Immunochromatographic test strip for quantitatively detecting troponin I in whole course and preparation method thereof
An immunochromatographic test strip, troponin technology, applied in biological tests, measuring devices, analytical materials, etc., to achieve accurate diagnosis of diseases and identification of infections, good labeling stability, and safe operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In the embodiment of the present invention, the troponin I antibody used is a monoclonal antibody prepared by conventional monoclonal antibody technology, and the specimen is detected using the principle of the double antibody sandwich method to detect the troponin I antigen.
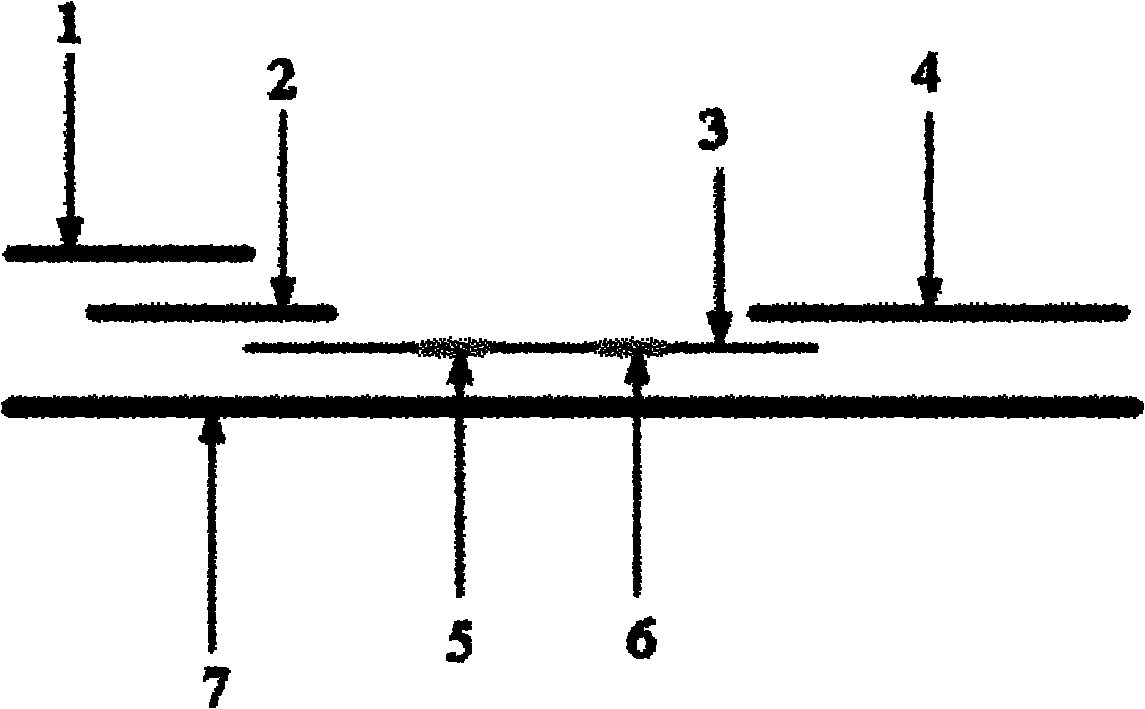
[0027] Such as figure 1 As shown, in this embodiment, the immunochromatographic test strip for quantitative detection of troponin I throughout the whole process includes a distal end and a proximal end. The sample pad 1 is located at the proximal end of the test strip, and it contains a hydrophilic hole. Diaphragm, sample pad 1 is the sample application area, which is used to draw samples for troponin I testing. Between the sample pad 1 and the distal end, a glass cellulose membrane marking pad 2, a nitrocellulose membrane coating membrane 3, and an absorbent paper 4 are sequentially overlapped. The sample pad 1, the glass fiber membrane marking pad 2, the nitrocellulose coating film 3 and the abso...
Embodiment 2
[0039] The structure of the test strip in this embodiment is the same as that of the first embodiment.
[0040] The concentration of the troponin I monoclonal antibody labeled with fluorescent latex particles is 0.1 mg / ml, and the spray film dosage on the test strip is 100 μl / 25 cm. The concentration of the troponin I monoclonal antibody coated in the detection area is 1 mg / ml, and the spray film dosage on the test strip is 100 μl / 25 cm. The concentration of the goat anti-mouse IgG is 1.5 mg / ml, and the spray film dosage on the test strip is 100 μl / 25 cm.
[0041] The preparation method of this embodiment except for the troponin I monoclonal antibody in step 2: the fluorescent latex is 15 μg / 100 μl. The rest are the same as in Example 1, and the method of use is also the same as in Example 1.
Embodiment 3
[0043] The structure of the test strip in this embodiment is the same as that of the first embodiment.
[0044] The concentration of the troponin I monoclonal antibody labeled with fluorescent latex particles is 0.8 mg / ml, and the spray film dosage on the test strip is 100 μl / 28 cm. The concentration of the troponin I monoclonal antibody coated in the detection area is 2 mg / ml, and the spray film dosage on the test strip is 100 μl / 28 cm. The concentration of the goat anti-mouse IgG is 2 mg / ml, and the spray film dosage on the test strip is 100 μl / 28 cm.
[0045] The preparation method of this embodiment except for the troponin I monoclonal antibody in step 2: the fluorescent latex is 100 μg / 100 μl. The rest are the same as in Example 1, and the method of use is also the same as in Example 1.
[0046] In an embodiment of the present invention, the immunochromatographic test strip for quantitative detection of troponin I throughout the entire process is assembled in a plastic shell f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com