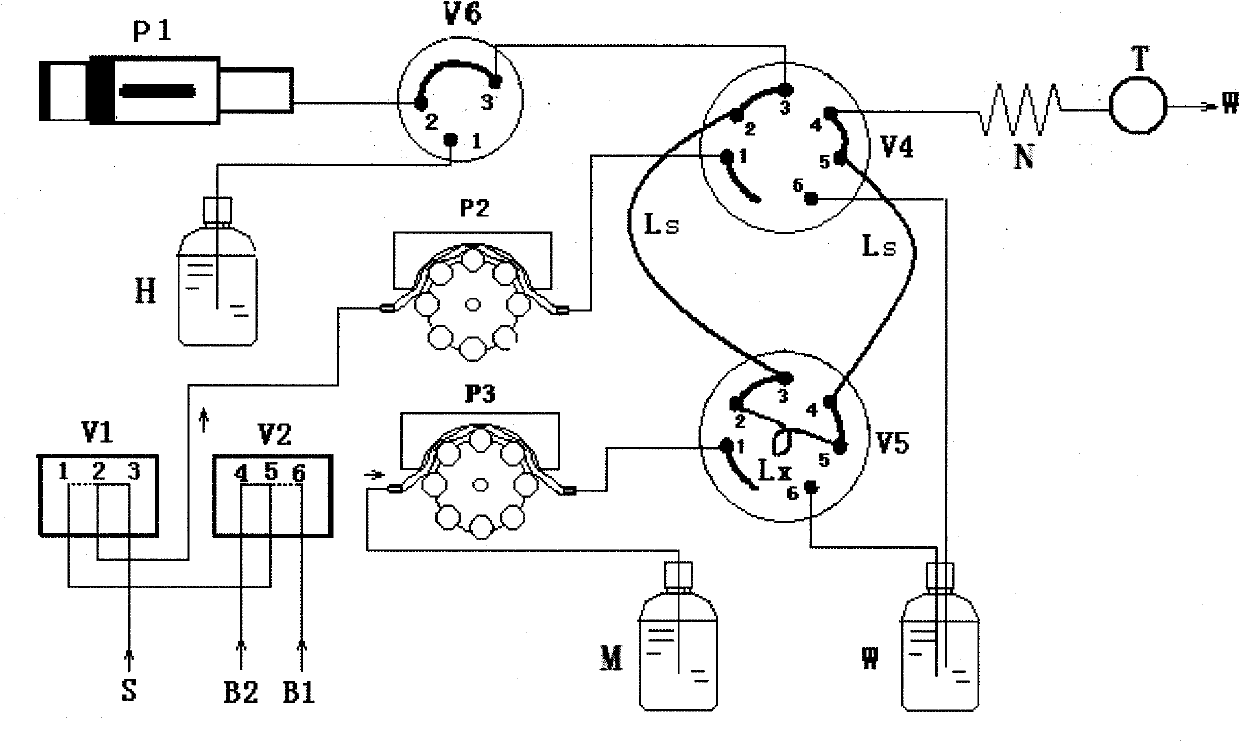
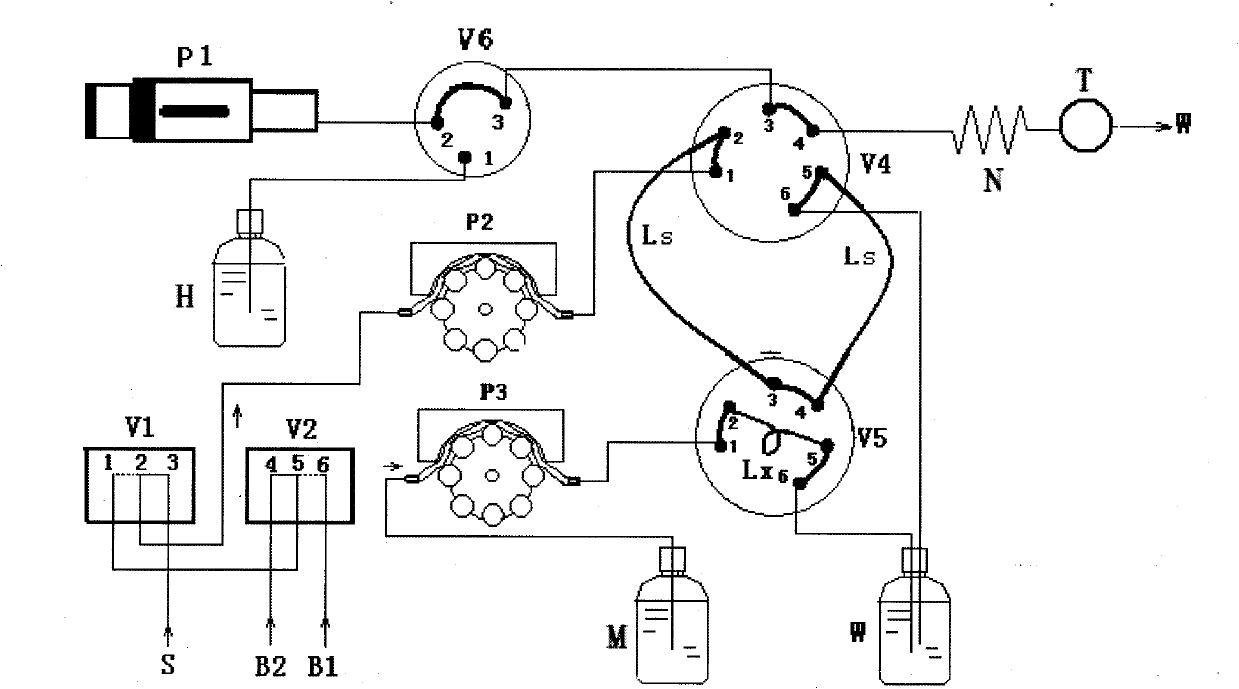
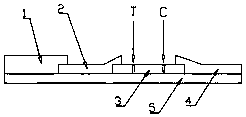
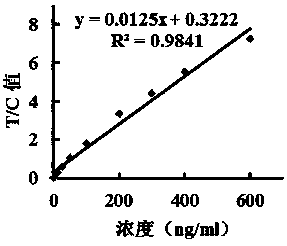
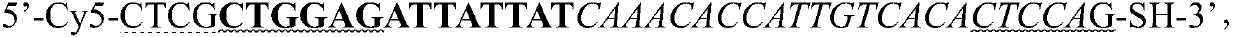Patents
Literature
1632results about How to "Quantitatively accurate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tobacco additive dropping pill wrapping material, dropping pill wrapped thereby and application of dropping pill
ActiveCN102108136AExpand the scope of applicationQuantitatively accurateTobacco smoke filtersGlycerolChemical composition
The invention discloses a tobacco additive dropping pill wrapping material, a dropping pill wrapped by the tobacco additive dropping pill wrapping material and application of the dropping pill and belongs to the technical field of cigarettes. A tobacco additive is wrapped in a special rubber sheet and then is prepared into capsules by a dropping method. The tobacco additive dropping pill wrapping material consists of the following raw materials in part by weight: 1 to 8 parts of sodium alga acid, 0.5 to 5 parts of glycerol and 85 to 100 parts of water; the tobacco additive dropping pill is prepared through a dripping machine in the prior art; the dropping pill is used for a filter tip rod and is wrapped in cut tobacco tows; and when used, the dropping pill is extruded by hands to release the additive or perfume. The additive can endow special fragrance and taste to cigarettes or can reduce the content of harm chemical components in the fume of the cigarettes. The preparation method of the tobacco additive dropping pill wrapping material has a simple and feasible technical process; equipment is simple; the method can be directly applied to processing of the cigarettes and is convenient to popularize and use; raw materials are wide in source and low in cost.
Owner:HUBEI CHINA TOBACCO IND +1
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Automatic detection meethod and system for smooth surface flaw
ActiveCN1563957AImprove accuracyQuantitatively accurateMaterial analysis by optical meansMathematical modelComputer-aided
Based on standard of faulty work and light scattering characteristic of faulty work, new type computer aided digitized testing device suitable to large caliber, sub aperture scattering imaging is built. Features of the testing device are as following: Kohler cold light source arranged in multiple optical fiber and multiple azimuth angles; reflecting imaging of scattered light from faulty work on surface to be tested; scattered light collected by micro zooming system and imaged on CCD; Movable operating table in X, Y directions controlled by computer through multiple sub apertures scans surface to be tested in large caliber; building up mathematical model pattern recognition based on mathematical morphology, and software for calibrating size of faulty work. The invention builds objective digital evaluation system, raises working efficiency. The device is suitable for recognizing and evaluating faulty work in size larger than several micros.
Owner:ZHEJIANG UNIV
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
A kit for early diagnosis of neoplastic disease, a method and applications of the kit
InactiveCN107893101ASimplify detection stepsImprove reliabilityMicrobiological testing/measurementDiseaseFluorescence
The invention belongs to the technical field of neoplastic disease diagnosis, and discloses a kit for early diagnosis of neoplastic disease, a method and applications of the kit. The kit includes a specific capturing and detecting system for a tumor exosome and a molecular beacon fluorescent detection system. The specific capturing and detecting system for the tumor exosome includes a solution ofa magnetic gold nanosphere composite modified by a tumor exosome specific ligand and adopting ferric ferrous oxide as a core, and a gold nanocage solution modified by the exosome specific ligand. Themolecular beacon fluorescent detection system includes a molecular beacon probe provided with a fluorescent group, fluorescent amplification substrate hybrid chains provided with fluorescent groups and a buffer liquid. The kit and the method combine tumor exosome specific capturing and analysis and a molecular beacon fluorescent detection method, and greatly increases accuracy and reliability of early diagnosis of tumor.
Owner:ZHENGZHOU UNIV
Immuno magnetic bead and producing method, and method and test plate for detection
An immune bead is made up of the magnetic carrier micro ball which combines at least one immune matching base. The micro ball is composed of the magnetic nm particle and the high molecular framework material which the core is the metal particle; the out of the core is high molecular framework, the out layer is the functional layer which can combines functional gene of different immune matching base. The manufacture method includes: the bead pretreatment, the bead activation, manufacture of the coupling antibody, closing the antibody with the confining liquid and purifying the immune bead. The detecting method is to detect the different things by the immunological response sandwich, the competition and the indirect method and set the control system on the testing board. The board is made up of the encrusting test paper, the coupling mat, the sample mat, the water suction mat, the coving film and the testing board outside calipers. It has the high sensitivity and accurate quantity; the regent is simple and cost low.
Owner:YANGTZE DELTA REGION INST OF TSINGHUA UNIV ZHEJIANG
Method for measuring alkaloid in tobacco and tobacco product
ActiveCN102004132AQuantitatively accurateGood reproducibilityComponent separationBiotechnologyGas liquid chromatographic
The invention relates to a method for measuring alkaloid in tobacco and tobacco products, which is characterized by dissociating alkaloid from the tobacco and tobacco products by using sodium hydroxide solution, extracting the alkaloid from samples by using triethylamine / trichloromethane solution, and detecting content of five kinds of alkaloid with GC-MS ( Gas Chromatograph-Mass Spectrum) quantitative analysis. Compared with other methods for analyzing the alkaloid, the invention has the advantages that alkaloid is separated from the tobacco by DB-35MS chromatographic columns so as to separate nornicotine and myosmine in the tobacco and accurately quantify target matters; batch sampling is carried out on the separation of nicotinamide and other alkaloid so as to detect the nicotinamide with high content in the tobacco and meet the quantitative analysis of other alkaloid with low content; the standard working curves of standard matters are separately compounded so as to avoid influence on quantization of other alkaloid by alkaloid catabolite existing in the standard samples. The method is simple and convenient and has the advantages of good repeatability, high analyzing and measuring sensitivity, and accurate quantization.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Method for simultaneously detecting multi-kind pesticide residues in bee products
InactiveCN101358953ASolve the problem of matrix effectFast wayComponent separationRetention timePhosphate
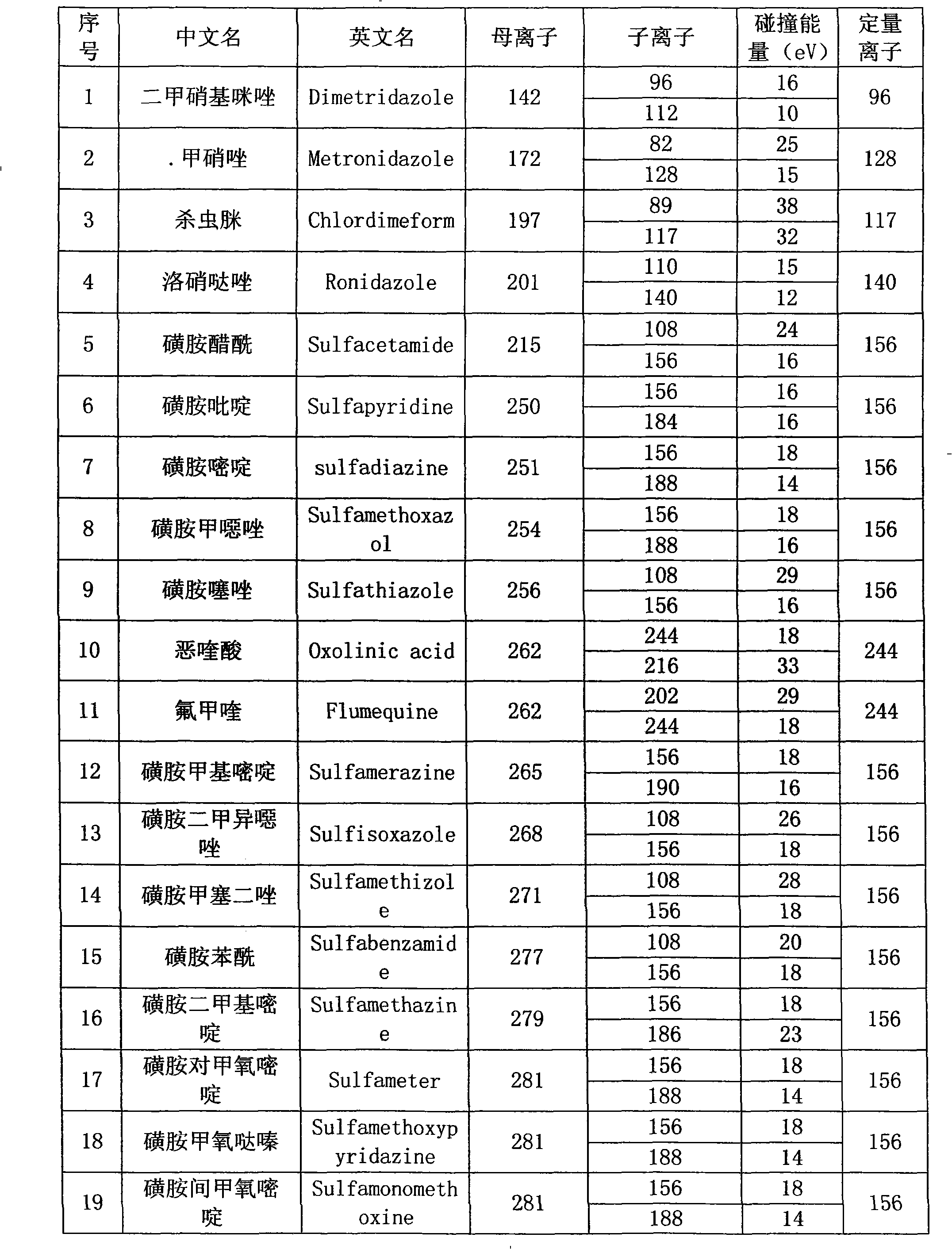
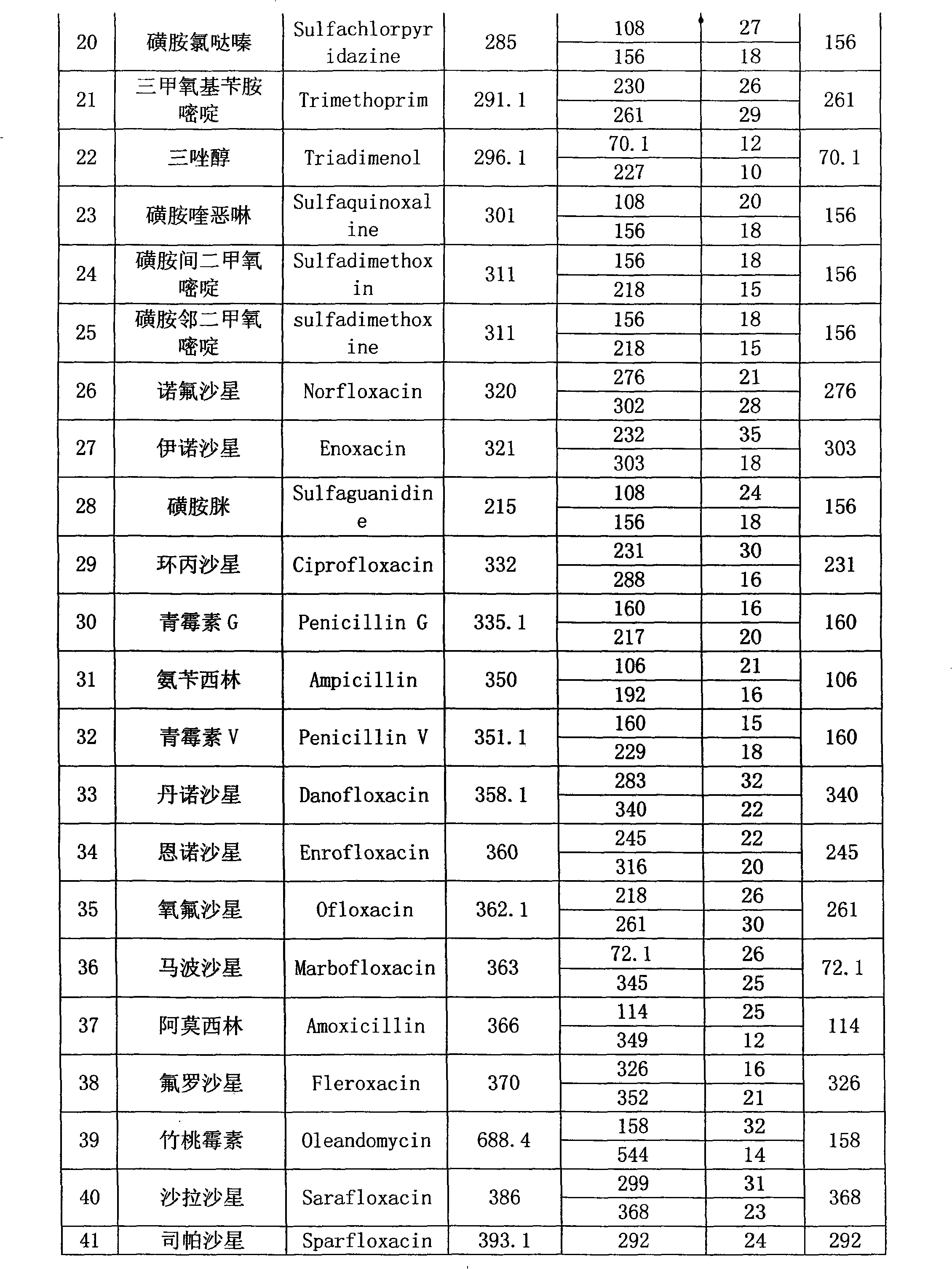
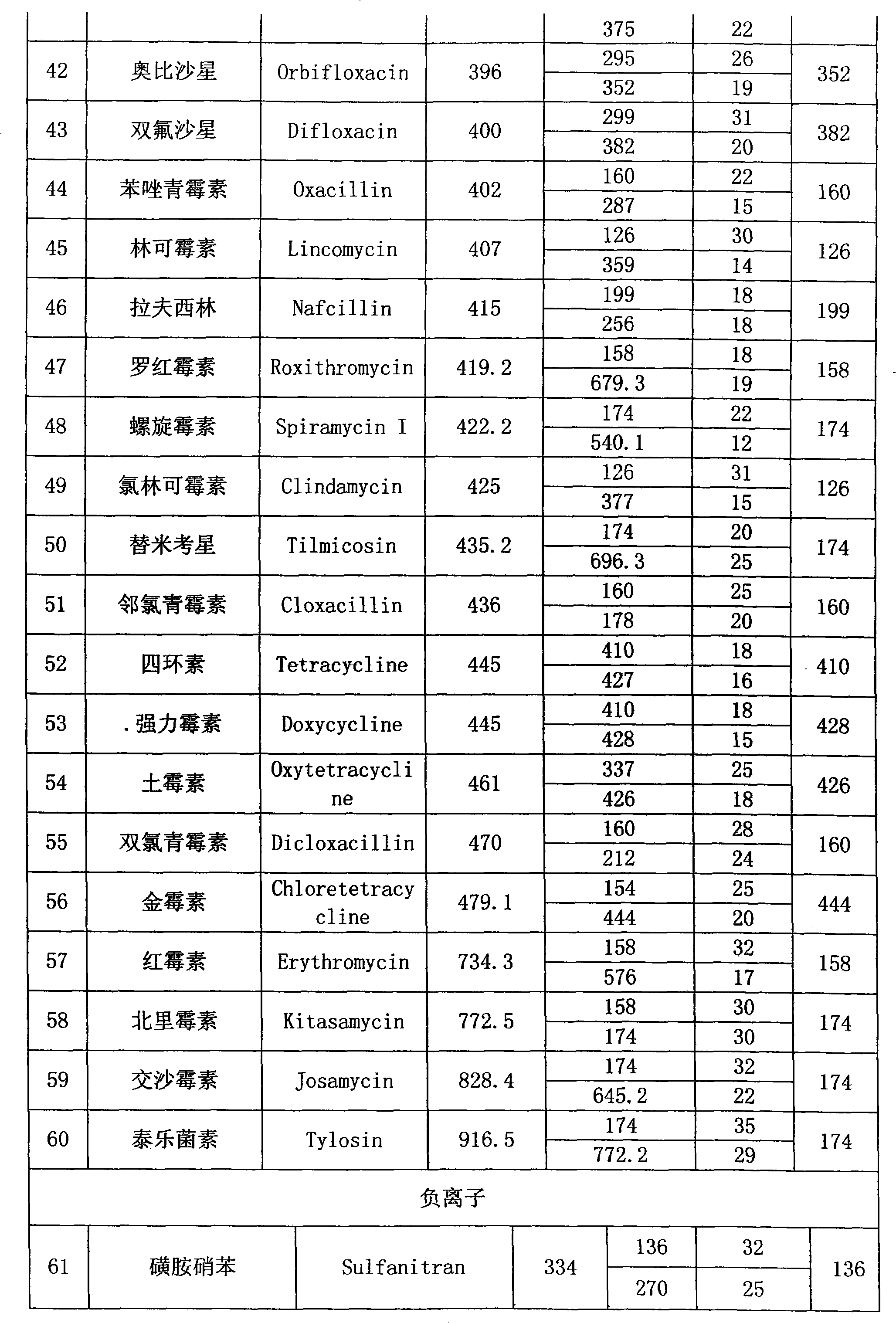
The present invention relates to a method of simultaneously detecting a plurality of agro-veterinary drug residues in bee products. The extracted liquid trichloroacetic acid or perchloric acid and the extracted liquid acetate, phosphate or borate solution are added into a sample; the pH value is controlled between 4.5 and 9.0; the mixed solution is centrifuged, the filtrate is added into a solid phase extraction column to be extracted, the extraction column is eluted and dried, the column is washed by oxalic acid-methanol solution, the volume of the eluent is defined by the aqueous solution of methanol, the eluent is added into liquid chromatography-tandem mass spectrometry to be analyzed and tested, the acquired chromatographic peak is contrasted with the known standard chromatographic peak of the drug, and according to the retention time and the abundance of the mass spectrum ions, the specific name of the detected drug is determined. The method only requires one pre-treatment of the sample, and thus can simultaneously extract 11 classes and more than 60 kinds of veterinary drug residues, such as sulfonamides, quinolones, macrolides, lincomycins, nitroimidazoles, beta-lactams, tetracyclines, chloromycetins, trinethoprims, chlordimeform, triadimenol and the like, the efficiency of analysis is high, and the detection cost is greatly reduced.
Owner:中华人民共和国江苏出入境检验检疫局
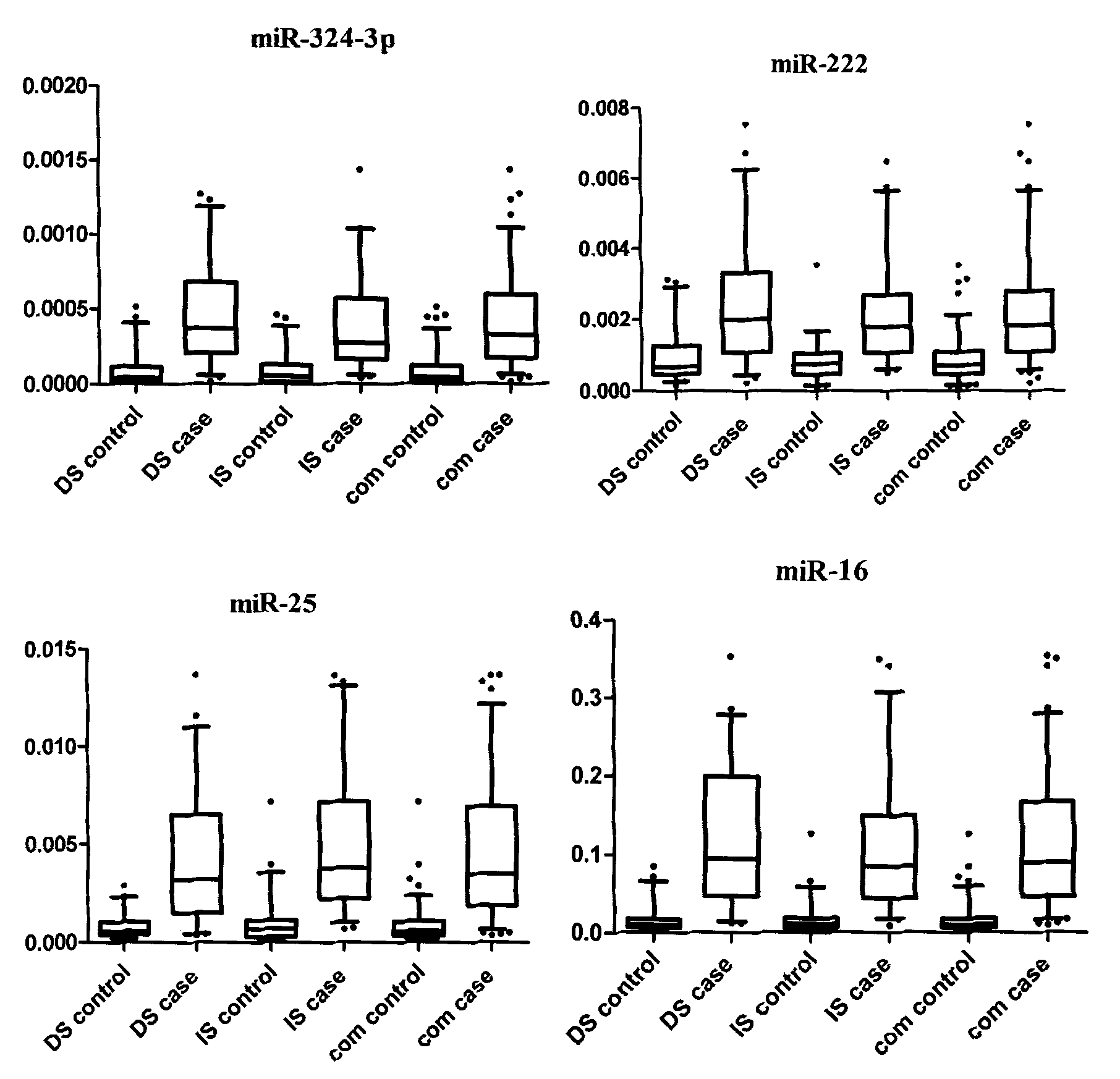
Serum/plasma miRNA marker associated with breast cancer and application thereof
InactiveCN101921760AAids in diagnosisEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationOncologyBlood plasma
The invention belongs to the fields of gene engineering and oncology and discloses a serum / plasma miRNA marker associated with breast cancer and application thereof. The marker is a combination of miR-16, miR-25, miR-222 and miR-324-3p. The maker and primers thereof can be used for preparing a diagnosis kit and assisting early diagnosis of the breast cancer.
Owner:NANJING MEDICAL UNIV
Method for detecting tobacco-specific nitrosamines
The invention relates to a method for measuring tobacco-specific nitrosamines (TSNAs for short) by using a super efficient liquid chromatogram-tandem mass spectrum combined method, and belongs to a method for detecting TSNAs. The method comprises the following steps of: adding internal standard-containing aqueous solution of ammonium acetate into a filter sheet after trapping smoke and cigarette filters smoked completely under standard conditions or tobacco shreds, performing ultrasonic extraction, transferring 1 to 2mL of extract into a small solid-phase extraction column taking N-vinyl pyrrolidone-benzene sulfonic acid copolymer as filler, flushing the small column by using aqueous solution of methanoic acid and methanol, finally eluting the small column by using solution of aqueous ammonia and methanol solution, analyzing the eluant by using an ultra performance liquid chromatography-mass spectrometer / mass spectrometer (UPLC-MS / MS), and quantifying according to the area ratio of a component peak to an isotope internal standard peak. The detecting method can be used for detecting the TSNAs in cured and hybrid cigarette mainstream smoke, cigarette side-stream smoke, filter tip intercepting smoke and tobacco shreds, and has the advantages of simple pretreatment, accurate quantification, high instrument analysis speed, low detection limit, high sample treatment flux and the like.
Owner:HONGTA TOBACCO GRP
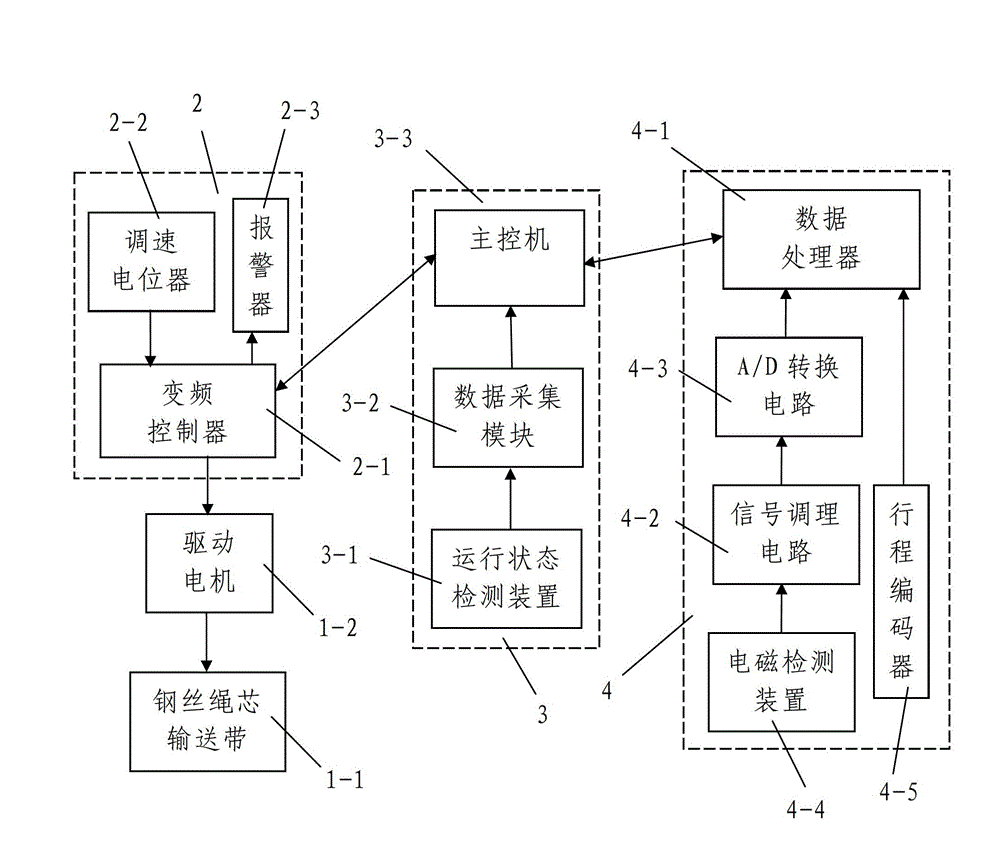
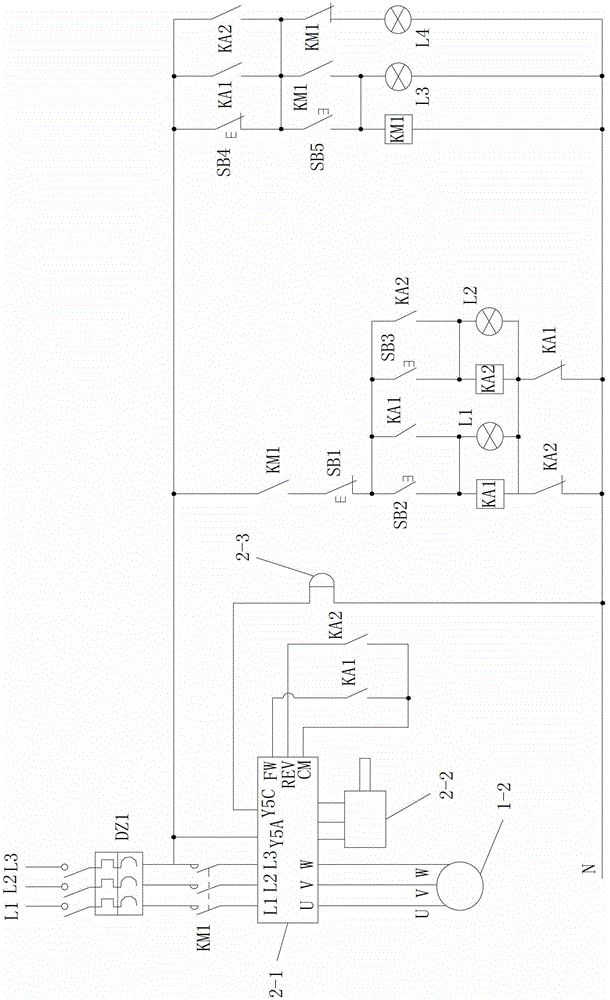
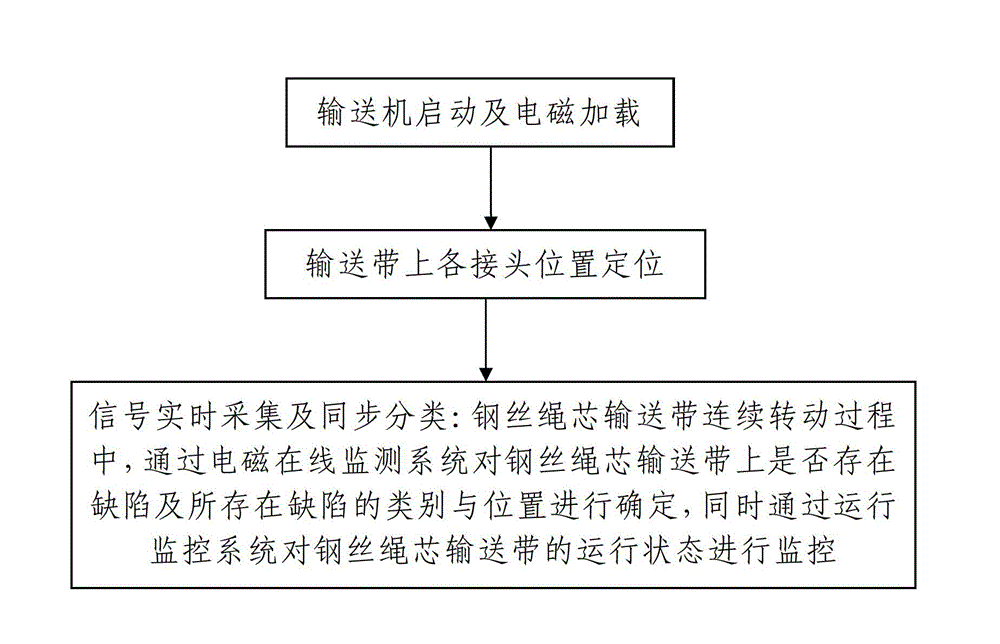
System and method for intelligently monitoring belt-type conveyer for coal mine steel wire rope core
ActiveCN103144937AReasonable designEasy wiringControl devices for conveyorsMonitoring systemEngineering
The invention discloses a system and a method for intelligently monitoring a belt-type conveyer for a coal mine steel wire rope core. A monitoring platform of the monitoring system comprises a variable frequency control system, a running monitoring system for monitoring the running state of a steel wire rope core conveying belt and an electromagnetic online monitoring system for monitoring whether a defect is existent in the steel wire rope core conveying belt or not and the type and the position of the existent defect. The monitoring method comprises the steps as follows: 1, starting the conveyer and electromagnetically loading; 2, positioning the connectors on the conveying belt; and 3, acquiring signals in real time and synchronously classifying the signals, namely in the continuous rotation process of the steel wire rope core conveying belt, whether the defect is existent in the steel wire rope core conveying belt or not and the type and the position of the existent defect are determined by the electromagnetic online monitoring system, and the running state of the steel wire rope core conveying belt is monitored by the running monitoring system at the same time. The system and the method are reasonable in design, simple and convenient to use and operate, convenient to realize, good in using effect and high in practical value, and integrate the functions of variable frequency control, running monitoring and electromagnetic online monitoring.
Owner:XIAN UNIV OF SCI & TECH
Cellular biological technique, reagent kits and preparation device
InactiveCN1920559AHigh sensitivityReduce manufacturing costMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementDiseaseMicrowell Plate
The invention relates to a new cell biological technique, agent box and prepare device, wherein said invention has high sensitivity, large information amount, simple production, and low cost, while it can analyze different nucleic acids, proteins, and molecules of sample at same time; the invention is characterized in that it adheres different cells on film base or plants them on different micro holes of porous plate, the tested sample is combined with cell or carried biology molecule, and the tested result can be watched by eye or recorded and analyzed by machine. The invention can be used in drug research, biology, etc.
Owner:赵翀
Immunochromatographic test strip for quantitatively detecting troponin I in whole course and preparation method thereof
The invention discloses an immunochromatographic test strip for quantitatively detecting troponin I in the whole course. The test strip is formed by sticking a sample pad, a glass fiber membrane labeling pad, a nitrocellulose coating membrane and absorbent paper to a substrate in sequence through lap joint, wherein a fluorescent latex particle labeled troponin I monoclonal antibody is coated on the glass fiber membrane labeling pad; the nitrocellulose coating membrane comprises a detection region and a quality control region; the detection region is coated by another troponin I monoclonal antibody with epitope different from that of the fluorescent latex particle labeled troponin I monoclonal antibody; and the quality control region is coated by goat anti-mouse IgG (immunoglobulin G). The test strip has the advantages of safety, simpleness and convenience in operation, suitability for single human / sample detection, rapidness and the like and can sensitively and quantitatively determine the troponin I in the whole course in 10 seconds, diagnose the diseases and identify infection more rapidly and accurately and detect the infection conditions and determine the curative effects of antibiotics.
Owner:GUANGZHOU WONDFO BIOTECH
Method for identifying aging degree of silicon rubber composite insulator
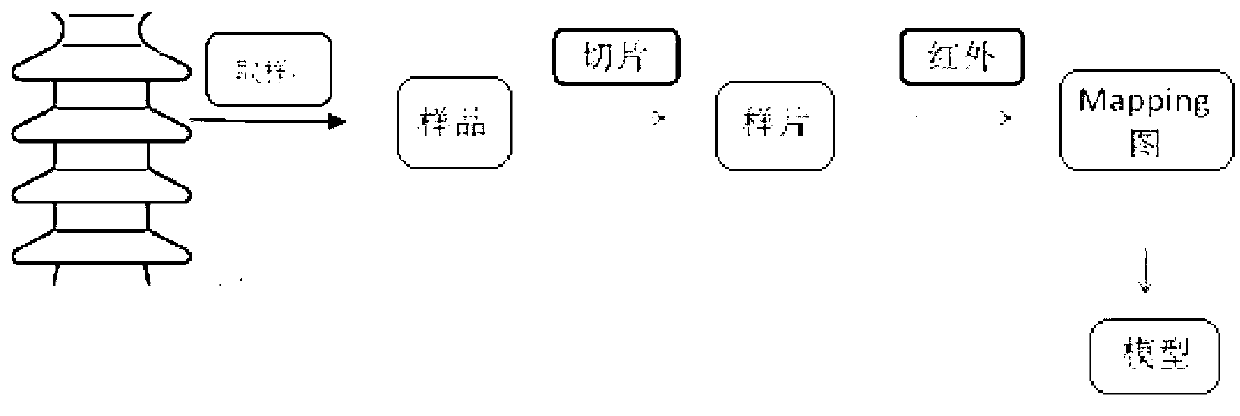
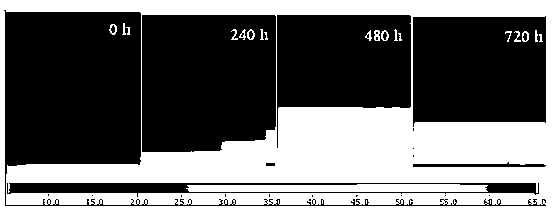
ActiveCN103344605ACan quantitatively analyze the degree of agingQuantitative analysis of aging degreeColor/spectral properties measurementsTest sampleSilicon rubber
The invention discloses a method for identifying the aging degree of a silicon rubber composite insulator. The method comprises the following steps: determining the states of umbrella skirt test samples of the silicon rubber composite insulator by taking the operating age limit as reference, and selecting a sample area of each state to obtain an average-value test sample of each state; sampling and cutting into slices; performing microscopic infrared scanning on the slices by taking silicon methyl 1296 cm<-1> as a calibrated peak to obtain microscopic infrared micro-area scanning images of the slices with different age limits; calculating the ageing depth H value from the microscopic infrared micro-area scanning images, fitting the relationship between the H value of the samples with different age limits and time to obtain an ageing model; quantitatively identifying the ageing degree of an unknown sample according to the ageing model. According to the method, the ageing degree of the silicon rubber composite insulator can be quantitatively analyzed by a microscopic infrared Mapping scanning technology; the change of the silicon rubber composite insulator from the surface to the center along with ageing can be observed; the visualization degree is high.
Owner:ELECTRIC POWER RES INST OF GUANGDONG POWER GRID +1
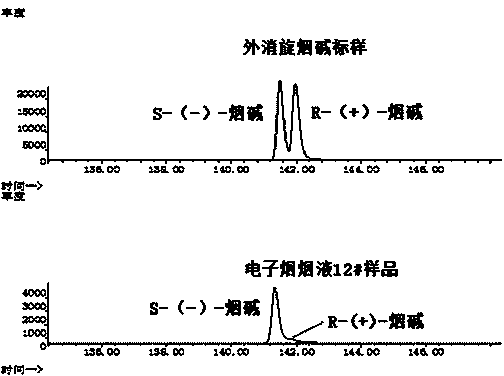
Chiral analysis method for nicotine in tobacco juice of electronic cigarette
InactiveCN104297409AEasy to handleLow detection limitComponent separationStrength propertiesAlcoholGas liquid chromatographic
The invention discloses a chiral analysis method for nicotine in tobacco juice of an electronic cigarette. The chiral analysis method comprises the steps of diluting the tobacco juice of the electronic cigarette by a methyl alcohol / methyl tertiary-butyl ether solution, analyzing S-(-)-nicotine and R-(+)-nicotine in the tobacco juice of the electronic cigarette by using a chiral stationary phase capillary column mthrough a gas chromatograph-mass spectrometer, performing chiral analysis on the S-(-)-nicotine and the R-(+)-nicotine by comparing the retention time of a chromatographic peak and characteristic ions of nicotine in a standard sample with the retention time of a chromatographic peak and characteristic ions of nicotine in an electronic cigarette tobacco juice sample, and normalizing areas of quantitative ion peaks of the S-(-)-nicotine and the R-(+)-nicotine to quantify the proportions of the S-(-)-nicotine and the R-(+)-nicotine to the total nicotine. The chiral analysis method has the advantages that the nicotine in the tobacco juice of the electronic cigarette is separated by a chiral stationary phase capillary column, so that the S-(-)-nicotine and the R-(+)-nicotine in the nicotine can be better separated, and accurate and quantitative analysis on the nicotine can be realized. The sample treatment is simple and convenient; the chiral analysis method is low in detection limit, high in sensitivity, high in reproducibility, accurate in quantification and suitable for chiral analysis on the nicotine in a large batch of electronic cigarette tobacco juice samples.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Methods and systems for detecting and/or sorting targets
InactiveCN101522915AHigh sensitivityMinimize denaturationMicrobiological testing/measurementCombined usePolynucleotide
Provided herein are methods and systems for detecting and / or sorting targets in a sample based on the combined use of polynucleotide-encoded protein and- substrate polynucleotides. The polyhucleotide-encoded protein is comprised of a protein that specifically binds to a predetermined target and of an encoding polynucleotide that specifically binds to a substrate polynucleotide, wherein the substrate polynucleotide is attached to a substrate.
Owner:CALIFORNIA INST OF TECH
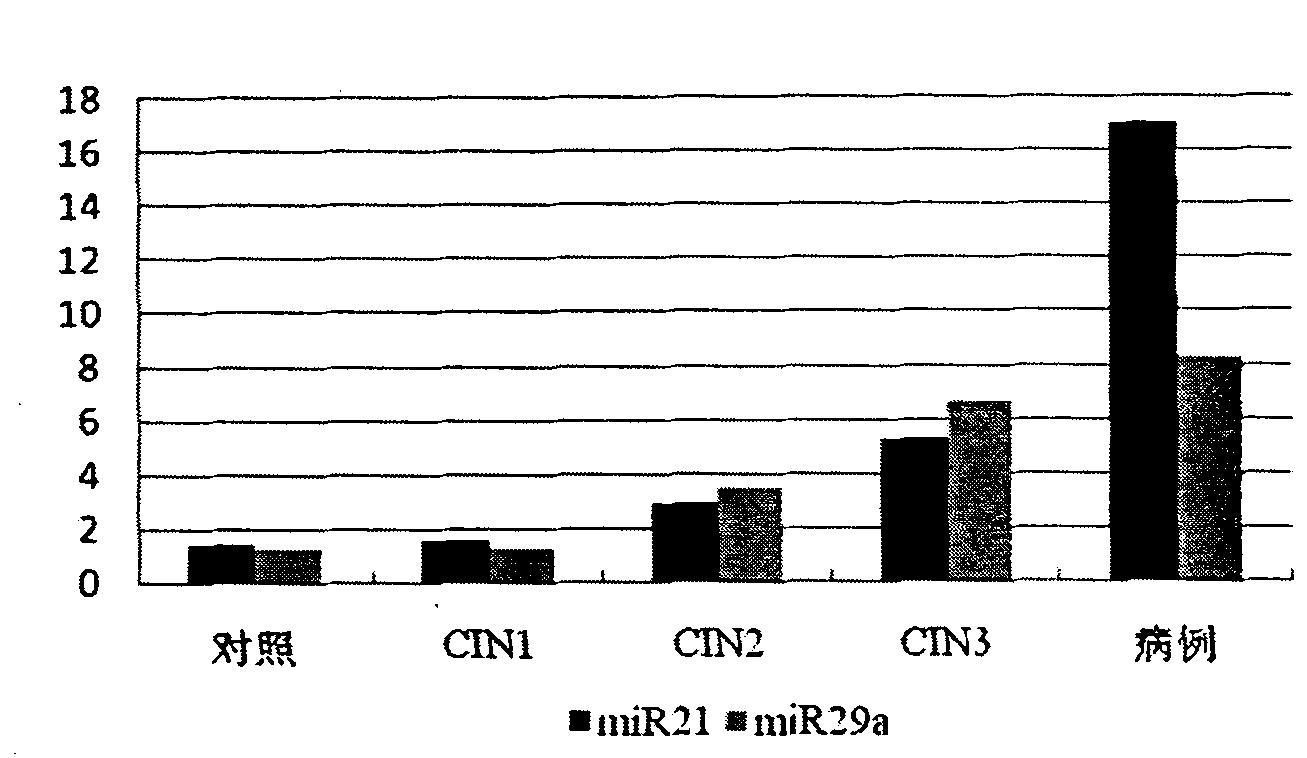
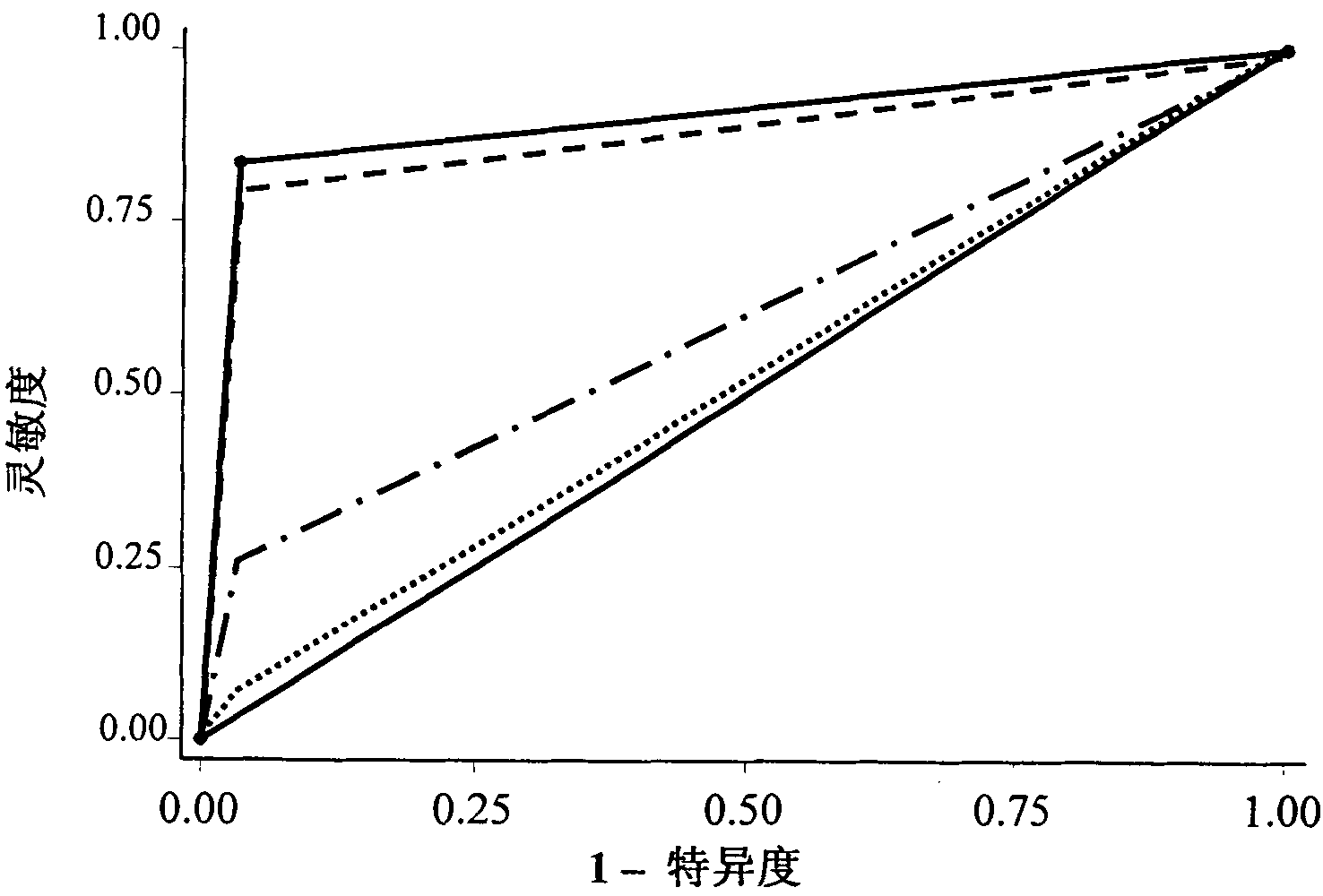
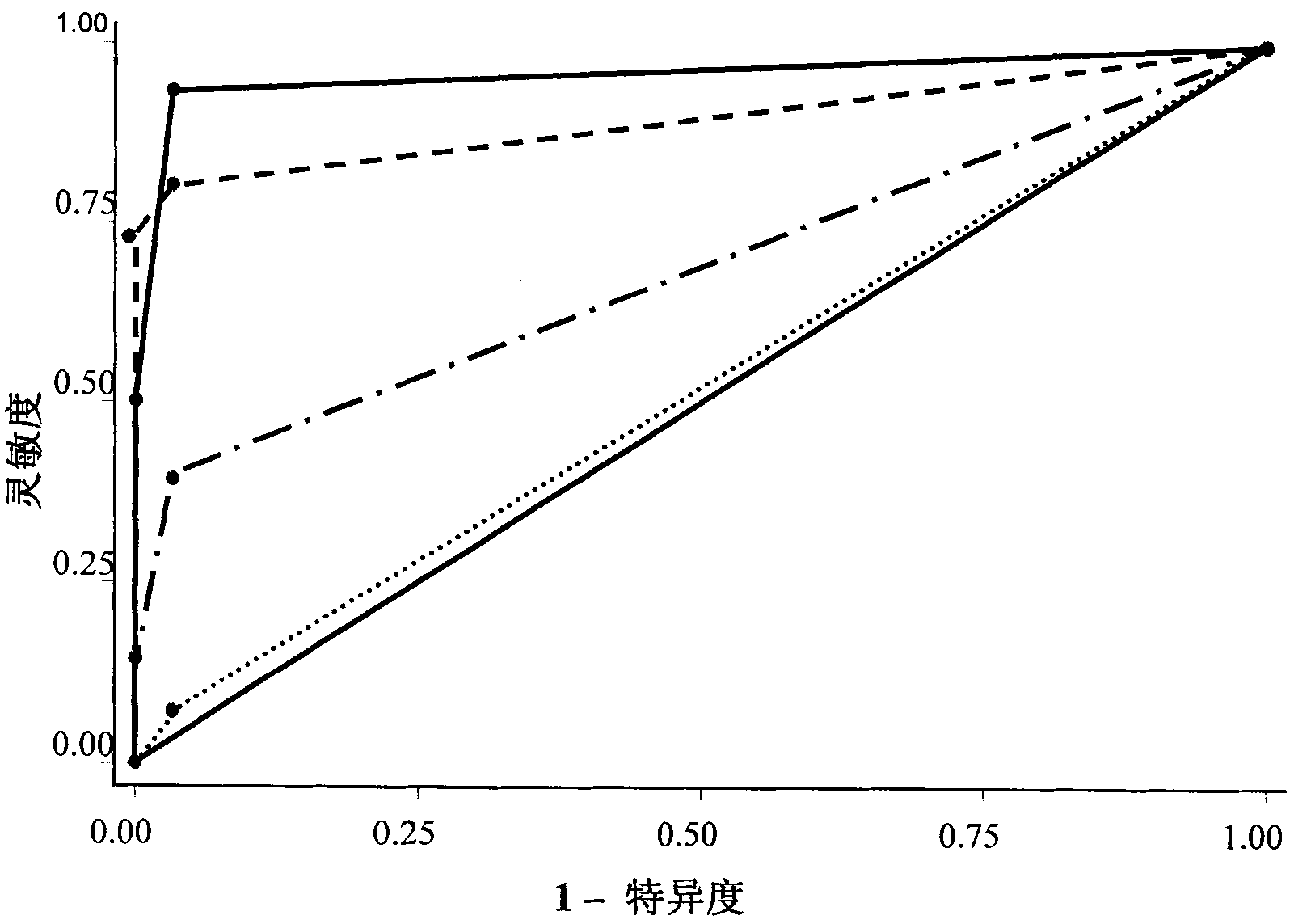
Serum/plasma miRNA serum marker related to cervical carcinoma and precancerous lesions thereof and application thereof
ActiveCN101921759AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationSerum markersBlood plasma
The invention belongs to the fields of gene engineering and oncology, and discloses a serum / plasma miRNA serum marker related to cervical carcinoma and precancerous lesions thereof and application thereof. The marker is single miR-21 or the combination of miR-21 and miR-29a. The marker and the primer thereof can be used for preparing diagnosis kits which are used for assistant early diagnosis of cervical carcinoma and precancerous lesions thereof.
Owner:NANJING MEDICAL UNIV
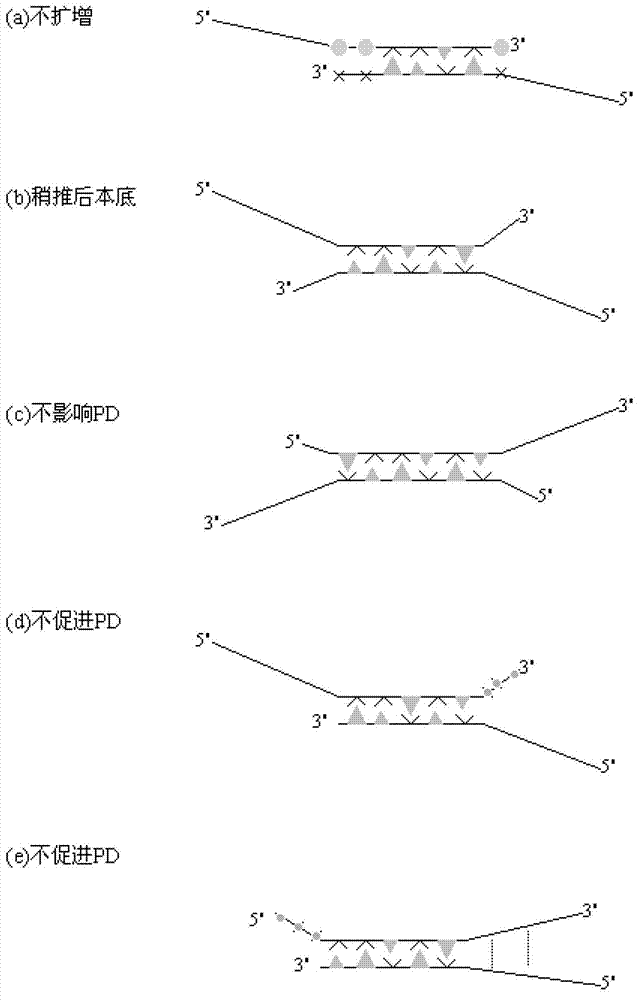
Primer middle sequence interference PCR (Polymerase Chain Reaction) technology
InactiveCN103114131AHigh Sensitivity FeaturesQuantitatively accurateMicrobiological testing/measurementAgricultural scienceFluorescence
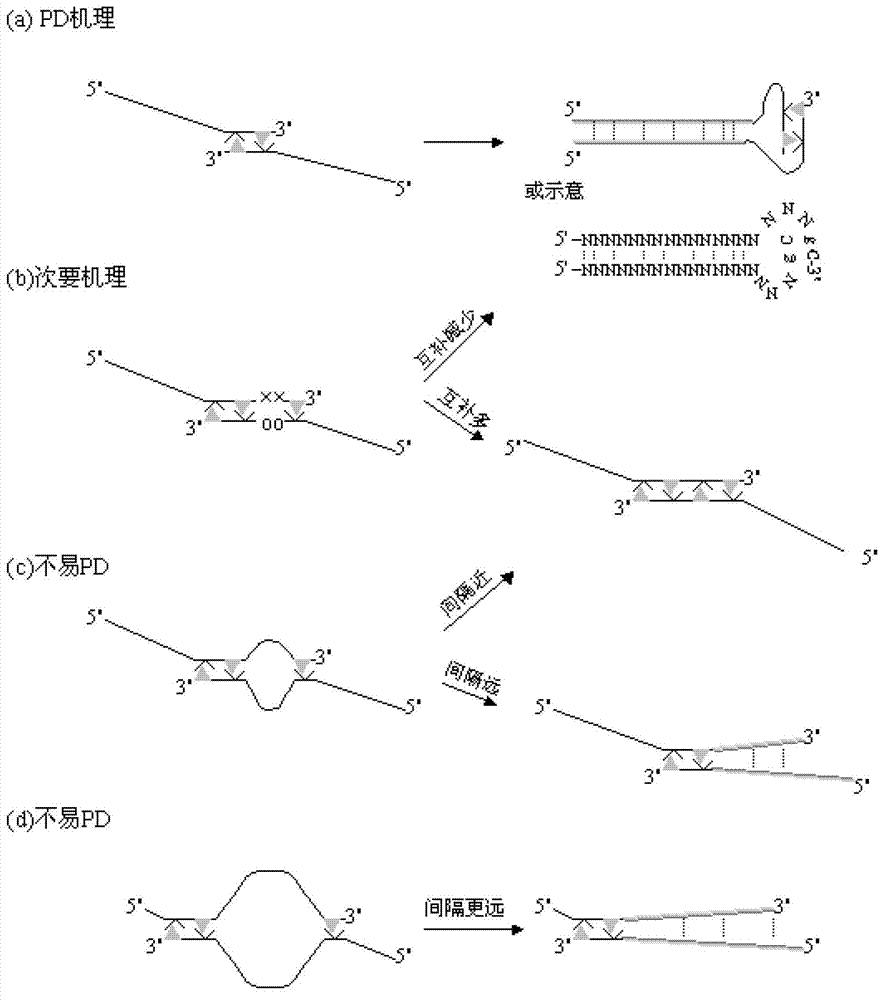
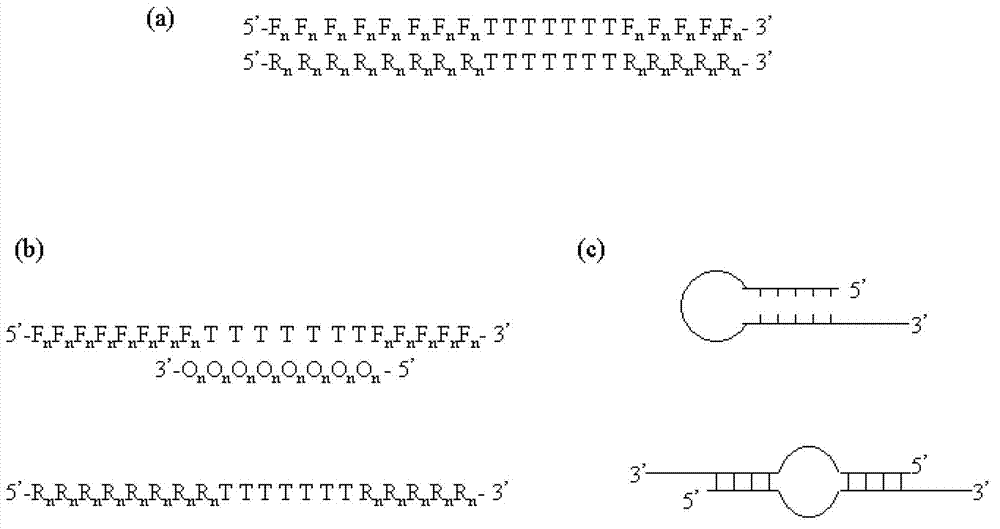
The invention relates to a primer middle sequence interference PCR (Polymerase Chain Reaction) technology. The improved PCR technology is characterized in that one segment of relatively non-complemented or same-sequence basic group primer molecules in the intermediate domain of primers perform the antisense interference inside and outside so as to competitively destroy the polymerization among the primers to selectively inhibit the primer dimer (PD) from being amplified. For the interference of the intermediate domain of the primers, based on the primers optimally selected by the conventional design principle, the technology that the intermediate domain (ID) of a pair of the primers are in parallel but are not complemented with each other or are in the same sequence or / and the technology that ID antisense oligonucleotides (Oligo) are added into the primers to perform the interference action or / and the Oligo antonymy is carried out in the primer molecules via the ID so as to perform the interference action are adopted, or the combined technology of the three types of the technologies is adopted. As a result, only the ID of the primers is interfered while the target specific amplification is not influenced; the combining force acting on the minority of base-group pairing hydrogen bonds at the tail end and the base-group hydrogen bonds outside the tail end due to the action of the primers is dispersed to a maximum extent, so that the PD is selectively inhibited. Therefore, the PD accumulation in the PCR system is avoided. If the mineral oil is additionally used, the sealed primers can slowly release the hot starting and the UDG pretreatment so as to prevent aerosol glue as a byproduct of the PCR system from causing the pollution. Consequently, the nucleic acid is amplified reliably and the real-time fluorescence PCR is quantified accurately.
Owner:珠海市坤元科技有限公司
25-hydroxy-vitamin D3 detection kit, as well as preparation method and applications thereof
ActiveCN102998468AEasy to operateIncreased sensitivityBiological testingAntiendomysial antibodiesActive agent
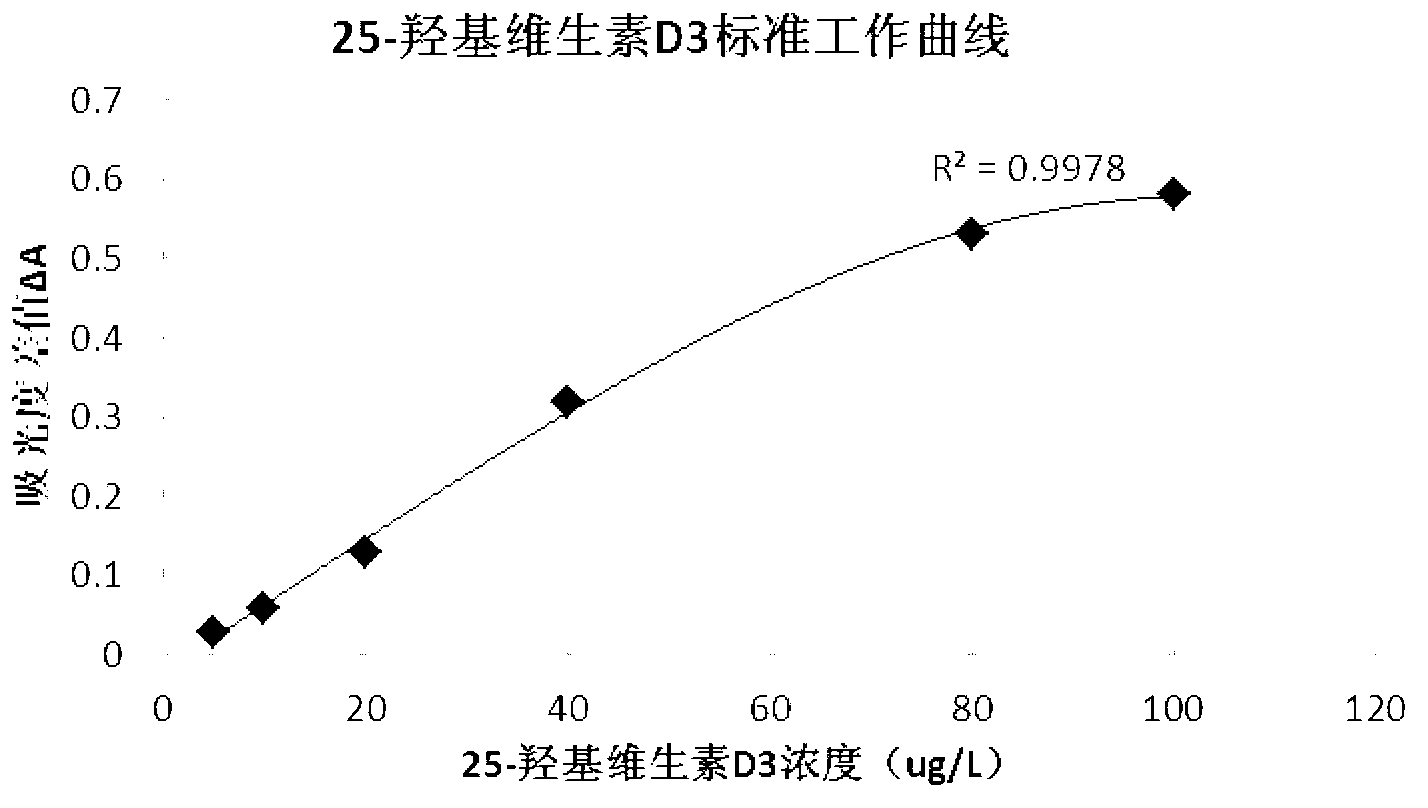
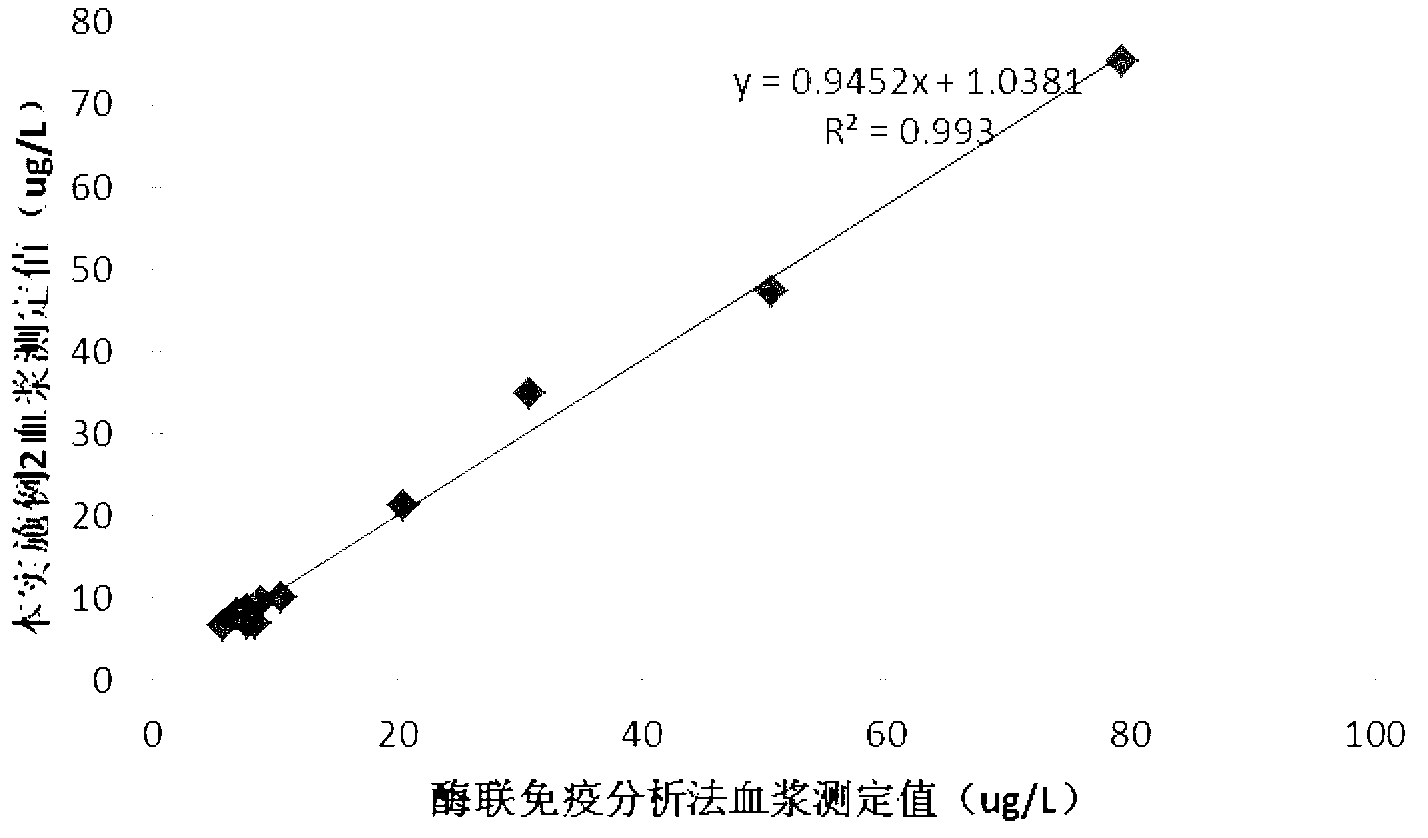
The invention discloses a 25-hydroxy-vitamin D3 detection kit, as well as a preparation method and applications thereof. The detection kit consists of a reagent I and a reagent II which are independent from each other, wherein the reagent I comprises the following components: a 25-hydroxy-vitamin D3-BSA conjugate, a biobuffer, a chelating agent, a surface active agent, a coagulant, a preservative, a stabilizer and water; the reagent II comprises the following components: rubber latex particles coated by a 25-hydroxy-vitamin D3 antibody, a biobuffer, a chelating agent, a surface active agent, a suspending agent, a preservative, a sealing agent, a stabilizer and water. The detection kit disclosed by the invention is high in detection sensitivity, high in specificity, accurate in quantification and wide in linear range, and is especially suitable for the detection of samples with extremely low 25-hydroxy-vitamin D3 content. In addition, the detection kit disclosed by the invention does not need to dilute the samples in advance in the detection process, facilitates the clinical use, and is easy to operate, low in detection cost and suitable for various full-automatic biochemical analyzers.
Owner:CUSABIO TECH LLC
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceBasic levelQuantum dot
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Detection method of multiresidue of 5 nitrofuran metabolites and chloramphenicol in shrimp
ActiveCN106124653AImprove extraction efficiencyOvercoming the limitations of separate assaysComponent separationMetaboliteQuantitative accuracy
A detection method of a nitrofuran metabolites and chloramphenicol multiresidue in shrimp belongs to the technical field of aquatic products detection. The method is as below: hydrolyzing a sample with hydrochloric acid, subjecting nitrofuran metabolites to derivatization by using 2-nitrobenzaldehyde, adjusting the pH value to 6.5-7.5, adding acetonitrile, adding an extraction salt package; conducting liquid-liquid extraction of a target compound by using acetonitrile; purifying and concentrating a supernatant; then determining by a liquid chromatography-tandem mass spectrometer, and quantifying by an internal standard method. The method uses a novel sample extraction and purification mode for simultaneous analysis and determination of two prohibited drugs with the highest detection rate in shrimp, overcomes the limitations of separate determination of two drugs in the detection method of the prior art, improves the extraction efficiency of chloramphenicol in actual positive samples, greatly improves the work efficiency, shortens the work time and saves reagent consumption and labor costs. The method adopts the isotope internal standard method for quantification, the determination result is more accurate and reliable, has high sensitivity, and good reproducibility and quantitative accuracy.
Owner:青岛菲优特检测有限公司
Anti-Golgi apparatus protein monoclonal antibody and use
InactiveCN101407544AHigh sensitivityQuantitatively accurateImmunoglobulins against animals/humansFermentationSerum igeSerum samples
The invention relates to an anti-Golgi protein antibody and an application thereof. The invention recombines human GP73 protein immunity animal to obtain an anti-GP73 polyclonal antibody and a monoclonal antibody which specifically aims at GP73 and builds a plurality of methods for detecting GP73 in clinical tissue sections and serum samples, such as immunohistochemical stain and double antibody sandwiched ELISA method and the like. Tests prove that the polyclonal and monoclonal antibodies can be used for preparing a plurality of GP73 detecting agents of different detecting methods.
Owner:曹伯良 +1
Apparatus and method for measuring distribution constant of dissolved gas in transformer oil
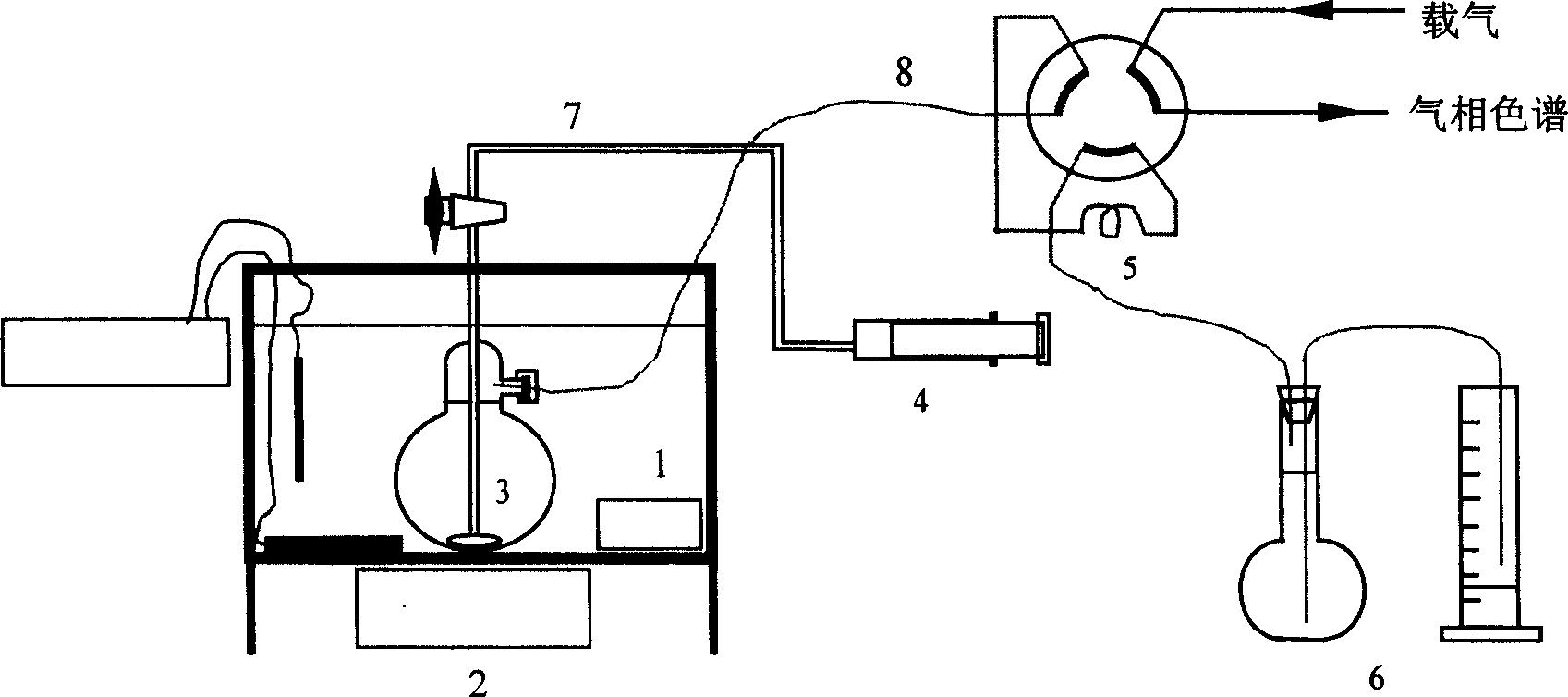
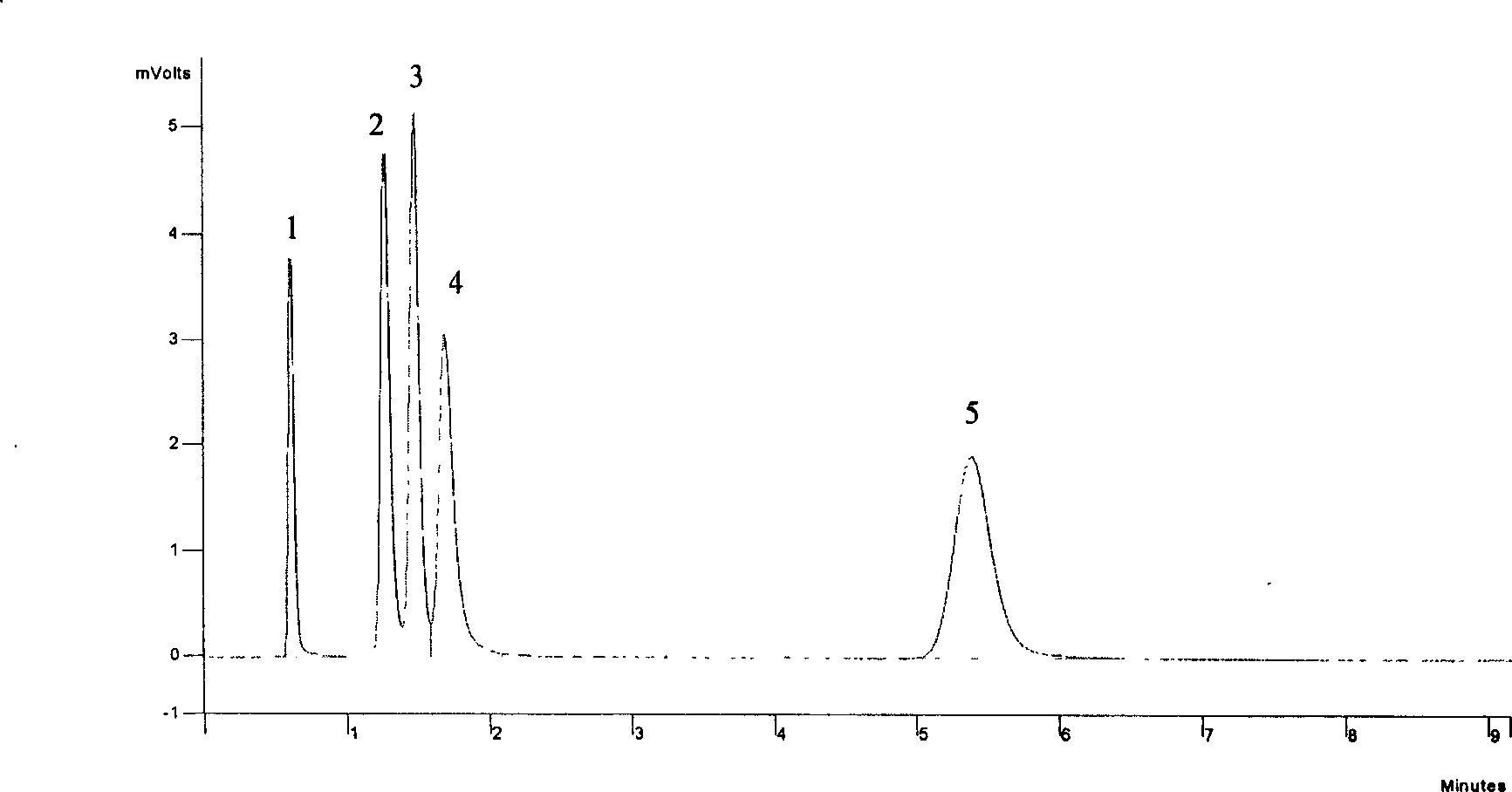
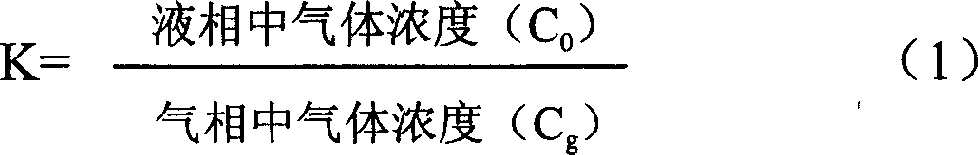
InactiveCN1654952AReliable methodQuantitatively accurateComponent separationWater bathsDistribution constant
A device for on-line testing solved gas distribution constant in transformer oil by gas-phase chromatography includes a constant temperature water-bath, a magnetic mixer, an oil-gas balance bottle, a six-way valve and an on-line gas-phase chromatograph to accurately test solved gas distribution constant in transformer oil under different temperatures.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Device for fluidly injecting and rapidly analyzing residual chlorine of water quality and analysis method thereof
InactiveCN101793902AQuantitatively accurateAccurate measurementMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsPeristaltic pumpReal time analysis
The invention discloses a device for fluidly injecting and rapidly analyzing residual chlorine of water quality and an analysis method thereof. The device comprises a photoelectric flow cell, a reacting tube, an indicator liquid storage vase, a current-carrying liquid storage vase, a first peristaltic pump, a current-carrying liquid injection pump, a second peristaltic pump, a current-carrying liquid three-way valve, a first sampling six-way valve, a second sampling six-way valve, a capillary tube, a sampling capillary tube, a first guide sample source, a second guide sample source, a first electromagnetic valve and a second electromagnetic valve, wherein the first electromagnetic valve and the second electromagnetic valve are switched with a water sample source. In the invention, the sampling capillary tube with a fixed length is adopted, the water sample ration is accurate, the dispersed state of the sample in the current-carrying liquid has high repeatability in a certain retained time, the measurement result is accurate, and the flow path is simple, thereby being easy for realizing automation, real-time and on-line monitoring and low failure rate. The invention can be used for on-line monitoring of the residual chlorine in drinking water and industrial recirculating cooling water, conveniently and rapidly obtaining real-time analysis data, and especially finding, reporting and handling water pollution accidents with serious idiopathic.
Owner:HOHAI UNIV
Test strip and test card for fluorescence immunochromatography of myeloperoxidase
InactiveCN108398557AHigh sensitivityGood value for moneyMaterial analysisControl lineMyeloperoxidase antibody
The invention discloses a test strip for fluorescence immunochromatography of myeloperoxidase. The test strip comprises a base plate, a sample pad, a binding pad, a nitrocellulose membrane and an absorbent pad, and the sample pad, the binding pad, the nitrocellulose membrane and the absorbent pad are assembled on the base plate in a sequential overlapping manner, wherein the absorbent pad and thebinding pad are respectively pressed on two ends of the nitrocellulose membrane in an overlapping manner, and a detection area is formed on the surface of the nitrocellulose membrane; the sample pad is pressed on the binding pad in an overlapping manner, and a myeloperoxidase antibody-fluorescent microsphere compound is immobilized on the binding pad; and the nitrocellulose membrane in the detection area is coated with a detection line formed by a monoclonal antibody for recognizing another epitope of myeloperoxidase and a control line formed by a goat anti-mouse IgG polyclonal antibody. The test strip has the advantages of high sensitivity, high stability, realization of the detection linearity being 3.125-600 ng / ml, no non-specificity, short detection time of 5 min, realization of bedside quick test, and great improvement of the clinical diagnosis efficiency.
Owner:河南省生物工程技术研究中心
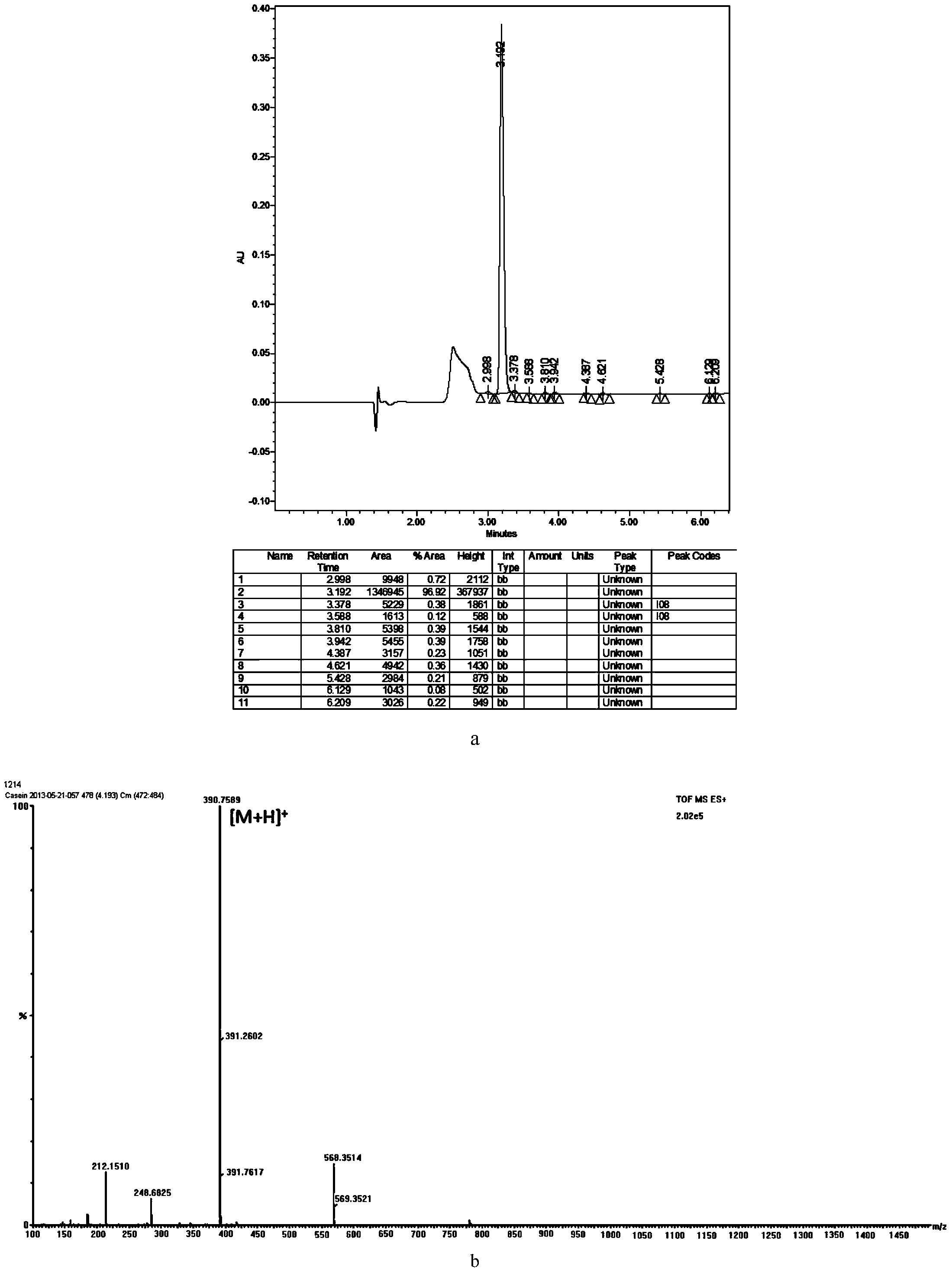
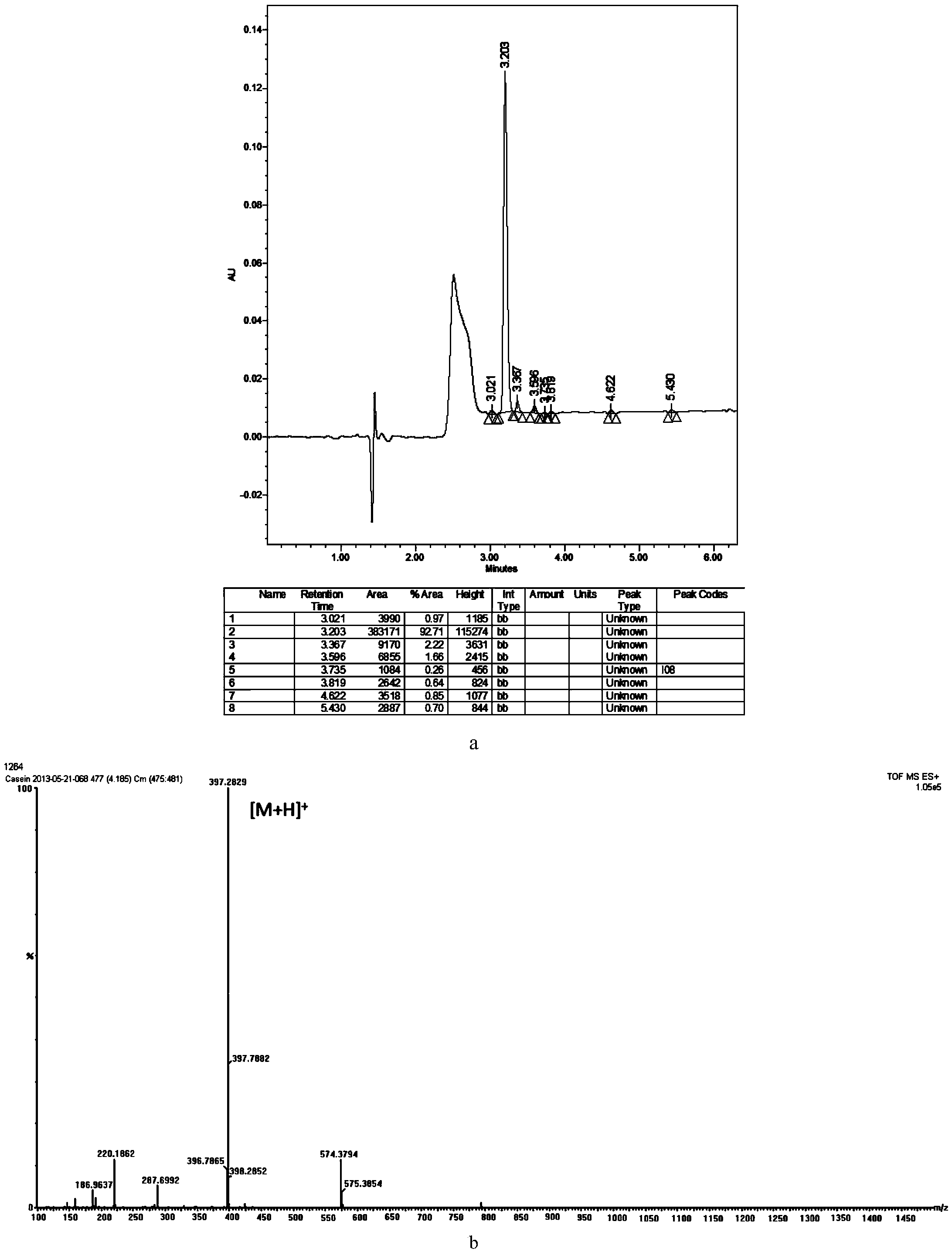
Bovine beta-casein quantitative determination kit and application thereof
ActiveCN103529138AAccurate quantitative detectionComplete range of configurationsComponent separationQuantitative determinationInternal standard
The invention provides a bovine beta-casein quantitative determination kit. The kit mainly comprises a bovine beta-casein specific peptide of which the amino acid sequence is VLPVPQK, an isotope labeled bovine beta-casein specific peptide (VL*PV*PQK) and an isotope labeled bovine beta-casein internal standard substance of which the amino acid sequence is QSVLSLSQSKVL*PV*PQKAVPYPQRD). According to the kit, the ration is limited to be 1mg / 100g, the reproducibility RSD less than 9.50 percent (n is equal to 11), the recovery in a food substrate is 73.41-93.88 percent (n is equal to 6), and the recovery in a powdered milk substrate is 91.62-107.28 percent (n is equal to 6); a sample is pre-treated simply and quickly, and is low in cost. The bovine beta-casein quantitative determination kit is applicable to multiple food substrates, and can be used for accurately quantitating macro and trace bovine beta-casein.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
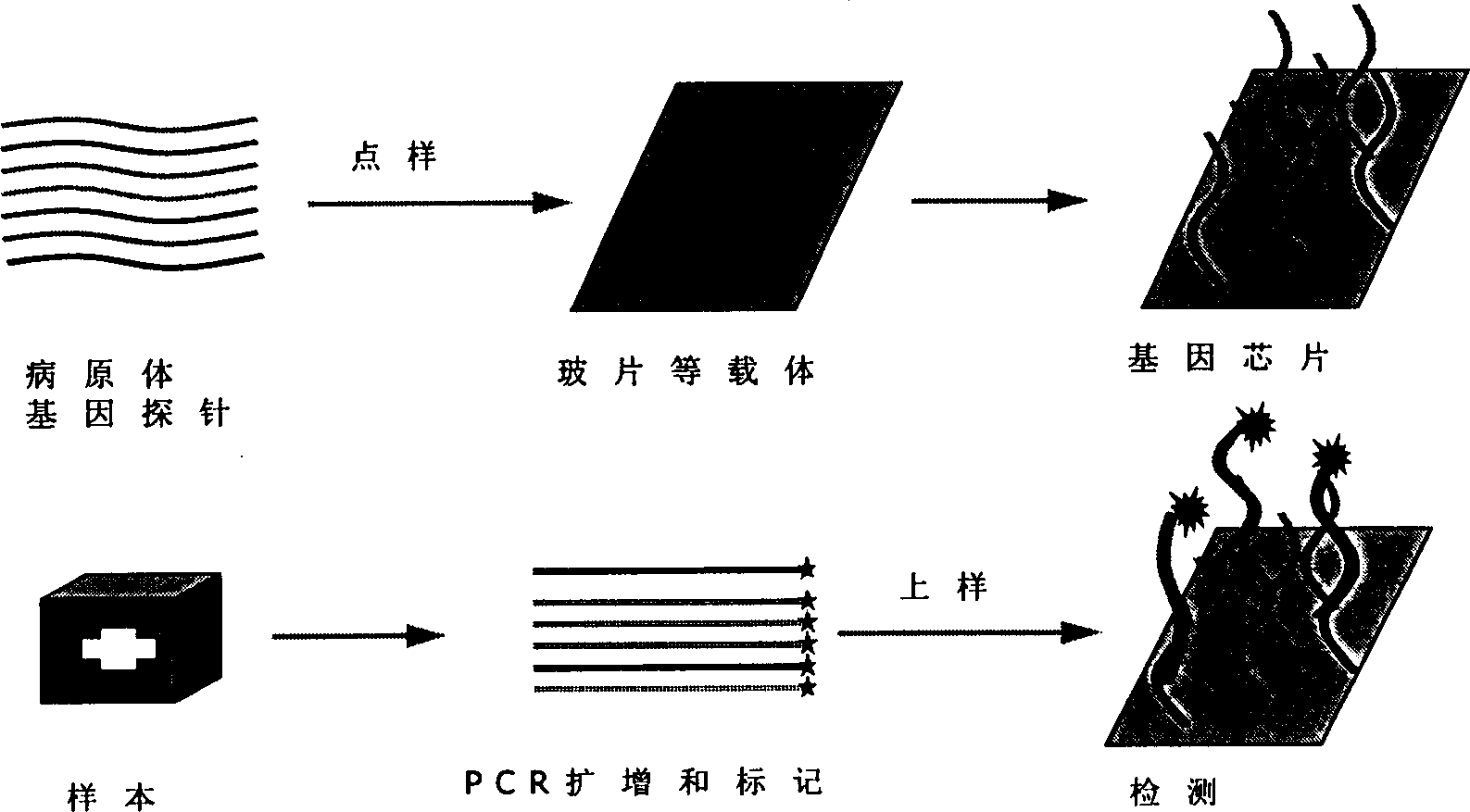
Detection type gene chip for detecting various infectious desease and use thereof
InactiveCN1450171AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic hemorrhagic fever virusMalaria
The present invention relates to a gene chip for detecting several infections lideases and its application. It can be used for detecting, typing and strain-identifying 7 main infections diseases of epidemic hemorrhagic fever, tsutsugamushi disease, leptospirosis, schistosomiasis, malaria, cholera and hemorrhagic enteritis due to 0157:H7. Said invention can select specific gene probe and PCR primer respectively from S gene of epidemic hemorrhagic fever virus, 56KD protein gene of oriental rickettsia, 23 SRNA gene of leptospirosis, 5D antigen gene of schistosomiasis, SSrRNA gene of malarial parasite, 0157 antigen gene of colibacillus 0157:H7, H7 antigen gene and toxic gene and outer membrane OWP protein gene of cholera vibrio and toxic gene.
Owner:陶开华 +1
Method for rapidly detecting pigment green in tea through using laser-Raman spectrum technology
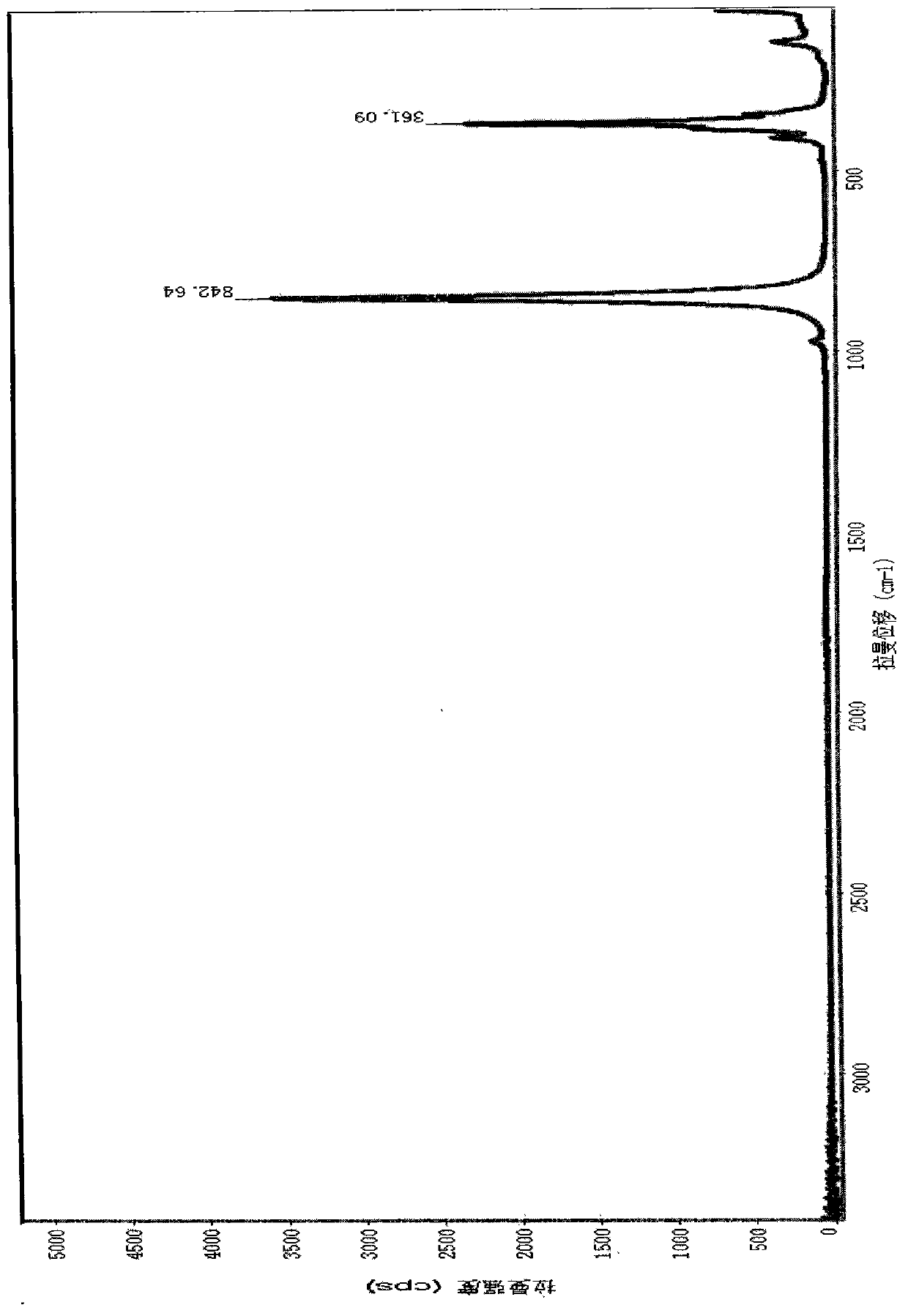
ActiveCN102890079ASolve qualitative problemsRapid Qualitative and Quantitative AnalysisRaman scatteringLaser ramanLead chromate yellow
The invention discloses a method for rapidly detecting pigment green in tea through uisng a laser-Raman spectrum technology. The method comprises the following steps of: performing laser-Raman spectrum chart scanning on tea to be detected, and generating any one group of the following three groups of absorption peaks: (1) 2150(+ / -3)cm<-1>, 842(+ / -3)cm<-1>, (2) 1524(+ / -3)cm<-1>, 1339(+ / -3)cm<-1>, 842(+ / -3)cm<-1>, and (3) 2150(+ / -3)cm<-1>, 1524(+ / -3)cm<-1>, 1339(+ / -3)cm<-1>, 842(+ / -3)cm<-1>, so as to obtain a qualitative result of pigment green in the tea, wherein when the first group of characteristic peaks occurs, the pigment green is formed by mixing iron blue and lead chromate yellow; when the second group of characteristic peaks occurs, the pigment green is formed by mixing phthalocyanine blue and lead chromate yellow; and when the third group of characteristic peaks occurs, the pigment green is formed by mixing the iron blue, the phthalocyanine blue and the lead chromate yellow. According to the method, the existence of the pigment green in the tea can be accurately, qualitatively and quantitatively determined.
Owner:江苏省理化测试中心
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Preparation method of mint essential oil burst beads for cigarettes
ActiveCN107723091AImprove compactnessImprove the airtightness of the cyst wallTobacco smoke filtersEssential-oils/perfumesWater soluble polymersIon
The invention provides a preparation method of mint essential oil burst beads for cigarettes, belonging to the technical field of cigarette processing. The preparation method of burst beads comprisesthe following steps: performing high-speed homogenization and emulsification on mint essential oil, a calcium ion water solution and a surfactant to form an emulsion, directly and dropwisely adding emulsion drops into a sodium alginate water solution through an orifice by controlling the flow velocity of the emulsion at 1-5mL / minute by using a peristaltic pump, enabling the emulsion drops to stayin the sodium alginate solution for 2-40 minutes to obtain calcium alginate beads, taking out the beads, washing the beads with distilled water, putting the beads into an oxidizer water solution, performing soaking for 1-20 minutes, taking out the beads, washing the beads with distilled water, soaking the beads in a water-soluble polymer solution for 5-30 minutes, taking out the beads, washing thebeads with distilled water, and performing natural air drying, thereby preparing the mint essential oil burst beads for cigarettes, of which the grain size is 1-20mm. The mint essential oil burst beads for cigarettes are applied to cigarette filters to achieve the goal of improving the quality of cigarettes.
Owner:WUHAN YELLOW CRANE TOWER NEW MATERIAL TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com