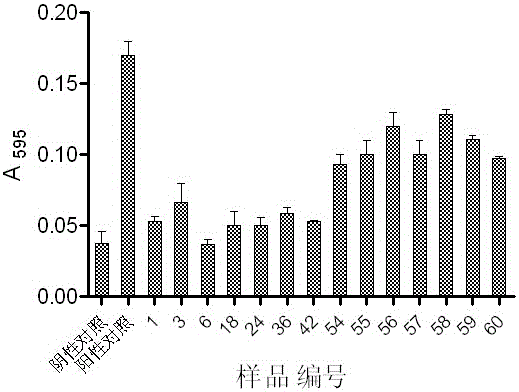
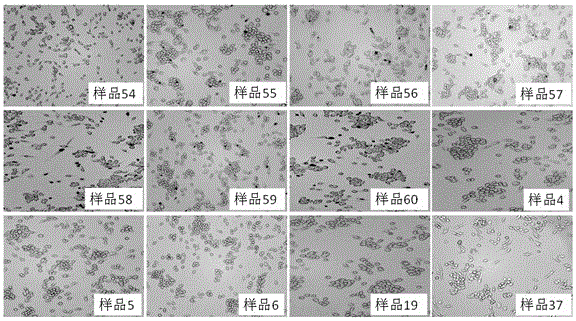
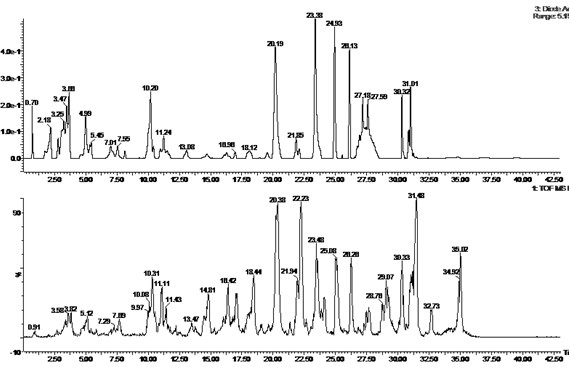
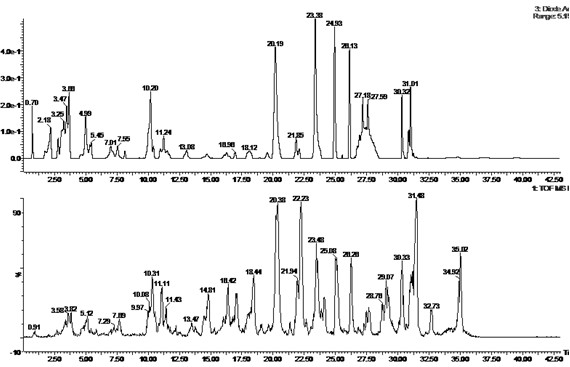
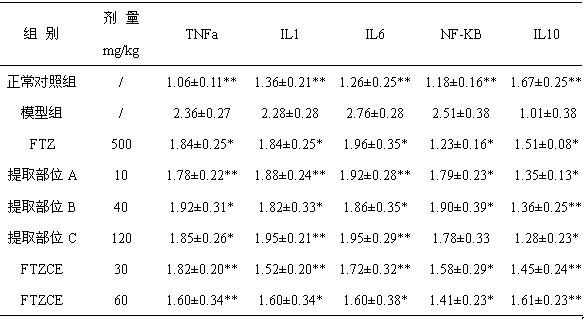
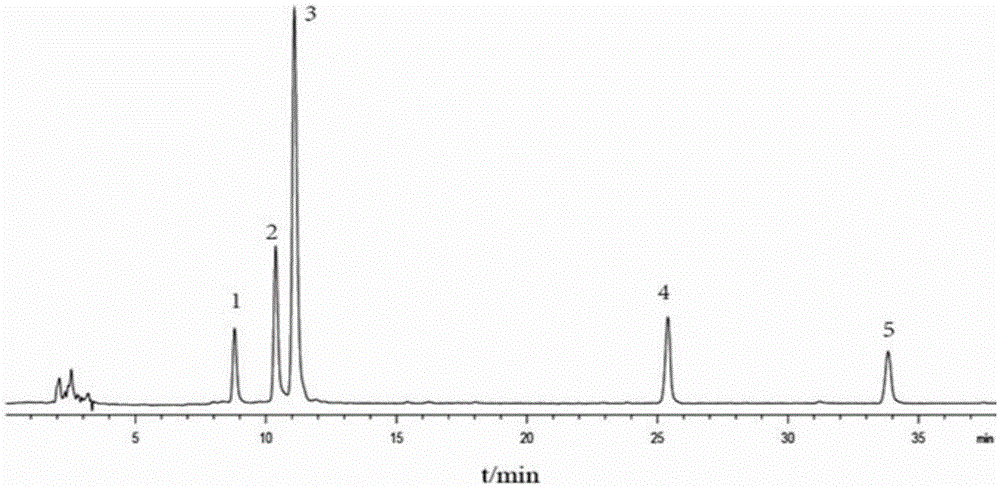
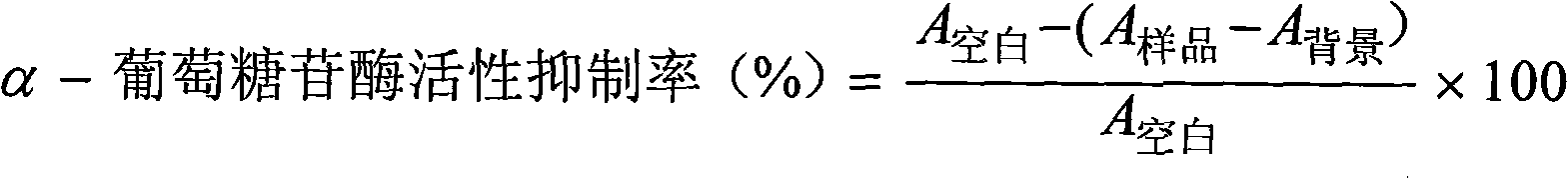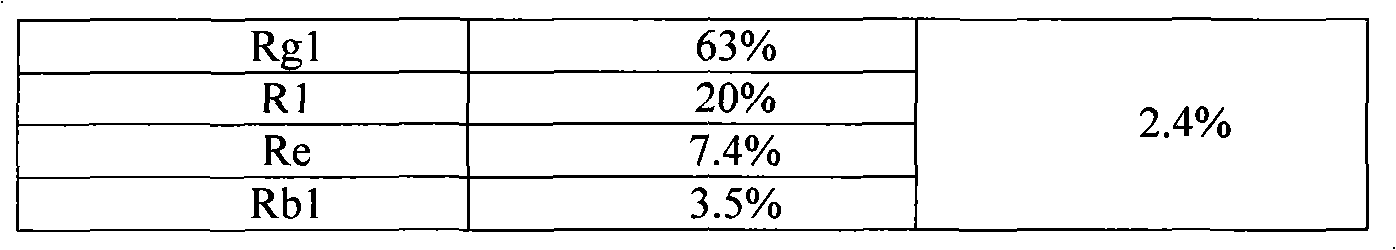Patents
Literature
141 results about "Notoginsenoside R1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
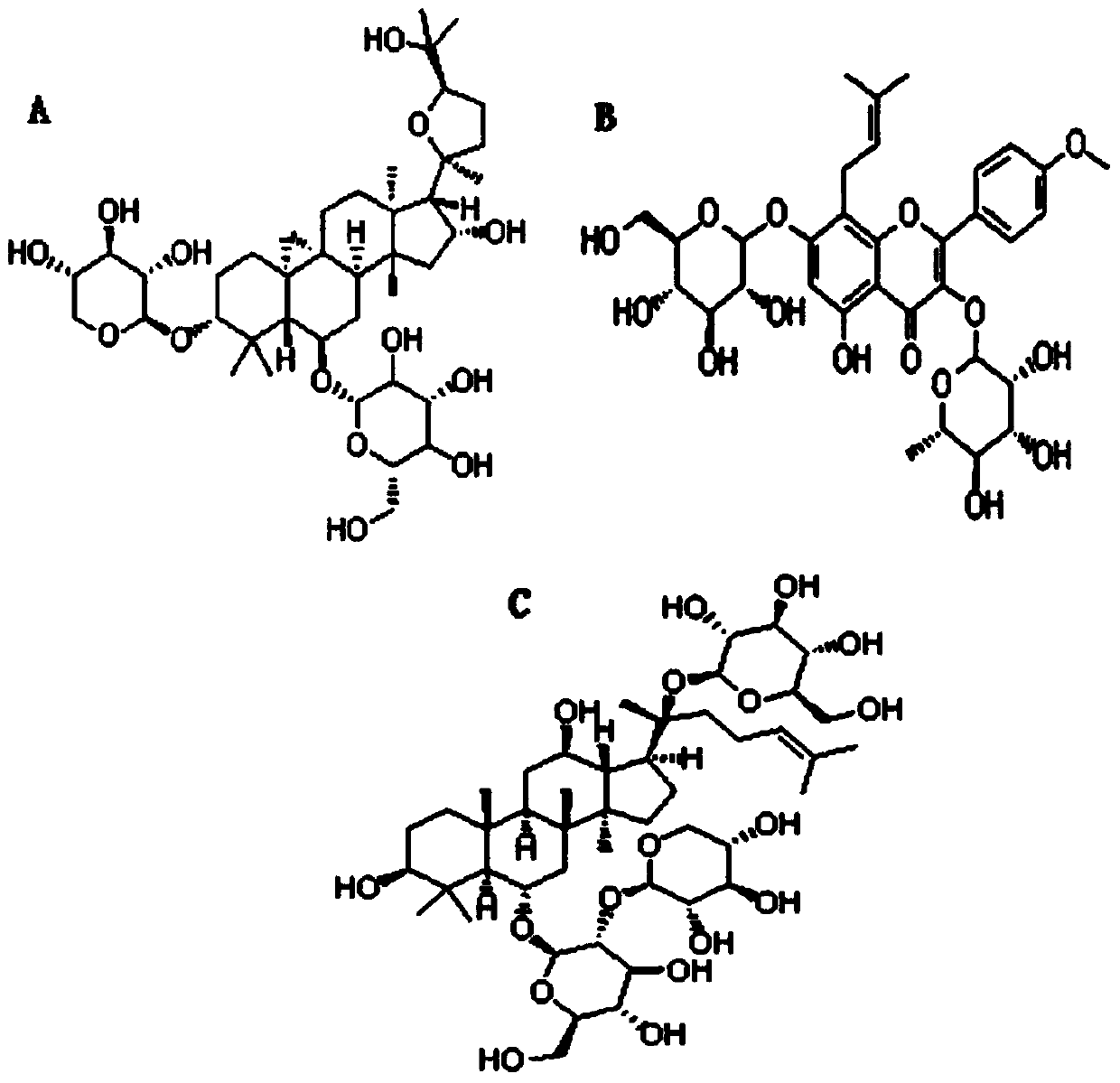
Notoginsenoside R1 is a ginsenoside found in Panax notoginseng that is dammarane which is substituted by hydroxy groups at the 3beta, 6alpha, 12beta and 20 pro-S positions, in which the hydroxy groups at positions 6 and 20 have been converted to the corresponding beta-D-xylopyranosyl-(1->2)-beta-D-glucopyranoside and beta-D-glucopyranoside respectively, and in which a double bond has been ...
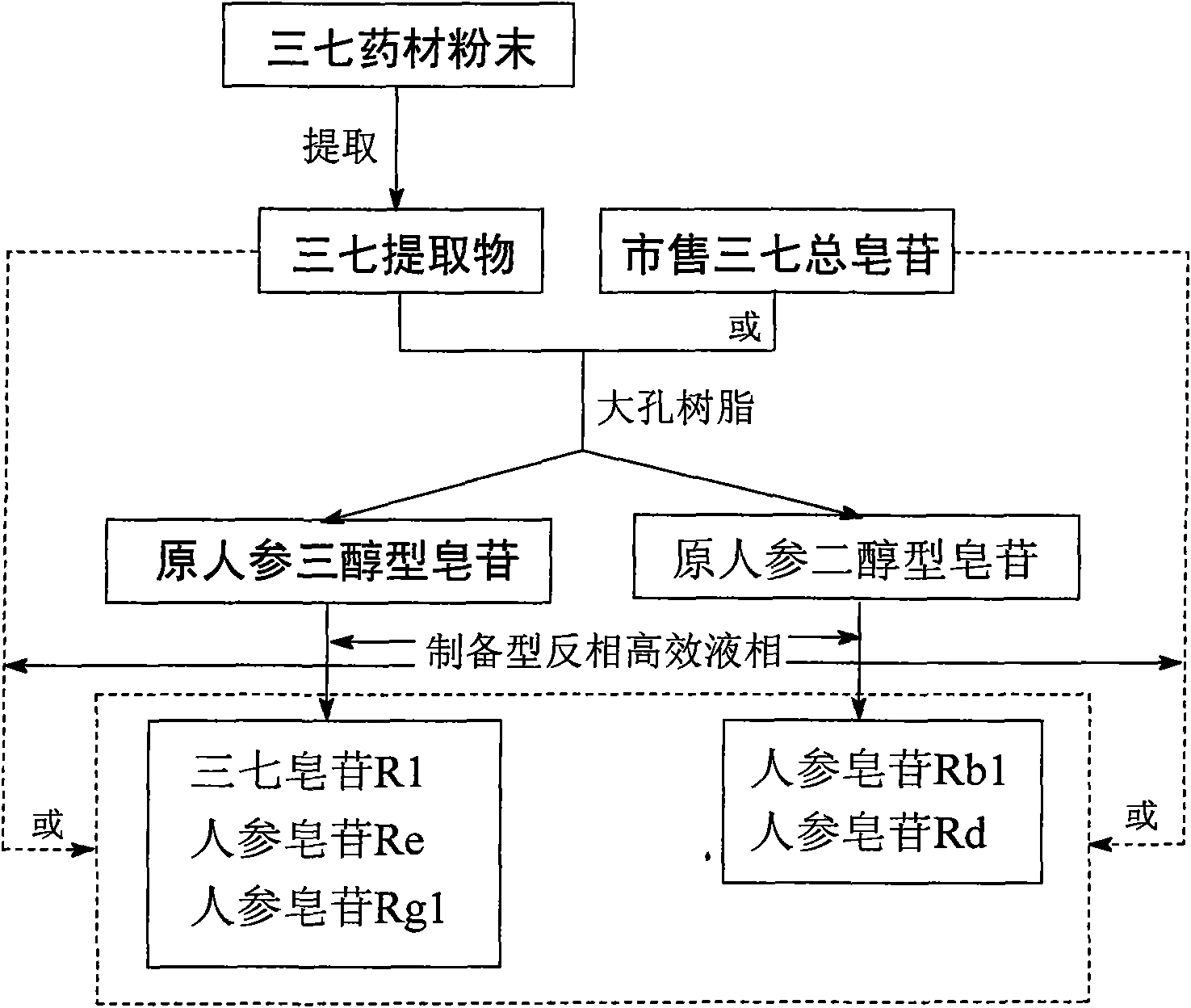
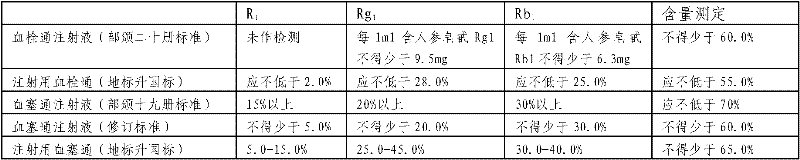
Method for preparing notoginsenoside R1 and ginsenoside Rg1, Re, Rb1 and Rd
InactiveCN101575357ASimple processShorten the production cycleSteroidsIsocratic elutionPanax notoginseng extract
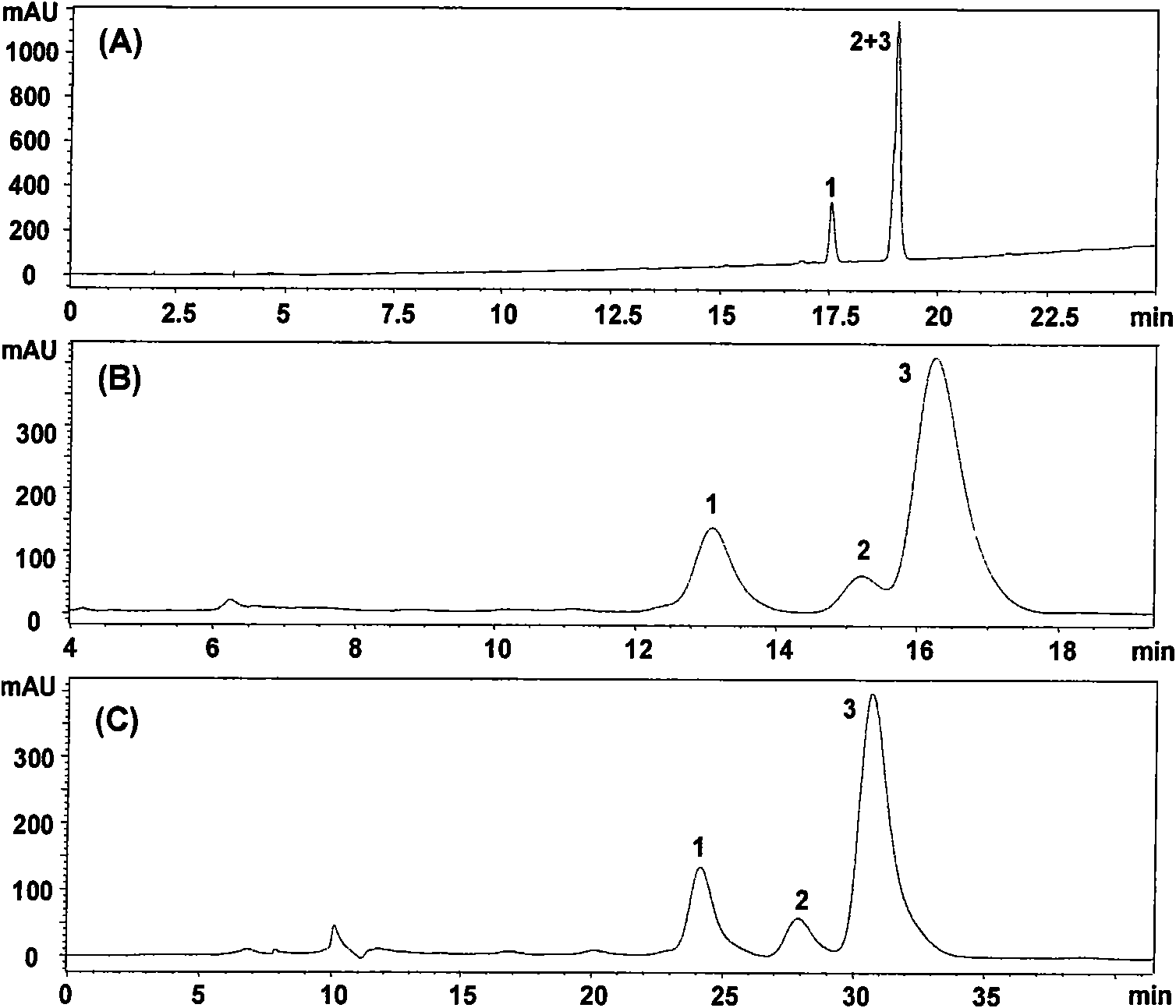
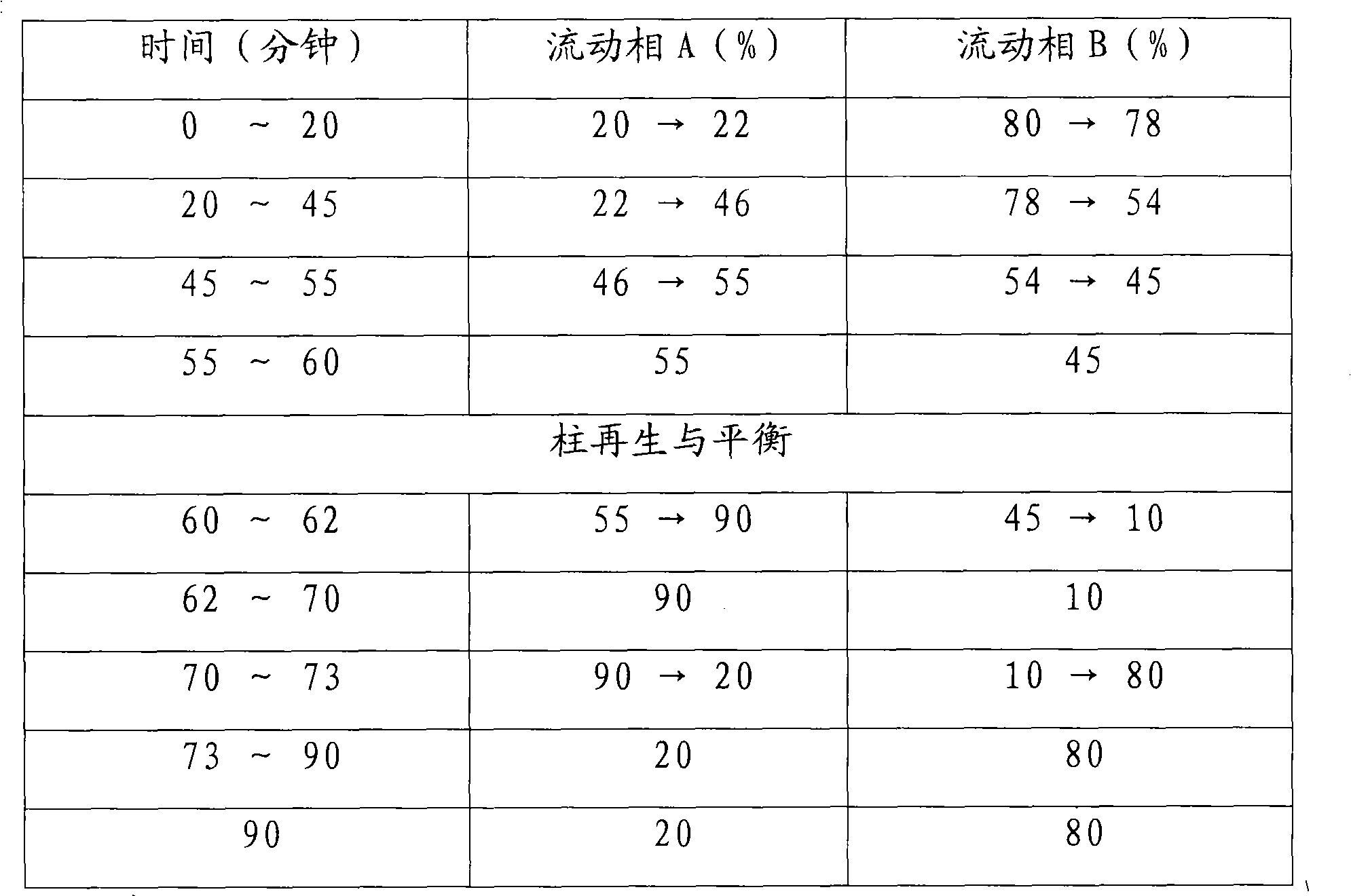
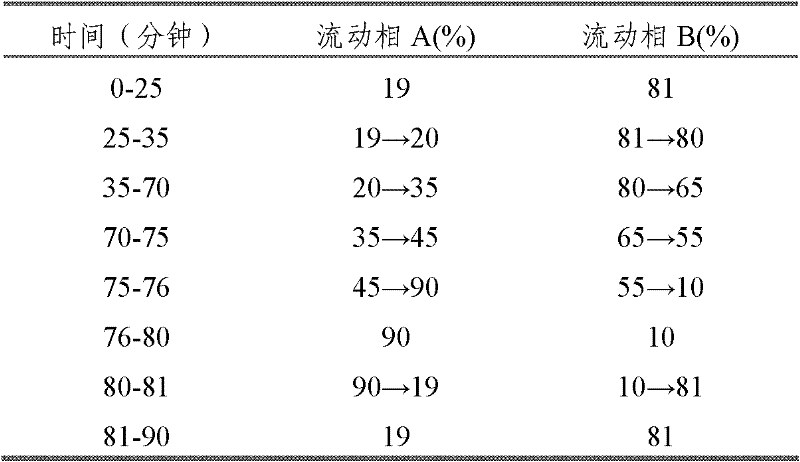
The invention provides a method for simply, conveniently and quickly preparing notoginsenoside R1 and ginsenoside Rg1, Re, Rb1 and / or Rd, which can meet the requirement of industrial production and is environment-friendly. In the method, notoginseng extract and arasaponin or notoginsenoside intermediates are used as original materials, a preparative scale reversed phase high-performance liquid chromatography is adopted, and an ethanol-water system is used as a flow phase to carry out isocratic elution or gradient elution, wherein the ethanol-water system is an ethanol-water solution of 30 percent to 80 percent (V / V). The method has the advantages of simple process, no pollution, low cost and high purity, wherein the purity of products produced by the method is more than 97 percent.
Owner:UNIVERSITY OF MACAU
Radix notoginseng extract and preparation thereof
ActiveCN101732378AHigh purity of ingredientsIncrease concentrationCardiovascular disorderPlant ingredientsGinsenoside RcPanax notoginseng extract
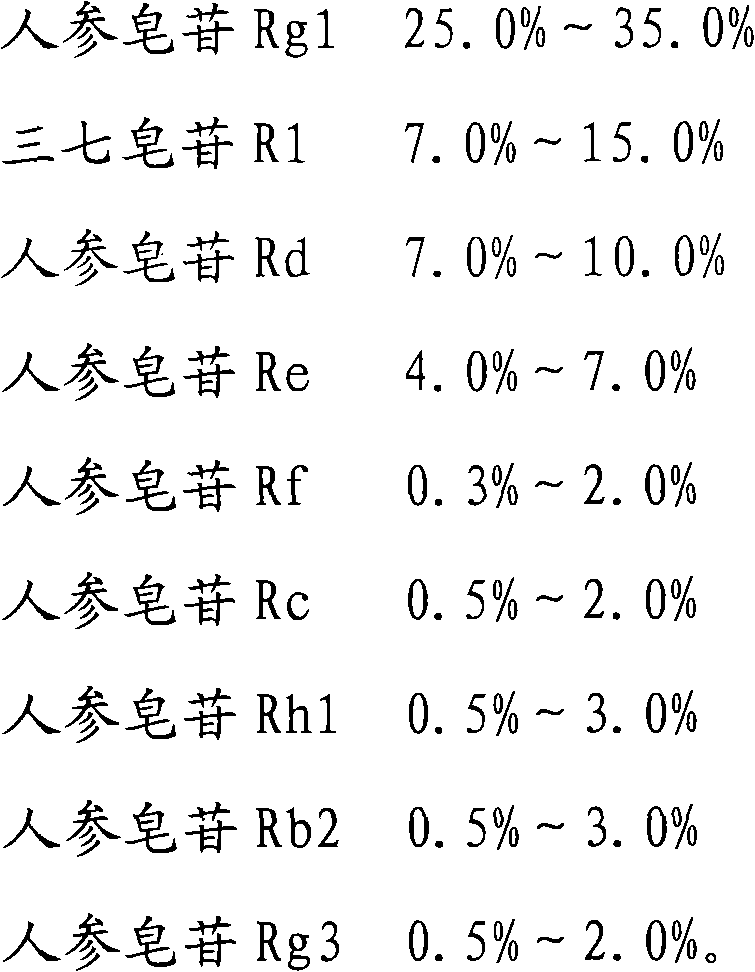
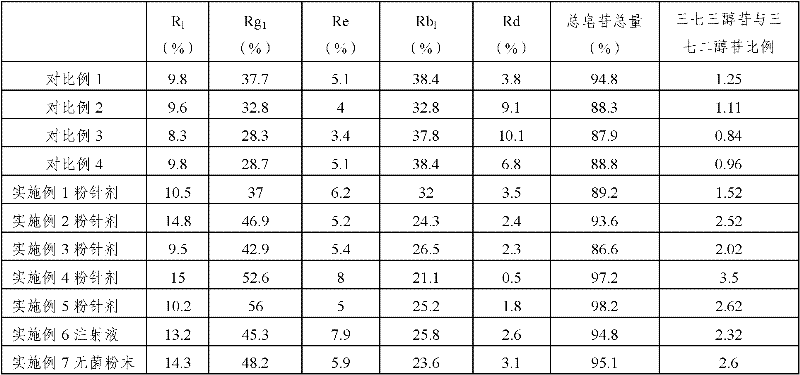
The invention provides a radix notoginseng extract which contains 5-10% of notoginsenoside R1, 25-36% of ginsenoside Rg1, 2.5-5% of ginsenoside Re, 30-39% of ginsenoside Rb1, 5-10% of ginsenoside Rd and at least 2% of ginsenoside Rf, ginsenoside Rh1, ginsenoside Rc, ginsenoside Rb2 and ginsenoside Rg3, wherein the ginsenoside R1, the ginsenoside Rg1, the ginsenoside Re, the ginsenoside Rb1 and the ginsenoside Rd account for 75-95% of the total weight. The invention also provides a preparation method of the radix notoginseng extract. The radix notoginseng extract prepared by the method has little impurities, the purity of the component of total saponin is higher, and especially, the components of the ginsenoside Rf, the ginsenoside Rh1, the ginsenoside Rc, the ginsenoside Rb2, the ginsenoside Rg3 and the like with very low content are purified. The medicinal preparation prepared by the radix notoginseng extract has better curative effect and higher safety.
Owner:HARBIN ZHENBAO PHARMA
Medicinal composition for preventing and treating cardiovascular diseases and application thereof
InactiveCN102526186ADrop in trustQuality is easy to controlOrganic active ingredientsCardiovascular disorderVascular diseaseCoronary heart disease
The invention discloses a medicinal composition for preventing and treating cardiovascular diseases, belonging to the technical field of Chinese medicines. The medicinal composition is prepared from salvianolic acids, total tanshinone and a notoginsenoside mixture in a certain weight ratio, wherein the notoginsenoside mixture is prepared by mixing ginsenoside Rb1, ginsenoside Rg1 and notoginsenoside R1; and the notoginsenoside mixture can be replaced by an equivalent amount of arasaponin. The medicinal composition has remarkable treatment effects on a coronary heart disease, angina and myocardial infarction.
Owner:GUANGANMEN HOSPITAL CHINA ACAD OF CHINESE MEDICAL SCI
Medicinal preparation for treating nerve-root cervical spondylopathy, and preparation method and quality detection method thereof
InactiveCN104887771AMeet needsVarious dosage formsNervous disorderComponent separationClinical efficacyCervical spondylopathy
The invention relates to a medicinal preparation for treating nerve-root cervical spondylopathy, and a preparation method and a quality detection method thereof. The invention is an extension of an original invention. The medicinal preparation comprises a granule, a tablet and a capsule, the preparation method is an optimized and screened production technology based on the original invention, a modern new device, a new process and a new technique are adopted to realize industrial production; and quality standard researches are completed and improved on the basis of original standards, HPLC is adopted to simultaneously determine the content of ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 in a finished product, and thin layer discrimination of all medicines is carried out to comprehensively control the quality, so the clinic curative effects are guaranteed.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Medicine composition of Panax notoginseng saponins
InactiveCN101390887AImprove protectionInhibit aggregationOrganic active ingredientsMetabolism disorderPANAX NOTOGINSENG ROOTTreatment effect
The invention relates to panax notoginseng saponins medicine combination and the application thereof, which are characterized in that the active ingredient is composed of panax notoginseng saponins which contains notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Re, ginsenoside Rd, ginsenoside Rb3 and ginsenoside Rb2; the combination with different contents and ingredients of saponins are prepared; the combinations have different treatment effects.
Owner:HEILONGJIANG ZBD PHARMA
Medicinal composition and preparation method thereof
ActiveCN102028700AIncrease the maximum tolerated doseRaise the median lethal doseOrganic active ingredientsPeptide/protein ingredientsHemolysisAcute toxicity testing
The invention provides a medicinal composition, which comprises the following components: ginsenoside Rb1, ginsenoside Rg1, notoginsenoside R1, ginsenoside Rd, ginsenoside Re, ginsenoside Rf, ginsenoside Rc, ginsenoside Rh1, ginsenoside Rb2 and ginsenoside Rg3. The invention also provides a preparation method for the medicinal composition. The medicinal composition has definite components; compared with the total notoginsenoside in the prior art, the medicinal composition has stable quality and good controllability; and results of experiments of acute toxicity, undue toxicity, hemolysis and the like show that the medicinal composition has higher safety and wide clinical application prospect.
Owner:KPC PHARM INC
Extract of panax notoginseng saponins and preparation method thereof
InactiveCN101829170AIncrease cerebral blood flowHigh trafficBlood disorderCardiovascular disorderPANAX NOTOGINSENG ROOTAdjuvant
The invention relates to an extract of panax notoginseng saponins, a preparation method thereof, a preparation prepared from the total saponins and a method for preparing the preparation. The extract comprises panaxtriol saponins (PTS) and panaxadiol saponins (PDS), wherein the PTS mainly comprises notoginsenoside R1 and ginsenoside Rg1; the PDS mainly comprises the ginsenoside Rb1 and the ginsenoside Rd; the extract is characterized in that the weight ratio of the PTS to the PDS is 1:0.5-2; and the total content of the notoginsenoside R1, the ginsenoside Rg1, the ginsenoside Rb1 and the ginsenoside Rd accounts for over 80 weight percent of the extract of the panax notoginseng saponins. The preparation of the extract of the panax notoginseng saponins comprises the therapeutically effective amount of the panax notoginseng saponins and pharmaceutically acceptable adjuvant.
Owner:北京中海康医药科技发展有限公司
Quality detection method for weinai'an tablet
ActiveCN104165962AHigh precisionQuantitatively accurateComponent separationBULK ACTIVE INGREDIENTContent determination
The invention discloses a quality detection method for a weinai'an tablet. The quality detection method adopts one or more of the following identification and content determination items: thin-layer identification of radix astragali; thin-layer identification of radix astragali, red ginseng and pseudo-ginseng; thin-layer identification of atificial cow-bezoar; high performance liquid chromatography qualitative and quantitative identification method of weinai'an tablet; and high performance liquid chromatography quantitative identification method of Astragaloside IV. The method provided by the invention improves and revises the original weinai'an capsule quality standards, revises and enlarges thin-layer identification of radix astragali's four flavonoid components and Astragaloside IV, simultaneously revises and enlarges thin-layer identification of red ginseng, and identifies the index components Astragaloside IV, ginsenoside Rg1, Rb1 and notoginsenoside R1 at the same time. The method employs an HPLC-PDA-ELSD combined technology to establish a main active ingredient content determination and characteristic spectrum to realize effective control of the weinai'an tablet quality. At the same time, the method has the advantages of high efficiency, accurate quantification, good stability, high precision and excellent repeatability.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Method for increasing content of notoginsenoside R1 in panax notoginseng
ActiveCN105009917AEasy to operateLower requirementSugar derivativesFertilising methodsPANAX NOTOGINSENG ROOTSalicylic acid
The invention discloses a method for increasing the content of notoginsenoside R1 in panax notoginseng. Before the panax notoginseng is harvested, a salicylic acid solution is sprayed to panax notoginseng plants. According to the method, due to the fact that the salicylic acid solution is used for processing the panax notoginseng plants before the panax notoginseng is harvested, the content of the notoginsenoside R1 in the panax notoginseng is increased, the method can be used on a large scale in a conventional panax notoginseng cultivating method so that the notoginsenoside R1 can be industrially produced on a large scale, and the requirement of the market for the notoginsenoside R1 is met.
Owner:GUANGXI BOTANICAL GARDEN OF MEDICINAL PLANTS
New use of notoginsenoside R1
InactiveCN103446169AGive full play to medicinal valueImprove the incidence of bloody stoolOrganic active ingredientsDigestive systemTreatment effectUlcerative colitis
The invention discloses a new use of notoginsenoside R1 in the preparation of medicines for preventing and / or treating the inflammatory diseases of the digestive system or healthcare foods as an active component. Experiment results show that the notoginsenoside R1 has an inhibition effect on the NF-kappa B signal pathway, and has substantial prevention and treatment effects on the NF-kappa B signal pathway mediated ulcerative colonitis, so the notoginsenoside R1 can be developed to medicines for preventing and / or treating the inflammatory diseases of the digestive system or healthcare foods. The new use urges the notoginsenoside R1 to fully perform its medicinal values, and is very important for researching and applying Panax Notoginseng.
Owner:SHANGHAI UNIV OF T C M
Method for preparing astragalus Sanxian soup flexible nano-liposome
InactiveCN104188908AUniform particle sizeHigh encapsulation efficiencyOrganic active ingredientsSkeletal disorderCholic acidAstragaloside
The invention discloses a method for preparing astragalus Sanxian soup flexible nano-liposome. The preparation method comprises the following steps: (1) weighing lecithin, cholesterol, astragaloside, icariin and notoginsenoside R1 according to a ratio, adding the raw materials into a container, and adding a methanol-chloroform mixed solvent, so that the raw materials are fully dissolved; (2) arranging the dissolved raw materials on a rotary evaporator, performing reduced pressure spin evaporation to remove an organic solvent in a constant temperature water bath, and performing vacuum drying overnight; (3) preparing a sodium cholate PBS solution, adding ethylene diamine tetramethylidene phosphoric acid into the sodium cholate PBS solution, adding the mixed solution into the dried raw materials, and performing normal pressure spin evaporation in the constant temperature water bath, thereby preparing primary suspension of the liposome; and (4) ultrasonically oscillating the primary suspension of the liposome, and finally sequentially squeezing the primary suspension to pass through microfiltration membranes with the pore diameters of 0.80mu m, 0.45mu m and 0.22mu m to obtain the flexible nano-liposome. The liposome disclosed by the invention has the advantages of uniform particle size, high encapsulation efficiency and high targeting property.
Owner:GUANGDONG MEDICAL UNIV
Traditional Chinese medicine composition of bone healing medicine, preparing method thereof and detecting method thereof
ActiveCN102579734AGood curative effectImprove qualityHeavy metal active ingredientsInorganic boron active ingredientsCurative effectBone healing
The invention provides a traditional Chinese medicine composition of a bone healing medicine, a preparing method thereof and a detecting method thereof. Each preparation unit of the traditional Chinese medicine composition contains pseudo-ginseng which is no less than 2.2mg by total content of notoginsenoside R1, ginsenoside Rg1 and ginsenoside Rb1 and / or Carthamus tinctorius which is no less than 0.03mg by content of hydroxysafflor yellow A, quality is more stable, and curative effect is more exact. According to the detecting method, the content of the notoginsenoside R1, the ginsenoside Rg1, the ginsenoside Rb1 and / or the content of the hydroxysafflor yellow A are detected through detecting methods including a high-performance liquid chromatography method. The method is simple and stable, high in accuracy and good in reproducibility, and capable of representing effective ingredient content of the traditional Chinese medicine composition of the bone healing medicine completely and effectively and benefiting monitoring product quality.
Owner:陕西天地人和药业有限公司
Method for decocting pseudo-ginseng root in gynaecologic revival pill
InactiveCN102429941AImprove clinical efficacyGood curative effectAntipyreticAnalgesicsMedicinal herbsLiver and kidney
The invention provides a method for decocting a pseudo-ginseng root medicinal material in a gynaecologic revival pill. The method comprises the following steps of: pouring carefully-chosen and sliced pseudo-ginseng root into heated hen oil for frying; taking out after frying to the crisp state; filtering oil out; and cooling naturally. In the method, the hen oil is used for decocting the carefully-chosen and sliced pseudo-ginseng root in a frying way. Due to the adoption of the decoction method, the utilization ratios of ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 serving as components of pseudo-ginseng root in the gynaecologic revival pill are increased greatly, and the clinical curative effects of tumor resistance, thrombus resistance, hemostasis, hematopoiesis, pain relieving and the like of the pseudo-ginseng root medicinal material are enhanced remarkably; and after the pseudo-ginseng root medicinal material is applied to the gynaecologic revival pill, the curative effects of nourishing the blood, regulating the menstrual function, tonifying the liver and kidney, warming uterus and relieving pain of the gynaecologic revival pill are enhanced remarkably.
Owner:贵阳德昌祥药业有限公司
Drug composition for preventing and treating related diseases of ischemic stroke as well as preparation method and applications thereof
ActiveCN104147032AClear compositionImprove auricle microcirculationOrganic active ingredientsCardiovascular disorderDiseaseAstragaloside
The invention is suitable for the field of medicines and provides a drug composition for preventing and treating related diseases of ischemic stroke. The drug composition comprises the following components in parts: 1.0-3.5 parts of notoginsenoside R1, 4.0-7.5 parts of ginsenoside Rg1, 3.5-7.5 parts of ginsenoside Rb1, 1.0-3.0 parts of ginsenoside Re, 0.03-0.15 part of astragaloside, 0.02-0.010 part of calycosin-7-O-beta-D-glucopyranoside, 0.01-0.10 part of harpagoside, 0.002-0.010 part of cinnamic acid, 0.45-1.5 parts of salvianolic acid B, 0.02-0.10 part of cryptotanshinone and 0.02-0.10 part of tanshinone IIA. The drug composition provided by the invention has the functions of promoting blood circulation and removing blood stasis, supplementing qi and nourishing yin, can be used for preventing and treating the related diseases of the ischemic stroke and is capable of overcoming the shortcomings of component complexity and the like of traditional compound traditional Chinese medicine preparations.
Owner:GUANGDONG ZHONGSHENG PHARMA
Panax notoginseng saponin composition and preparation method and application thereof
ActiveCN105816471AClear validityClear contentOrganic active ingredientsBlood disorderGinsenoside RdLow toxicity
The invention provides a panax notoginseng saponin composition and a preparation method and application thereof.The composition is prepared from, by weight, 30-50% of ginsenoside Rg1, 25-40% of ginsenoside Rb1, 7-16% of notoginsenoside R1, 2.7-8% of ginsenoside Re, 0.5-7.0% of ginsenoside Rd, 0.5-2% of ginsenoside Rf, 0.3-2.0% of ginsenoside Rh2, 1-3% of ginsenoside Rc, 0.3-2.5% of ginsenoside Rb3 and 0.5-2.5% of ginsenoside Rg3, wherein ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 account for 70-95% of the total weight of the active components, and the sum of the contents of ginsenoside Rb1 and notoginsenoside R1 is not smaller than 8 times of the content of ginsenoside Rd.Compared with commercially available Xueshuantong injections and Xueshuantong preparations, the panax notoginseng saponin composition has a more obvious arterial and venous thrombosis resistant effect, low toxicity and high safety.
Owner:HARBIN ZHENBAO PHARMA
Compound Chinese medicine extract preventing arteriosclerosis and preparation method thereof
ActiveCN102133222AReservedHigh content of active ingredientsAnhydride/acid/halide active ingredientsCardiovascular disorderSalvianolic acid BAklanonic acid
The invention discloses a compound Chinese medicine extract preventing arteriosclerosis, comprising the following effective ingredients: dried alcohol, Beta-sitosterol, hexacosanoic acid, butenolide III, oleanolic acid, berberine, jateorhizine, coptisine, salvianic acid A, salvianolic acid B, ring-tetracosane, 9,12-octadecadienoic acid, 5,7-dimethoxy coumarin, specnuezhenide, ginsenoside Rb1 and Rg1, notoginsenoside R1, encommiol and the like. A preparation method of the extract is as follows: taking salvia miltiorrhiza, fructus ligustri lucidi, rhizoma coptidis, cirsium japonicum, eucommia bark, atractylodes macrocephala koidz, radix pseudo-ginseng and bergamot as raw materials, conducting C1-3 alcohol extraction and / or water extraction on a total extract, then extracting the total extract by organic solvents with different polarities so as to obtain all the effective ingredients, and finally mixing the effective ingredients so as to obtain the compound Chinese medicine extract preventing arteriosclerosis. As for the compound Chinese medicine extract, a large number of ineffective chemical ingredients are removed, so that the content of effective ingredients is improved greatly, the influence on product processing and preparation quality caused by the ineffective ingredients is reduced, the preparation process is stable, the product quality is controllable, and the mass production is facilitated.
Owner:QINGDAO BAILI CAIXIN MEDICAL TECH CO LTD
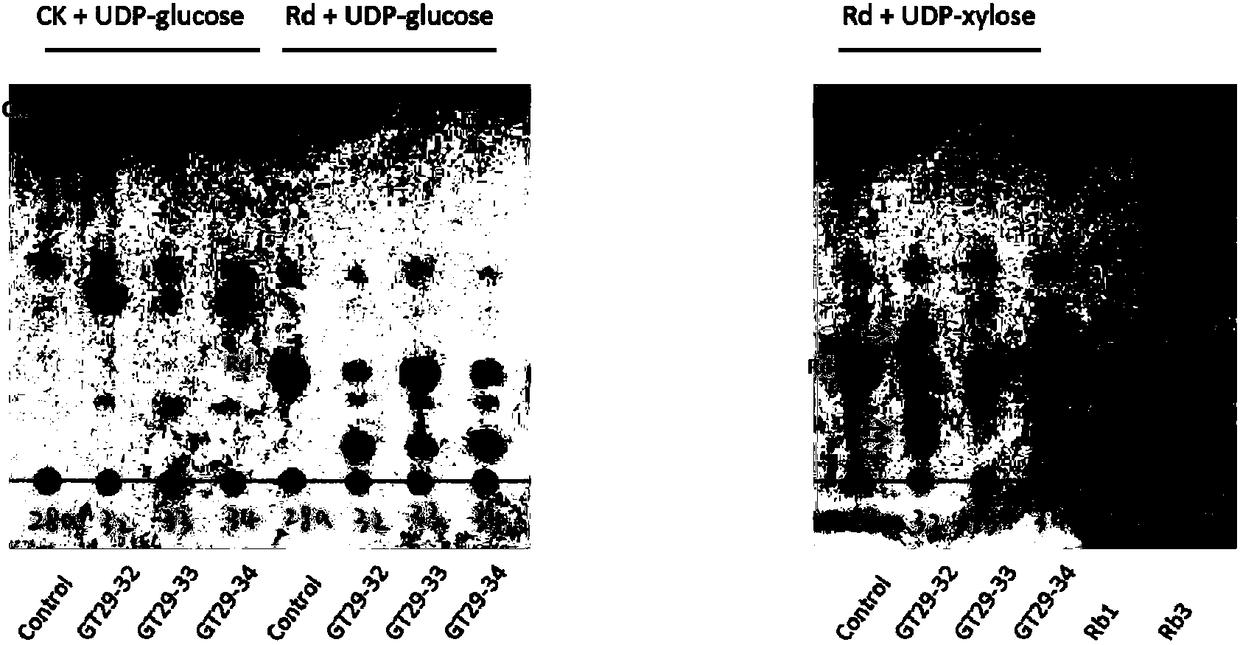
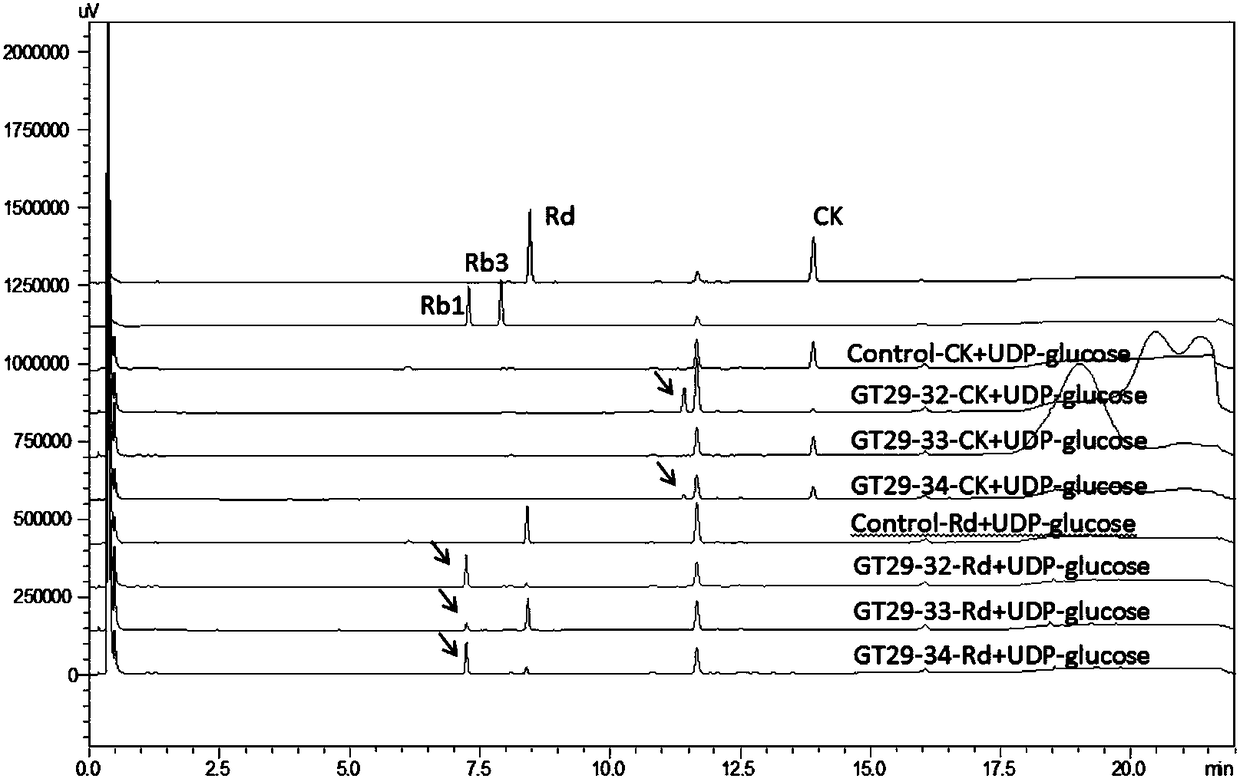
UDP-glycosyl transferases capable of catalyzing carbohydrate chain extension, and applications thereof
InactiveCN108949711AHas neuroprotective propertiesHas anti-inflammatory propertiesTransferasesFermentationTriterpeneTriterpenoid
The invention relates to a set of UDP-glycosyl transferases capable of catalyzing carbohydrate chain extension, and applications thereof, and more specifically provides a catalytic reaction used for obtaining ginsenoside products including ginsenoside Rb1, ginsenoside Rb3, gypenoside LXXV, gypenoside XVII, notoginsenoside U and, notoginsenoside R1, and notoginsenoside R2 through catalyzing of C-20site 1th glycosyl and C-6 site 1th glycosyl of tetracyclic triterpene substances with glycosyl transferases GT29-32, GT29-33, GT29-34, GT29-4, GT29-5, GT29-7, GT29-9, GT29-11, GT29-13, GT29-17, GT29-18, GT29-24, and GT29-25, and derived peptides thereof through carbohydrate chain extension. The glycosyl transferases can be used for construction of artificially synthesized ginsenosides and a plurality of novel ginsenosides and derivatives of ginsenosides.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
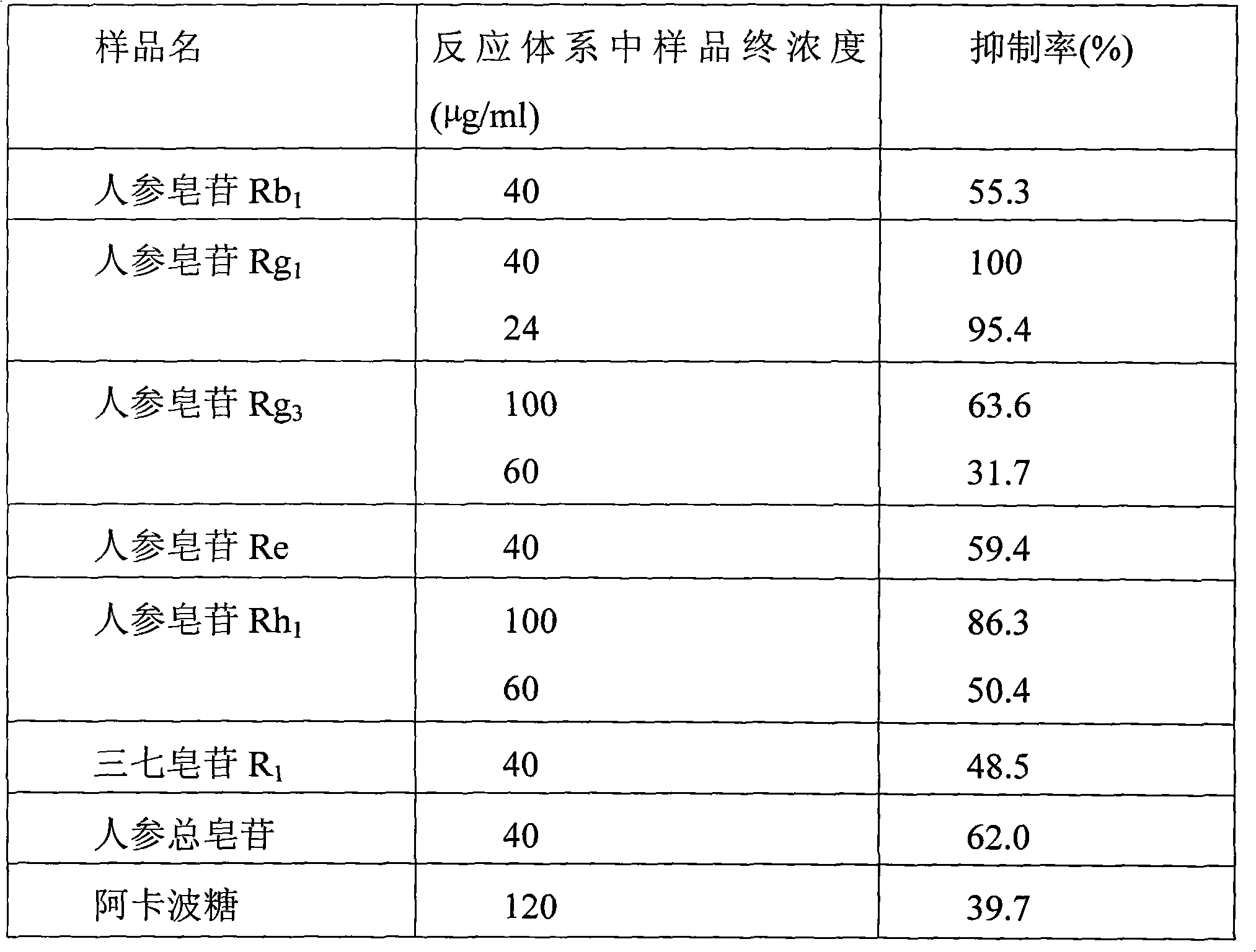
Application of ginsenoside components to preparation of medicaments for inhibiting activity of alpha-glucosidase
InactiveCN101991627AEnhanced inhibitory effectOrganic active ingredientsMetabolism disorderAdditive ingredientDiabrezide
The invention belongs to the technical field of application of traditional Chinese medicines, relates to application of ginsenoside components to the preparation of medicaments for preventing and treating diabetes, in particular to application of the ginsenoside components to the preparation of medicaments for inhibiting the activity of alpha-glucosidase. The ginsenoside components at least comprise total ginsenosides, total saponins of American ginseng, total saponins of notoginseng root, ginsenoside Rb1, ginsenoside Rg1, ginsenoside Rg3, ginsenoside Re, ginsenoside Rh1 and notoginsenoside R1. Experiments prove that the ginsenoside components have obvious effect of inhibiting the activity of the alpha-glucosidase.
Owner:上海新康制药厂有限公司 +1
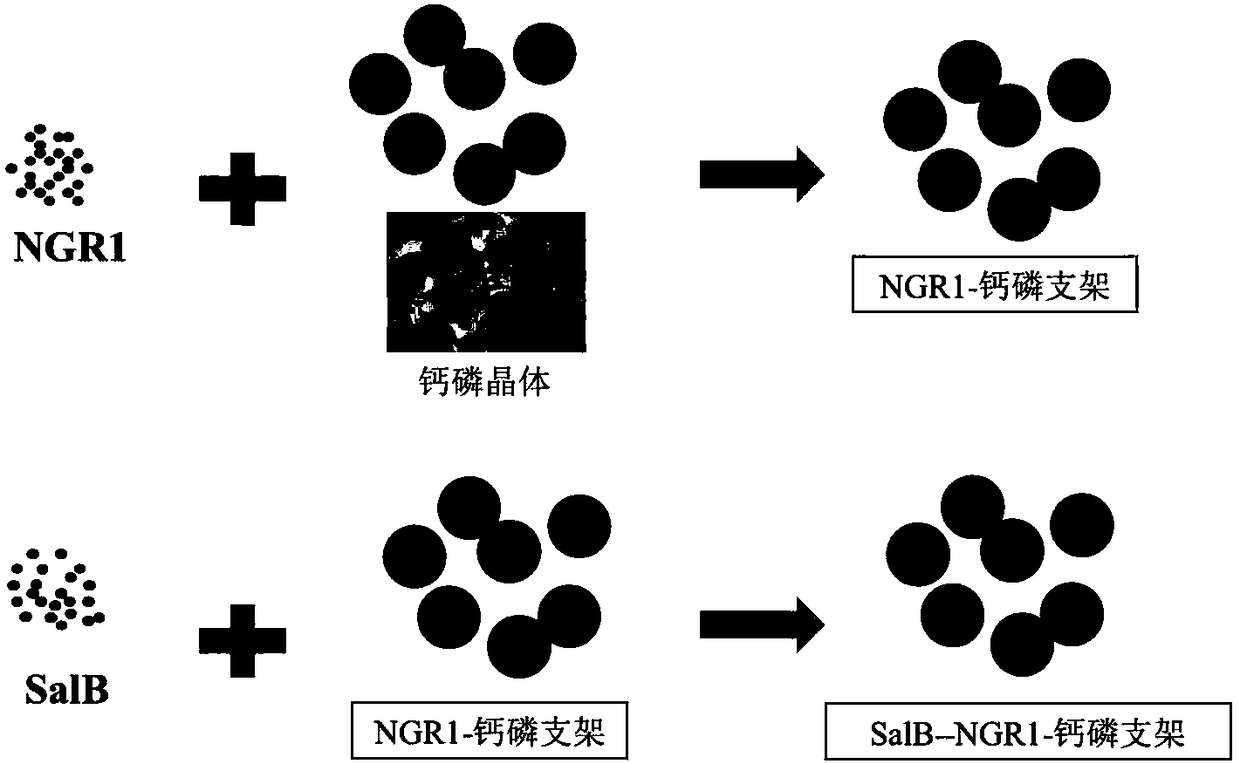
Preparation method of traditional Chinese medicine monomer sequence slow-released calcium-phosphorus support material for osteogenesis and angiogenesis
The invention relates to a preparation method of a traditional Chinese medicine monomer sequence slow-released calcium-phosphorus support material for osteogenesis and angiogenesis. The method comprises the steps of 1, preparing calcium-phosphorus crystal supports; 2, slowly releasing an osteogenesis coating; 3, slowly releasing an angiogenesis coating; 4, repeating functional particles for 2-5 cycles and conducting crystallization and freeze-drying for use, wherein the operation is carried out in a sterile environment, and the calcium-phosphorus supports in the desired size and shape are prepared according to different clinical needs. The method has the advantages that a low-temperature micro-nano-deposition technique is used for sequentially and circularly carrying traditional Chinese medicine monomer components including salvianolic acid B and notoginsenoside R1 to construct the composite biomimetic calcium-phosphorus support material capable of promoting osteogenesis and angiogenesis at the same time. According to the low-temperature micro-nano-deposition technology, a biomimetic coating is prepared, calcium-phosphorus micro-nano crystals are formed in a buffer system, and thecrystals can be condensed into the supports with certain strength at normal temperature.
Owner:HANGZHOU HUIBO SCI & TECH CO LTD
Medicinal composition for treating Parkinson disease
InactiveCN102000096ARaw materials are easy to getImprove securityOrganic active ingredientsNervous disorderApoptosisDisease injury
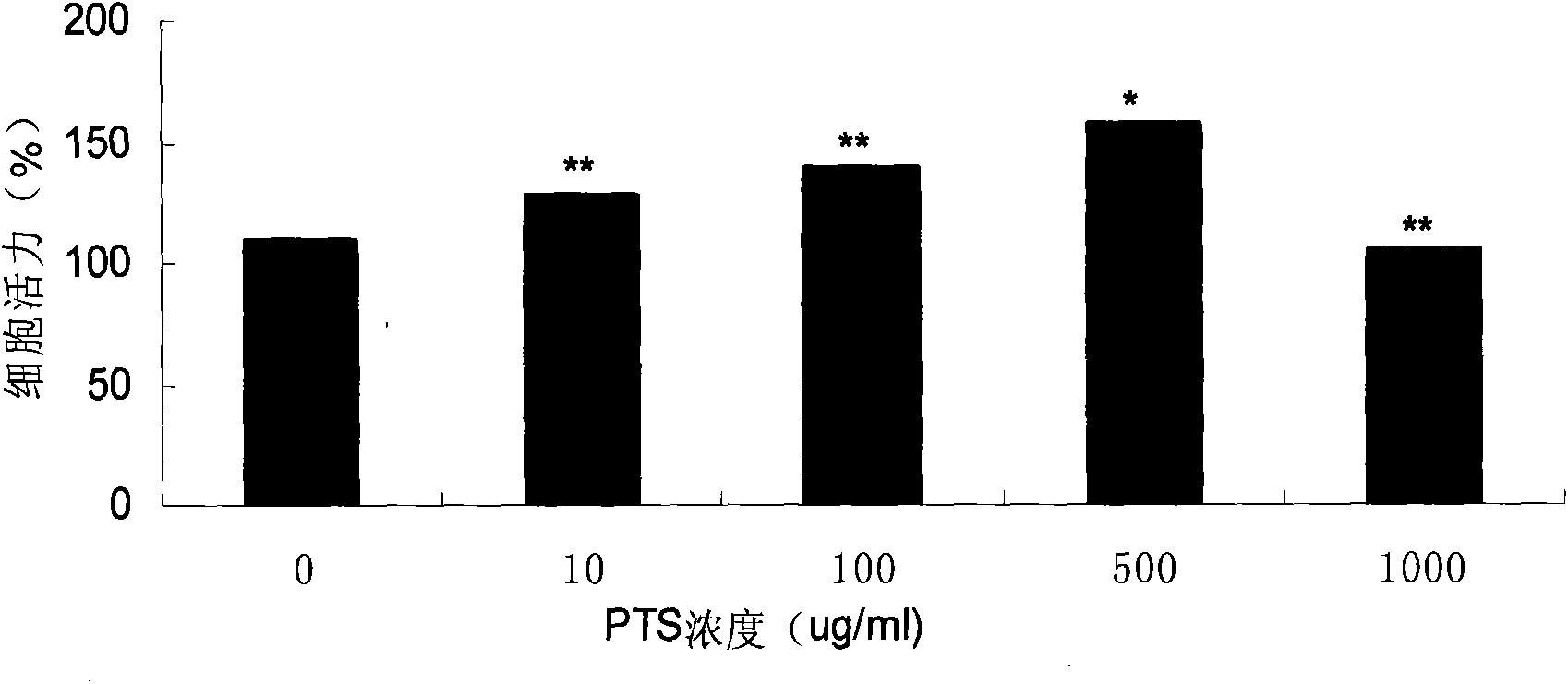
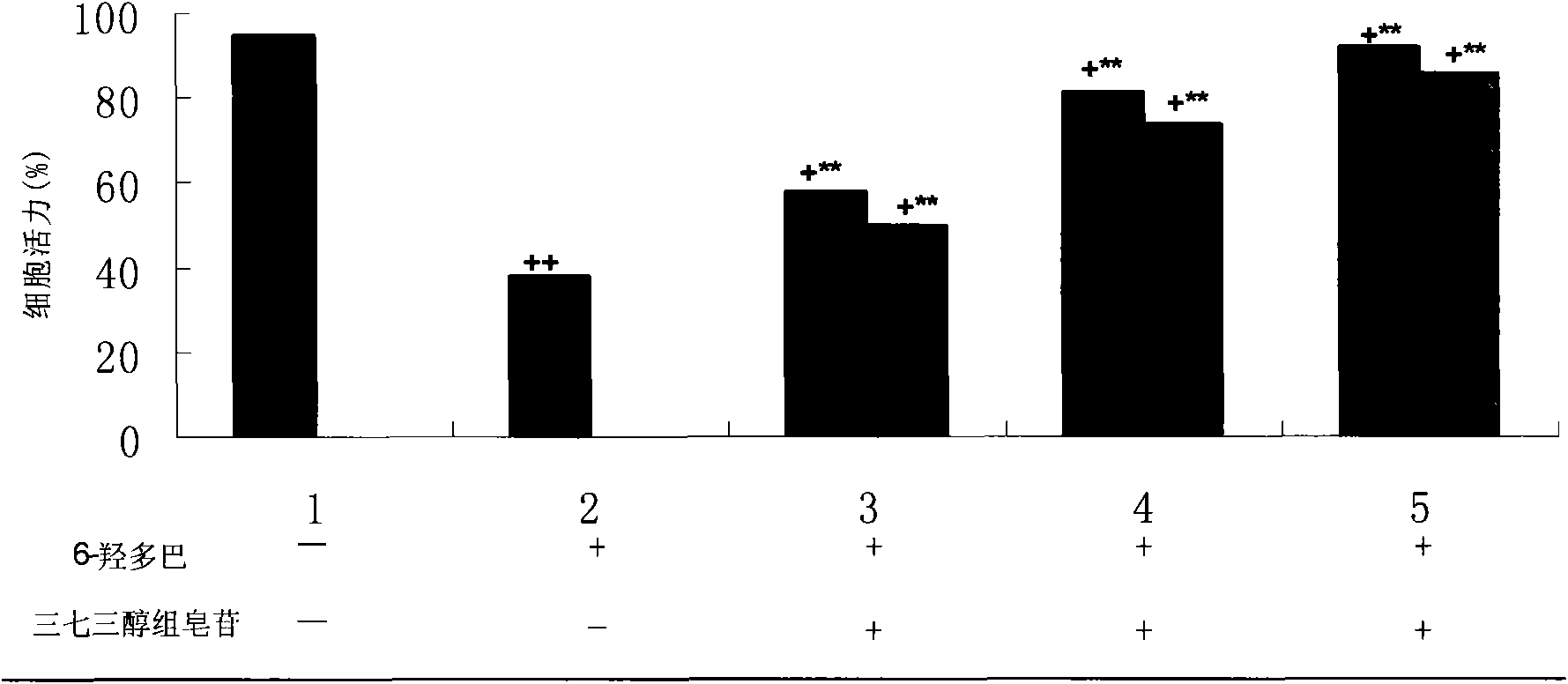
The invention relates to the field of medicines, and discloses a medicinal composition for treating the Parkinson disease. The medicinal composition comprises the active component of panaxtrial saponins. Preferably, the panaxtrial saponins comprises 60-80wt% of ginsenoside Rg1, 5-15wt% of ginsenoside Re, 10-25wt% of notoginsenoside R1 in content. The panaxtrial saponins has effects on inhibiting SH-SY5Y cell viability loss caused by 6-hydroxydopamine in a dosage dependency mode and inhibiting PC12 cell apoptosis induced by MPP+, and has an obvious effect on inhibiting SH-SY5Y cell viability loss caused by 6-OHDA. Animal experiments show that the dopamine content is reduced in the corpus striatum of a Parkinson mouse model by the inhibition effect of the panaxtrial saponins in a dosage dependency mode. The results show that the panaxtrial saponins have a new application in preparing the medicine for treating the Parkinson disease and have a favorable clinical application prospect.
Owner:KPC PHARM INC
Pharmaceutical composition for improving immunity
InactiveCN101773548AImprove bioavailabilityImprove the immunityPill deliveryCapsule deliveryPANAX NOTOGINSENG ROOTMedicine
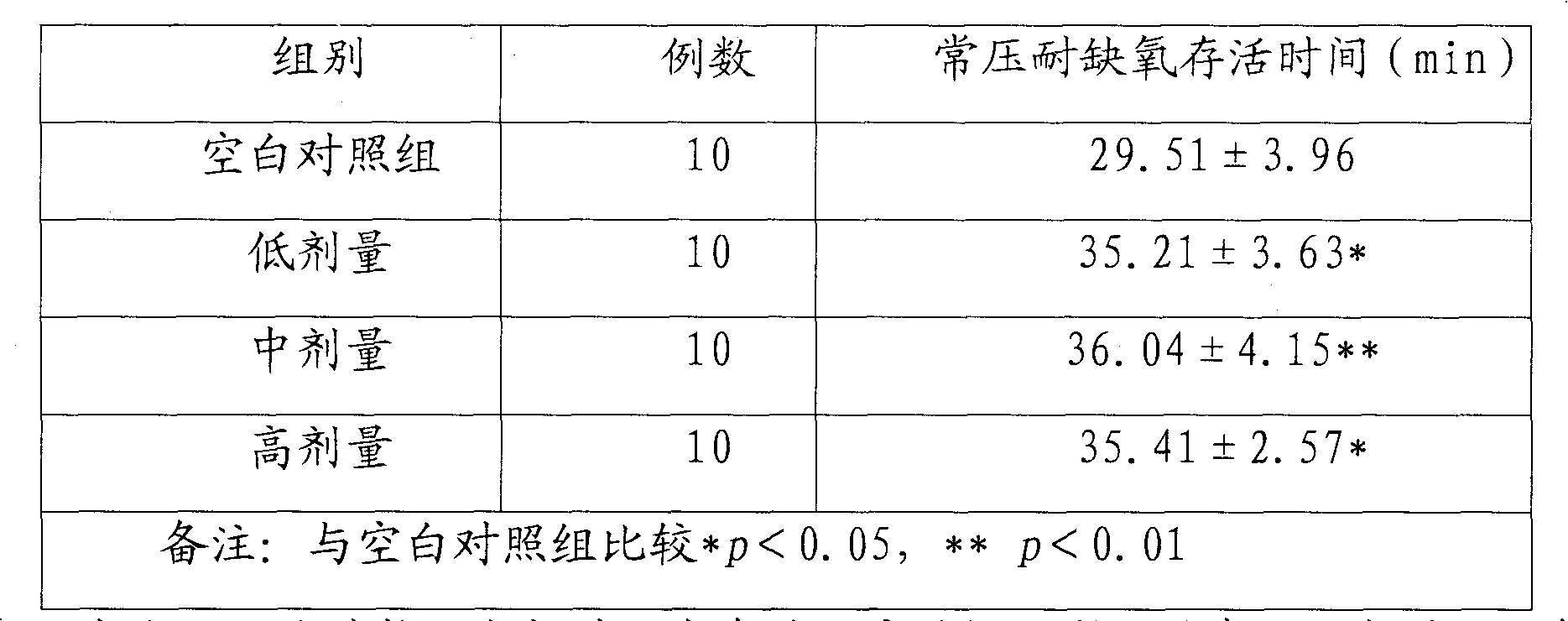
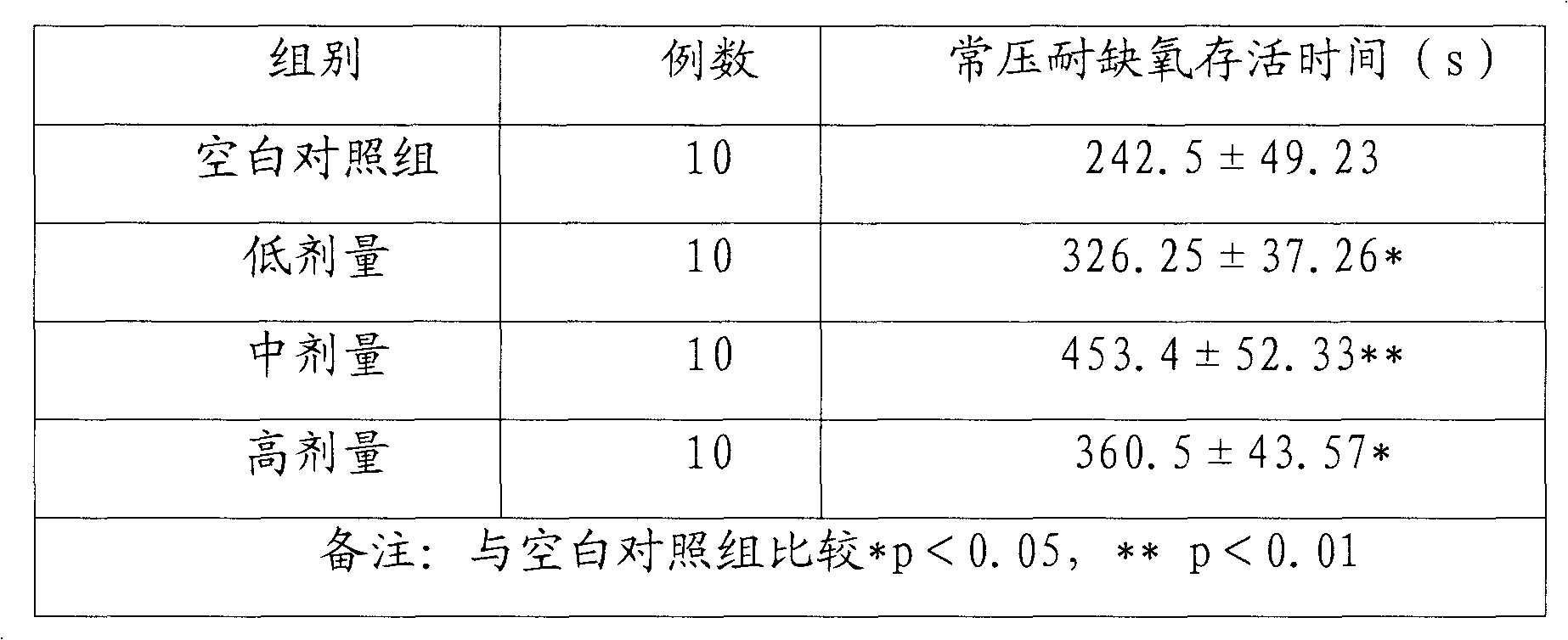
The invention relates to the medical field, and discloses a pharmaceutical composition for improving immunity, comprising: 0.1-60wt percent of star anise oil and 0.5-10wt percent of panax notoginseng saponins, wherein the panax notoginseng saponins comprises the components by weight percent: 5-40 percent of ginsenoside diol Rb1, 20-75 percent of ginsenoside triol Rg1 and 5-8 percent of notoginsenoside R1. The invention also provides various formulations of the pharmaceutical composition. The mouse oxygen-poor-resistance and anti-fatigue capability experiment proves that a tested mouse taking the pharmaceutical composition survives obviously longer than that of a compared group under the oxygen-poor environment and in the experiment of swimming with a load, so that the pharmaceutical composition has remarkable enhancing oxygen-poor-resistance and anti-fatigue capability, higher development and utilization values as well as market application prospect.
Owner:KUNMING PHARMA GRP JINTAIDE PHARMA
Compound traditional Chinese medicine extract preventing glucose metabolism disturbance and preparation method thereof
ActiveCN102133221AHigh content of active ingredientsActive ingredients are clearHydroxy compound active ingredientsMetabolism disorderBiotechnologySalvianolic acid B
The invention discloses a compound traditional Chinese medicine extract preventing glucose metabolism disturbance, comprising the following effective ingredients: dried alcohol, Beta-sitosterol, hexacosanoic acid, butenolide III, oleanolic acid, berberine, jateorhizine, coptisine, salvianic acid A, salvianolic acid B, ring-tetracosane, 9,12-octadecadienoic acid, 5,7-dimethoxy coumarin, specnuezhenide, ginsenoside Rb1 and Rg1, notoginsenoside R1 and encommiol. A preparation method of the extract is as follows: extracting raw material medicines by C1-3 alcohol and / or water, combining a total extract, extracting the total extract by organic solvents with different polarities so as to obtain all effective ingredients, and finally mixing the ingredients so as to obtain a product. As for the compound traditional Chinese medicine extract, a large number of ineffective chemical ingredients in Chinese medicine are removed, so that the content of effective ingredients is increased greatly, and the influence on product processing and preparation quality caused by the ineffective ingredients is reduced; and simultaneously, the preparation process is stable, the product quality is controllable, the mass production is facilitated, and the drug effect of the compound traditional Chinese medicine is improved.
Owner:青岛百里才鑫医药科技有限公司
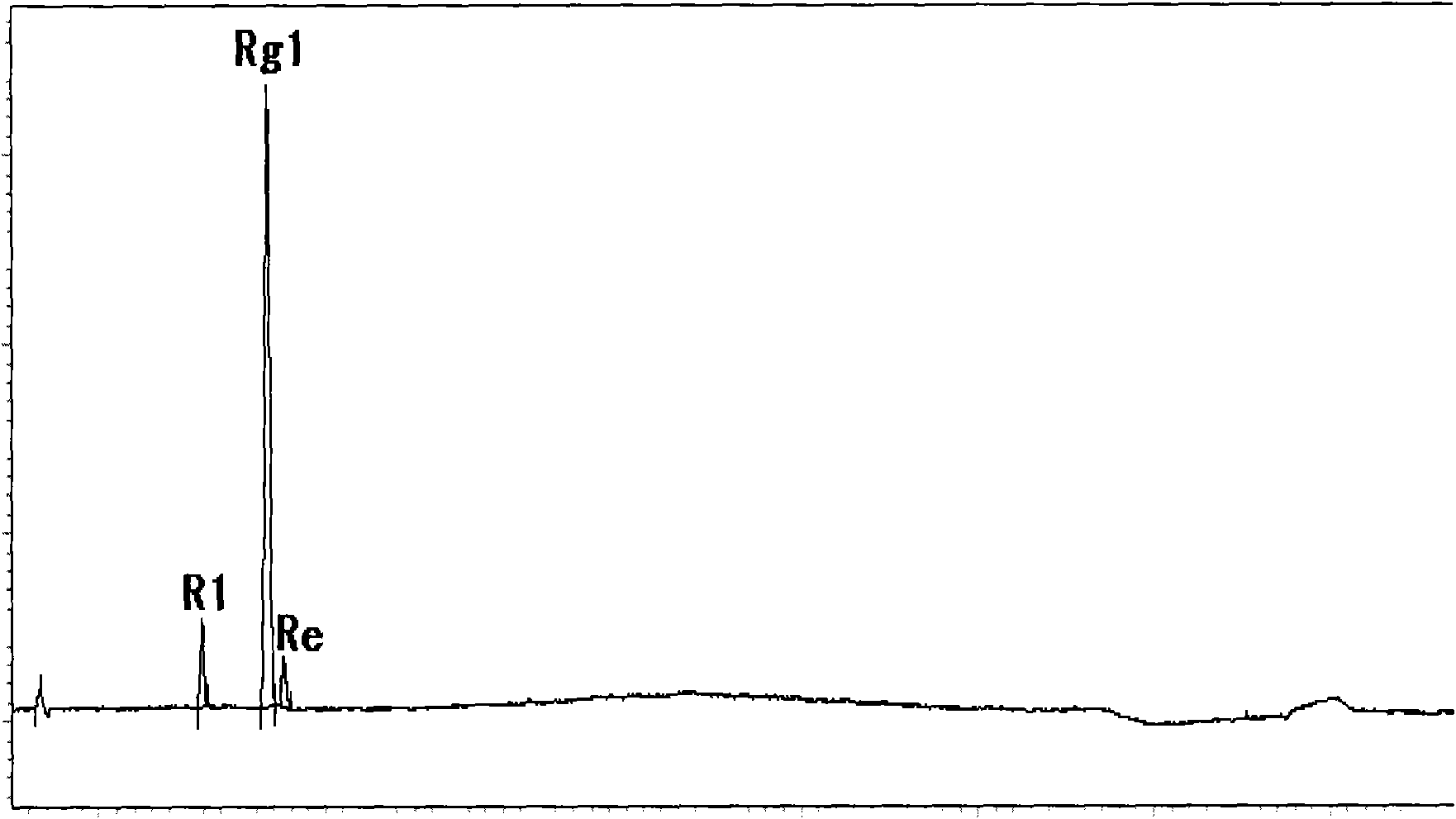
Panaxtriol saponin extract and preparation process thereof
InactiveCN101966218AIncrease contentHigh purityCardiovascular disorderPlant ingredientsCurative effectTriol
The invention discloses a panaxtriol saponin extract and a preparation process thereof. An extract Rg1 (C42H72O14) accounts for 40 to 70 percent of a total extract, notoginsenoside R1 (C47H80O18) accounts for 10 to 30 percent of the total extract, and ginsenoside Re (C48H82O18) accounts for 5 to 10 percent of the total extract. The preparation method of the extract comprises the following steps of: percolating raw materials through ethanol, concentrating percolate for centrifuging, processing supernate through macroporous weak polar resin, collecting 30 to 40 percent ethanol eluent, concentrating the ethanol eluent and processing the concentrated ethanol eluent through decolorizing resin to obtain the notoginsenoside triol extract. The extract of the invention is high in purity, good in curative effect and stable in quality, and simultaneously, the preparation method is excellent in separation effect, high in content, simple in process, low in cost and convenient to operate and is suitable for industrial production.
Owner:TIANJIN TASLY PHARMA CO LTD
Detection method of traditional Chinese preparation for treating stomach illness
InactiveCN105974025AStrong specificityGood reproducibilityComponent separationMedicineQuality control
The invention relates to a detection method of a traditional Chinese preparation for treating a stomach illness. The detection method of the traditional Chinese preparation comprises qualitative identification and content determination conducted on a monarch drug pseudo-ginseng, namely,pseudo-ginseng identification by adopting a thin-layer chromatography and content determination of notoginsenoside R1, ginsenoside Rg1 and ginsenoside Rb1 in the pseudo-ginseng by adopting high efficiency liquid chromatography. Compared with an existing quality control method, the quality control method has the advantages of being accurate and good in reproducibility. By adopting the method, the related medicine quality can be accurately controlled, the medicine usage risk can be reduced, and the product quality can be improved.
Owner:JIANMIN PHARMA GRP CO LTD
Compound injection formulation compose dof borneol and pseudo-ginseng total saponin and its preparing method
The present invention relates to a Chinese medicine compound injection preparation for curing angiocardiopathy and serebrovascular disease and its preparation method. Said compound preparation is made up by using Chinese medicinal material (synthetic or natural) borneol and notoginseng total saponin raw material (containing ginsenoside Rg1 and Rb1 and notoginsenoside R1 total content is 50-90%) according to weight mixing ratio of 1:5-20, and can be made into its compound injection (containing liquid injection, powder injection and infusion fluid). Said Chinese medicine compound injection preparation can be used for curing cerebral infarction, cerebral ischemia, coronary heart disease, angina pectoris and cardiac infraction, etc.
Owner:JIANGSU CAREFREE PHARM CO LTD
Efficient panax notoginseng saponins injection and preparation method thereof
InactiveCN102335214ARaise the ratioGood effectOrganic active ingredientsPowder deliveryDiseasePANAX NOTOGINSENG ROOT
The invention discloses an efficient panax notoginseng saponins injection and a preparation method thereof. The injection comprises active ingredients in percentage by weight: 9.5-15.0% of notoginsenoside R1, 37.0-56.0% of ginsenoside Rg1, 5.0-8.0% of ginsenoside Re, 21.0-32.0% of ginsenoside Rb1 and no less than 0.45% of ginsenoside Rd; and a weight ratio of panaxtriol saponins to panaxadiol saponins is 1.5-3.5:1. By verifying through a pharmacodynamic experiment, the efficient panax notoginseng saponins injection, according with the ratio of the panaxtriol saponins to the panaxadiol saponins in the invention, has good effects in the treatment of cardio-cerebrovascular diseases, such as obstruction of collaterals by blood stasis, apoplectic hemiplegia, chest stuffiness and pains, central retinal vein occlusion and the like.
Owner:GUANGXI WUZHOU PHARMA GRP
Method for measuring content of multiple active ingredients in xiaoshuan tongluo tablets
InactiveCN105259264AThe method is simple and feasibleAccurate and reliable measurement resultsComponent separationAstragalosideInjection volume
The invention belongs to the field of traditional Chinese medicine and particularly relates to a method for measuring the content of main active ingredients in xiaoshuan tongluo tablets. According to the measurement method, the content of ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 in the xiaoshuan tongluo tablets can be measured simultaneously with an HPLC (high performance liquid chromatography) method, a reference substance mixed solution I and a test solution I are injected into a liquid chromatograph respectively according to chromatographic conditions, the injection volume of the reference substance mixed solution I is 10 mu L, the injection volume of the test solution I is 5 mu L, the chromatographic peak area is recorded, and the content is calculated with an external standard method. Meanwhile, according to the measurement method, the content of astragaloside is measured with an ELSD (evaporative light-scattering detector) method, a reference substance mixed solution II and a test solution II are injected into the liquid chromatograph respectively according to chromatographic conditions, the injection volumes of the reference substance mixed solution II are 5 mu L and 10 mu L respectively, the injection volume of the test solution II is 10-20 mu L, the chromatographic peak area is recorded, and the content is calculated according to a two-point external standard method logarithmic equation.
Owner:HARBIN KANGLONG PHARM CO LTD
Oral spray used for daily oral health maintenance and preparation method of oral spray
ActiveCN108635378AGrowth inhibitionGood removal effectHydroxy compound active ingredientsAntipyreticPropolisFusobacterium nucleatum
The invention provides an oral spray used for daily oral health maintenance and a preparation method of the oral spray. The oral spray is prepared from the following raw materials: epigallocatechin gallate, procyanidine, erythritol, propolis extract, notoginsenoside R1, menthol, lactitol, alcohol and sterile water. The oral spray prepared by adopting the method provided by the invention has the clearing and the obvious inhibiting effects for various main pathogenic bacteria of the oral cavity, including fusobacterium nucleatum, porphyromonas gingivalis and the like, has the obvious inflammation-diminishing efficacy for various inflammations of the oral cavity, is beneficial for the wound healing of the oral cavity, and can reduce gum bleeding and the like.
Owner:SHANDONG UNIV
High-performance liquid phase detection method for heart-calming granules
ActiveCN105548425AIncrease contentComprehensive quality control indicatorsComponent separationGinsenoside RdGinsenoside Rb1
The invention provides a high-performance liquid phase detection method for heart-calming granules. The method comprises the step of adopting a high-performance liquid phase method for measuring the content of notoginsenoside R1, the content of ginsenoside Rg1 and the content of ginsenoside Rb1, and the step of adopting the high-performance liquid phase method for measuring the content of ginsenoside Rd and the content of lobetyolin. On the basis of utilizing the high-performance liquid phase method for measuring the content of notoginsenoside R1, the content of ginsenoside Rg1 and the content of ginsenoside Rb1, the high-performance liquid phase method is additionally adopted for measuring the content of ginsenoside Rd and the content of lobetyolin, and therefore the quality control index for the heart-calming granules is more comprehensive, and the detection method is good in precision, stability and repeatability.
Owner:SHANDONG BUCHANG PHARMA +1
Method of improving identification for pseudo-ginseng medicinal material based on thin-layer chromatography
The invention provides a method of improving identification for a pseudo-ginseng medicinal material based on a thin-layer chromatography. The method comprises the following steps that step 1), 0.5 g of medicinal material powder is taken, weighed out accurately, and placed in a conical flask with a plug, 20 mL of n-butyl alcohol saturated by water is added, after close plugging, ultrasonic processing and filtering, 40 mL of water saturated by n-butyl alcohol is added into filtered liquid, and the mixture is shaken uniformly and subjected to layering; n-butyl alcohol liquid is taken and evaporated to be dry, 1 mL of methyl alcohol is added for dissolving residues, and the mixture is taken as a test solution; step 2), a ginsenoside Rg1 reference product, a ginsenoside Re reference product, aginsenoside Rb1 reference product and a notoginsenoside R1 reference product are taken, methyl alcohol is added, and a reference product solution is obtained; step 3), according to the thin-layer chromatography method experiment, 1 microliter of the test solution and 1 microliter of the reference product solution are sucked and respectively dotted on the same silica gel G thin layer plate, dichloromethane-absolute ethyl alcohol-water-glacial acetic acid is taken as a developing agent, and the solutions are developed, taken out and dried; step 4), a color developing agent is sprayed, and heating is conducted until the developed color of spots is clear.
Owner:ZHEJIANG JOLLY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com