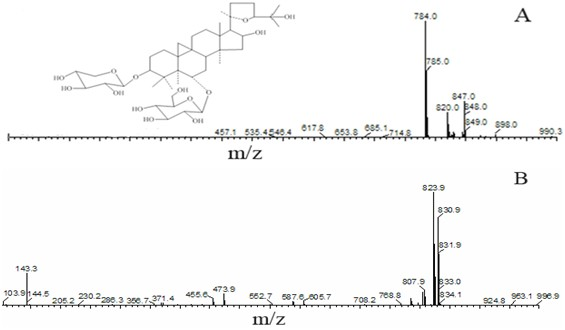
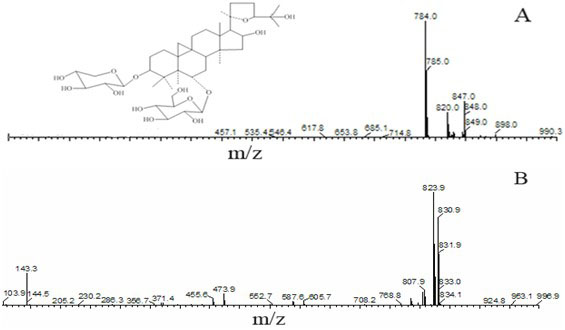
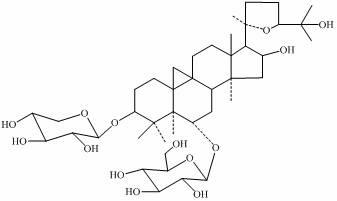
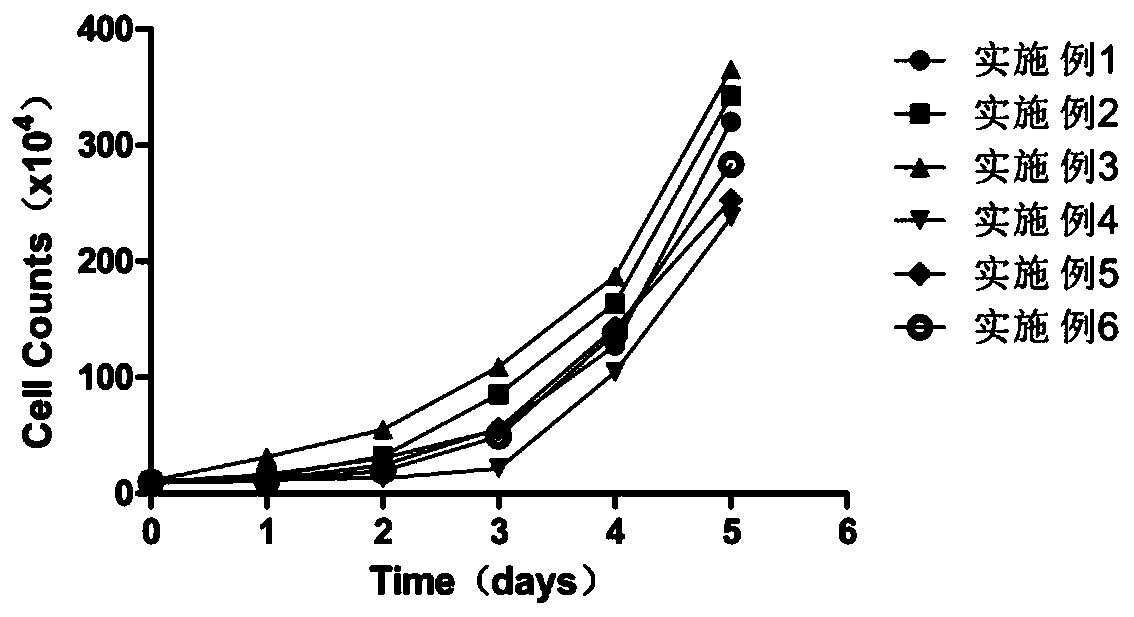
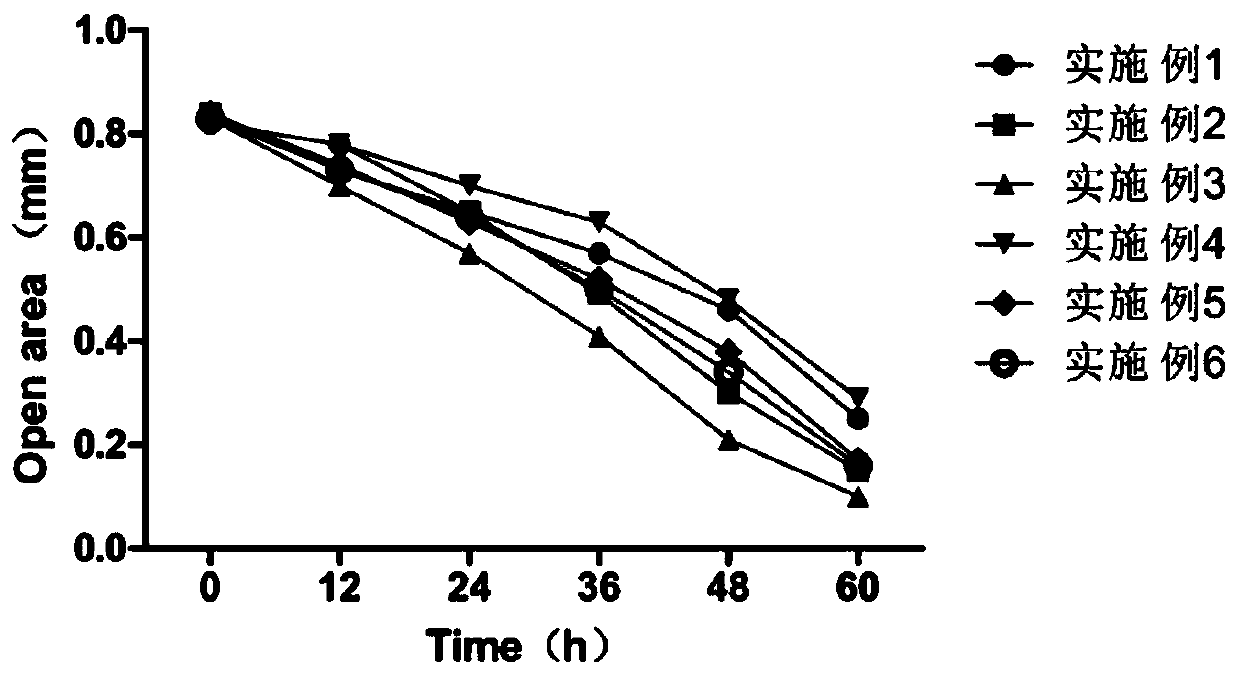
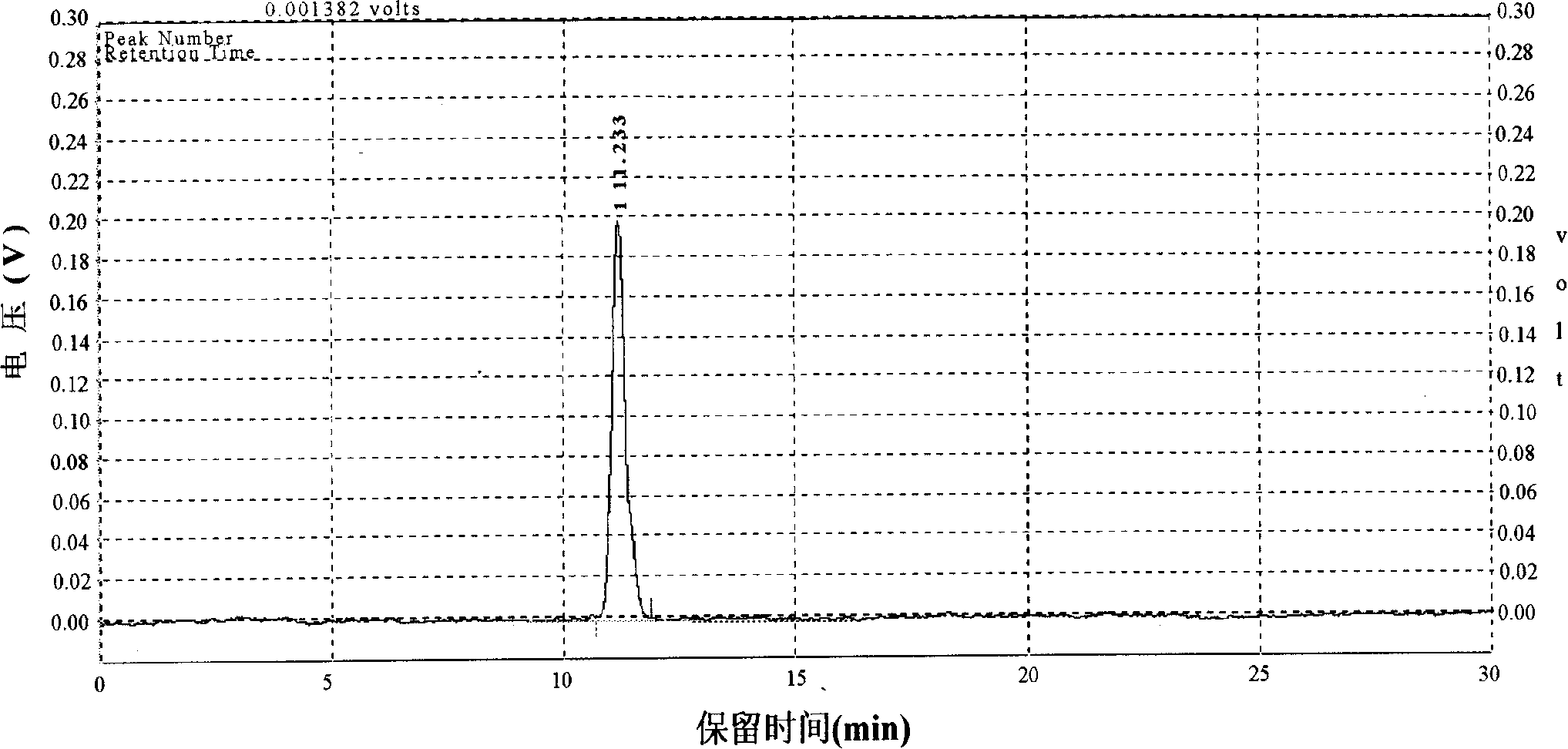
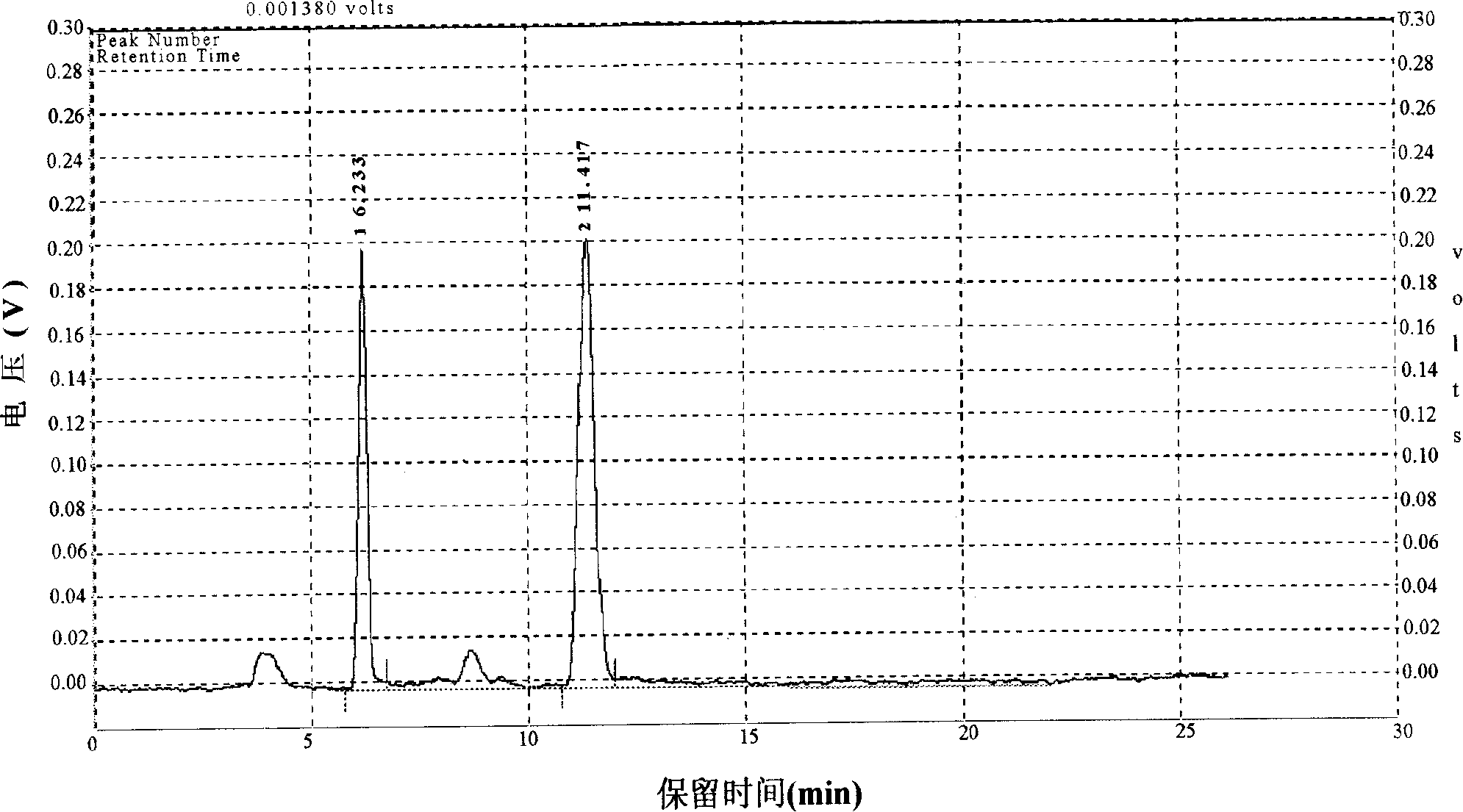
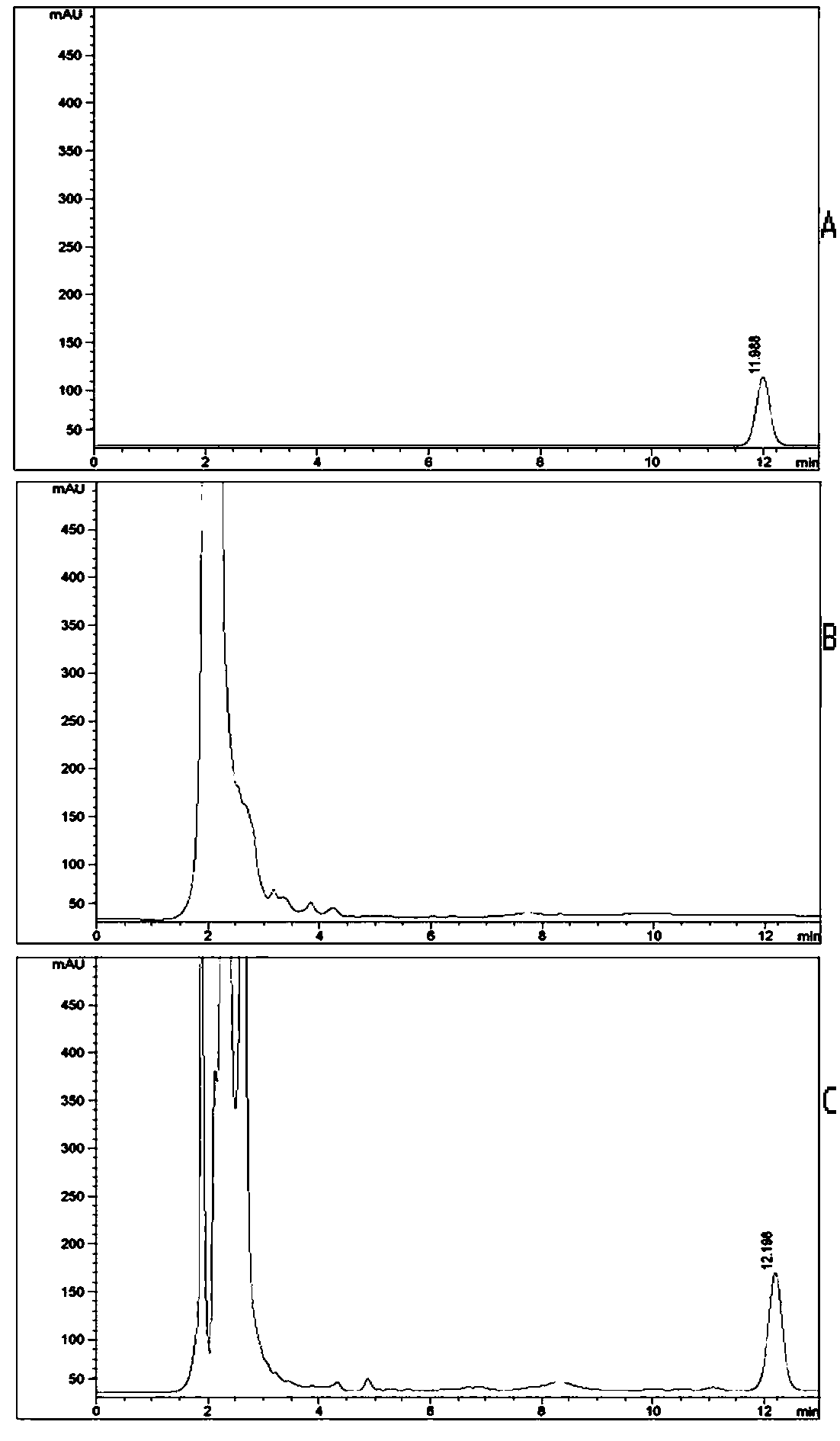
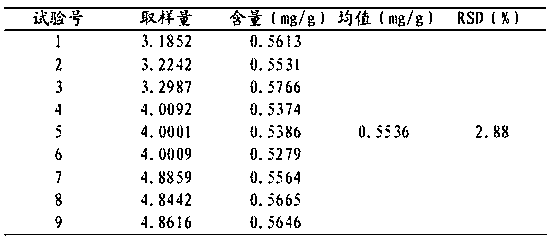
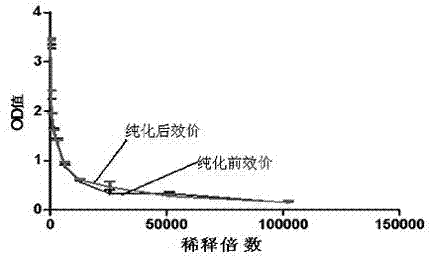
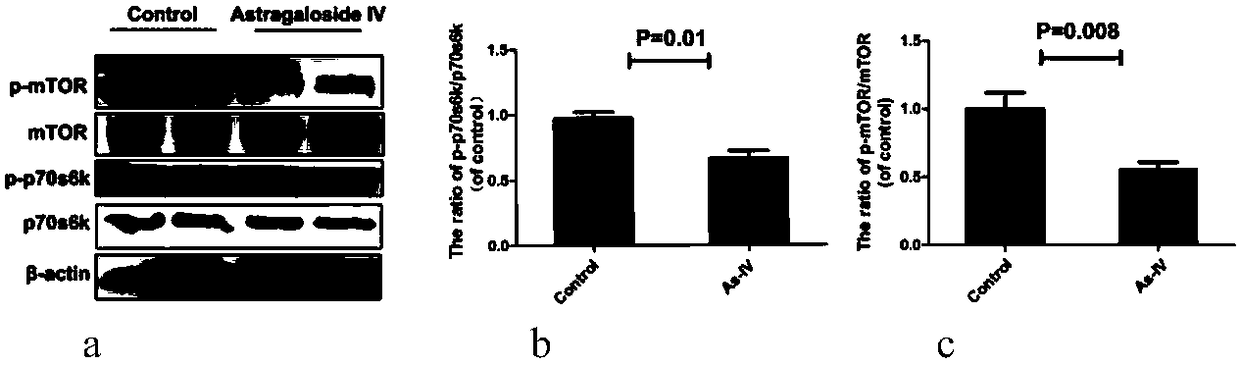
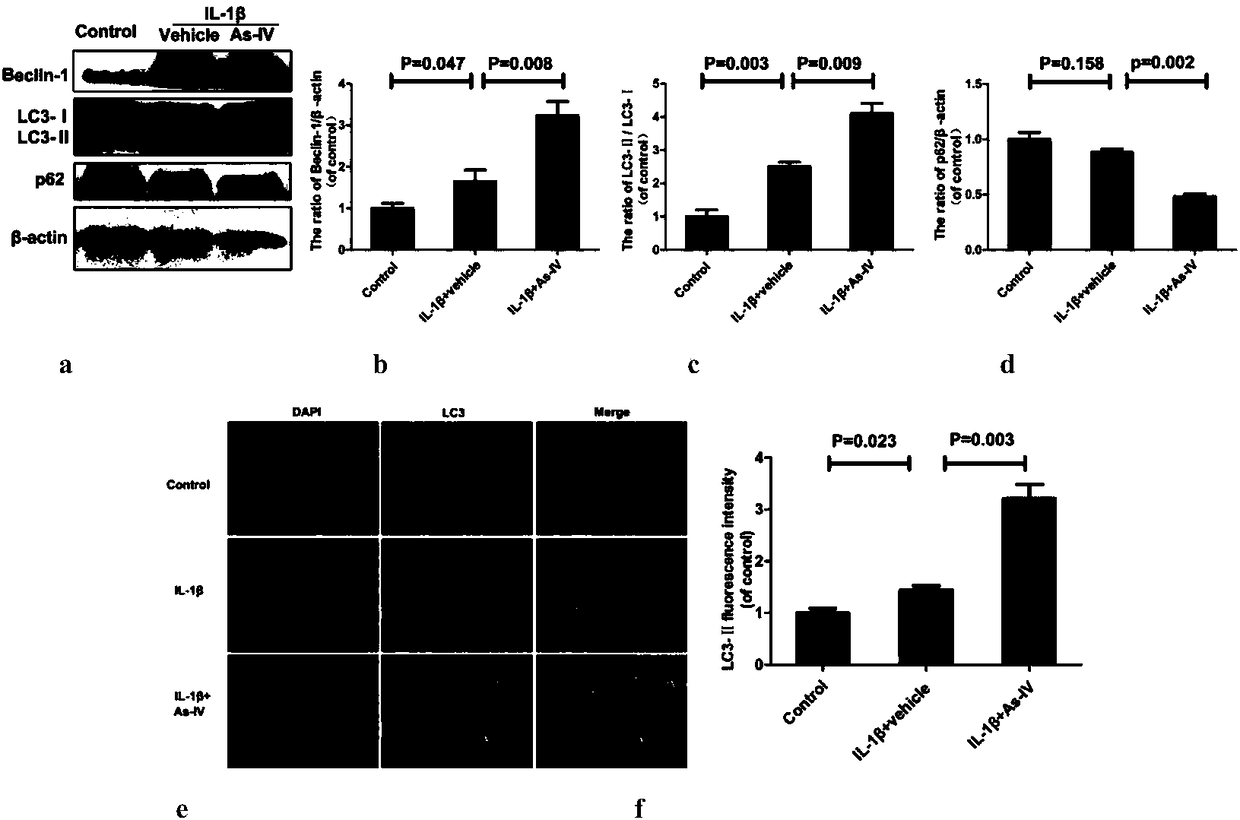
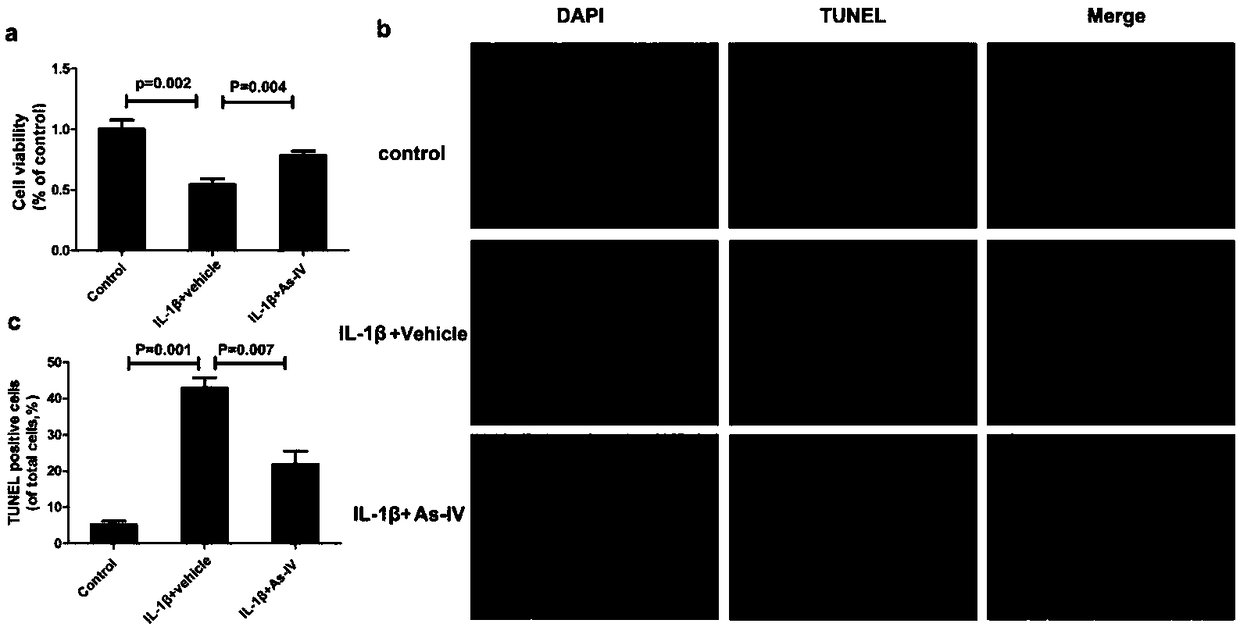
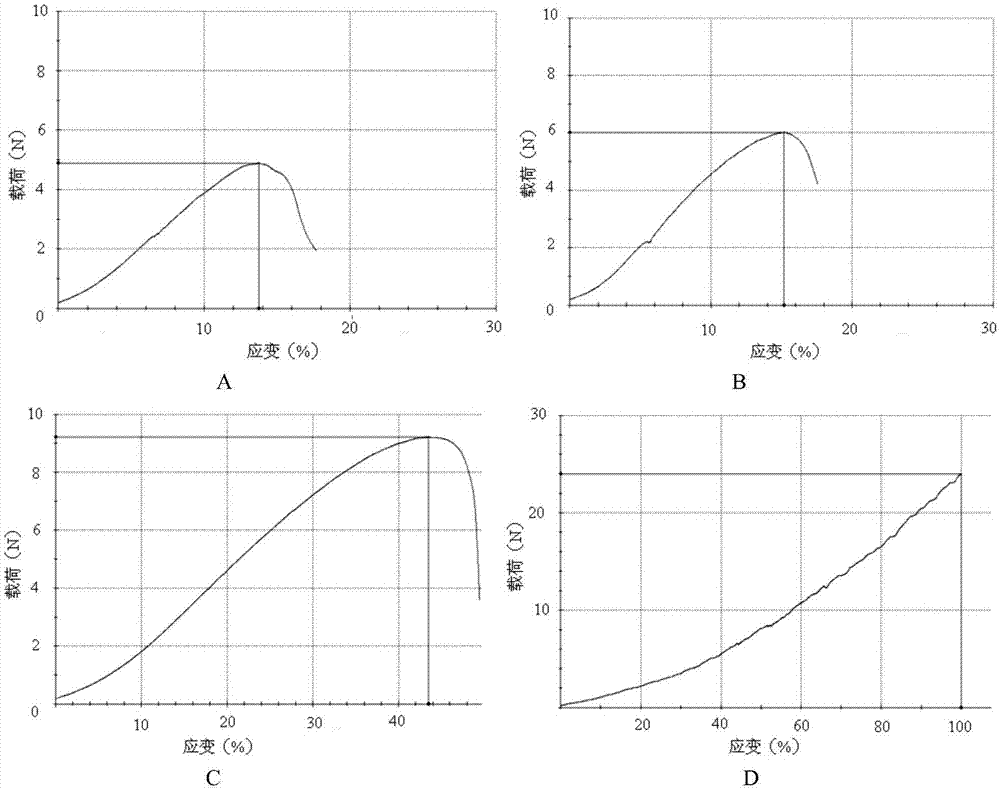
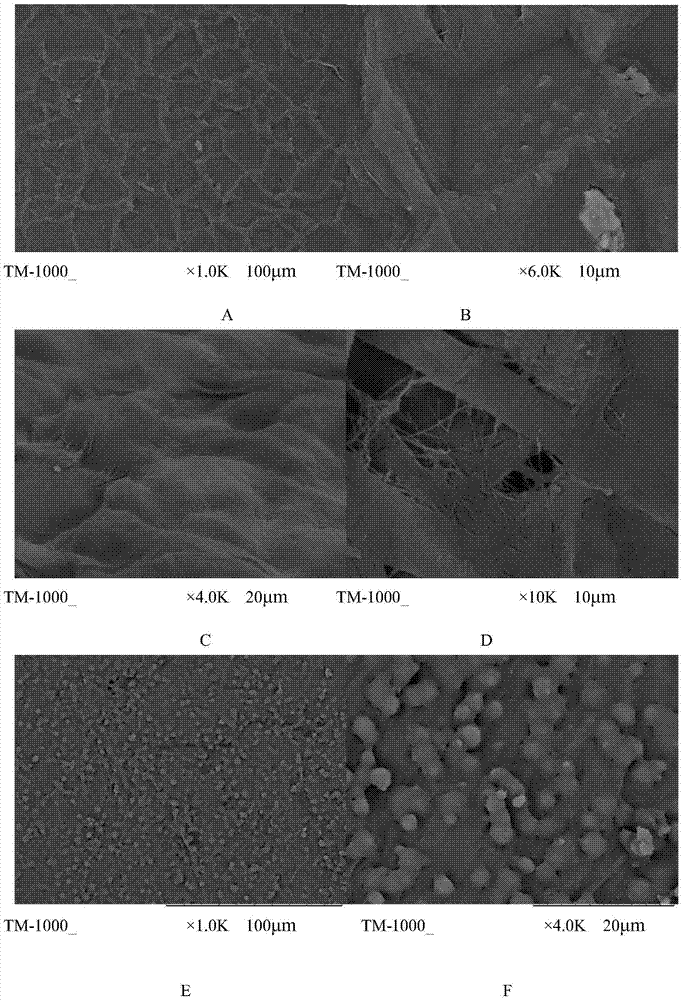
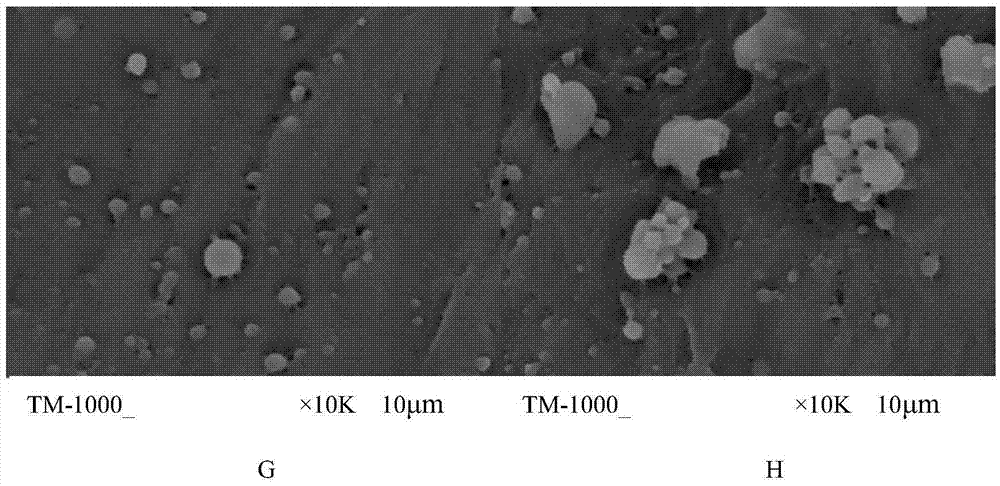
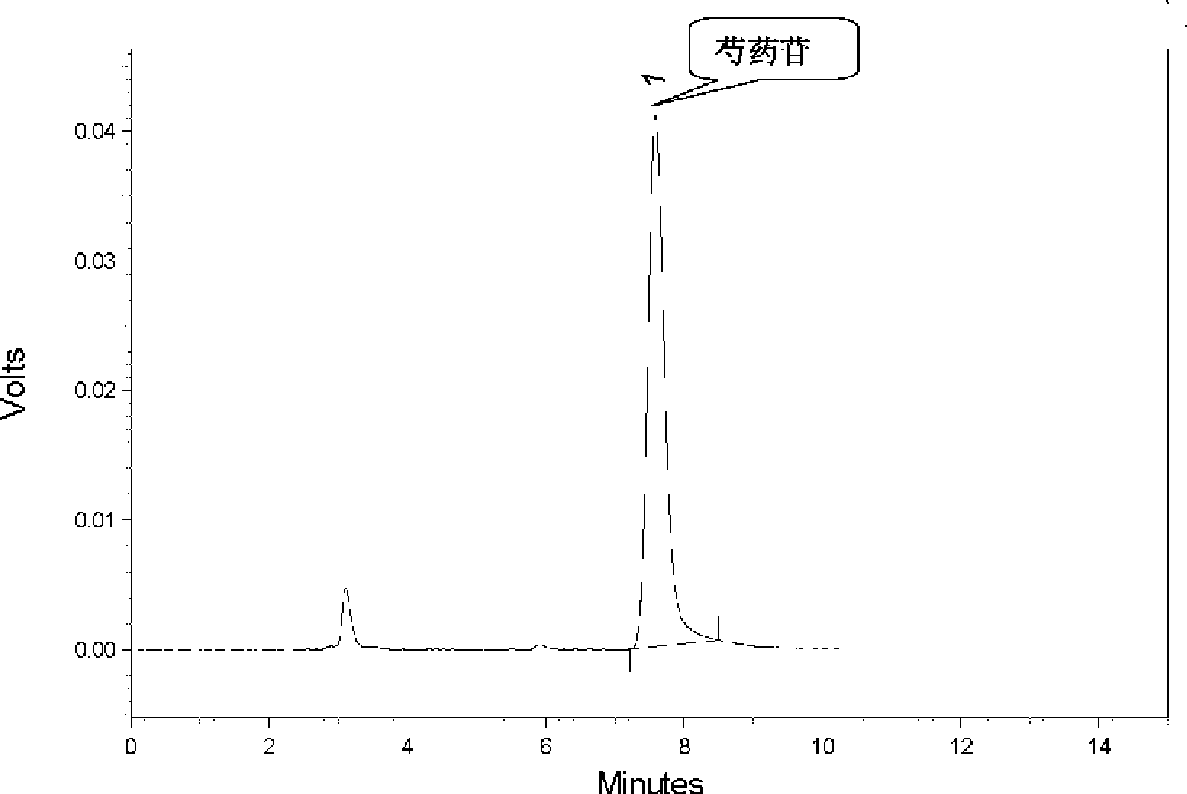
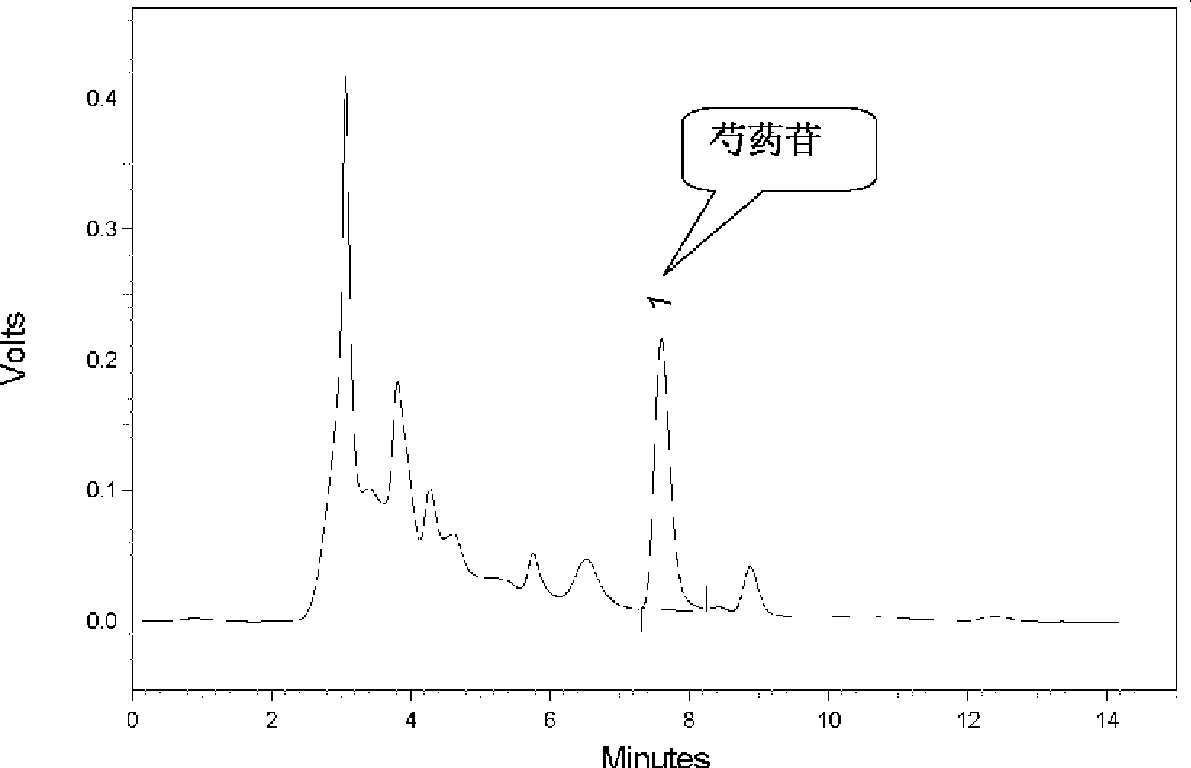
Patents
Literature
93 results about "Astragaloside IV" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
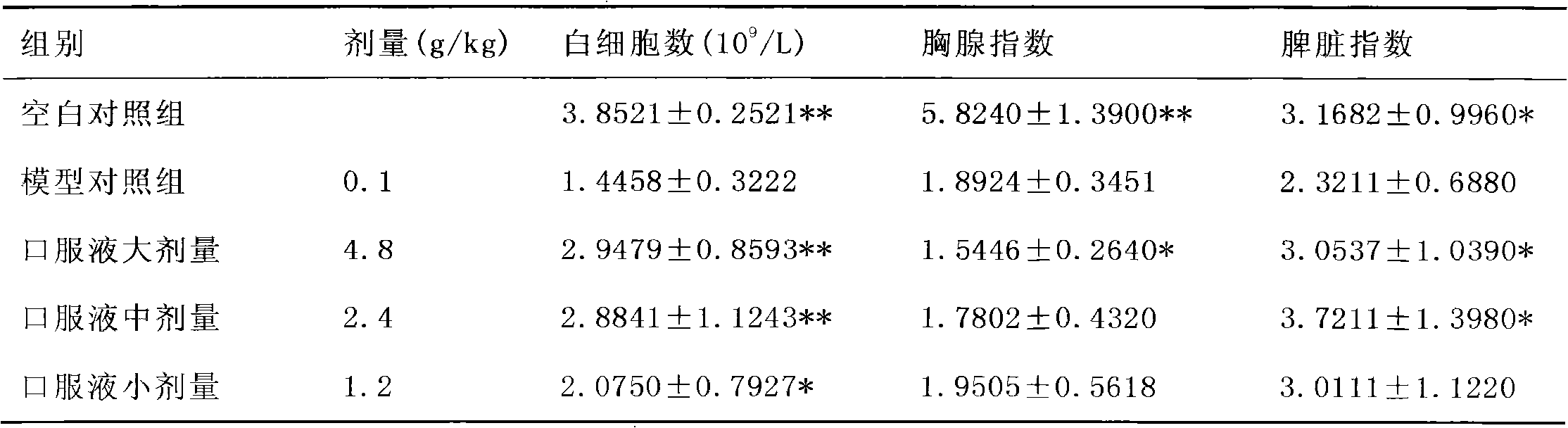
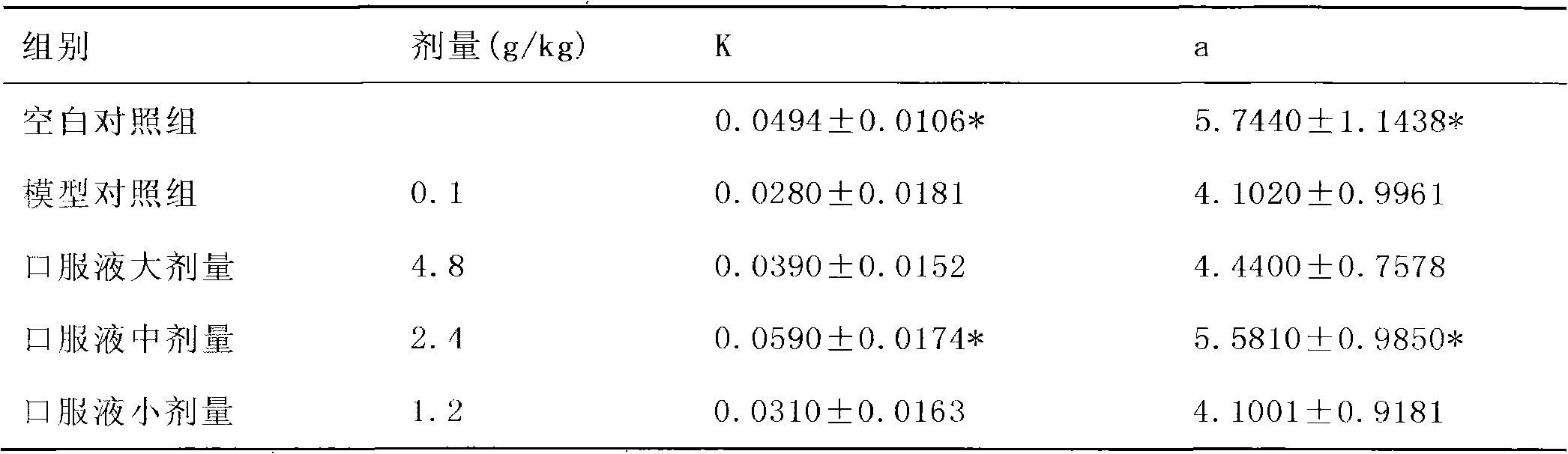
Method for detecting the quality of the medicine combination for treating cerebral apoplexy and vasculitis
The invention provides a quality detection method for pharmaceutical compositions for treating stroke and vasculitis through a TLC qualitative identification method for Dendrobium nobile, pilose asiabell root, scrophularia root, licorice root, astragalus root, glycyrrhizic acid,wherein a HPLC content determination process and content regulation are conducted to sweet-scented osmanthus glycoside and astragalus root saponins. The invention discloses the research development of the preparation fingerprint pattern and optimization for conditions of the fingerprint pattern apparatus including chromatographic column, mobile phase and detecting wave length, as a result the liquid phase fingerprint spectrogram standard for the oral liquid preparations is drafted.
Owner:JIANGSU KANGYUAN SUNSHINE PHARMA CO LTD +1
Method for preparing astragaloside iv by enzymic hydrolysis for astragalus saponin glycosyl
InactiveCN101130802AHigh extraction rateOvercoming destructiveMicroorganism based processesFermentationEnzymatic hydrolysisIsoflavones
The present invention discloses a method for preparing astragalus methylglucoside by utilizing enzymatic hydrolysis of astragalus saponin glycosyl. It is characterized by that it uses an enzyme method to hydrolyze astragalus saponin glycosyl to prepare astragalus methylglucoside. The described enzyme is prepared by using astragalus saponin or flavone or isoflavone as fermentation enzyme-producing inductor and utilizing bacteria, streptomycete, mold, saccharomycetes and basidiomycetes to make fermentation. Said invention can be used for preparing astragalus tablet and powder products with high astragalus methylglucoside content.
Owner:金凤燮
Quality detection method for weinai'an tablet
ActiveCN104165962AHigh precisionQuantitatively accurateComponent separationBULK ACTIVE INGREDIENTContent determination
The invention discloses a quality detection method for a weinai'an tablet. The quality detection method adopts one or more of the following identification and content determination items: thin-layer identification of radix astragali; thin-layer identification of radix astragali, red ginseng and pseudo-ginseng; thin-layer identification of atificial cow-bezoar; high performance liquid chromatography qualitative and quantitative identification method of weinai'an tablet; and high performance liquid chromatography quantitative identification method of Astragaloside IV. The method provided by the invention improves and revises the original weinai'an capsule quality standards, revises and enlarges thin-layer identification of radix astragali's four flavonoid components and Astragaloside IV, simultaneously revises and enlarges thin-layer identification of red ginseng, and identifies the index components Astragaloside IV, ginsenoside Rg1, Rb1 and notoginsenoside R1 at the same time. The method employs an HPLC-PDA-ELSD combined technology to establish a main active ingredient content determination and characteristic spectrum to realize effective control of the weinai'an tablet quality. At the same time, the method has the advantages of high efficiency, accurate quantification, good stability, high precision and excellent repeatability.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Method for preparing bolus for reinforcing middle-jiao and replenishing qi
InactiveCN101181619AFast absorptionAccurate doseDigestive systemPill deliveryMedicineAstragaloside IV
The invention provides a method for preparing Buzhong Yiqi pill, comprising the steps as follows: the eight Chinese medical materials of broil radix astragali, radix codonopsitis, baked liquoric root, largehead atractylodesrhizome (fried), angelica, largetrifoliolious bugbane rhizome, bupleurum and tangerine peel are milled into fine powders respectively and then mixed evenly; an adhesive agent is added into the mixture to prepare gains; the grains are pressed into pills; and the adhesive agent is the boiling liquid of ginger and fructus zizyphi. The preparation method has the advantages of adopting a method of pressing preparation, simple technique, and easy operation. The product prepared with the method has stable quality, and the dissolving time of the product is shortened by over 70 to 95 percent when compared with the original Buzhong Yiqi pill, with quicker medicine absorption and higher content of astragaloside IV. The invention solves the problem of uneven size of bills preparing with the water pill method. Therefore, the dosage of the medicine can be more accurate, while the rate of finished products at one time can be increased.
Owner:上海复星临西药业有限公司 +1
Method for preparing astragaloside IV by converting total saponins of astragalus by microorganisms
InactiveCN102559828AIncrease contentSolve the sourceOrganic active ingredientsMetabolism disorderMicrobial transformationInstability
The invention discloses a method for preparing astragaloside IV by converting total saponins of astragalus by microorganisms, which comprises the following steps of: preparing bacteria, mould and yeast into bacterium suspension or spore suspension and inoculating the bacterium suspension or the spore suspension into a seed culture medium to form seed solution; then inoculating the seed solution into a fermentation medium to carry out culturing; after the seed solution grows well, adding the total saponins of astragalus to carry out conversion; carrying out scanning and analysis by a TLC (Thin Layer Chromatography) to convert other astragaloside into the astragaloside IV, so that the amount of the astragaloside IV is improved by over four times; and extracting the conversion solution by an organic solvent and separating and purifying the conversion solution by macroporous resin to obtain a pure product of the astragaloside IV. According to the method disclosed by the invention, the defects of damage to the saponins, serious pollution, poor purposiveness and the like in the process of hydrolyzing the total saponins of astragalus by a chemical method can be overcome and the problem of enzyme instability in the process of hydrolyzing the total saponins of astragalus by an enzyme method can be solved. The method has the advantages of no pollution, strong specificity and high conversion rate. The purity of the prepared astragaloside IV can reach over 99.9 percent.
Owner:FUDAN UNIV
Method for extracting astragaloside IV from astragalus
The invention relates to a method for extracting astragaloside IV from astragalus and belongs to the technical field of medicines. From an economic prospective, the convention method greatly increases production cost and period, which is unfavorable for the competition of enterprises on market. The method provided by the invention comprises the following steps: a, soaking an astragalus medicinal material in alcohol, heating, extracting and concentrating the extract to a certain volume; b, adding alcohol into the concentrate; c, adding petroleum ether, normal hexane or ethyl acetate into the solution obtained by the step b for extraction, and collecting a bottom phase; d, concentrating the lower phase till the lower phase contains no ethanol, adding alkaline solution and dehydrating for 0.5 to 10 hours at 30 to 100 DEG C; and e, adding n-butyl alcohol and one or several of ethyl acetate, normal hexane or petroleum ether for mixed extraction, collecting an upper phase, washing the upper phase, evaporating the upper phase to dryness, and crystallizing to obtain astragaloside IV. The purity of the astragaloside IV prepared by the method can reach 90 to 99 percent.
Owner:NANJING UNIV OF TECH
Detection method of Chinese preparation mixture for invigorating the spleen and replenishing qi
The invention relates to a detection method of a Chinese preparation mixture for invigorating the spleen and replenishing qi. The Chinese preparation mixture comprises the following ingredients: 280 g of astragalus, 84 g of Codonopsis pilosula, 140 g of liquorice, 84 g of rhizoma atractylodis macrocephalae, 84 g of Chinese Angelica, 84 g of cimicifugae foetidae, 84 g of radix bupleuri, 84 g of tangerine peel, 28 g of ginger, and 56 g of Chinese date. The detection method comprises the following steps: identifying the liquorice, Chinese Angelica and tangerine peel in the mixture, and determining the content of Astragaloside IV in the mixture.
Owner:JIANGXI JEMINCARE GRP CO LTD +1
Detection method of pharmaceutical composition Xianyu for treating epileptoid convulsions, infantile convulsions and facial spasms
ActiveCN104569166AProtection against convulsionsProtection Sedation HypnosisNervous disorderComponent separationAstragalosideConvulsion
The invention provides a detection method of pharmaceutical composition Xianyu for treating epileptoid convulsions, infantile convulsions and facial spasms. The detection method comprises steps as follows: detecting the content of gastrodin in the pharmaceutical composition with a liquid chromatography; detecting the content of astragaloside in the pharmaceutical composition with an HPLC (high performance liquid chromatography); detecting salvianic acid A sodium in the pharmaceutical composition with a TLC (thin-layer chromatography). With the adoption of the detection method, the quality of the pharmaceutical composition can be controlled more accurately and can be stable, controllable, efficient and safe, defects in the prior art can be overcome, and the powerful guarantee can be provided for well satisfaction of medical requirements.
Owner:XIAN CHIHO PHARMA
Culture medium for promoting growth of mesenchymal stem cells and preparation method thereof
ActiveCN109762782AAvoid reactionAvoid cytotoxicitySkeletal/connective tissue cellsCytokineCulture mediums
The invention discloses a culture medium capable of promoting the division growth of mesenchymal stem cells, which comprises a serum-free basic culture medium and an additive added on the basis of theserum-free basic culture medium, wherein the additive comprises a hibiscus mutabilis extract, seaweed polysaccharide, astragaloside IV, human serum albumin, transferrin, glutamine, platelet-derived factor, epidermal growth factor, fibroblast growth factor, human insulin growth factor and vitamin A; the hibiscus mutabilis extract has antioxidant and cell activating activity, and can effectively promote the growth of cell metabolic by compounding seaweed polysaccharide and astragaloside IV; human serum albumin, transferrin, glutamine and vitamin A provide essential nutrient substances for the growth of stem cells; platelet-derived factor, epidermal growth factor, fibroblast growth factor, human insulin growth factor and other cytokines together promote the rapid growth and proliferation ofstem cells; the culture medium not only can improve the growth activity of mesenchymal stem cells, shorten the culture time, promote the expression of cell growth factors, but also can maintain the stem cell activity of the differentiation potential of mesenchymal stem cells; stem cells are not differentiated in daily culture, thereby providing convenience for scientific research.
Owner:嘉文丽(福建)化妆品有限公司
Method for making chinese medicine radix astragali
InactiveCN101176745AThe effect of reducing lossesThe effect of loss is obviousDigestive systemDermatological disorderAstragaloside IVTraditional medicine
The invention relates to a processing method of the Chinese herbal medicine astragalus, which is characterized in that: the Chinese herbal medicine astragalus is put into the refine honey under normal temperature and evenly mixed; the astragalus is then covered closely until the refine honey is fully absorbed by the Chinese herbal medicine astragalus; then the astragalus is put in an oven for drying and is taken out when dried; thus, the processed honey-made astragalus is obtained. With the method of putting the astragalus into the refine honey and mixing the two, covering the astragalus closely until the refine honey is fully absorbed by the Chinese herbal medicine astragalus, the invention has the advantages that: the function of invigorating spleen to replenish qi of the Chinese herbal medicine astragalus is reinforced; the astragaloside IV content is 48.8% to 752.5% higher than the prior art, proving than the invention can significantly reduce the loss of the astragaloside IV after the Chinese herbal medicine astragalus is processed; with the invention adopting drying method, the processing conditions such as processing temperature can be well controlled, and the unification and quality controllability of honey-made astragalus can be reinforced.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
A method of measuring the content of astragaloside in heart-tonifying pulse-restoring granules
InactiveCN106033075AMeet the requirements of method validationSimple and fast operationComponent separationAstragalosideWater methanol
The invention relates to a method of measuring an effective component in a traditional Chinese medicine, and particularly relates to a method of measuring the content of astragaloside in heart-tonifying pulse-restoring granules. The method includes a step of preparing a reference substance solution, namely a step of measuring a proper amount of an astragaloside reference substance and adding water-containing methanol or a mobile phase to prepare into a solution containing 0.16-0.24 mg1 / mL of astragaloside; a step of preparing a sample solution to be measured, namely a step of weighting the heart-tonifying pulse-restoring granules, extracting with an alkali water solution having a concentration of 2-5%, adding the extract liquid into a solid phase extraction column, and eluting with methanol, and a step of measuring, namely a step of separately injecting 5-20 muL of the reference substance solution and the sample solution to be measured into a high performance liquid chromatograph.
Owner:TIANJIN TASLY PHARMA CO LTD +1
Method for extracting and purifying astragaloside IV in Astragalus root
The invention discloses a method for extracting and purifying astragaloside IV in Astragalus root. The method comprises the steps of crushing, pretreatment, extraction, concentrating, adsorption separation, decolorizing, concentrating crystallization, centrifugation, recrystallization, centrifugation and drying. The method has the advantages of easily available raw material, extraction from the Astragalus root having rich sources, high yield of above 0.1% and high purity of above 99% of the obtained product astragaloside IV, high extraction efficiency, short treatment time, and suitableness for large-scale industrial production.
Owner:安徽龙津生物科技有限公司
Method for determining content of Astragaloside IV in traditional Chinese medicinal composition
InactiveCN104280464AAids in quality controlImprove securityComponent separationMedicineAstragaloside IV
The invention discloses a method for determining the content of Astragaloside IV in a traditional Chinese medicinal composition. The method concretely comprises the steps of preparing a tested substance solution, preparing a reference substance solution and determining. The determination method is helpful for controlling the quality of medicines, and improves the safety and the reliability of the medicines.
Owner:HEBEI YILING MEDICINE INST
Immunoaffinity chromatographic column adopting astragaloside IV as ligand and application of immunoaffinity chromatographic column
InactiveCN105435758AFully crosslinkedImprove purification efficiencyOther chemical processesSolid sorbent liquid separationAcetic acidSorbent
The invention discloses a technical scheme of an immunoaffinity chromatographic column adopting astragaloside IV as a ligand and an application of the immunoaffinity chromatographic column. The immunoaffinity chromatographic column adopts the astragaloside IV as the ligand and is prepared through specific steps as follows: an astragaloside IV hapten is prepared; an immunoaffinity adsorbent is prepared from the astragaloside IV hapten and a solid-phase carrier through cross-linking; the immunoaffinity adsorbent is taken as packing to be charged into a chromatographic column; the packing is washed alternately with an acetic acid buffer solution and a Tris-HCl buffer solution for three times, and the immunoaffinity chromatographic column is obtained. A method adopting the prepared immunoaffinity chromatographic column to purify an astragaloside IV antibody comprises steps as follows: a to-be-purified sample is added to the immunoaffinity chromatographic column and is eluted by an eluent and neutralized by a neutralization solution and the like. According to the invention, the purification efficiency of the astragaloside IV monoclonal antibody is effectively improved, the purity can be higher than 99%, besides, the operation is simple, the cost is low, and the pollution is low.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Method for analyzing all components of angelica sinensis six-yellow decoction
PendingCN112229934AAccurate analysisEasy to analyzeComponent separationAstragalosideChlorogenic acid
The invention provides a method for analyzing all components of an angelica sinensis six-yellow decoction, and belongs to the technical field of pharmaceutical analysis. An ultra-high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) technology is adopted to detect an angelica sinensis six-yellow decoction sample, and 15 chemical reference substances are adopted at the same time: baicalin, wogonoside, berberine hydrochloride, epiberberine hydrochloride, coptisine hydrochloride, palmatine hydrochloride, phellodendrine hydrochloride, astragaloside, calycosin-7-glucoside, ferulic acid, chlorogenic acid, ligustilide, verbascoside, baicalein and wogonin are adopted to confirm a detection result of the angelica sinensis six-yellow decoction andclearly identify 68 components; comprehensive analysis of the components of the angelica sinensis six-yellow decoction is realized, and the reliability of quality control is improved.
Owner:山东宏济堂制药集团股份有限公司
Application of astragaloside IV in preparation of drugs for treating and delaying intervertebral disc degeneration
InactiveCN108186659AModerate anti-apoptotic effectImprove biological activityOrganic active ingredientsSkeletal disorderDiseaseWestern medicine
The invention relates to application of astragaloside IV in the preparation of drugs for treating and delaying intervertebral disc degeneration. Astragaloside IV serving as a plant extract with good biological activity has the advantages that the drug dosage is low and the anti-apoptotic effect on nucleus pulposus cells is obvious; compared with Western medicine, astragaloside IV serving as traditional Chinese medicine is mild in anti-apoptotic effect, and an alternative treatment drug can be provided for the comprehensive treatment of intervertebral disc degeneration diseases.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Preparation method of venation-dredging preparation
InactiveCN110123968AExtract comprehensiveFully extractedPharmaceutical non-active ingredientsBlood disorderChlorogenic acidClinical efficacy
The invention belongs to the field of traditional Chinese medicine preparations, and particularly relates to a preparation method of a venation-dredging preparation. The preparation method improves the content of three kinds of iconic medicinal components of chlorogenic acid, astragaloside IV and berberine hydrochloride in the venation-dredging preparation, the extraction amount of Chinese angelica and rhizoma atractylodis volatile oil is improved, and the clinical efficacy of the product is ensured; at the same time, the three kinds of precious animal medicinal materials of leeches, centipedes and scorpion are extracted by a double extraction method of alcohol-refluxing and water-adding decoction, which can ensure the comprehensive and full extraction of water-soluble and alcohol-solublecomponents in animal medicinal materials, the preparation reduces the content of ineffective impurities in the preparation, reduces the dosage, improves the taste, and improves the compliance of patient administration.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method and application of biological nanopatch
ActiveCN105435306AGood biocompatibilityStrong anti-infection effectProsthesisAstragalosideMicro structure
The invention discloses a method for preparing a biological nanopatch constructed by astragaloside induced BMSC compound polylactic acid-glycolic acid nano-particle modified small intestinal submucosa matrix. The method comprises the following steps: preparing polylactic acid-glycolic acid nano-particles (PLGA) by adopting a multiple emulsion method; preparing a small intestinal submucosa matrix (SIS) having a natural three-dimensional ultra micro-structure by adopting a decellation technology to adsorb the PLGA nano-particles on the surface of SIS and favor adsorption and growth of host cells by means of the SIS after PLGA nano-particle surface modification; inducing BMSC by astragaloside to promote proliferation and differentiation of BMSC and enable the BMSC to compound and grow on the PLGA nano-particle surface modified small intestinal submucosa matrix (PLGA-SIS) of pigs. Therefore, the AS-BMSC-PLGA-SIS biological patch has stable physicochemical property, good biocompatibility and high mechanical strength, and is beneficial to tissue regeneration.
Owner:HANGHZOU HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Process for extracting astragaloside IV
The invention relates to the field of medicament extraction and refining, which is a process for extracting astragaloside IV and solves the problems that the products of the prior art has low yield or needs special devices and technology, is not beneficial to generalization and production and the like. The invention provides a simple, effective, high-yield and new extracting process capable of realizing the industrial large-scale production. The process is operated by the following steps of: cutting raw materials in to pieces or coarse grains, adding enzyme for enzymolysis, and after the enzymolysis, extracting the raw materials by water; extracting and refilling the astragaloside IV. The invention combines the enzymolysis method with a water extraction method and an ethanol extraction method, such that the astragaloside IV in the raw materials can be extracted in maximum; the waste of the raw materials is avoided, the production rate of the astragaloside IV is improved, and the yieldof the astragaloside IV is high. In addition, a solution mixing butyl acetate and tert-butyle alcohol is used for refilling, such that the finally obtained product has good quality, stable formation and high yield; and the raw materials are repeatedly and fully utilized in the whole production process, thus the economic efficiency is improved, the environment is protected, and the invention is suitable for the industrial production.
Owner:张守力
Qi tian dropping pill extract and Qi tian dropping pill as well as method for producing the same
ActiveCN101371877ASimple production methodEasy to operatePill deliveryPharmaceutical non-active ingredientsAdditive ingredientCardiac muscle
The invention relates to a Qitian drop pill extract, a Qitian drop pill and a production method thereof. The Qitian drop pill extract comprises the following ingredients: astragalus hoangtchy, radices paeoniae rubra, integripetal rhodiola herb and safflower. The Qitian drop pill consists of the Qitian drop pill extract and a pharmaceutical base. The Qitian drop pill extract comprises improved quantities of hydroxysafflor yellow, peoniflorin, integripetal rhodiola herb and astragaloside IV, and the production method of the Qitian drop pill extract is simple. Pharmacodynamic studies of the Qitian drop pill prove that the Qitian drop pill of different dosage teams has different degrees of protective functions to rat myocardial ischemia injury, and the curative effects of the dosage teams of Qitian drop pill is similar with that of Compound Danshen Drop Pill. The production method of the Qitian drop pill is simple.
Owner:XINJIANG JINSHIKANG PHARMA
Method for detecting astragalus-chickpea particles
InactiveCN102353731AQuality improvementQuality is easy to controlComponent separationParticle size analysisThin layer chromatographicLactose
The invention relates to a method for detecting astragalus-chickpea particles. Raw materials of the astragalus-chickpea particles mainly comprise astragalus, red-rooted salvia root, sealwort, chickpea, cicada shell and lactose. The detection method comprises content measurement: measuring the content of astragalus and red-rooted salvia root in the particles with a high performance liquid chromatography method, wherein every bag of astragalus-chickpea particles contains not less than 35.0 mg of red-rooted salvia root counted by danshinolic acid BC36H30016 and not less than 1.0 mg of astragalus counted by astragaloside A C41H68014. The detection method can further comprise thin layer chromatography, character detection and particle detection. By establishing a detection method with high accuracy, high repeatability and high stability, the weight of the astragalus-chickpea particles can be controlled effectively, and the quality of the astragalus-chickpea particles is stable, controllable, efficient and safe.
Owner:山西太行药业股份有限公司
Novel traditional Chinese medicine for treating hypoleucocytosis caused by radiotherapy and chemotherapy for treating cancers and preparation method thereof
InactiveCN102552380AThe composition of the prescription is simpleActive ingredients are clearAntineoplastic agentsBlood disorderDiseaseWhite blood cell
The invention discloses a novel traditional Chinese medicine for treating hypoleucocytosis caused by radiotherapy and chemotherapy for treating cancers and a preparation method thereof, and also discloses pharmacological effects and clinical application of the novel traditional Chinese medicine. The novel traditional Chinese medicine provided by the invention comprises pearl ginseng and astragalus mongholicus. The preparation method of the novel traditional Chinese medicine comprises the following steps of adding water into pearl ginseng and astragalus mongholicus, respectively carrying out decoction extraction, merging the extract, carrying out concentration, carrying out alcohol precipitation, standing, recovering ethanol of supernate, carrying out condensation, and mixing the concentrated solution and one or more pharmaceutically acceptable carriers / auxiliary materials to obtain an oral solution, wherein the oral solution is the novel traditional Chinese medicine. The oral solution named as a pearl ginseng-astragalus mongholicus oral solution has active components of total saponins and plant polysaccharides, wherein the total saponins comprise ginsenoside Ro, panax japonicus saponin IVa and astragaloside IV. The oral solution can be utilized for treating diseases of hypoleucocytosis, lusterless complexion, mental and physical fatigue, palpitaition, shortness of breath, insomnia, dry throat and inappetence after radiotherapy and chemotherapy for treating cancers.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
Medicine for treating cardiovascular and cerebrovascular disease
InactiveCN1919252AConvenient sourceEasy to industrializePowder deliveryHydroxy compound active ingredientsSalvianolic acid BRosewood oil
The invention discloses a medicament for treating cardiovascular and cerebrovascular diseases, which comprises astragalus root saponin extract 35.0-75%, red sage root extract 15.0-55.0%, panaxtriol saponins extract 2.5-15.0%, and natural borneol or rosewood oil 2.5-15.0%. The content of danshensu in the salvia miltiorrhizae extract is 3-10%, the content of total savianolic acid is 50-75%, the content of salvianolic acid B is 20-45%, the content of total saponins in panaxtriol saponins extract is 80-90%, the content of total saponins in astragalus root saponin extract is 70-98%, the content of Astragaloside IV is 5-15%. The composition has evident functions in resisting cerebral ischemia and myocardial ischemia, the curative effect is better than the application of single extract of red sage root or panaxtriol saponins, thus providing a more effective and more convenient Chinese medicinal composition and preparation clinically.
Owner:TIANJIN TASLY PHARMA CO LTD
Medicine for treating cardiovascular and cerebrovascular disease
InactiveCN1919238AConvenient sourceEasy to industrializePowder deliveryHydroxy compound active ingredientsSalvianolic acid BBlood vessel
The invention discloses a Chinese medicinal composition for treating cardiovascular and cerebrovascular diseases, which comprises astragalus root saponin extract 35.0-75%, red sage root extract 15.0-55.0%, pseudo-ginseng saponin extract 2.5-15.0%, and natural borneol or rosewood oil 2.5-15.0%. The content of danshensu in the salvia miltiorrhizae extract is 3-10%, the content of total savianolic acid is 50-75%, the content of salvianolic acid B is 20-45%, the content of total saponins in pseudo-ginseng saponin extract is 70-98%, the content of total saponins in astragalus root saponin extract is 70-98%, the content of Astragaloside IV is 5-15%. The composition has evident functions in resisting cerebral ischemia and myocardial ischemia, the curative effect is better than the application of single extract of red sage root or notoginseng saponin, thus providing a more effective and more convenient Chinese medicinal composition and preparation clinically.
Owner:TIANJIN TASLY PHARMA CO LTD
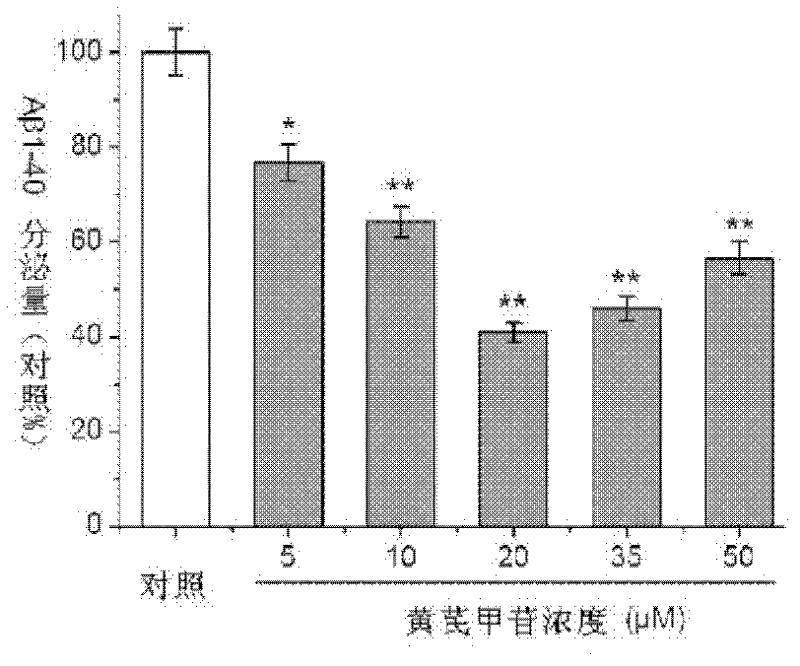
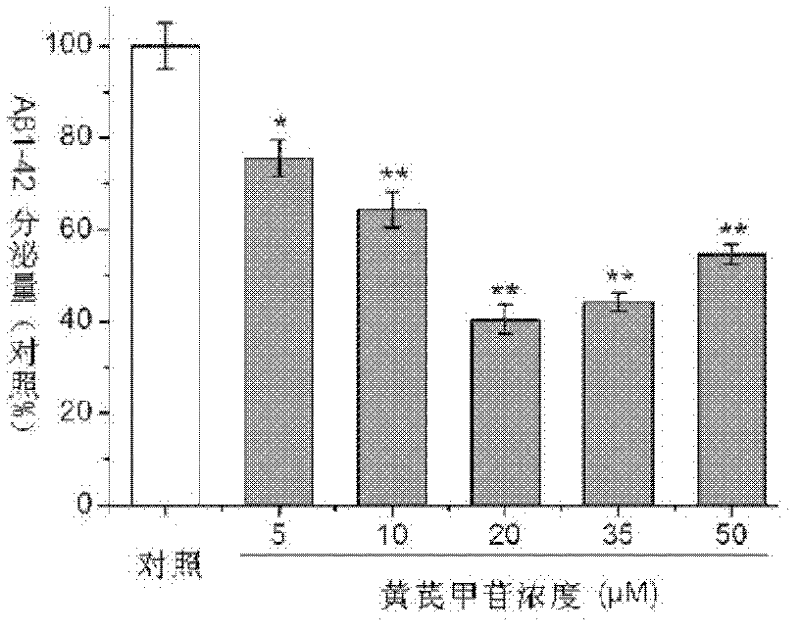
Application of Astragaloside IV
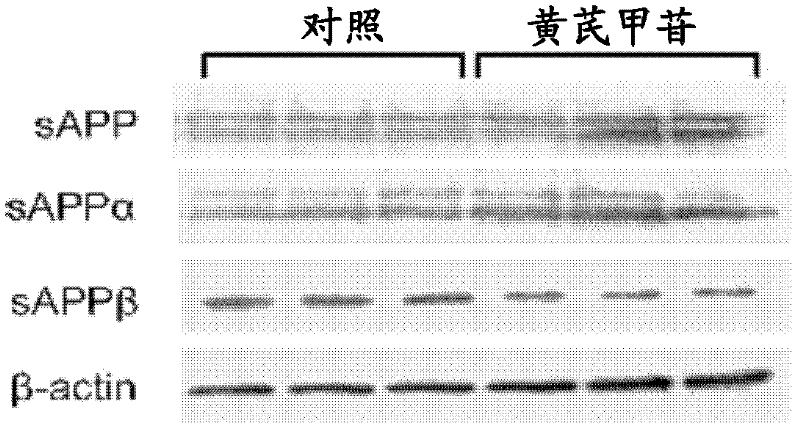
The invention relates to the field of traditional Chinese medicines, and discloses application of Astragaloside IV in the preparation of medicaments for preventing and treating Alzheimer disease. Tests show that the Astragaloside IV can inhibit the secretion of Abeta proteins and beta-secretases, the enzyme activity of the beta-secretases and the enzyme cutting effect of the beta-secretases in SH-SY5Y-APP695sw cells, thereby inhibiting the accumulation of the Abeta proteins. Meanwhile, tests show that the Astragaloside IV can promote oligomerized Abeta degradation, thereby reducing the toxic effect of the Abeta proteins and achieving the purpose of preventing and treating Alzheimer disease. Thus, the invention provides the application of the Astragaloside IV in the preparation of medicaments for preventing and treating Alzheimer disease.
Owner:BEIJING NORMAL UNIVERSITY +1
Radix astragali seedling culture method of radix astragali high in astragaloside IV content
InactiveCN111296261AImprove germination rateImprove permeabilityAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersBiotechnologyRadix Astragali seu Hedysari
The invention provides a radix astragali seedling culture method of radix astragali high in astragaloside IV content. Radix astragali seeds are subjected to soaking in an alkaline solution, soaking inhigh-concentration ethanol and ultrasonic treatment, so that the germinating rate of the radix astragali seeds can be increased, and the vitality of seedlings is high; through applying 5-8% of a sodium hypochlorite solution or 1% of a mercuric chloride solution, selecting bacteria-free water, and performing sterilization treatment on culture mediums, sterile growing environment is provided, and the survival rate of the seedlings is greatly increased; and besides, through applying different MS culture mediums at different stages of radix astragali seed germination and radix astragali seedlinggrowth and particularly applying N elements and trace organic matter (myo-inositol) at the seedling growth stage, the content of the astragaloside IV is greatly increased.
Owner:内蒙古自治区生物技术研究院
Method for preparing cycloastragenol through microorganism mixed fermentation, transformation and degradation of astragaloside iv
InactiveCN109609581AHigh purityImprove conversion rateMicroorganism based processesFermentationMicroorganismHigh volume manufacturing
The invention relates to a method for preparing cycloastragenol through microorganism mixed fermentation, transformation and degradation of astragaloside iv. Two strains of fusarium proliferatum 0821(CGMCC NO.16482 ) and Fusarium verticillioides 0213 (CGMCC NO.16481 ) which are screened out are used, the astragaloside iv is used as a substrate, and through mixed fermentation of the two strains, the cycloastragenol is prepared. A technique for mixed fermentation with two microorganisms is adopted to degrade the astragaloside iv so as to obtain the cycloastragenol high in purity. The technological flow is simple, the cost is low, and the entire technological process is performed at normal temperature and normal pressure. The required equipment condition is low, the method is suitable for mass production, the conversion rate of the final cycloastragenol is as high as 80% or above, and the purity is as high as 95% or above.
Owner:BEIJING UNIV OF CHEM TECH
A detection method for Ruhe Sanjie capsules
ActiveCN102274467AStrong process controllabilityReflect stabilityComponent separationCapsule deliveryDiseaseMedicine
The invention provides a method for detecting Chinese medicinal capsules for treating breast disease, such as capsules for treating nodule in breast by dissolving stasis. The detection method comprises: 1, detecting active ingredients from Chinese angelica by thin-layer chromatography; 2, detecting astragaloside IV and icariin by thin-layer chromatography; 3, detecting saikoside alpha by thin-layer chromatography; and / or 4 detecting active ingredients from Chinese pyrola herb by thin-layer chromatography. The detection method can accurately and completely reflect the content of medicinal components of the capsules for treating nodule in breast by dissolving stasis and therefore can provide a guarantee for the industrial production and clinic use of the capsules for treating nodule in breast by dissolving stasis.
Owner:刘坤
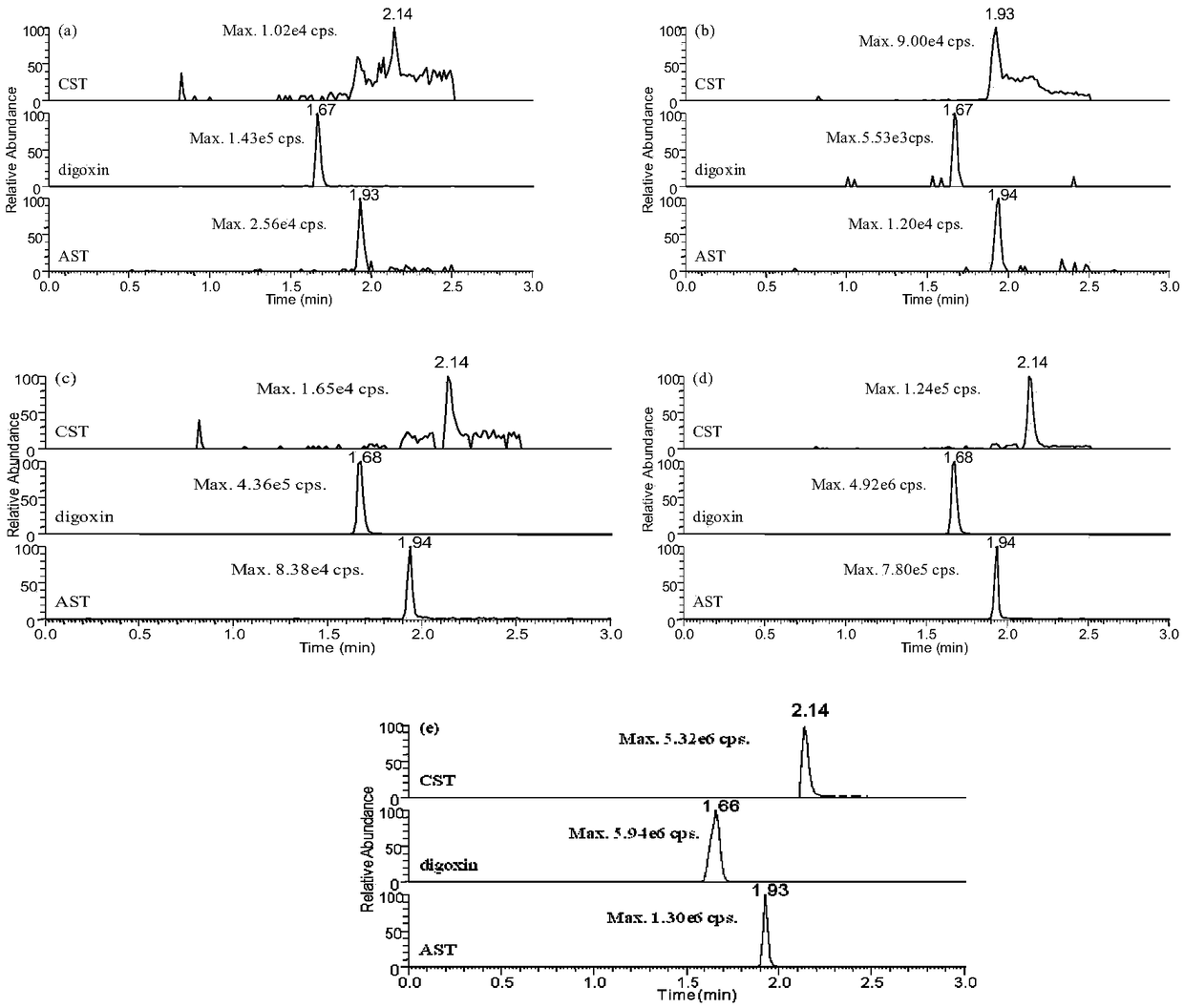
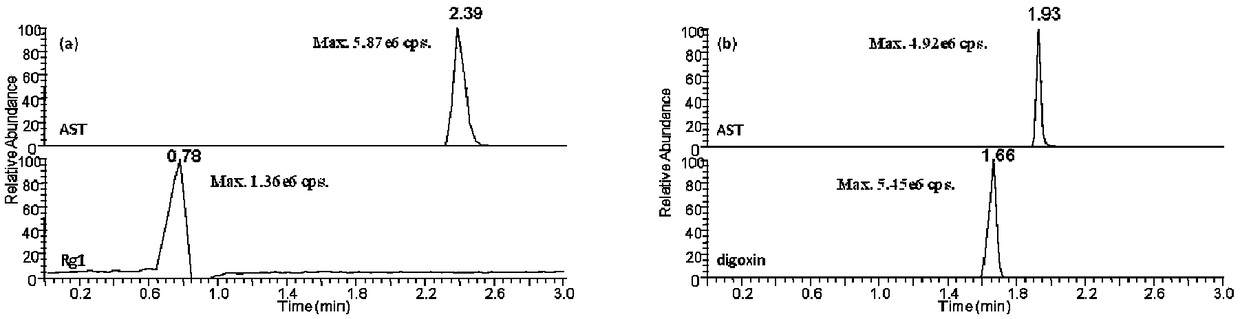
Method for simultaneously quantitatively detecting astragaloside-IV and cycloxanthine in mouse plasma
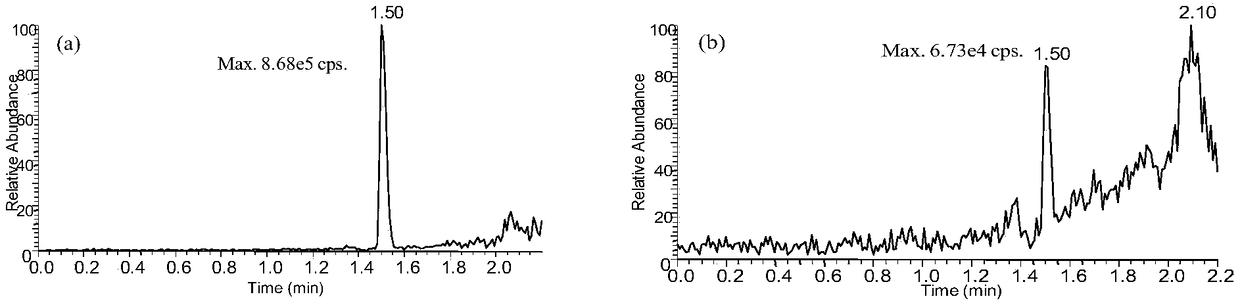
The present invention establishes a simultaneous quantification method for mouse plasma based on UPLC-HRMS, in which the method targets astragaloside IV and cycloxanthine that is the main metabolite of astragaloside IV. The quantitative time of the method is 3 mins, digoxin is used as an internal standard, and only 20 [mu]L of mouse plasma is needed, thus having the advantages of rapidity, high sensitivity and strong specificity. After being precipitated by the protein, the sample is filtered by dephospholipidation plate, which effectively reduces the matrix effect of endogenous metabolites ofphospholipids in plasma on the analyte. Ultra-high performance C18 column is used as the analytical column to detect two kinds of analytes and internal standards in the electrospray ion source positive ion selective ion monitoring mode. The linear range of the two analytes is 1-200ng / mL, the intra-day and inter-day precision is <=8.6%, and the precision is <=8.8%, which indicates that the methodhas good precision and accuracy. The method for simultaneously quantitatively detecting astragaloside-IV and cycloxanthine in mouse plasma was successfully applied to the pharmacokinetic study of astragaloside IV mice.
Owner:MINZU UNIVERSITY OF CHINA
Quality control method of 'xuhanting' preparation
ActiveCN101167974APerfect quality control methodQuality improvementComponent separationDrug compositionsQuality controlAstragaloside IV
The invention discloses a quality control method of Xuhanting medicament, which emends the quality control standard of the original Xuhanting granule and increases the content determination of astragaloside IV. The quality control method of Xuhanting preparation of the invention doesn't have disturbance in the negative comparison, has stronger exclusivity and better repeatability, is advantageous to realize effective controlling of the quality of Xuhanting preparation finished product, and insures the security and the efficiency of human body. The invention can be applicable to not only the quality control of Xuhanting granule but also the quality control of other prescriptions such as Xuhanting capsule, oral liquid, and the like.
Owner:GUANGZHOU BAIYUNSHAN QIXING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com