Extract of panax notoginseng saponins and preparation method thereof
A technology of Panax notoginseng saponins and Panax notoginseng saponins, which is applied in the direction of drug combinations, pharmaceutical formulas, plant raw materials, etc., can solve problems such as hidden safety hazards, high toxicity, and clinical application of Panax notoginseng saponins, and achieve high safety , The effect of less extraction process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
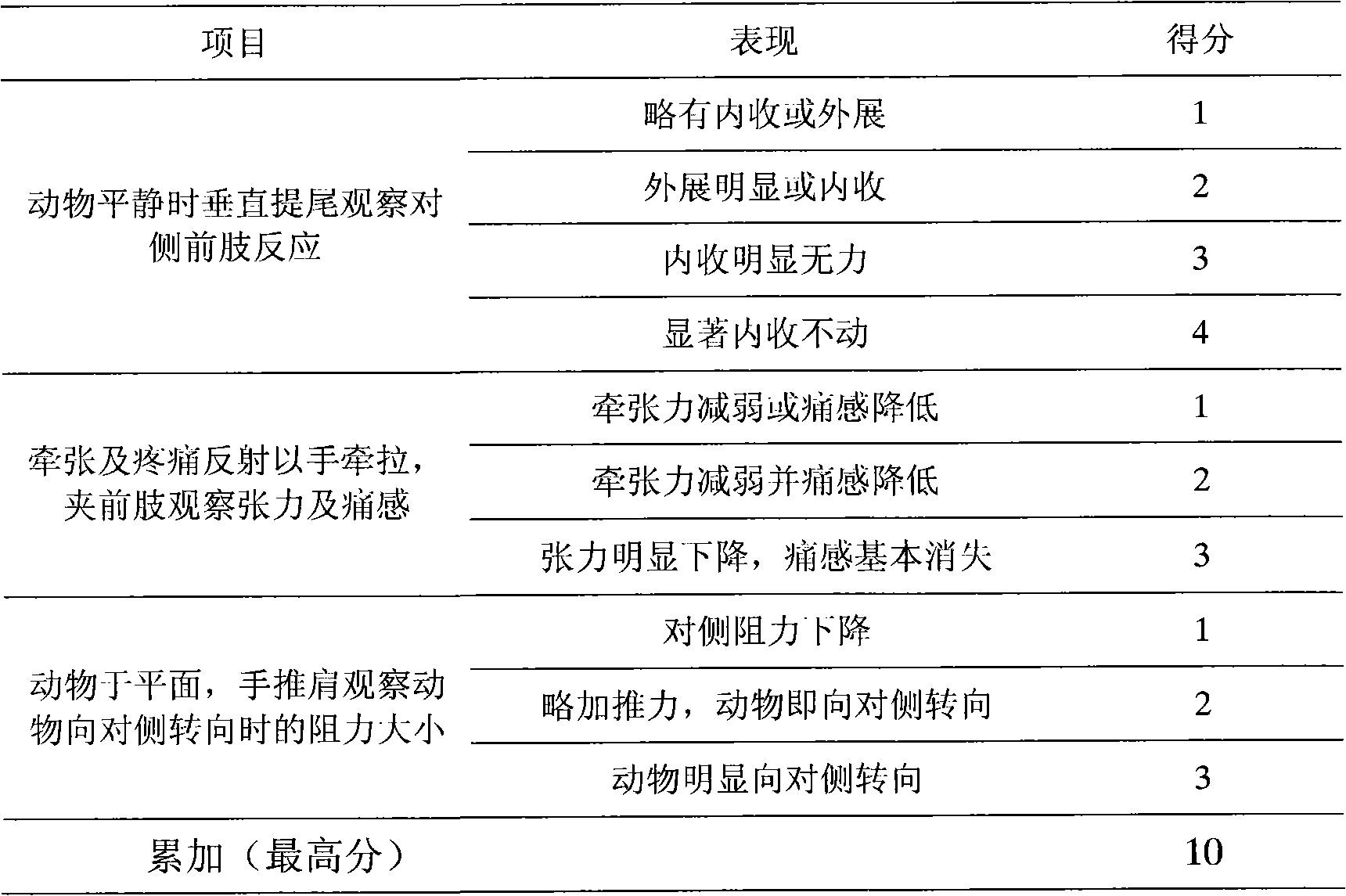
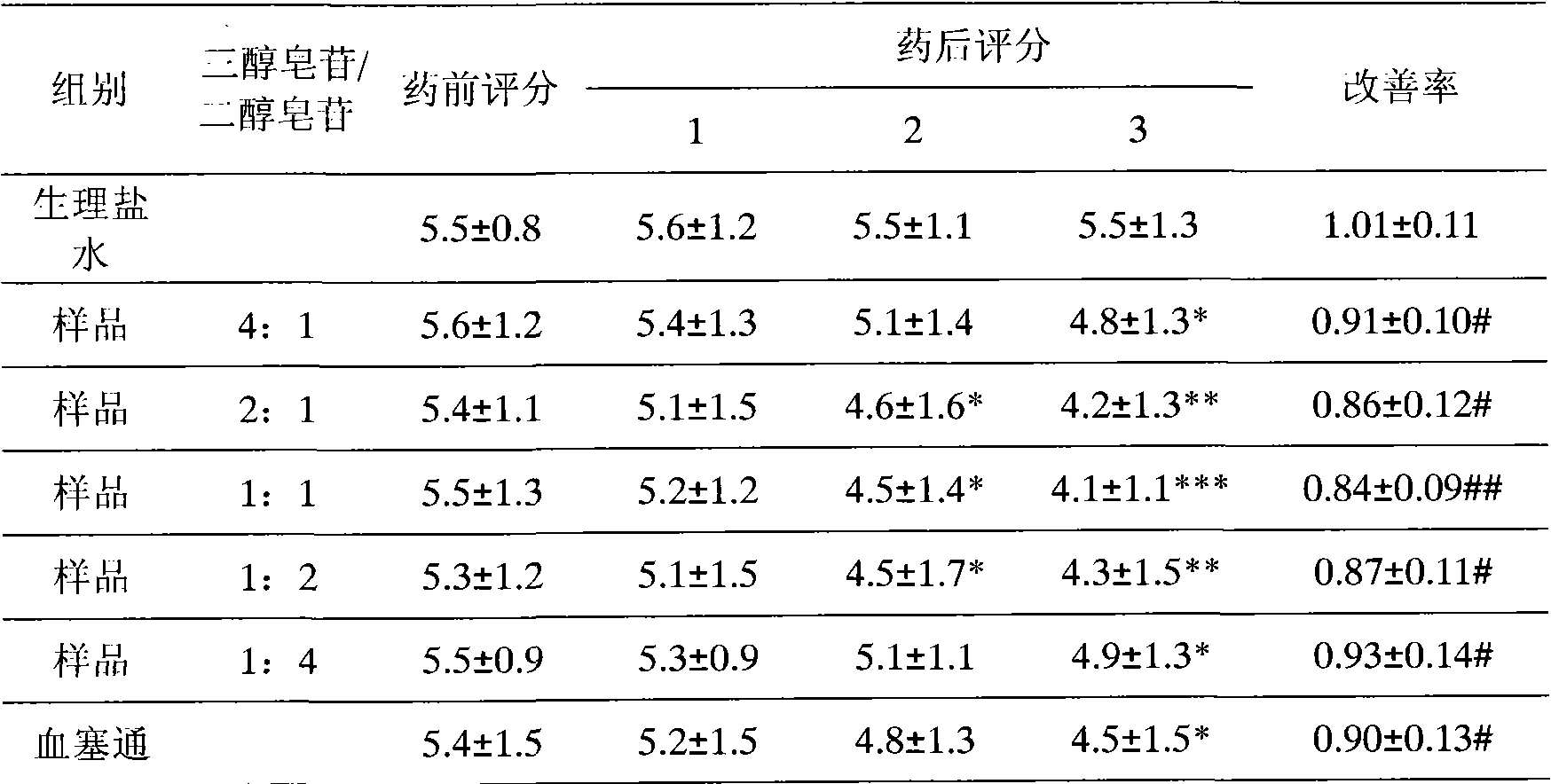
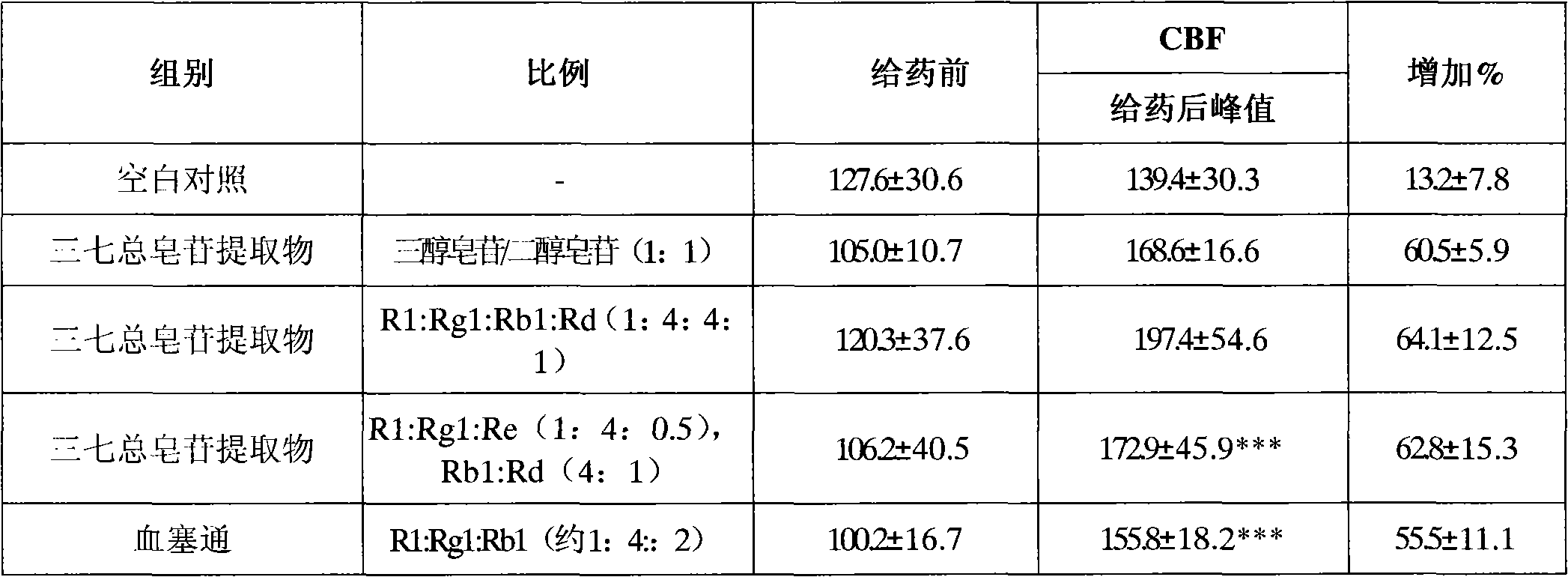
[0125] Take the crude extract of Panax notoginseng total saponins, put it on a macroporous adsorption resin column; elute with deionized water, discard the eluent; elute with 15% ethanol solution, discard the eluent; elute with 40% ethanol solution , collecting the eluent, concentrating under reduced pressure, and drying to obtain the saponins of the notoginsengtriol group; continuing to elute with 70% ethanol solution, collecting the eluent, concentrating the eluent under reduced pressure, and drying to obtain the saponins of the notoginsengdiol group;
[0126] The detection of notoginseng saponin R 1 , Ginsenoside Rg 1 , the content of ginsenoside Re accounted for 15.0%, 69.0% and 6.4% of the saponins of the notoginsenoside group respectively, and the ginsenoside Rb 1 and ginsenoside Rd accounted for 70.0% and 18.4% of the saponins in the notoginsengdiol group, respectively.
[0127] The saponins of the notoginseng triol group and the saponins of the notoginseng-diol group...
Embodiment 2
[0134] Take the crude extract of Panax notoginseng total saponins, put it on a macroporous adsorption resin column; elute with deionized water, discard the eluent; elute with 5% ethanol solution, discard the eluent; elute with 30% ethanol solution , collect the eluate, concentrate under reduced pressure, and dry to obtain the saponins of the notoginseng triol group; continue to elute with 80% ethanol solution, collect the eluate, concentrate the eluent under reduced pressure, and dry to obtain the saponins of the notoginseng triol group.
[0135] The detection of notoginseng saponin R 1 , Ginsenoside Rg 1 , the content of ginsenoside Re accounted for 13.8%, 66.7% and 9.7% of the saponins of the notoginseng triol group respectively, and recorded ginsenoside Rb 1 The contents of ginsenoside Rd and ginsenoside accounted for 64.0% and 21.1% of the saponins in the notoginsengdiol group, respectively.
[0136] The saponins of the notoginseng triol group and the saponins of the not...
Embodiment 3
[0138] Take the crude extract of Panax notoginseng saponins, put it on a macroporous adsorption resin column; elute with deionized water, discard the eluent; elute with 10% ethanol solution, discard the eluent; elute with 50% ethanol solution , collect the eluent, concentrate under reduced pressure, and dry to obtain the saponins of the notoginseng triol group; continue to elute with 60% ethanol solution, collect the eluate, concentrate the eluent under reduced pressure, and dry to obtain the saponins of the notoginseng triol group.
[0139] The detection of notoginseng saponin R 1 , Ginsenoside Rg 1 , the content of ginsenoside Re accounted for 20.5%, 61.7% and 12.7% of the saponins of the notoginseng triol group respectively, recorded ginsenoside Rb 1 The contents of ginsenoside Rd and ginsenoside accounted for 67.5% and 13.1% of the saponins in the notoginsengdiol group, respectively.
[0140] The saponins of the notoginseng triol group and the saponins of the notoginseng...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com