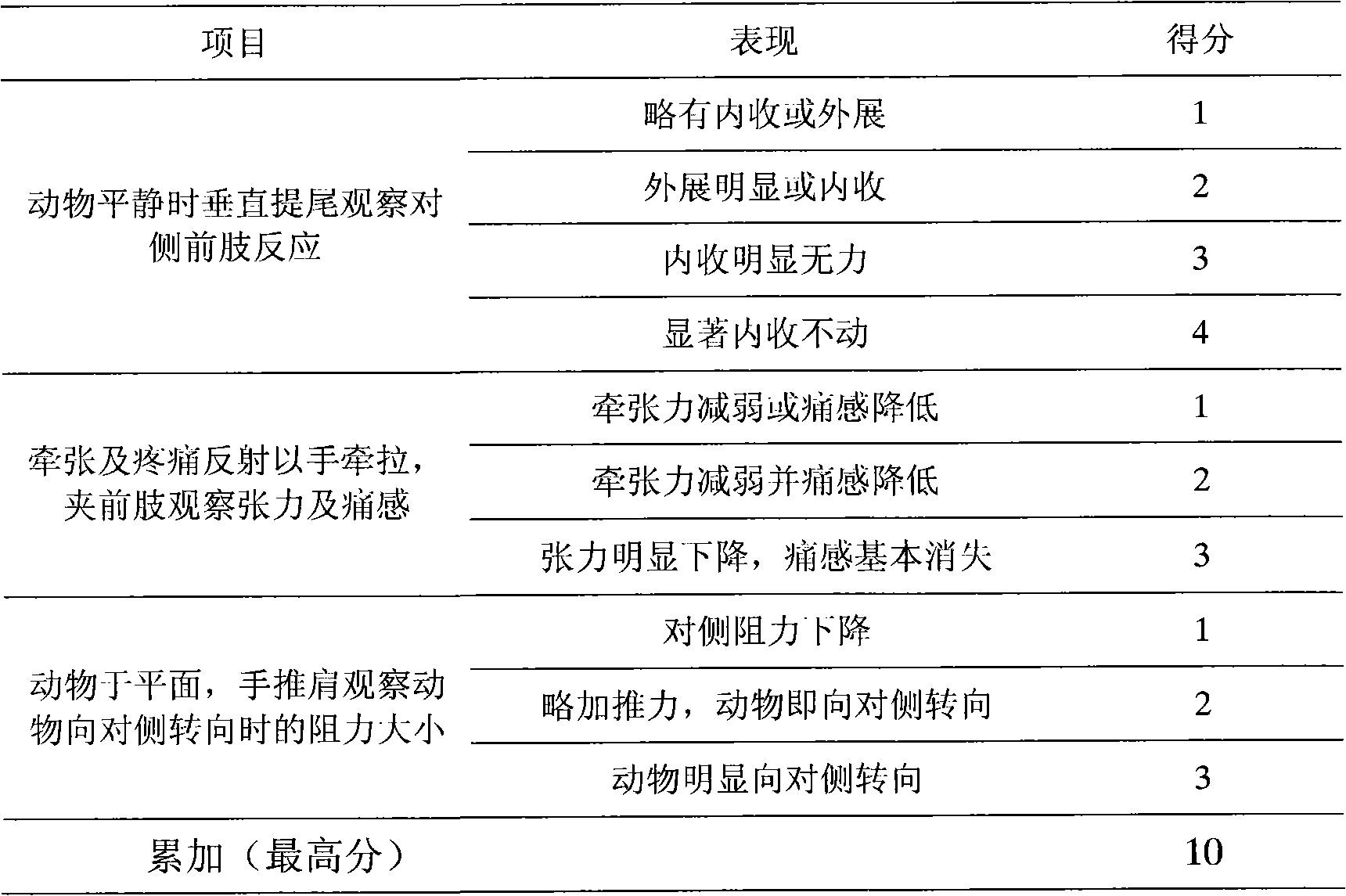
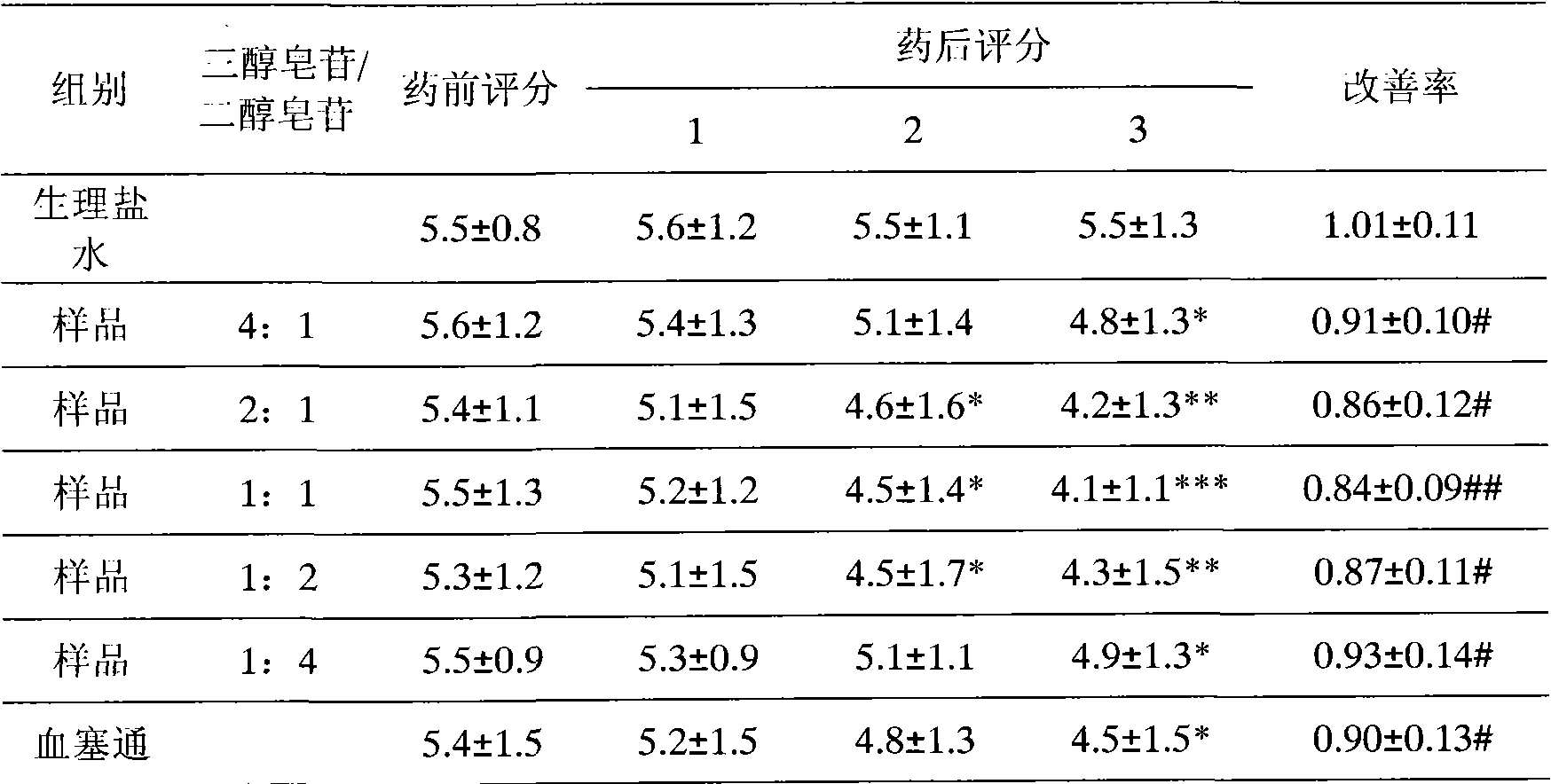
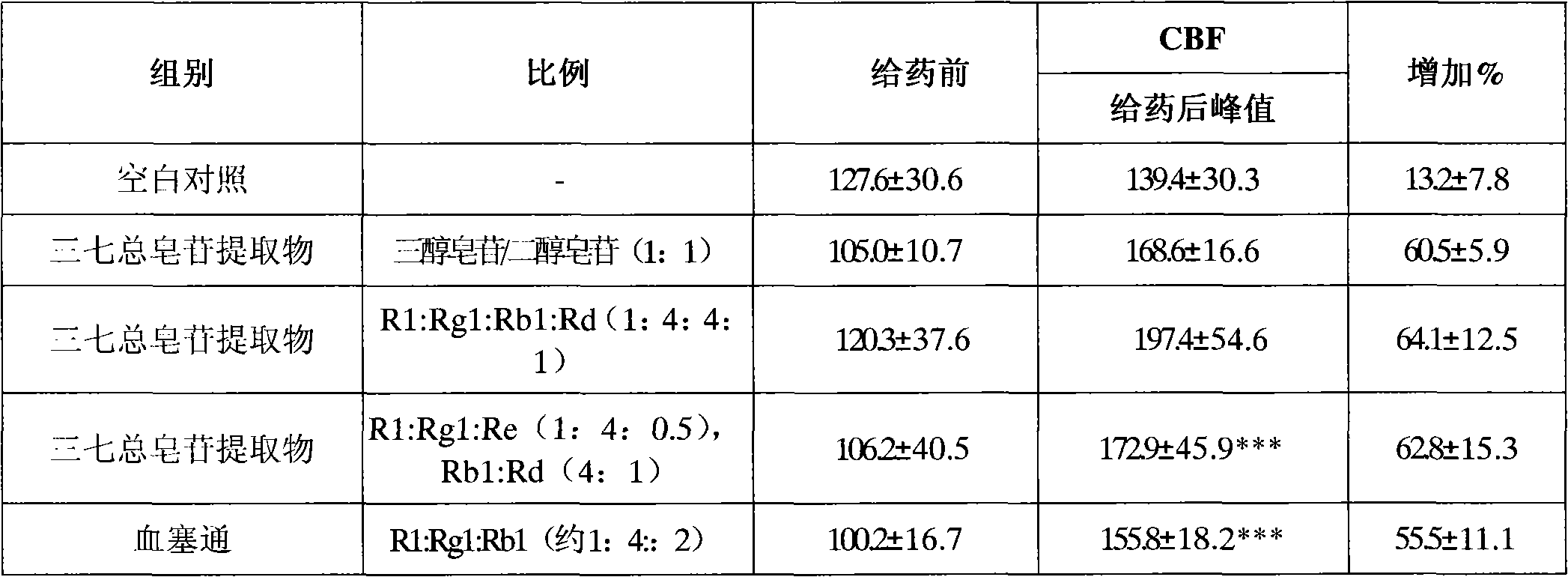
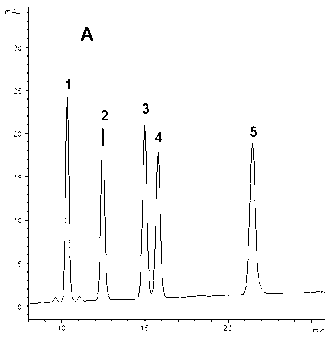
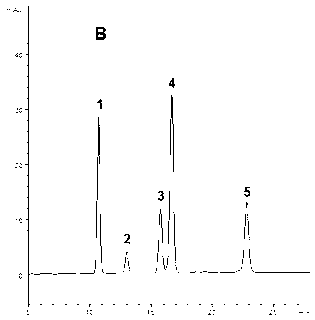
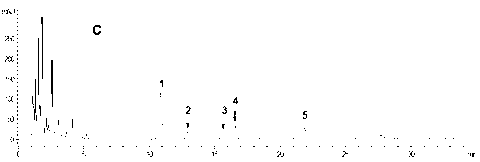
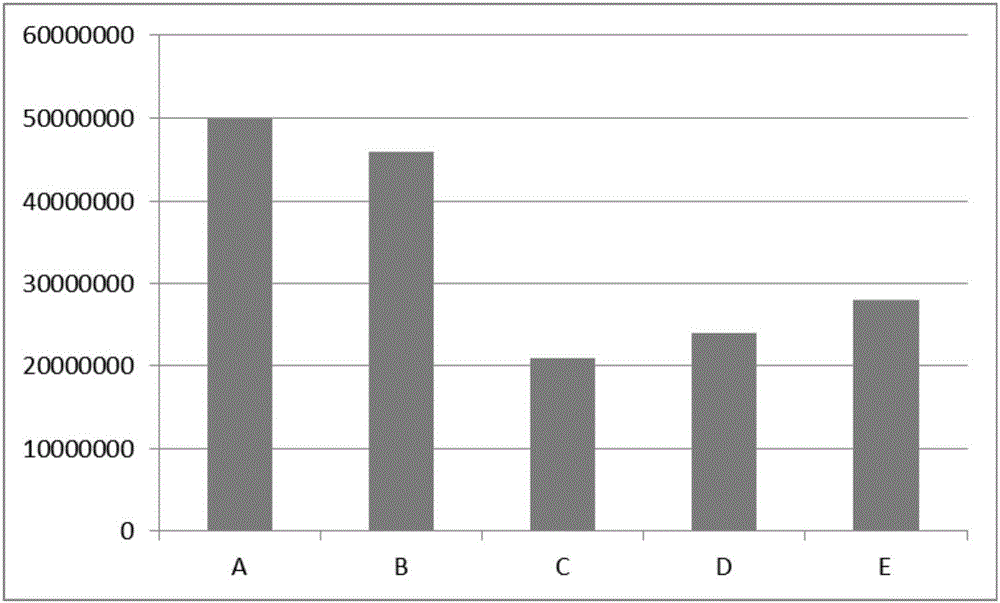
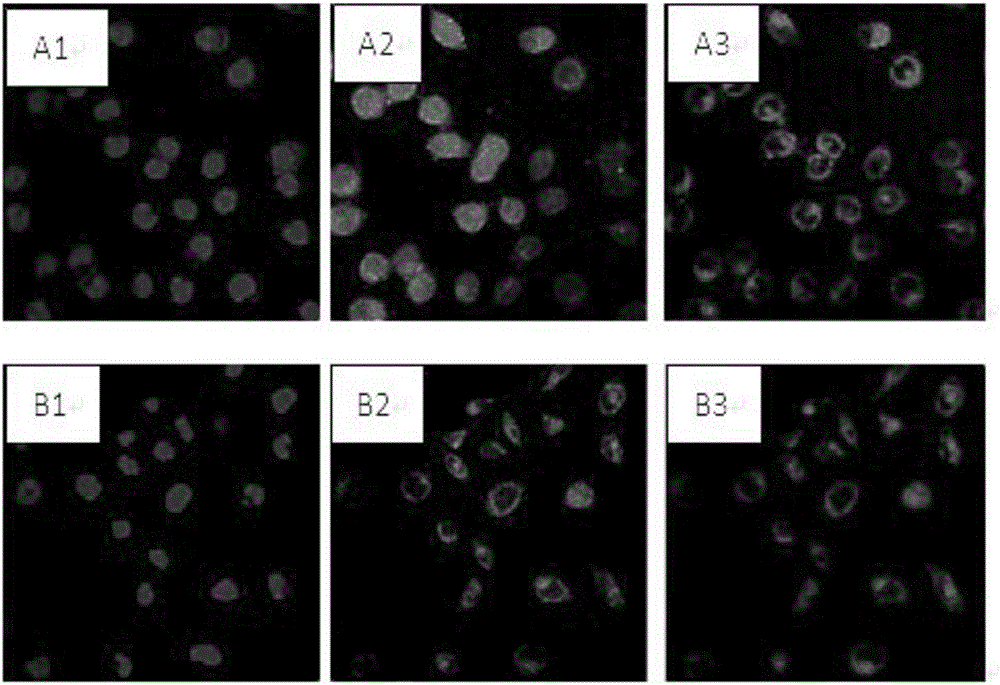
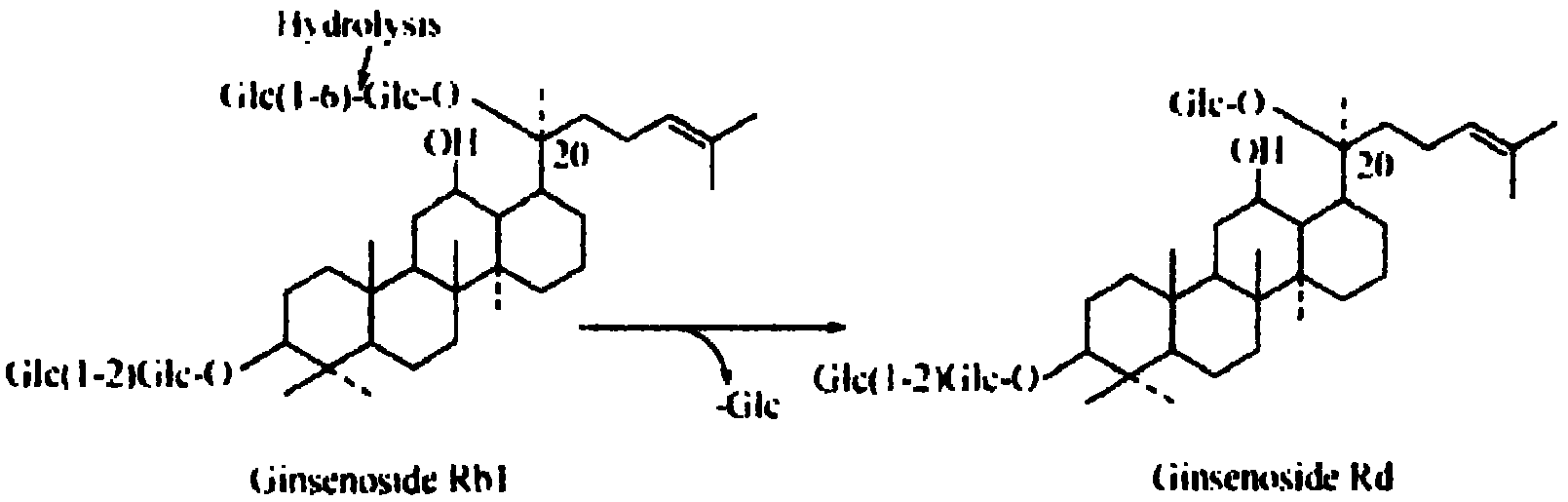
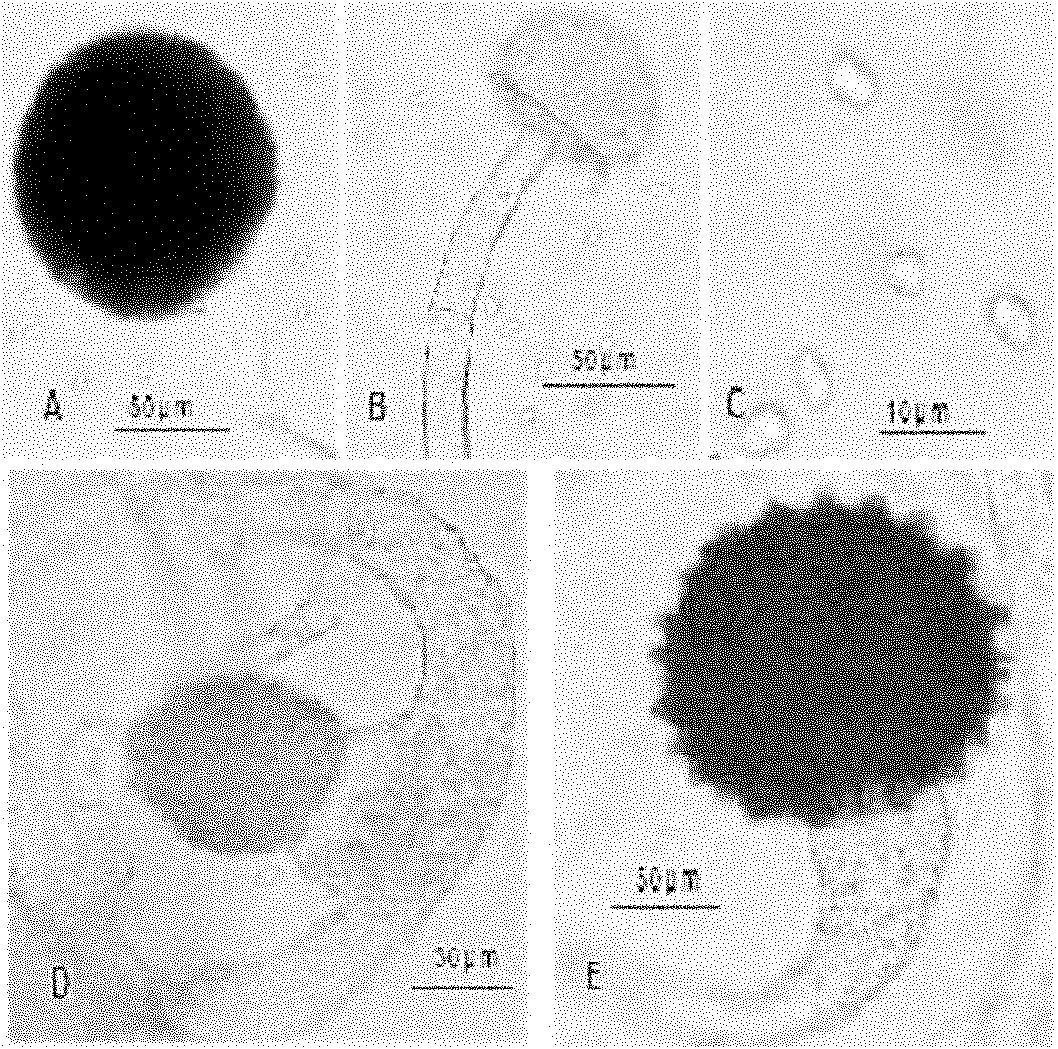Patents
Literature
367 results about "Ginsenoside Rb1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
Composition of traditional Chinese medicine effective constituent for preventing and treating diseased associated with cerebral ischemia injury
ActiveCN101357136AAvoid gatheringPrevent thrombosisOrganic active ingredientsCardiovascular disorderSequelaAdditive ingredient
The invention relates to a composite of active ingredients of Chinese herb medicine used for preventing the damage of cerebral ischemia and relevant diseases, in particular to a preparation which is prepared by the active ingredients of the Chinese herb medicine; the preparation is prepared by the active ingredients of the Chinese herb medicine and the conventional preparation technique which is used by carriers permitted by pharmacy, and comprises the following components with the parts by weight: 2 to 10 parts of ginsenoside Rb, 2 to 10 parts of ginsenoside Rg1, 1 to 5 parts of ginsenoside Rd, 1 to 5 parts of ginsenoside Re, 2 to 10 parts of stilbene glycoside, 1 to 5 parts of ginkgolide and 1 to 5 parts of flavonoid mixture which consists of kaempferol and quercetin with the weight rate to be 1:1. The invention has the advantages that the defects that in the traditional compounded Chinese medicine, the composition is complex, the product quality is not controlled effectively and the curative effect is not stable are overcome, has clear ingredients, definite effect, stable quality and obvious control effect on ischemic stroke, sequela, cerebral arteriosclerosis and vascular dementia, can improve the functions of motor nerve, and learning and memory of the patients, has low toxicity and simple preparation method without obvious side effect and is suitable for industrial mass production.
Owner:GUANGDONG PHARMA UNIV
Ginseng saponin Rg1 and Rb1 in pseudo-ginseng and preparation of total saponin thereof
The invention belongs to the medicine technical filed, in particular to a preparation method of monomeric compound ginsenoside Rg1, ginsenoside Rb1 and total arasaponin and the application in the medicine field thereof. The fresh medicinal material, the dried medicinal material and the medicinal material on the market of Panax notoginseng are taken as the raw materials; according to the polarity and the solubility property of a compound, separation and purification are carried out by adopting the solvent extraction method, the crystallization process and the chromatography and total arasaponin powder is prepared by combining the common drying means, such as decompression concentration drying, freeze drying, vacuum drying and the like; by carrying out one or more methods of recrystal, normal phase, opposite phase silica gel column chromatography, daiamid column chromatography and sephadex chromatography and the like on the powder, the ginsenoside Rg1 and the ginsenoside Rb1 monomers are prepared. The medicines which take the ginsenoside Rg1 and the ginsenoside Rb1 monomers or the total arasaponin as the active ingredients can be used for preventing and / or curing the senile dementia, the neurodegenerative diseases, the cerebrovascular disorder, various dysmnesia, the central lesion and other diseases.
Owner:YUNNAN JECUI BIOTECH
Radix notoginseng extract and preparation thereof
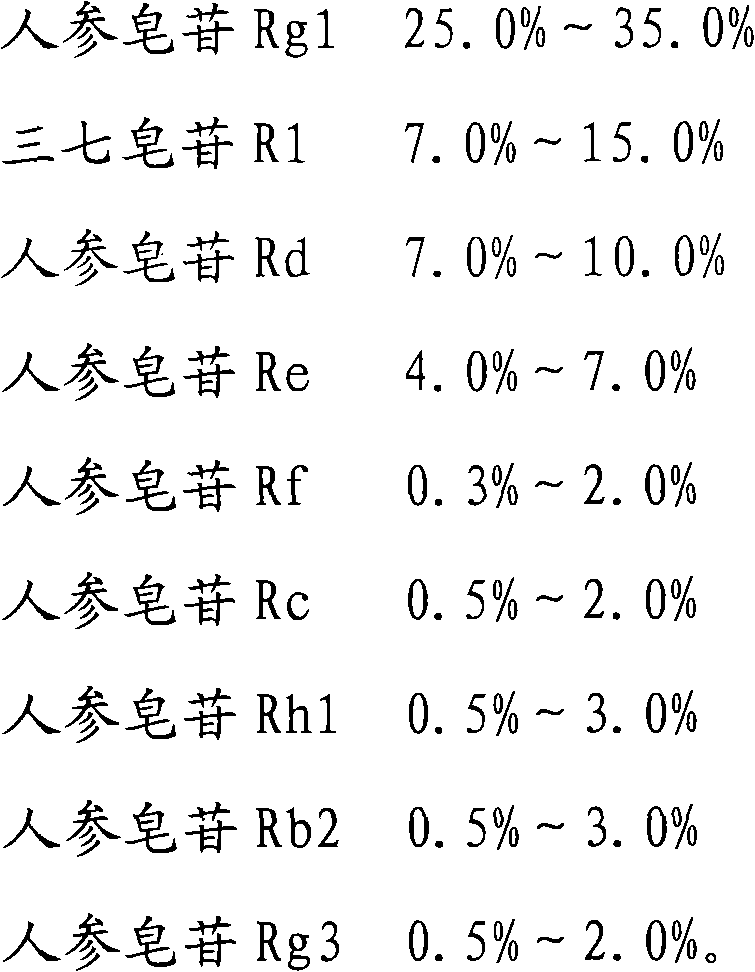
ActiveCN101732378AHigh purity of ingredientsIncrease concentrationCardiovascular disorderPlant ingredientsGinsenoside RcPanax notoginseng extract
The invention provides a radix notoginseng extract which contains 5-10% of notoginsenoside R1, 25-36% of ginsenoside Rg1, 2.5-5% of ginsenoside Re, 30-39% of ginsenoside Rb1, 5-10% of ginsenoside Rd and at least 2% of ginsenoside Rf, ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2 and ginsenoside Rg3, wherein the ginsenoside R1, the ginsenoside Rg1, the ginsenoside Re, the ginsenoside Rb1 and the ginsenoside Rd account for 75-95% of the total weight. The invention also provides a preparation method of the radix notoginseng extract. The radix notoginseng extract prepared by the method has little impurities, the purity of the component of total saponin is higher, and especially, the components of the ginsenoside Rf, the ginsenoside Rh1, the ginsenoside Rc, the ginsenoside Rb2, the ginsenoside Rg3 and the like with very low content are purified. The medicinal preparation prepared by the radix notoginseng extract has better curative effect and higher safety.
Owner:HARBIN ZHENBAO PHARMA
Extracting purified ginsenoside from leaves of Panax quinquefolium and ginseng at the same time and the preparing method thereof
InactiveCN101032535ASimple processLow costOrganic active ingredientsSteroidsSide effectGinsenoside Rc
The present invention relates to Chinese medicine and its extracting and processing technology, and is especially the effective part extracted from American ginseng leaf and ginseng leaf and its preparation process. The extracted effective part contains six kinds of ginsenoside substances, including ginsenoside Re, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. Its preparation process includes water extracting American ginseng leaf and ginseng leaf in the weight ratio of 1 to 0.3, macroporous resin adsorption of the water extract liquid, water eluting to eliminate impurity, further eluting with two kinds of elutents, collecting the eluted liquid, and refining the total solid matter to reach purity up to 63.45 %. The extracted effective part has less side effects, and may be used widely in compound medicine preparation and functional food and for separating ginsenoside substance.
Owner:吉林人参研究院
Method for preparing rare ginsenoside CK from transformed ginsenoside Rb1 and use thereof
ActiveCN105296587AImprove conversion rateRich in enzyme productionMicroorganism based processesFermentationBiotechnologyBifidobacterium
The invention discloses a method for preparing rare ginsenoside CK from transformed ginsenoside Rb1 and a use thereof. Ginsenoside Rb1 is transformed into rare ginsenoside CK by beta-glucosidase produced by bifidobacteria and a conversion rate is in a range of 62-68%. The transformed rare ginsenoside CK has very polarity, can be easily absorbed by the human body, and can enter into liver by blood circulation so that metabolism is finished and unique pharmacological effects are obtained. Bifidobacteria are edible probiotics and can produce glycosidase with high catalysis activity, selectivity and stability. The beta-glucosidase produced by bifidobacteria can transform ginsenoside Rb1 into rare ginsenoside CK, and has the advantages of high conversion rate, less side effect, safety, reliability, no pollution and industrial production easiness. The method has a low cost, simple processes and a short conversion period. Beta-glucosidase produced by bifidobacteria has an important application value in preparation of rare ginsenoside CK and the method utilizes the application value.
Owner:HUNAN INSTITUTE OF ENGINEERING
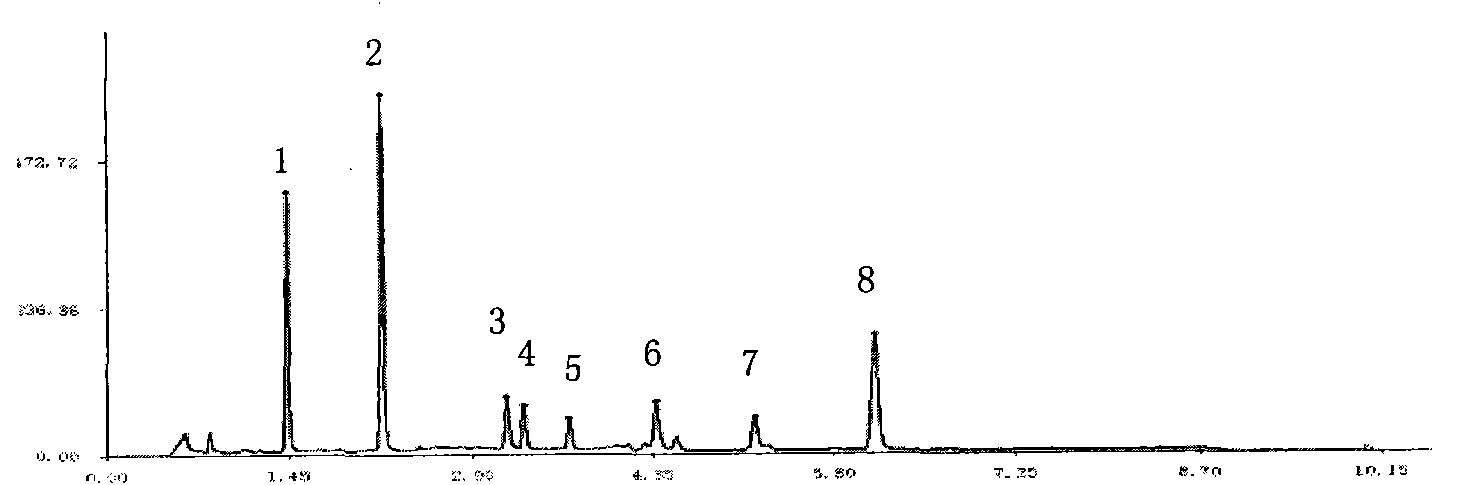
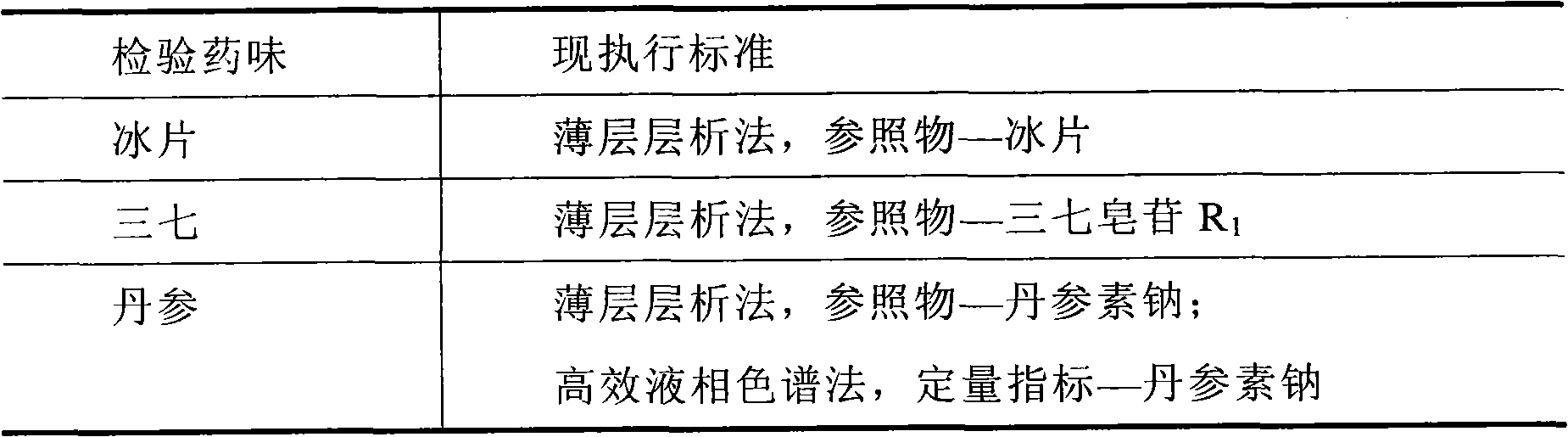
Quality detection method for compound prepn of red sage and notoginseng
InactiveCN1772041ASimple methodGood precisionComponent separationBlood disorderHplc dadSalvianolic acid B
The present invention relates to Chinese medicine quality detecting technology, and is especially the quality detection method for compound preparation of red sage and notoginseng. HPLC-DAD process is adopted to measure the contents of protocatechuic aldehyde, salvianolic acid B, cryptotanshinone, neotanshinone IIA, arasaponin R1, ginsenoside Rg1 and ginsenoside Rb1 in compound red sage preparation simultaneously. The process is simple, precise, repeatable and reliable, and may be used in the quality control of compound red sage preparation.
Owner:CHINA PHARM UNIV
Notoginseng medicine composition for treating cardiac and cerebral vascular diseases
The present invention relates to a kind of total arasaponin composition, which has the active component of total arasaponin comprising arasaponin R1, ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 not less than 55.0 wt% and ginsenoside Rd not more than 2.5 wt%. The total arasaponin composition is prepared into injection, powder for injection, enteric coated tablet, bolus, medicine powder and other preparation forms. The medicine of the present invention has the functions of promoting blood circulation to disperse blood clots and activating collateral flow, and is used in treating blood stasis to block collateral channels, apoplexy, hemiplegia and other cardiac and cerebral vascular diseases.
Owner:GUANGXI WUZHOU PHARMA GRP
Medicinal composition for preventing and treating cardiovascular diseases and application thereof
InactiveCN102526186ADrop in trustQuality is easy to controlOrganic active ingredientsCardiovascular disorderVascular diseaseCoronary heart disease
The invention discloses a medicinal composition for preventing and treating cardiovascular diseases, belonging to the technical field of Chinese medicines. The medicinal composition is prepared from salvianolic acids, total tanshinone and a notoginsenoside mixture in a certain weight ratio, wherein the notoginsenoside mixture is prepared by mixing ginsenoside Rb1, ginsenoside Rg1 and notoginsenoside R1; and the notoginsenoside mixture can be replaced by an equivalent amount of arasaponin. The medicinal composition has remarkable treatment effects on a coronary heart disease, angina and myocardial infarction.
Owner:GUANGANMEN HOSPITAL CHINA ACAD OF CHINESE MEDICAL SCI
Composition for treating cardiovascular disease and preparation thereof
The invention discloses a composition for treating cardiovascular diseases and a preparation method thereof. The composition comprises (by weight part) Radix Ginseng Rubra saponin 2-20, Ophiopogon japonicus saponin 0.2-3, and Ophiopogon japonicus flavone 0.02-3; wherein the Radix Ginseng Rubra saponin extract contains (by dry weight) ginsenoside Rg1 not less than 9.0%, ginsenoside Re not less than 3.0%, ginsenoside Rb1 not less than 12.0%, and Panax ginseng total saponins not less than 80%; the Ophiopogon japonicus saponin extract contains (by dry weight) ophiopogonin D not less than 6.0%, ophiopogonin D' not less than 6.0%, and Ophiopogon japonicus total saponins not less than 80%; and the Ophiopogon japonicus flavone extract contains (by dry weight) methylophiopogonone A and methylophiopogonone B in total not less than 80%. Animal experiments showed that the composition has good therapeutic action on myocardial ischemia and has better anti-shock effect.
Owner:巩洪刚 +1
Medicinal preparation for treating nerve-root cervical spondylopathy, and preparation method and quality detection method thereof
InactiveCN104887771AMeet needsVarious dosage formsNervous disorderComponent separationClinical efficacyCervical spondylopathy
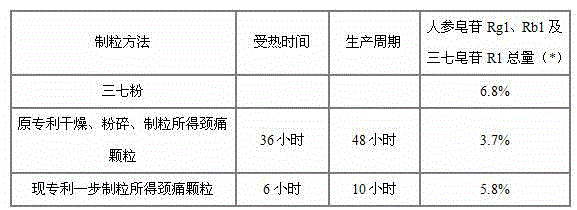
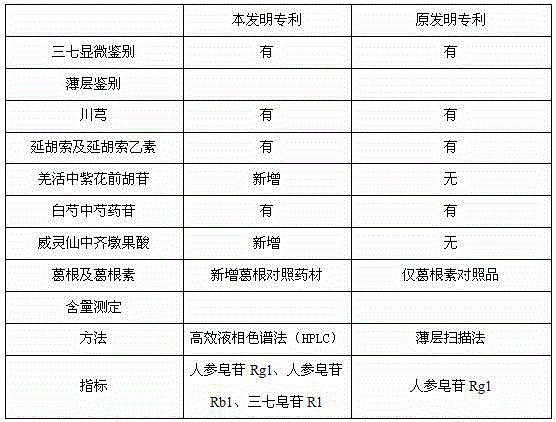
The invention relates to a medicinal preparation for treating nerve-root cervical spondylopathy, and a preparation method and a quality detection method thereof. The invention is an extension of an original invention. The medicinal preparation comprises a granule, a tablet and a capsule, the preparation method is an optimized and screened production technology based on the original invention, a modern new device, a new process and a new technique are adopted to realize industrial production; and quality standard researches are completed and improved on the basis of original standards, HPLC is adopted to simultaneously determine the content of ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 in a finished product, and thin layer discrimination of all medicines is carried out to comprehensively control the quality, so the clinic curative effects are guaranteed.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Medicine composition of Panax notoginseng saponins
InactiveCN101390887AImprove protectionInhibit aggregationOrganic active ingredientsMetabolism disorderPANAX NOTOGINSENG ROOTTreatment effect
The invention relates to panax notoginseng saponins medicine combination and the application thereof, which are characterized in that the active ingredient is composed of panax notoginseng saponins which contains notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Re, ginsenoside Rd, ginsenoside Rb3 and ginsenoside Rb2; the combination with different contents and ingredients of saponins are prepared; the combinations have different treatment effects.
Owner:HEILONGJIANG ZBD PHARMA
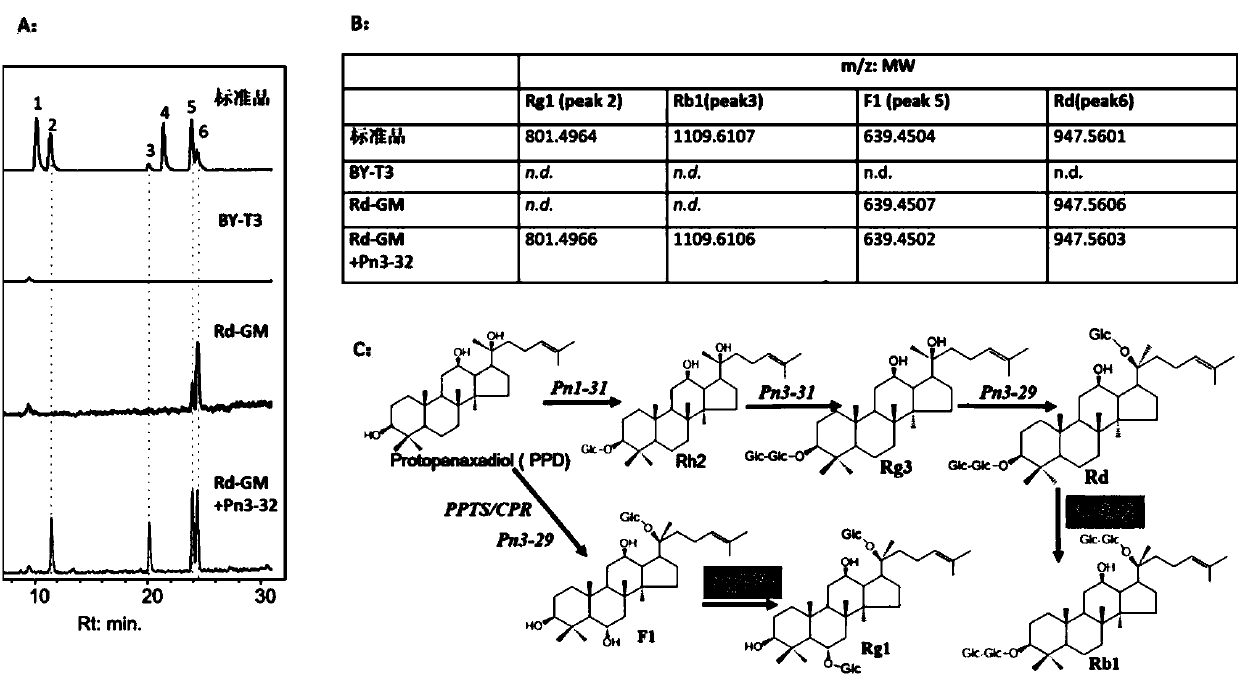
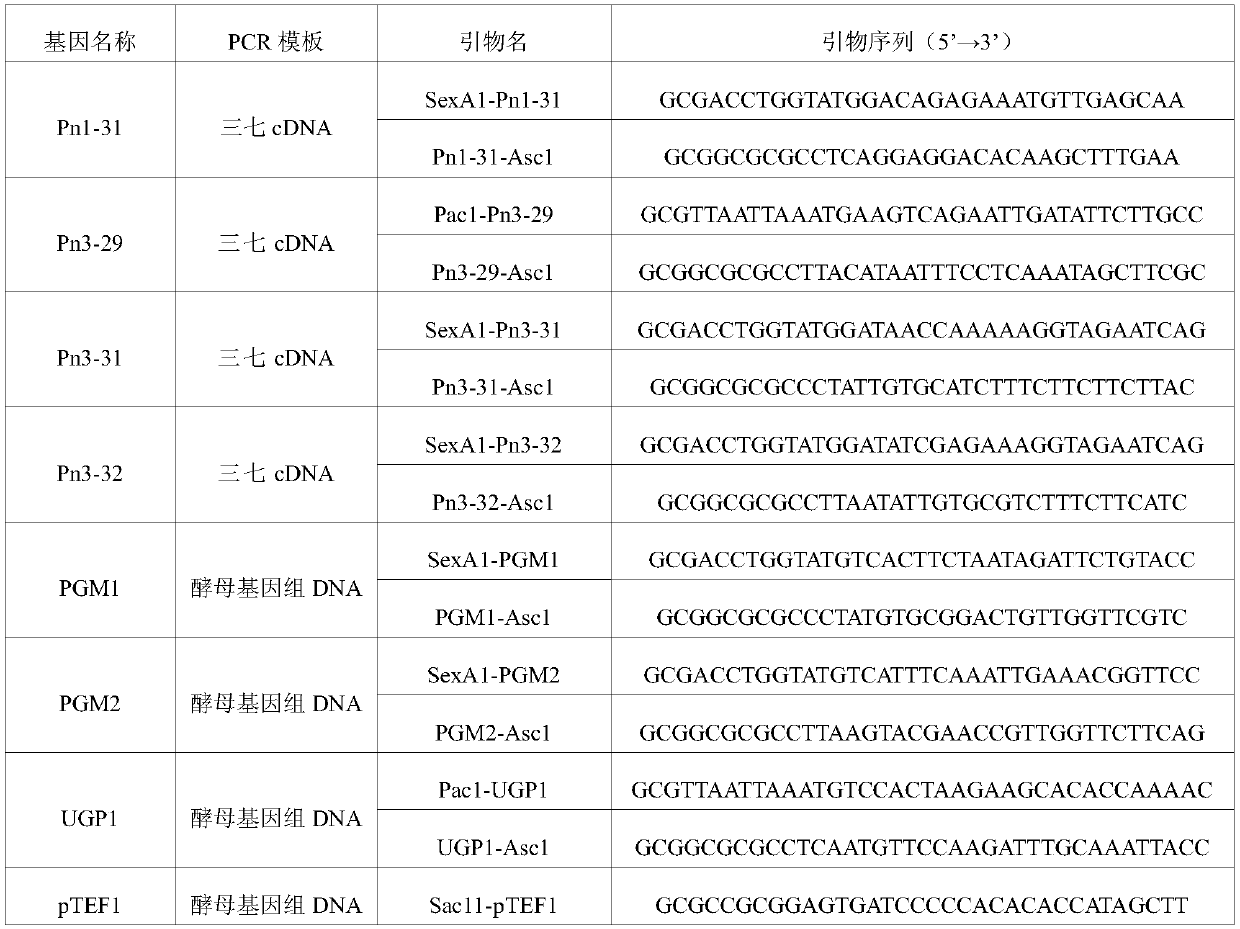
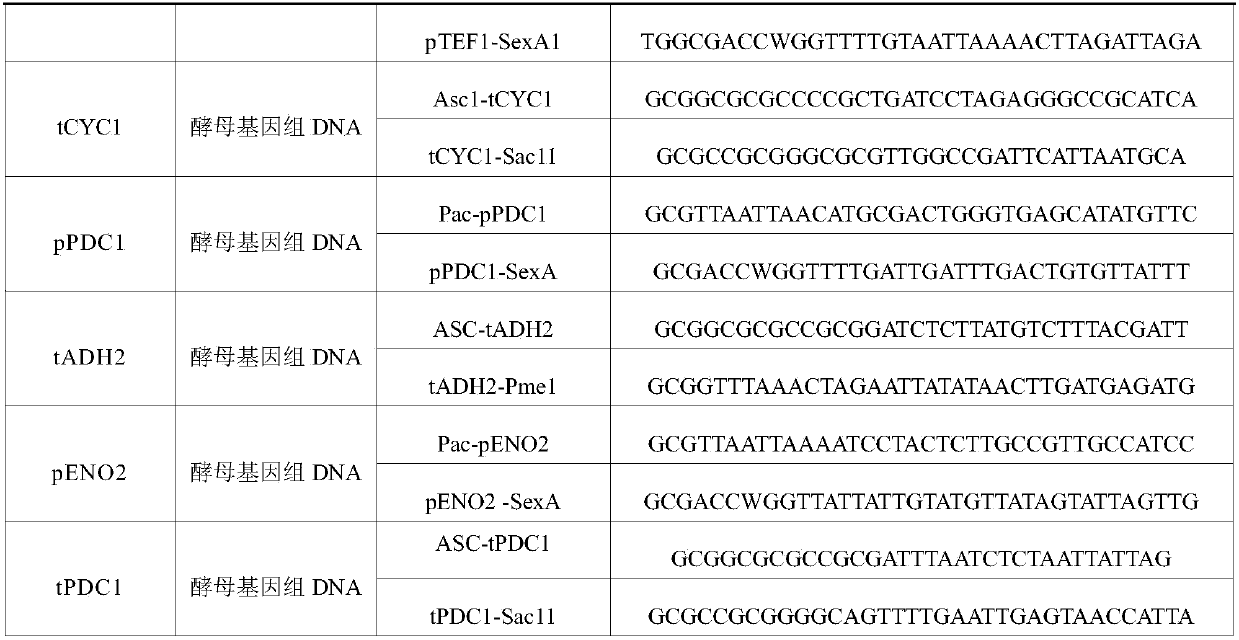
Applications of glycosyltransferase and related materials thereof in construction of engineering bacteria for producing ginsenoside Rb1 and Rg1
The invention discloses applications of glycosyltransferase and related materials thereof in the construction of engineering bacteria for producing ginsenoside Rb1 and Rg1. A glycosyltransferase genePn3-32 which can catalyze ginsenoside Rd to generate ginsenoside Rb1 can be successfully identified through a synthetic biological method; and the gene can simultaneously catalyze ginsenoside F1 to generate ginsenoside Rg1 and construct recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1. Through experiments, the constructed recombinant yeast producing the ginsenoside Rb1 and the ginsenoside Rg1 can simultaneously generate the ginsenoside Rb1 and the ginsenoside Rg1. Pn1-31, Pn-3-29, Pn3-31 and Pn3-32 glycosyltransferase genes in medicinal plant radix notoginseng are firstly utilized to continuously catalyze protopanaxadiol and protopanaxatriol to synthetize the ginsenoside Rb1, the ginsenoside Rg1 and corresponding intermediates, so that novel cases can be provided formicrobial strains to produce natural products.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Medicinal composition and preparation method thereof
ActiveCN102028700AIncrease the maximum tolerated doseRaise the median lethal doseOrganic active ingredientsPeptide/protein ingredientsHemolysisAcute toxicity testing
The invention provides a medicinal composition, which comprises the following components: ginsenoside Rb1, ginsenoside Rg1, notoginsenoside R1, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rc, ginsenoside Rh1, ginsenoside Rb2 and ginsenoside Rg3. The invention also provides a preparation method for the medicinal composition. The medicinal composition has definite components; compared with the total notoginsenoside in the prior art, the medicinal composition has stable quality and good controllability; and results of experiments of acute toxicity, undue toxicity, hemolysis and the like show that the medicinal composition has higher safety and wide clinical application prospect.
Owner:KPC PHARM INC
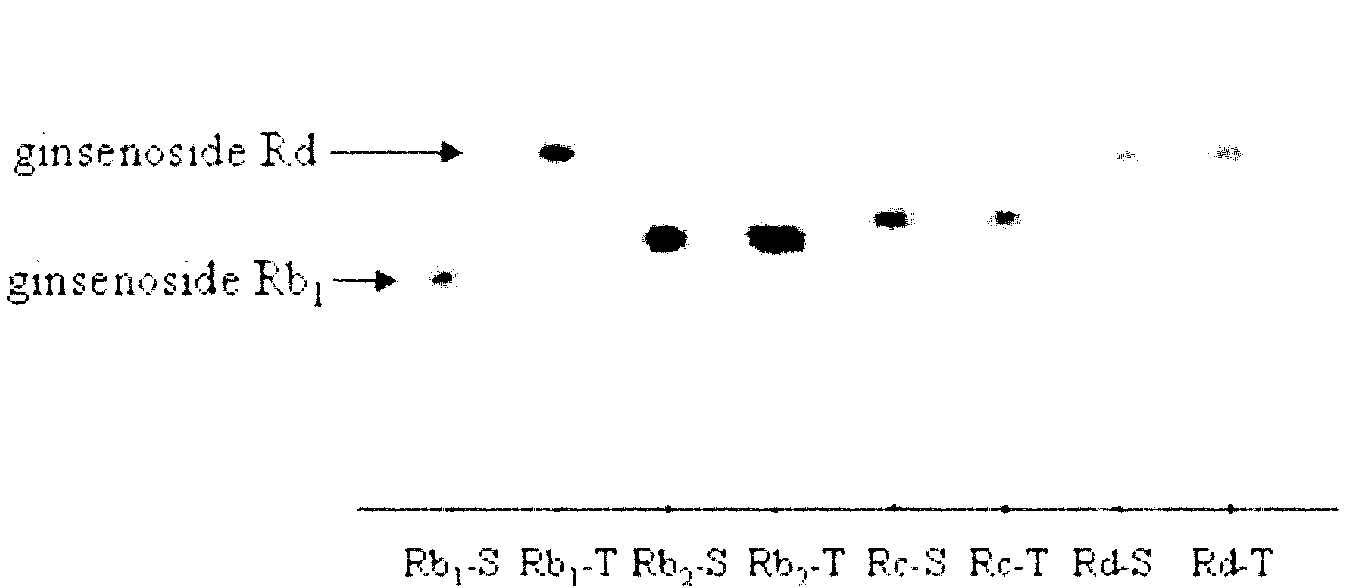
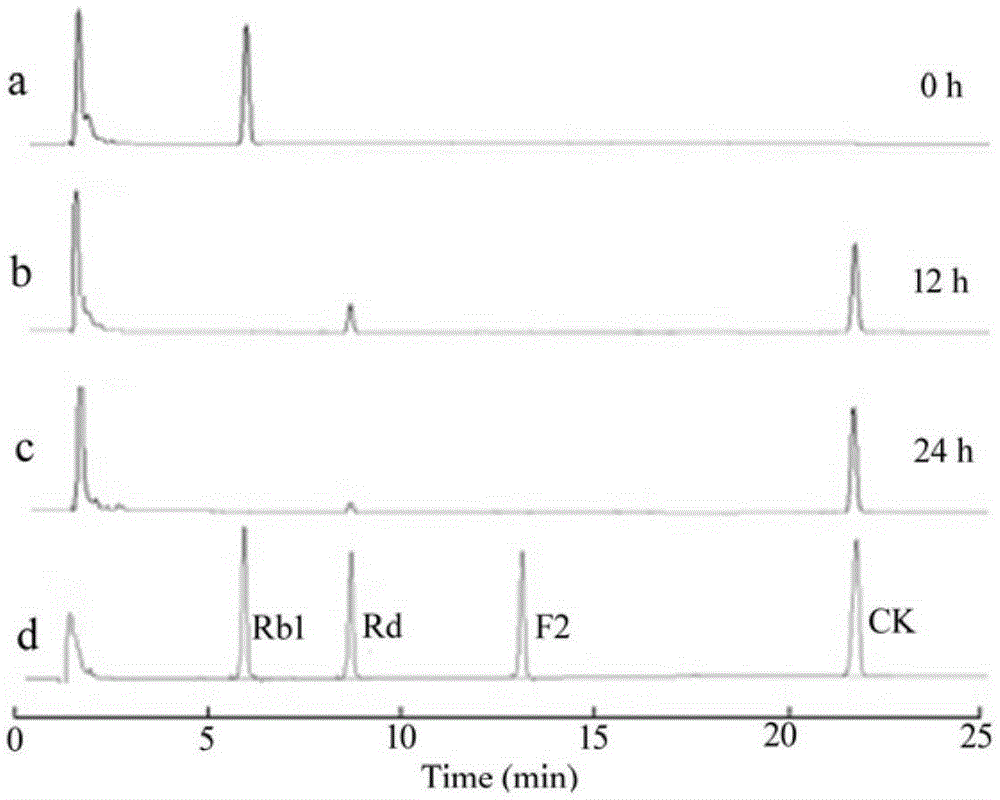
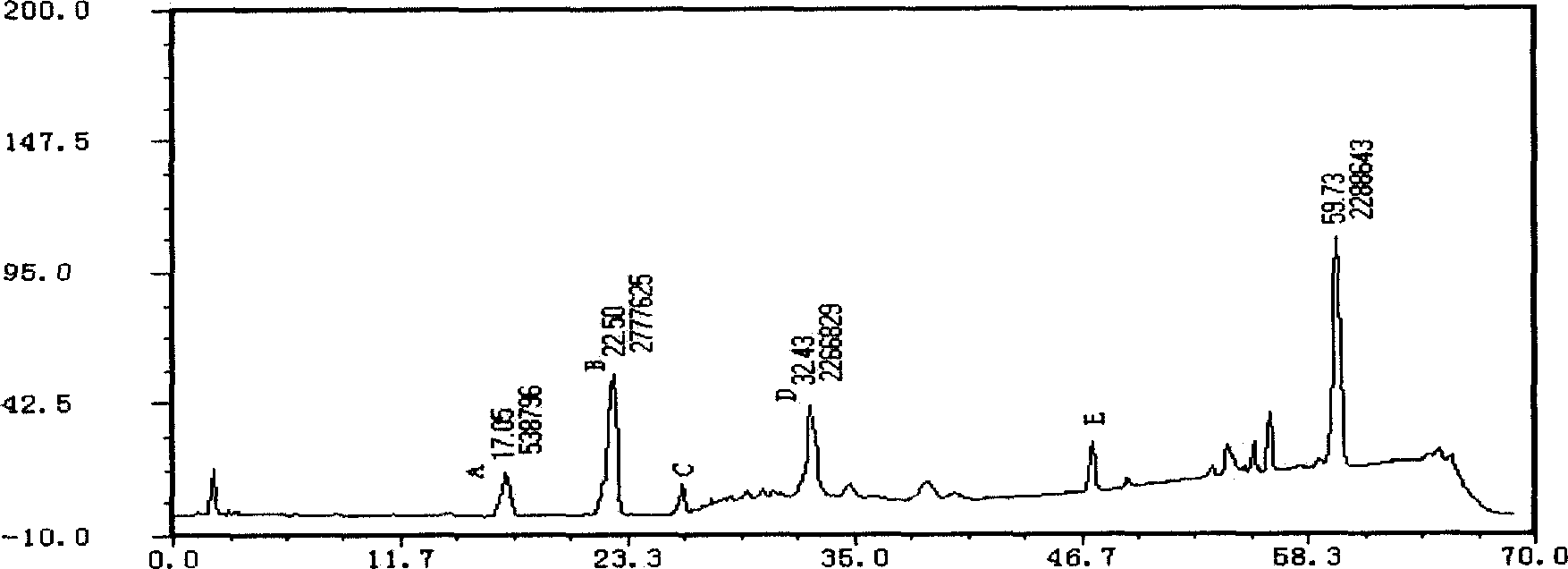
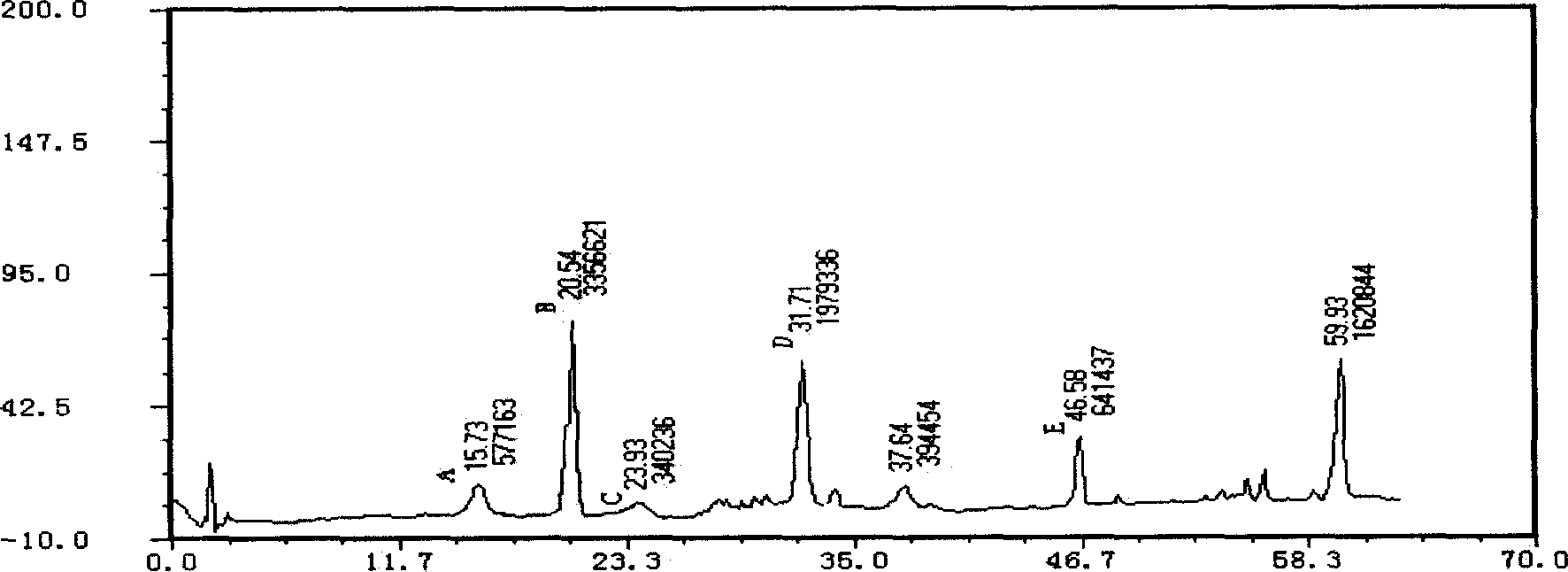
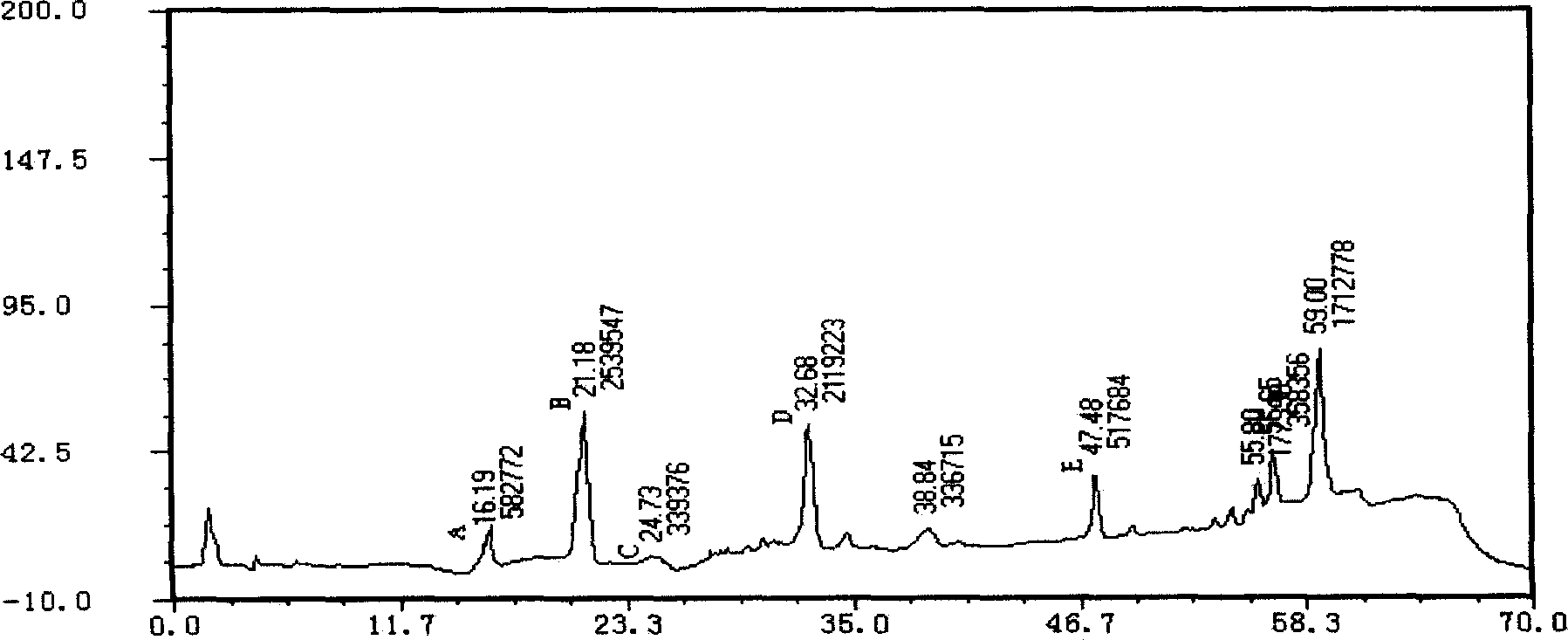
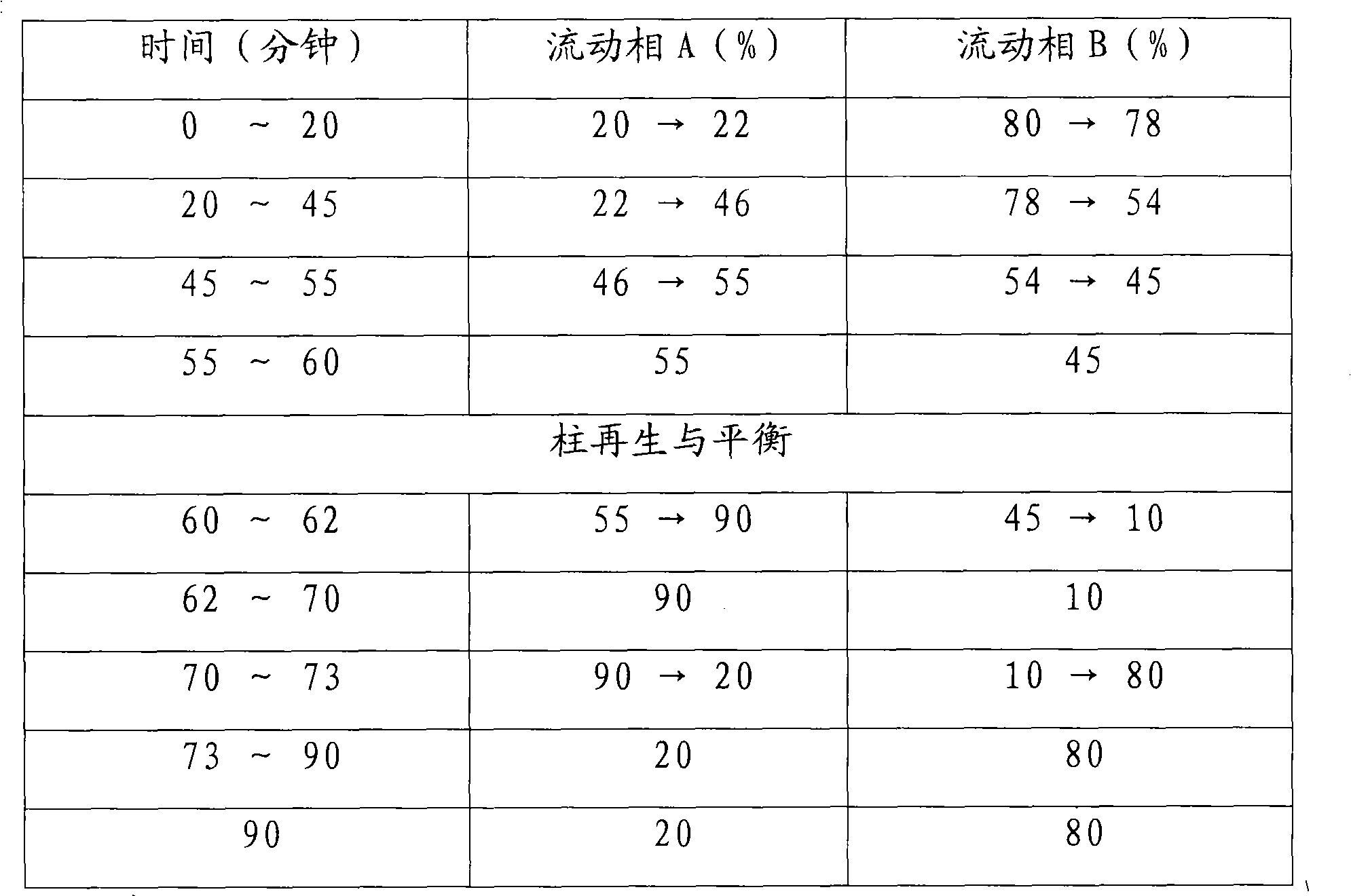
[Beta]-glucosidase, preparation method and application thereof
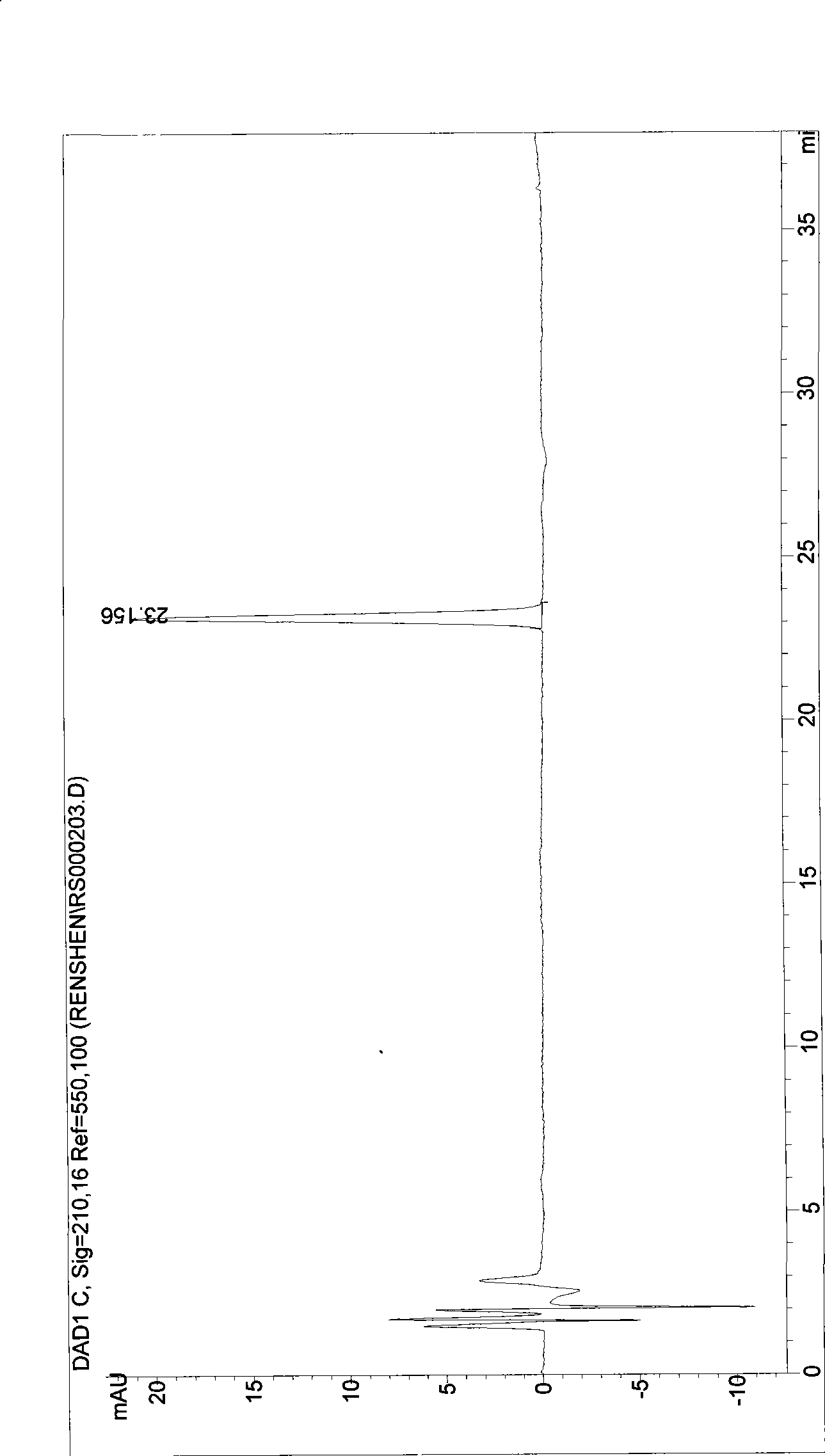
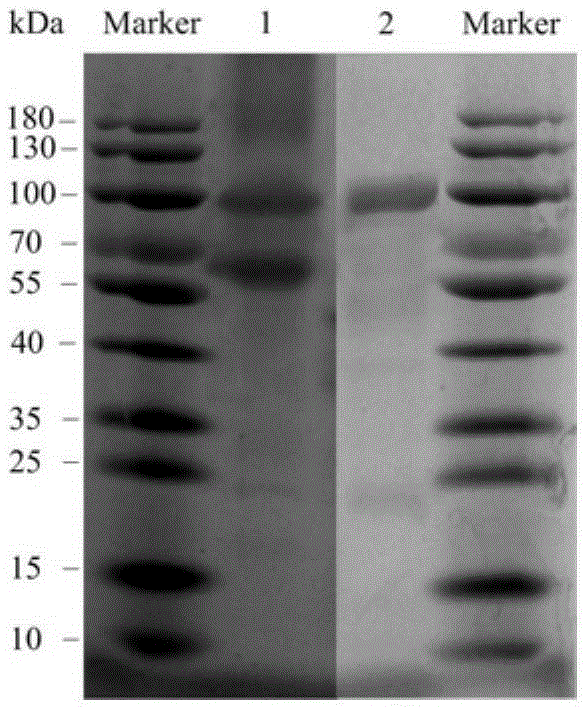
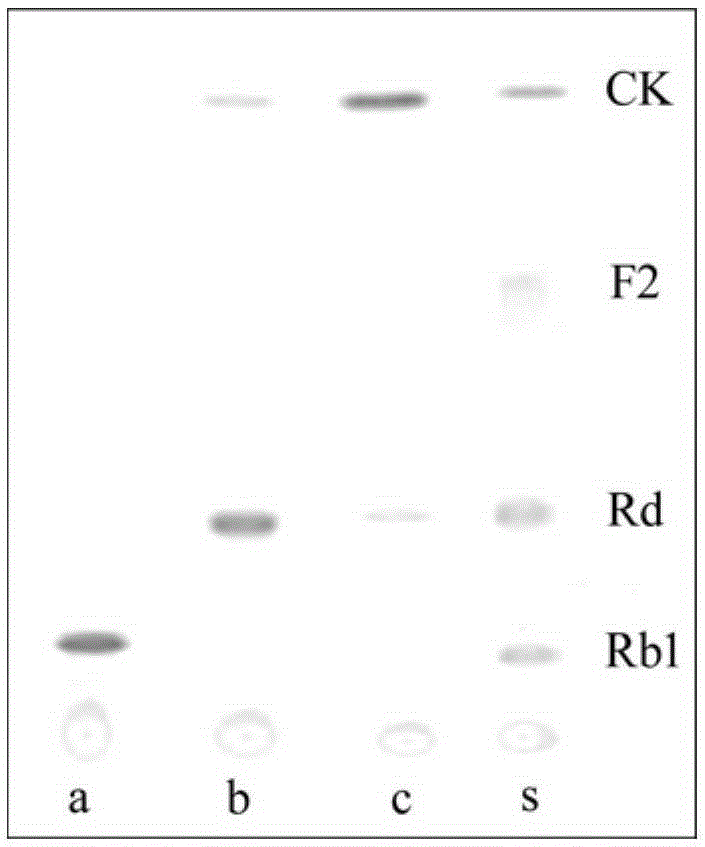
ActiveCN104328098AImprove conversion abilityHigh purityFermentationVector-based foreign material introductionAlgluceraseGene engineering
The invention belongs to the fields of gene engineering technology and biological medicines, particularly relates to a [beta]-glucosidase, a preparation method and an application thereof and especially relates to an application in enzymic-method conversion for preparing ginsenoside 20(S)-Rg3. The [beta]-glucosidase is high-temperature-resistant and can maintain an enzyme activity to be constant almost at 70 DEG C for 3h. The [beta]-glucosidase has a strong conversion capability on ginsenoside Rb1. When being used for incubation with the ginsenoside Rb1 for 60 min, the [beta]-glucosidase enables the ginsenoside Rb1 to be converted almost into the ginsenoside 20(S)-Rg3. The method for preparing the ginsenoside 20(S)-Rg3 is less in intermediate product in an enzymatic conversion reaction and the ginsenoside 20(S)-Rg3 is high in purity after conversion.
Owner:JIANGSU KANION PHARMA CO LTD +1
Cooked pseudo-ginseng and preparation method thereof
ActiveCN106138141AEnhance immune functionImprove anti-tumor effectOrganic active ingredientsImmunological disordersLower gradeLow graded
The invention discloses cooked pseudo-ginseng. By weight, the total saponin content is 6.0-12.0%, and the sum of the content of the low-grade saponin in the transformation accounts for 40-90% of the total saponins of the cooked pseudo-ginseng. Inherent saponins in pseudo-ginseng contain ginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1, the low-grade saponins generated by transformation contains ginsenoside 20 (S)-Rh1, ginsenoside 20 (R)-Rh1, ginsenoside Rd, ginsenoside Rk3, ginsenoside Rh4, ginsenoside 20 (S)-Rg3, ginsenoside 20(R)-Rg3, ginsenoside Rk1, and ginsenoside Rg5. The invention also discloses a preparation method of the cooked pseudo-ginseng. By precisely controlling the water addition, processing temperature and processing time, the cooked pseudo-ginseng having a certain saponin and the proportion thereof is obtained. The cooked pseudo-ginseng is rich in low-grade saponins that rarely exists in the dried pseudo-ginseng and can improve the immunity and the anti-tumor effect.
Owner:四川仟源中药饮片有限公司 +1
Detection method of compound danshen dripping pills
ActiveCN102119961AQuality improvementImprove product qualityHydroxy compound active ingredientsComponent separationSalvia miltiorrhizaChromatographic fingerprint
The invention relates to a detection method of compound danshen dripping pills, comprising the following contents of: observation of characters, discrimination of contents, inspection of contents, comparison of finger prints and assaying of contained components. The detection method provided by the invention comprises a discriminating method of the medicinal component panax notoginseng, the discriminating method adopts thin-layer chromatography, the reference materials adopt a panax notoginseng reference medicinal material, a ginsenoside Rg1 reference substance, a ginsenoside Re reference substance, a ginsenoside Rb1 reference substance and a panax notoginseng saponin R1 reference substance. The detection method provided by the invention also comprises a discriminating method and an assaying method of the medicinal component danshen, wherein the discriminating method adopts a sub-2 mu m liquid chromatography technology to perform chromatographic fingerprint discrimination, and adopts sodium danshensu as a reference material; and the assaying method adopts a sub-2 mu m liquid chromatography technology to perform assaying on the components such as danshensu, panax notoginseng saponin R1, ginsenoside Rg1 and ginsenoside Rb1 in the danshen.
Owner:TIANJIN TASLY PHARMA CO LTD
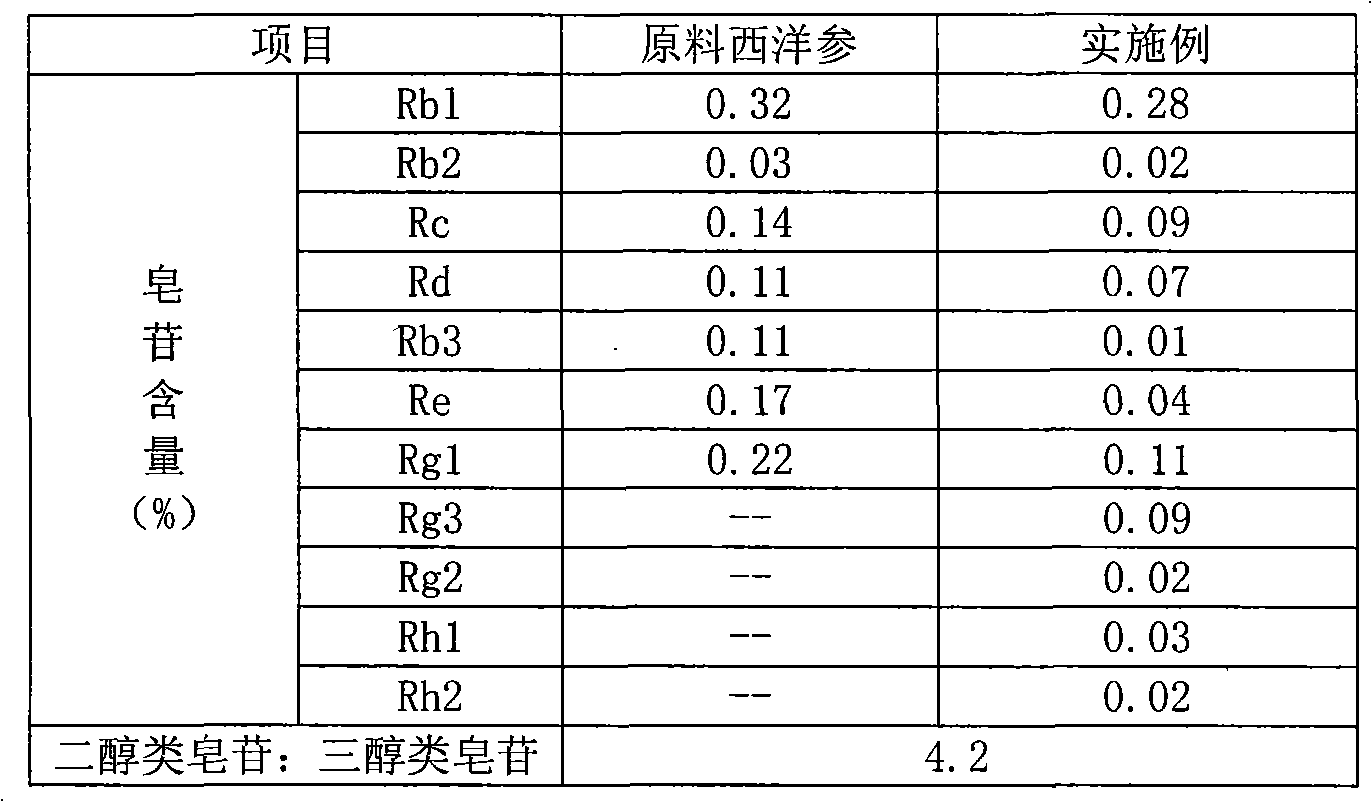
Method for extracting and separating total saponins of panax ginseng from american ginseng
ActiveCN102772462AHigh purityHigh yieldNervous disorderAntinoxious agentsAMERICAN GINSENG ROOTGinsenoside Rc
The invention discloses a method for extracting and separating total saponins of panax ginseng from american ginseng. The method employs a series of high-efficiency extraction, separation and purification technical measures of alcohol-backflow extracting, water precipitating, purifying by a macroporous adsorption resin, decolorizing with an ion exchange resin, etc. The obtained total saponins of panax ginseng are white powder and comprise ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, ginsenoside Rc and ginsenoside Rd, wherein the total purity of ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 is more than 74.0%. The production process is simple and practical, has no pollution, and is suitable for large-scale production.
Owner:HEBEI YILING MEDICINE INST
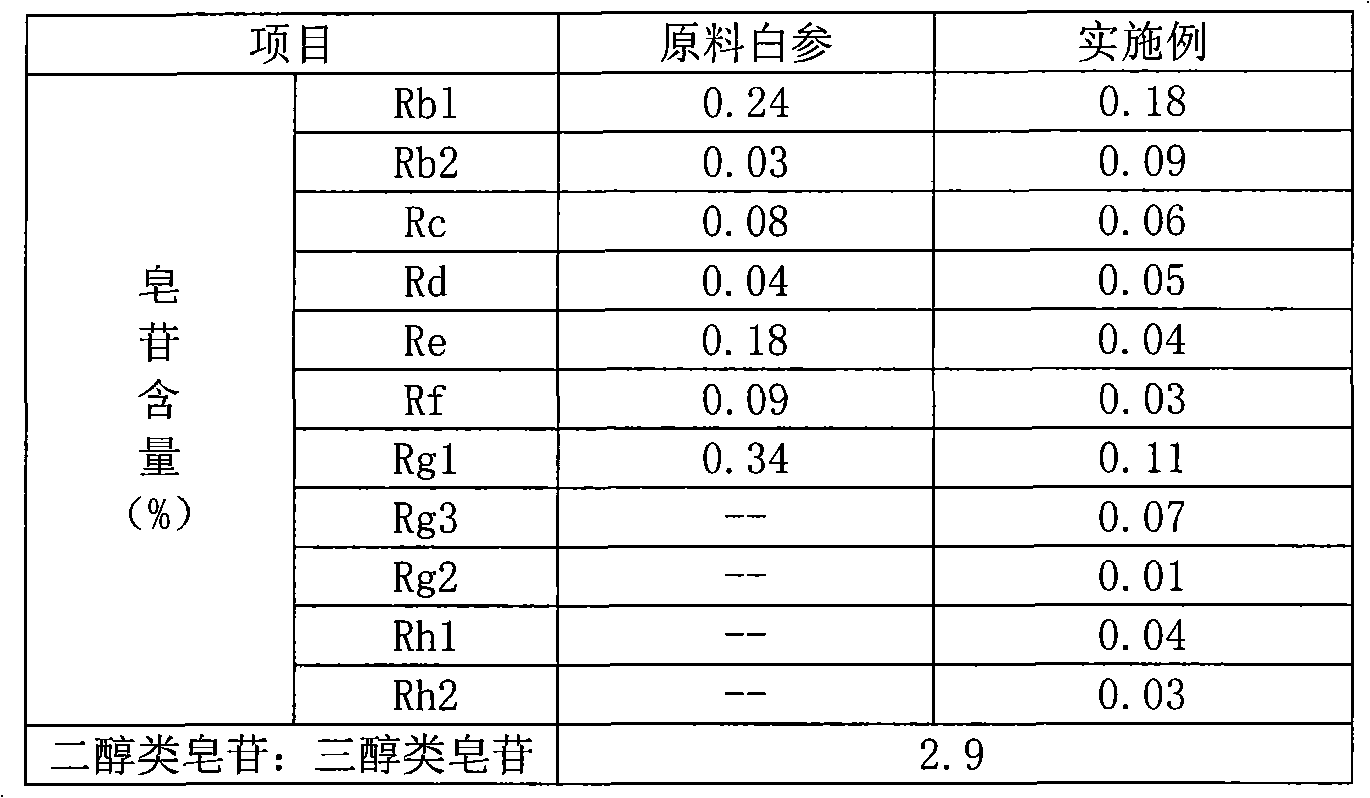
Production method of flavor panax ginseng
The invention relates to a preparation method of flavor ginseng; after ginseng is added with nature fruit juice or organic acid for reaction, the content of the precious ginsenoside in products is enhanced, the efficacies of ginseng is improved, while the specific flavor of ginseng is maintained. The main quality characteristics is that: the flavor ginseng includes at least one of the following ingredients: ginsenoside Rg3, ginsenoside Rh1 and ginsenoside 20R-Rh2; wherein, the ratio of the total amount of precious ginsenoside group, ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rd and ginsenoside Rc to the total amount of ginsenoside Re, ginsenoside Rg1 and ginsenoside Rf is more than 2.5; the preparation method can convert part of the component causing internal heat, namely, panaxatriol ginsenoside, into precious ginsenoside.
Owner:JILIN HONGJIU BIO TECH
Determining method for contents of twelve components in traditional Chinese medicine composition preparation
ActiveCN104914199AQuality improvementGood repeatabilityComponent separationBiotechnologyAstragaloside
The present invention discloses a UPLC-MS quantitative method for the contents of twelve components in a traditional Chinese medicine composition preparation, specifically determination of the contents of calycosin-7-O-beta-D-glucoside (1), isoquercitrin (2), narirutin (3), hesperidin (4), ginsenoside Re (5), ginsenoside Rg1(6), periplocoside (7), ginsenoside Rf (8), ginsenoside Rb1 (9), astragaloside (10), ginsenoside Rd (11) and periplocin H1 (12) in the traditional Chinese medicine composition, and belongs to the field of traditional Chinese medicine composition preparation component detection. The determining method of the present invention has characteristics of short period, good reproducibility and high sensitivity.
Owner:HEBEI YILING MEDICINE INST
Method of preparing ginseng saponine monomer from ginseng leaf
A process for preparing ginsenoside monomer from ginseng leaf includes such steps as preparing general ginsenoside from ginseng leaf, chromatography with alumina column to obtain several groups of ginsenosides, and chromatography by column to obtain different ginsenoside monomers.
Owner:HAINAN ASIA PHARM CO LTD
Extract of panax notoginseng saponins and preparation method thereof
InactiveCN101829170AIncrease cerebral blood flowHigh trafficBlood disorderCardiovascular disorderPANAX NOTOGINSENG ROOTAdjuvant
The invention relates to an extract of panax notoginseng saponins, a preparation method thereof, a preparation prepared from the total saponins and a method for preparing the preparation. The extract comprises panaxtriol saponins (PTS) and panaxadiol saponins (PDS), wherein the PTS mainly comprises notoginsenoside R1 and ginsenoside Rg1; the PDS mainly comprises the ginsenoside Rb1 and the ginsenoside Rd; the extract is characterized in that the weight ratio of the PTS to the PDS is 1:0.5-2; and the total content of the notoginsenoside R1, the ginsenoside Rg1, the ginsenoside Rb1 and the ginsenoside Rd accounts for over 80 weight percent of the extract of the panax notoginseng saponins. The preparation of the extract of the panax notoginseng saponins comprises the therapeutically effective amount of the panax notoginseng saponins and pharmaceutically acceptable adjuvant.
Owner:北京中海康医药科技发展有限公司
Application of panaxadiol saponins fraction in preparing medicine for preventing dermatitis and scar
ActiveCN102743402AHigh purityStable efficacyOrganic active ingredientsCosmetic preparationsMedicinal herbsGlucocorticoid
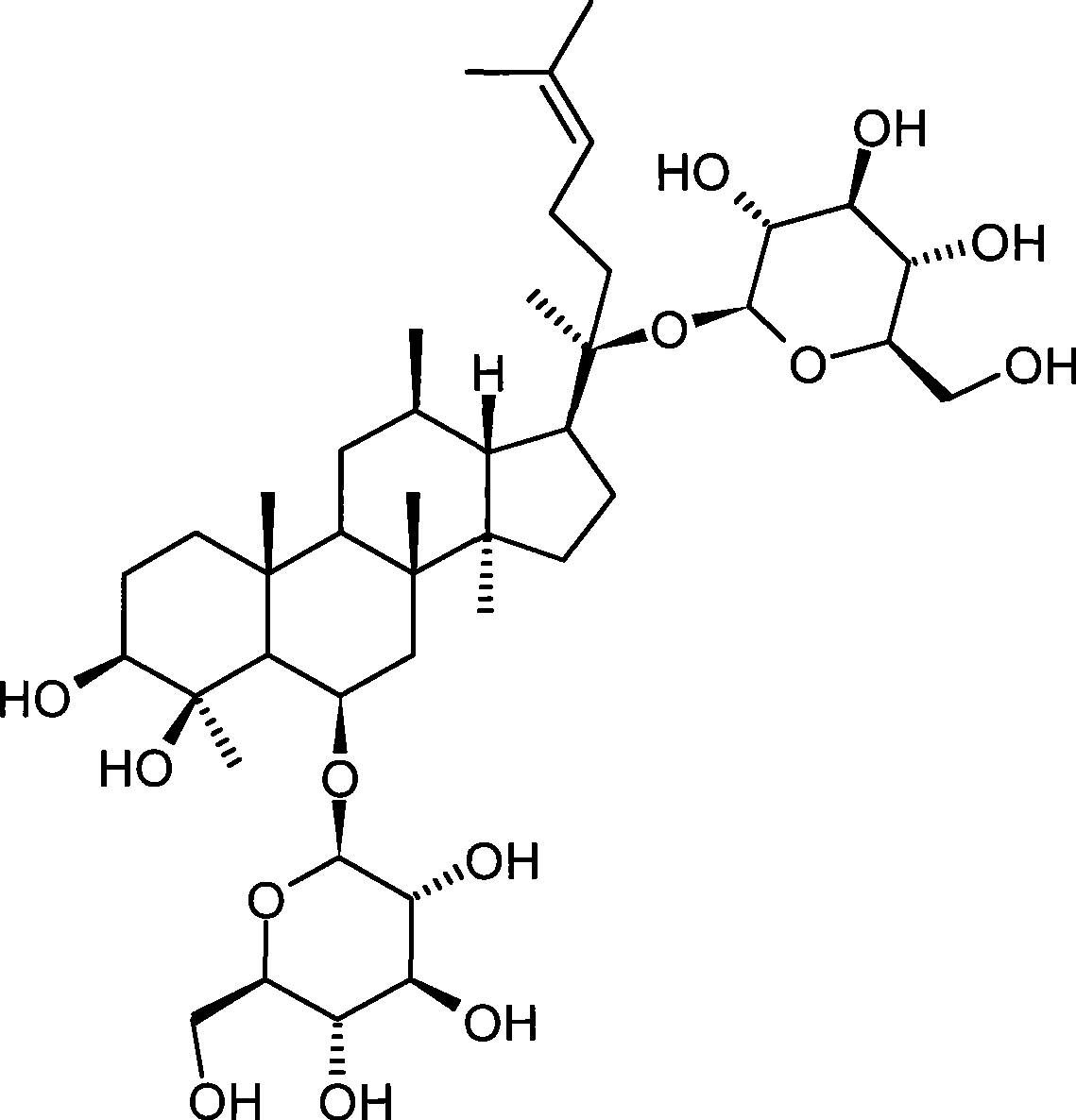
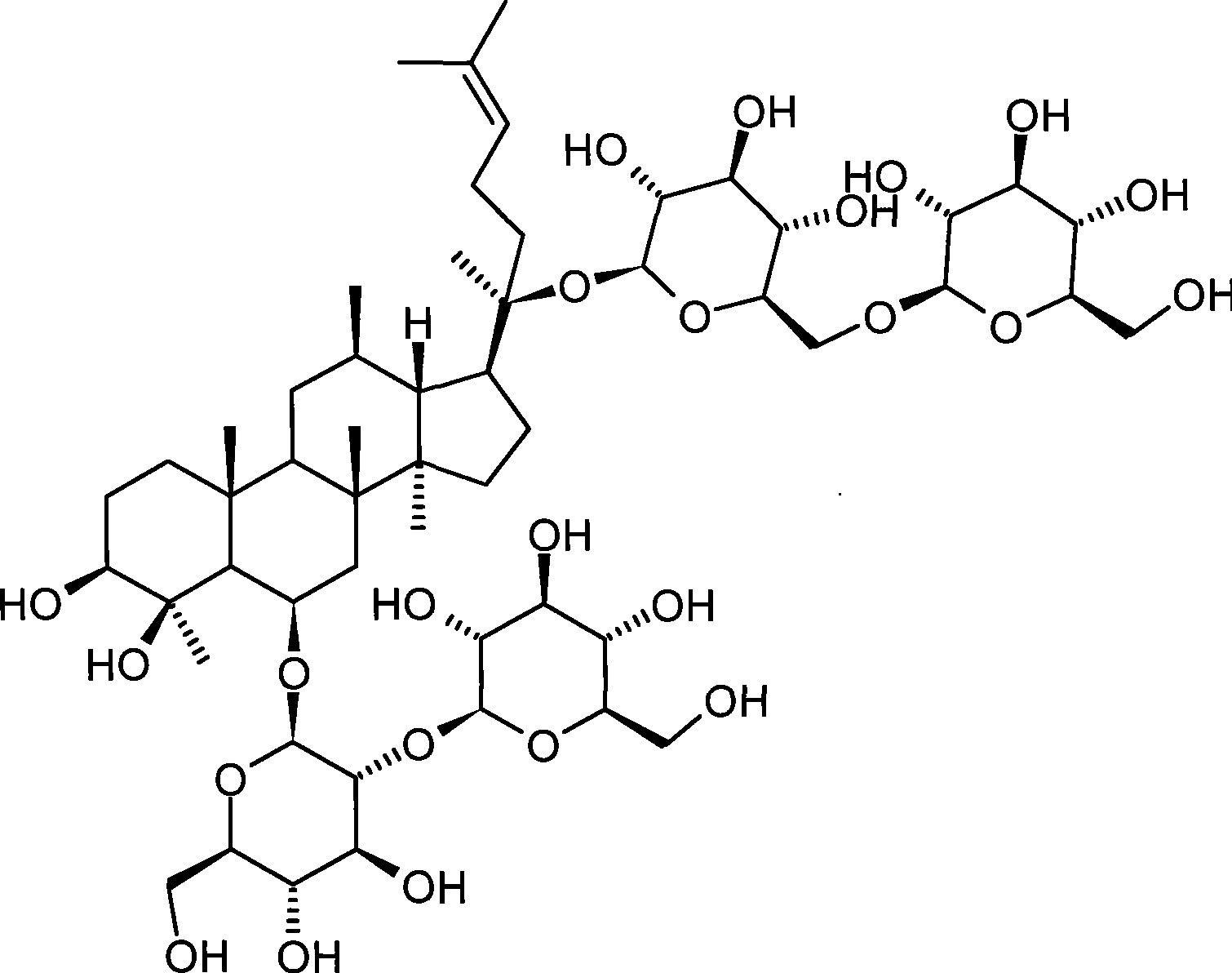
The invention provides application of a panaxadiol saponins fraction in preparing a medicine for preventing dermatitis and scar and a health-care cosmetic. The panaxadiol saponins fraction comprises the following main constituents: ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. The medicine or the cosmetic is prepared from active constituents in the single panaxadiol saponins fraction or / and other medicines together and a pharmaceutically acceptable or cosmetic acceptable carrier. The medicine or the cosmetic is prepared by utilizing raw materials: ginseng rhizome medicinal materials, American ginseng rhizome medicinal materials, ginseng stem leaf medicinal materials, American ginseng leaf medicinal materials and total extractives or total saponins of the ginseng rhizome medicinal materials, the American ginseng rhizome medicinal materials, the ginseng stem leaf medicinal materials and American ginseng leaf medicinal materials through a chromatographic separation and purification method combining macroporous resin column chromatography and octadecylsilane chemically bonded silica column chromatography. The scar can be prevented from forming while the tissue regeneration and repair are promoted by the medicine or the cosmetic. Compared with glucocorticoids, cellular immunity is integrally regulated, the drug action is stable after the medicine is suspended, and the drug action advantage is obvious. The medicine or the health-care cosmetic is highly safe. The structural formula of panaxadiol saponins is shown in the specification.
Owner:ZHEJIANG UNIV
Simple production method of rare ginsenoside IH-901
The invention discloses a simple production method of rare ginsenoside IH-901. The method comprises the following steps: evenly mixing 1 unit mass of a ginsenoside Rb1 monomer, 0.1-0.3 unit mass of domestic crude snail enzyme and 1 unit volume of phosphoric acid-citric acid buffer solution with the pH value of 3-5, performing heat preservation at the temperature of 35-50 DEG C for 10-20 hours, and then terminating the reaction; adding 0.5 unit mass of ethanol into the mixed solution for extraction, centrifugally collecting an ethanol layer, and then decompressing and concentrating to obtain a sample; placing the sample on the silicagel column, taking a lower CHCl3-AcOEt-MeOH-H2O layer as an eluent for eluting 0.8 unit mass of the sample, collecting the obtained elution solution, and then decompressing and concentrating to obtain a condensed extract; and dissolving the condensed extract in a mixed solution of ethanol and water at a volume ratio of 100:70, and staying overnight at room temperature so as to finally obtain IH-901 crystal with the purity more than 95%. In the method, as the ginsenoside Rb1 monomer is taken as a reaction substrate and the cheap domestic crude snail enzyme is taken as the reaction enzyme, the separation and purification process of the product is very simple; and in addition, by utilizing the single substrate and the cheap domestic crude snail enzyme, the simple production method has the advantages of rich enzyme source, low raw material cost, simple product extraction process and the like.
Owner:HUAQIAO UNIVERSITY
Human mesenchymal stem cell preservation transportation liquid and application thereof
The invention discloses human mesenchymal stem cell preservation transportation liquid which is prepared from 1 to 15 mg / ml of sodium chloride, 1 to 15 mg / ml of sodium gluconate, 1 to 10 mg / ml of sodium acetate, 0.1 to 9.9 v / v% of human AB serum, 0.1 to 25 mg / ml of ginsenoside Rb1, 1 to 100 ng / ml of transferring, 1 to 100 ng / ml of reduced glutathione and 0.01 to 1 mmol / l of glutamine. The invention further discloses application of the liquid in preservation and transportation of human mesenchymal stem cells. The human mesenchymal stem cell preservation transportation liquid can reduce the clustering phenomenon of the human mesenchymal stem cells in a transportation process and keep high attachment rate and activity, so that the human mesenchymal stem cell preservation transportation liquid can be conveyed to all parts of the country and surrounding countries through modern vehicles such as an airplane, and the clinical use range of the human mesenchymal stem cells is greatly enlarged.
Owner:杭州哈佛赛尔干细胞技术有限公司
Traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill detection method
InactiveCN104483315AStrong process controllabilityImprove quality controlComponent separationMaterial analysis by optical meansControllabilityHigh-performance liquid chromatography
The invention discloses a traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill detection method, according to the method, rehmannia glutinosa, yam, tuckahoe, schisandra, chrysanthemum and astragalus in a lumbus-strengthening kidney-tonifying pill can be microscopically identified, a radix angelicae sinensis referenee crude herb, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, dipsacus asper saponin VI, astragaloside and deoxyschizandrin are used as reference substances, whether the lumbus-strengthening kidney-tonifying pill contains the radix angelicae sinensis ,ginseng, dipsacus asper, astragalus and schisandra can be identified by thin-layer chromatography; the dipsacus asper saponin VI content in the lumbus-strengthening kidney-tonifying pill preparation can be detected by HPLC, according to the detection method, the quantitative index is that the dipsacus asper saponin VI (C47H76O18) content in every 1g of the traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill may not be less than 0.60mg, the controllability of quality standards of the traditional Chinese medicine preparation lumbus-strengthening kidney-tonifying pill can be improved to further ensure the product intrinsic quality and effect, so that the quality standard is more perfect, and the drug quality control level is improved.
Owner:JINGFUKANG PHARMA GRP CHIFENG DANLONG PHARMA CO LTD
Method for increasing content of ginsenoside Rb1 in cultured pseudo-ginseng
InactiveCN107278590AIncrease contentPromote growthBioloigcal waste fertilisersHorticulture methodsEnzyme GeneSalicylic acid
The invention discloses a method for increasing content of ginsenoside Rb1 during pseudo-ginseng cultivation. The method comprises the following steps: utilizing a jasmonic acid methyl ester solution and a salicylic acid solution to spray pseudo-ginseng plants in an alternating mode 2-10 days before pseudo-ginseng harvest. By spraying the jasmonic acid methyl ester solution and the salicylic acid solution on pseudo-ginseng leaves in an alternating mode before pseudo-ginseng harvest, high expressions of key enzyme genes of metabolism of ginsenoside Rb1 in cultured pseudo-ginseng are induced, thereby rapidly increasing the content of ginsenoside Rb1 in the short time.
Owner:周运德
Ginseng endogenesis zygorhynchus moelleri mildew as well as method for preparing ginsenoside Rd by utilizing same
The invention relates to ginseng endogenesis zygorhynchus moelleri mildew (CGMCC (China General Microbiological Culture Collection): No.4315). The mildew has the capability for preparing Rd by converting a substrate ginsenoside Rb1 and has higher substrate unicity and product unicity, and the preparation of the ginsenoside Rd by utilizing the mildew can adopt in-site conversion. A method for preparing the ginsenoside Rd comprising the following steps of: dibbling the mildew in a PDA (Potato Dextrose Agar) culture medium containing the ginsenoside Rb1, standing still at 25 DEG C and culturing for 5-7 days or vaccinating the mildew on an enzyme production culture medium by adopting a microorganism enzymic method and culturing at 28 DEG C for 5-7 days, collecting enzyme liquid, mixing with the ginsenoside Rb1 and reacting at 40 DEG C for 24h. The ginsenoside Rd produced by adopting the technical scheme of the invention has the advantages of strong specificity, simplicity, convenience, safety, reliability and low cost without side products, the purity of the fermentation product Rd is higher than 90 percent, and the conversion rate can be higher than 60 percent.
Owner:DALIAN NATIONALITIES UNIVERSITY
Extracting method of ginsenoside
ActiveCN105663195ASimple and fast operationReduce manufacturing costOrganic active ingredientsNervous disorderAlcoholVitamin C
The invention relates to total ginsenoside rich in ginsenoside Rg3 and an extracting method thereof. The extract comprises, by mass, 1.0-3.0% of ginsenoside Rb1, 0.2-1.0% of ginsenoside Rb2, 0.5-2.5% of ginsenoside Rb3, 0.5-2.5% of ginsenoside RC and 28-40% of ginsenoside Rg3. The extracting method comprises the following steps that ethyl alcohol is used as an extracting solvent for extracting ginseng through a microwave extracting method, a certain amount of vitamin C is added into the extracting solution, a crude extracting solution of the total ginsenoside is extracted through microwave heating within a certain period of time, and the crude extracting solution of the total ginsenoside is further enriched and purified through a macroporous resin adsorption process to obtain the extract of total ginsenoside.
Owner:江西樟树市庆仁保健品有限公司
Prepared pseudo-ginseng, as well as preparation method and application thereof
ActiveCN104523790AHas anti-tumor effectImprove immunityOrganic active ingredientsImmunological disordersGinsenoside Rb1Chemistry
The invention provides prepared pseudo-ginseng, as well as a preparation method and application thereof. Based on weight percentage, the total amount of notoginsenoside R1, ginsenoside Rg1 and ginsenoside Rb1 in the prepared pseudo-ginseng is not less than 5.0 percent, wherein the content of notoginsenoside R1 is not less than 0.4 percent, the content of ginsenoside Rg1 is not less than 2.0 percent and the content of ginsenoside Rb1 is not less than 1.5 percent; the total amount of ginsenoside Rh4, ginsenoside 20(S)-Rg3 and ginsenoside 20(R)-Rg3 is not less than 0.5 percent, wherein the content of ginsenoside Rh4 is not less than 0.3 percent, the content of ginsenoside 20(S)-Rg3 is not less than 0.06 percent, and the content of ginsenoside 20(R)-Rg3 is not less than 0.03 percent.
Owner:雅安迅康药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

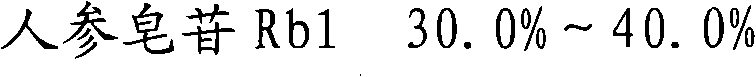
![[Beta]-glucosidase, preparation method and application thereof [Beta]-glucosidase, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/93e53ac3-03b3-4f99-9eff-2e5a46b634c5/HDA0000624842180000011.PNG)
![[Beta]-glucosidase, preparation method and application thereof [Beta]-glucosidase, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/93e53ac3-03b3-4f99-9eff-2e5a46b634c5/HDA0000624842180000012.PNG)
![[Beta]-glucosidase, preparation method and application thereof [Beta]-glucosidase, preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/93e53ac3-03b3-4f99-9eff-2e5a46b634c5/HDA0000624842180000021.PNG)