Quality detection method for weinai'an tablet
A quality detection method and detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of incomplete quality detection and backward quality detection standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
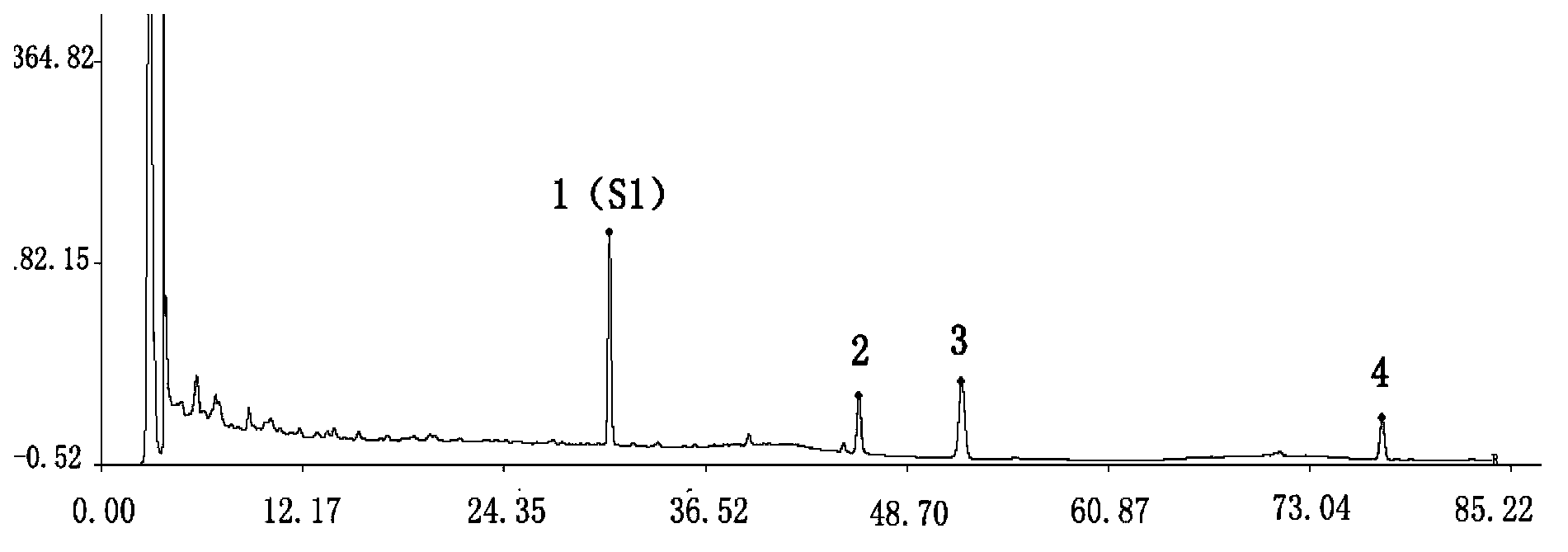
[0048] Example 1 Thin-layer identification of Astragalus in Weinaian tablets
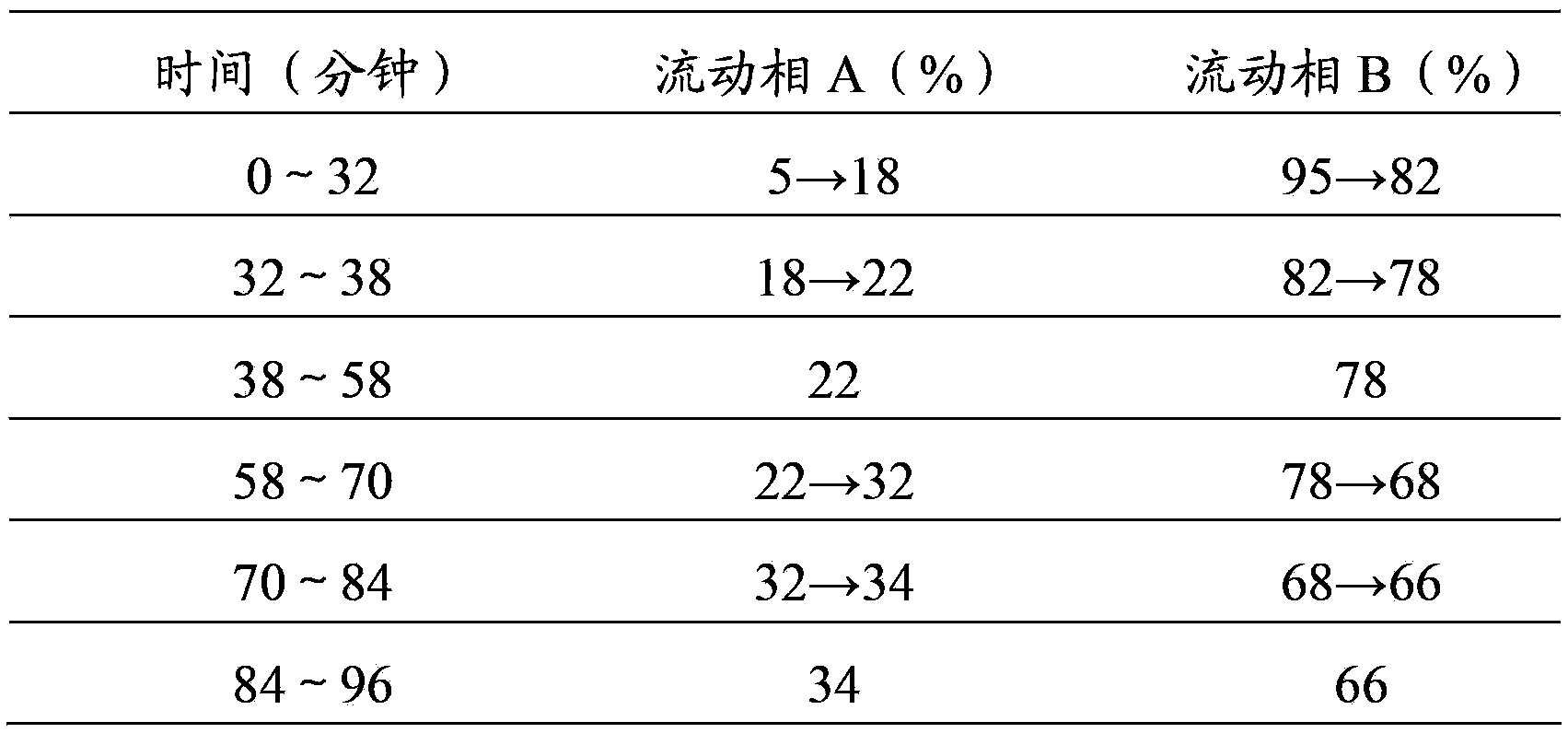
[0049] A detection method for Weinaian tablets, the detection method adopts a thin-layer chromatography identification method to determine astragalus, comprising the following steps:
[0050] a Prepare the test solution: grind the finished product of Wei Naian tablets, take 2 g, add 50 ml of methanol, sonicate, filter, evaporate the filtrate, add 20 ml of water to the residue to dissolve, then extract 3 times with ethyl acetate, 30 ml each time , Combine the obtained upper ethyl acetate solution, evaporate the ethyl acetate solution, and add 1ml methanol to the residue to dissolve it as the test solution;
[0051] b Prepare astragalus reference medicinal solution: take 2g of astragalus reference medicinal material, add 50ml methanol, sonicate, filter, evaporate the filtrate, add 20ml of water to the residue to dissolve, and then extract 3 times with ethyl acetate, 30ml each time, and combine the upper acet...
Embodiment 2
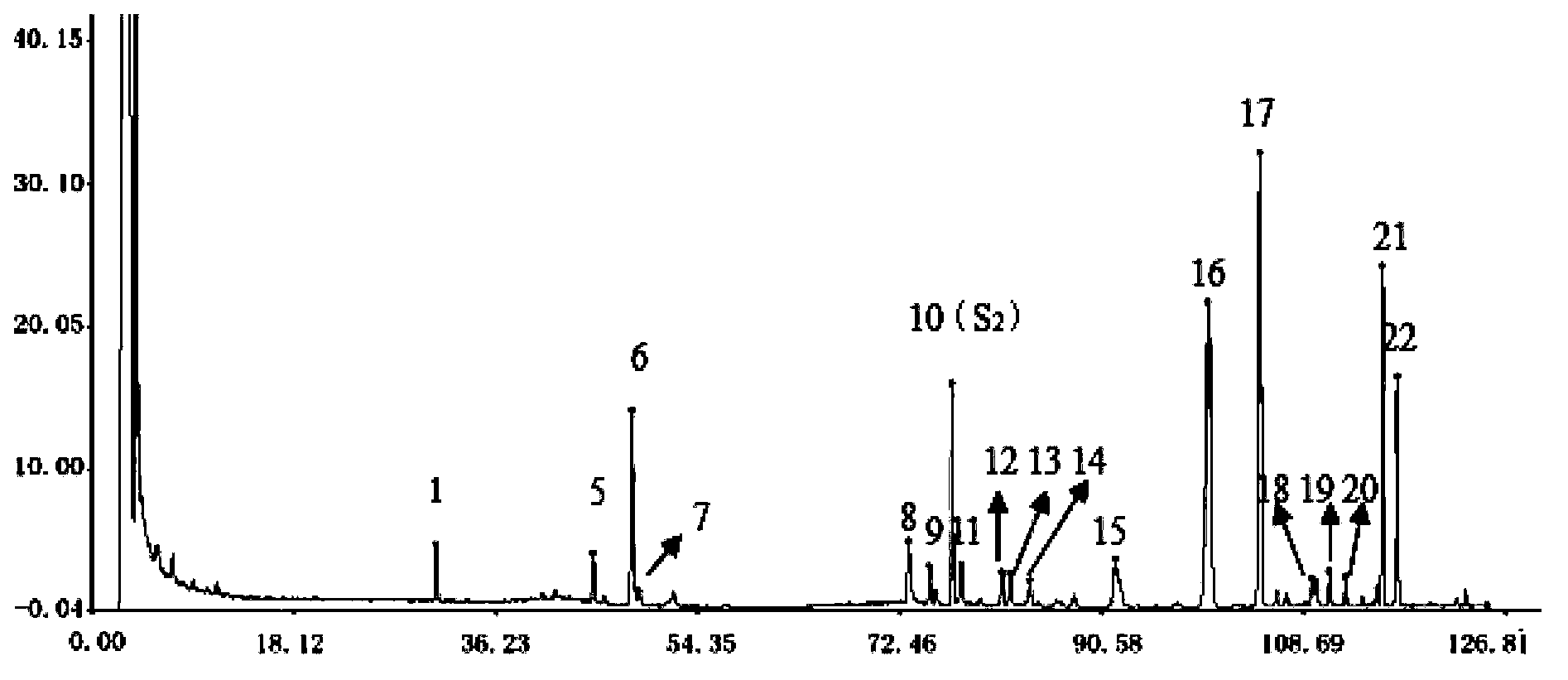
[0054] Example 2 Simultaneous TLC identification of Astragalus, Red Ginseng and Panax notoginseng in Weinaian tablets
[0055] A detection method for Weinaian tablets. The detection method adopts a thin-layer chromatography identification method to simultaneously determine astragalus, red ginseng and Panax notoginseng in Weinaian tablets, comprising the following steps:
[0056] a Prepare the test solution: grind the finished product of Wei Naian tablets, take 2 g, add 50 ml of methanol, sonicate, filter, evaporate the filtrate, add 20 ml of water to the residue to dissolve, then extract 3 times with ethyl acetate, 30 ml each time , Combine the lower aqueous solution, shake and extract with water saturated n-butanol 3 times, 30ml each time, combine the n-butanol solution, wash 2 times with ammonia test solution, 30ml each time, discard the ammonia solution, and distill the n-butanol solution Dry, add 0.5ml methanol to the residue to dissolve it as the test solution;
[0057] b Prepa...
Embodiment 3
[0060] Example 3 Identification of Artificial Bezoar in Weinaian Tablets by Thin Layer Chromatography
[0061] A detection method for Weinaian tablets. The detection method adopts a thin-layer chromatography identification method to determine artificial bezoar, which comprises the following steps:
[0062] a Prepare the test solution: Grind the finished product of Wei Naian'an tablets, take 0.2 g, add 20 ml of methanol, sonicate, filter, evaporate the filtrate, and add 1 ml of methanol to the residue to dissolve it as the test solution;
[0063] b Preparation of control medicinal material solution: take 10 mg of artificial bezoar control medicinal material, add 2 ml of methanol, ultrasonicate, centrifuge, and take the supernatant as the control medicinal material solution;
[0064] c Prepare the reference substance solution: take the bile acid reference substance and the hyodeoxycholic acid reference substance, and add methanol to make a solution containing 1 mg of the above reference ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com