Method for preparing astragaloside IV by converting total saponins of astragalus by microorganisms
A technology for the transformation of total astragalosides and microorganisms, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of difficult separation and extraction, low content of astragaloside IV, and overcome saponin damage, regional and Strong stereoselectivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Using Absidia corymbifera Preparation of Astragaloside IV by Conversion of Total Astragalus Saponins
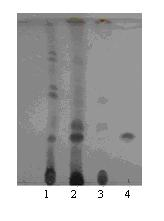
[0028] Two Umbrella Branches Absidia corymbifera ) test tube slant, add 50 mL sterile water to make a spore suspension, inoculate (1 mL per bottle) in a 250 mL shaker flask filled with 50 mL liquid medium, and the liquid medium is potato medium (200 g potato plus water 1 L, boil for half an hour, filter to remove the residue, add 20 g glucose to the filtrate, natural pH). 28°C, 220 rpm, cultured for 24 hours to obtain seed solution. Inoculate 250 mL of seed liquid into a 5 L fermentation bottle containing 1 L of the above liquid medium, culture at 28 °C, 220 rpm for 24 h, add crude total saponins of astragalus (feeding concentration 10 g / L), and transform under the same conditions 72 h. For the results of TLC analysis of the fermentation broth, see figure 1 , Absidia corymbifera It can convert other astragalosides in total astragalosides into astra...
Embodiment 2
[0034] Embodiment 2: Preparation of Astragaloside IV Injection
[0035] Prescription: Astragaloside IV 1.0 g
[0036] Ethanol 200 mL
[0037] Propylene glycol 200 mL
[0038] Glucose 100g
[0039] Make up to 1000 mL with water for injection
[0040] Preparation method: Take the prescribed amount of astragaloside IV, dissolve it in the mixed solution of the prescribed amount of ethanol and propylene glycol at 40°C, stir and add part of the water for injection, then add the prescribed amount of glucose, and make up the water for injection to 1000 mL after completely dissolving , filtered through a 0.22 μm filter, potted, and sterilized to obtain 200 vials of 2 mL specifications of astragaloside IV injection.
[0041]
Embodiment 3
[0042] Example 3 Astragaloside IV Injection Treating Cardiovascular and Cerebrovascular Examples
[0043] 1 Experimental purpose Coxsackie B 3 virus (CVB 3 ) infection-induced viral myocarditis model in mice, evaluate the therapeutic effect of the astragaloside IV injection prepared in the present invention on viral myocarditis, and provide experimental basis for the development and clinical application of new drugs for the treatment of viral myocarditis.
[0044] 2 Experimental method Astragaloside IV was divided into two dose groups, and 50 Balb / c mice were randomly divided into 4 groups. Normal control group (5 rats): no astragaloside IV, no virus inoculation, only the same amount of vehicle; virus control group (15 rats): each rat was intraperitoneally inoculated with 0.1 mL CVB 3 Virus fluid (TCID 50 =10 -7.5 , 10 5 Doubling dilution); administration group I - high dose (15 rats): CVB inoculated with the same method as above 3 Astragaloside IV injection 3 mg / kg by t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com