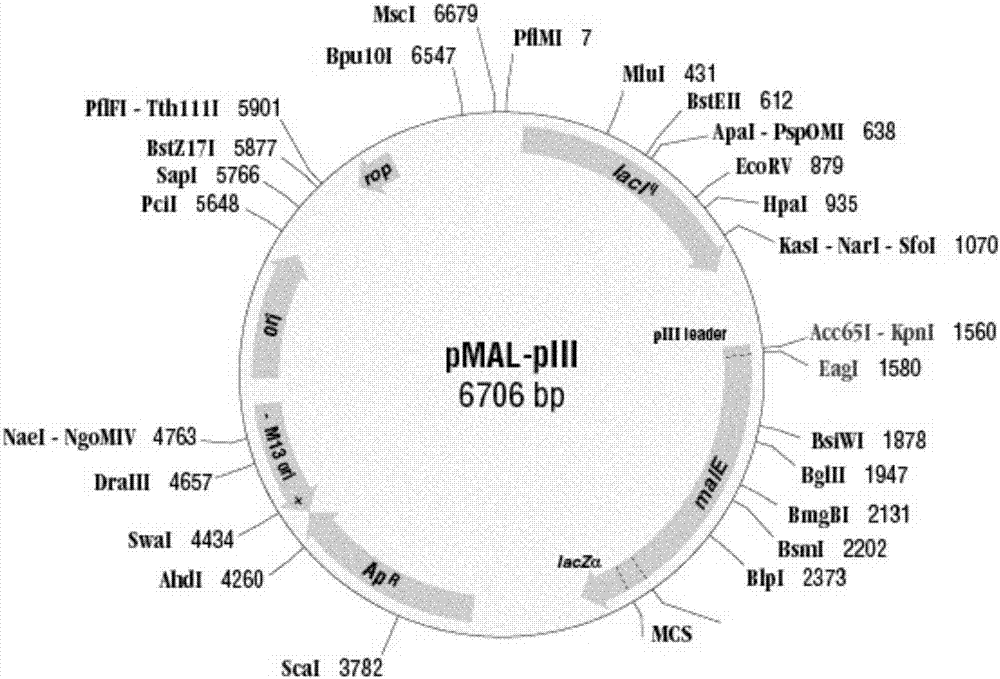
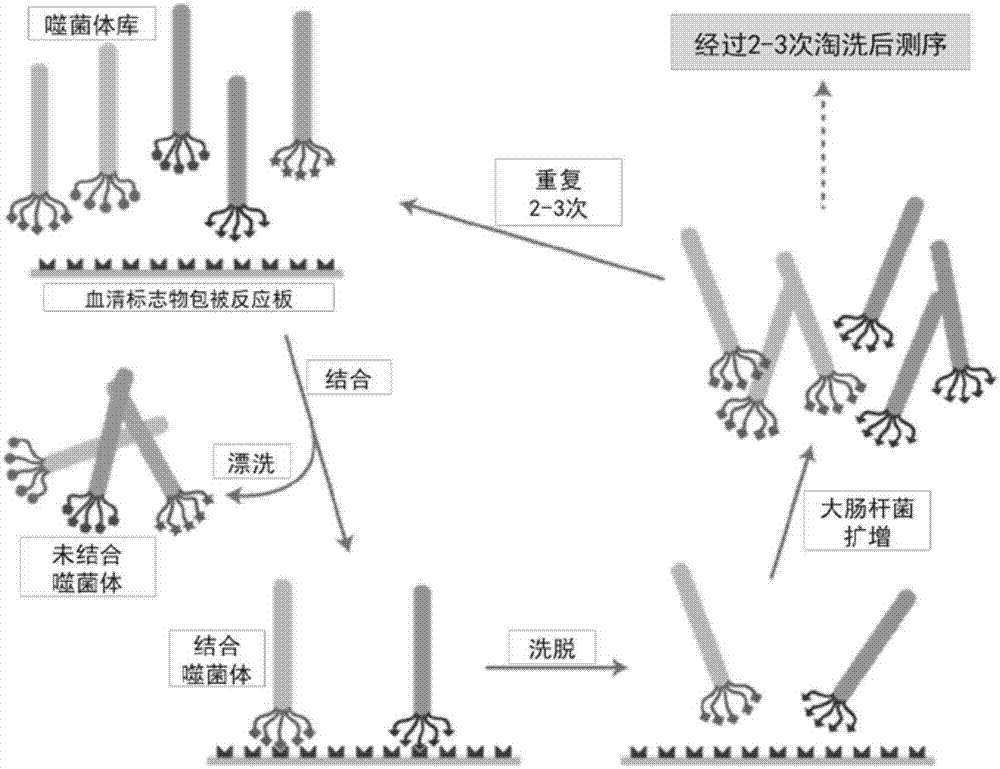
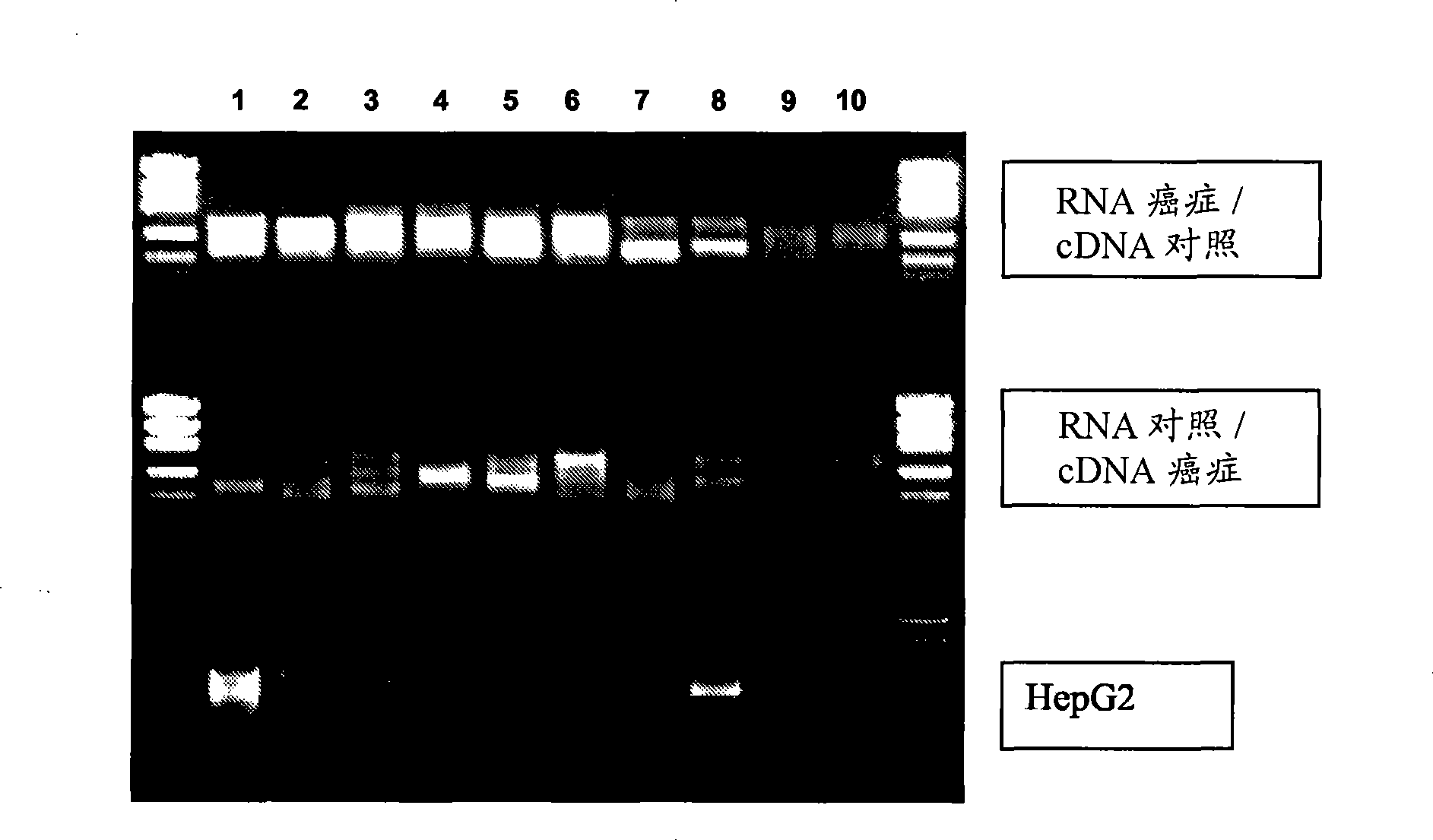
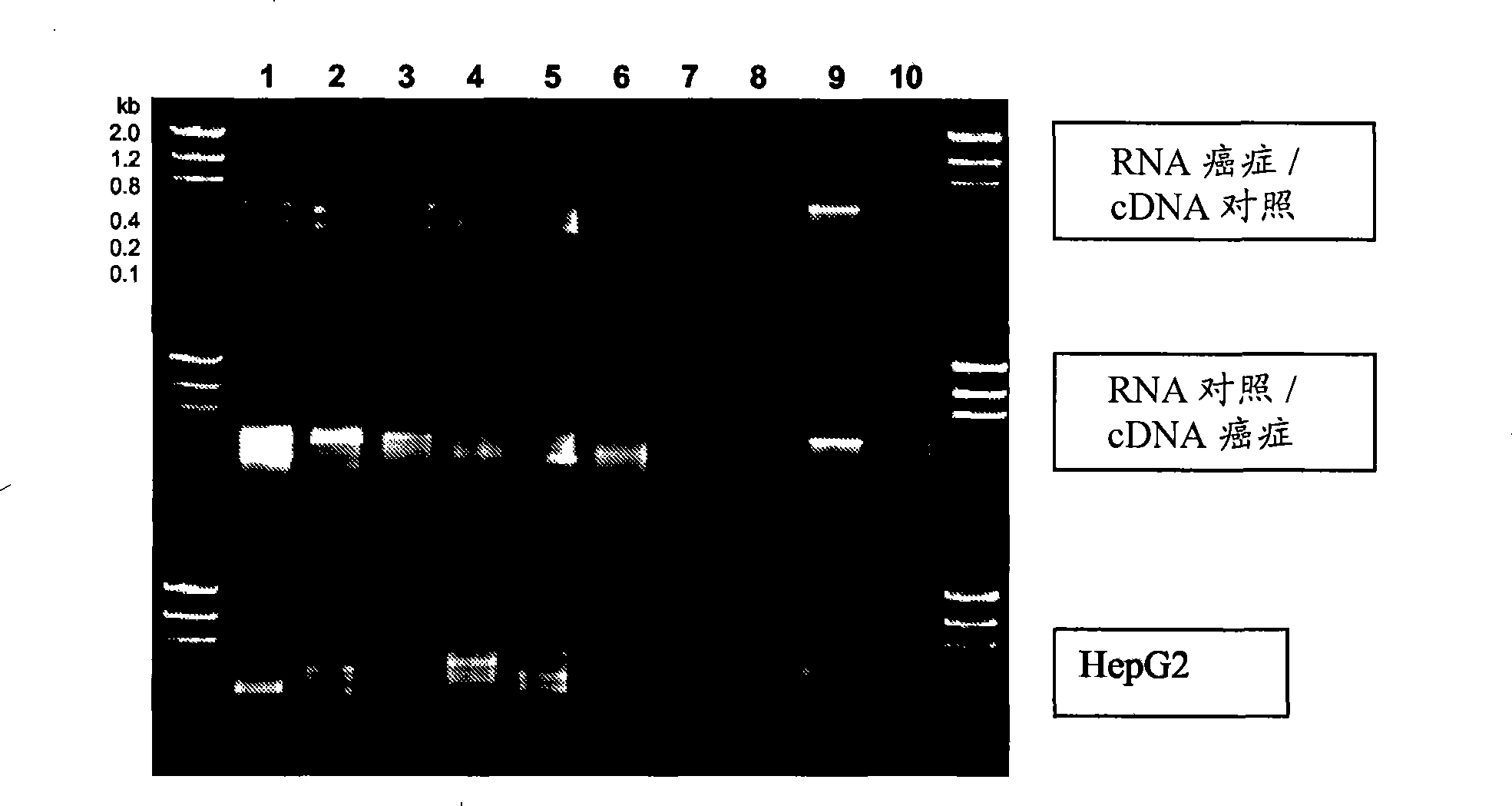
Patents
Literature
249 results about "Serum markers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serum Tumor Markers for Gastrointestinal Cancer. DESCRIPTION. Serum tumor markers are substances produced by cells in response to cancer or certain noncancerous conditions. Most tumor markers are made by normal cells as well as cancer cells; however, they are produced at much higher levels in cancerous conditions.
Methods for diagnosing, prognosing, or theranosing a condition using rare cells
InactiveUS20100233693A1Microbiological testing/measurementPreparing sample for investigationSerum markersRare cell
The invention encompasses methods for diagnosing, theranosing, or prognosing a condition in a patient based on the results of one or more analysis methods. The methods can comprise enriching a sample obtained from the patient for one or more rare cells. The analysis methods can include performing enumeration of the one or more rare cells or cell subtypes, performing nucleic acid analysis, or detecting a serum marker.
Owner:ON Q ITY
SERUM MARKERS PREDICTING CLINICAL RESPONSE TO ANTI-TNFa ANTIBODIES IN PATIENTS WITH ANKYLOSING SPONDYLITIS
InactiveUS20110251099A1Sustained responseBioreactor/fermenter combinationsBiological substance pretreatmentsSerum markersAnkylosing spondylitis
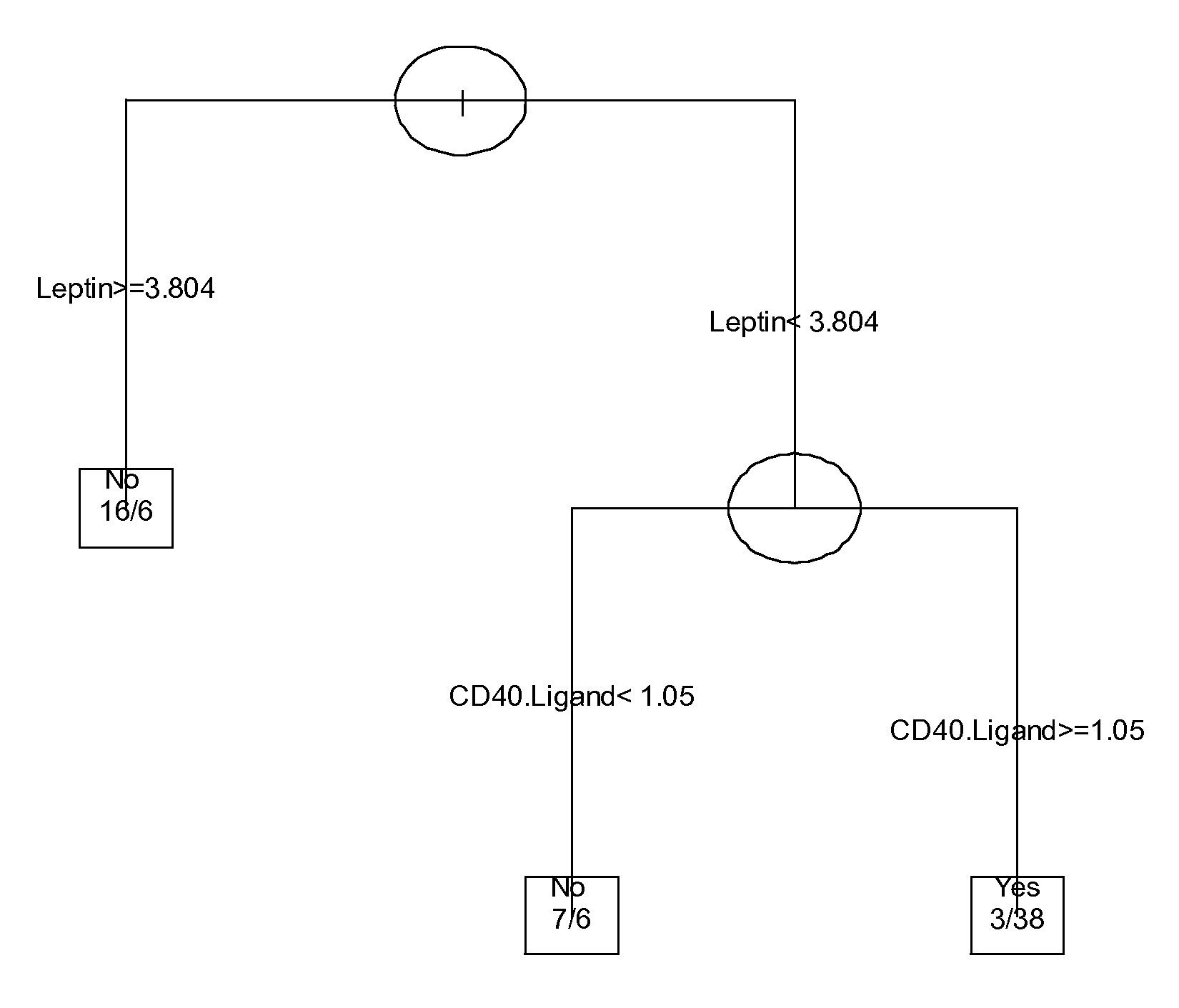
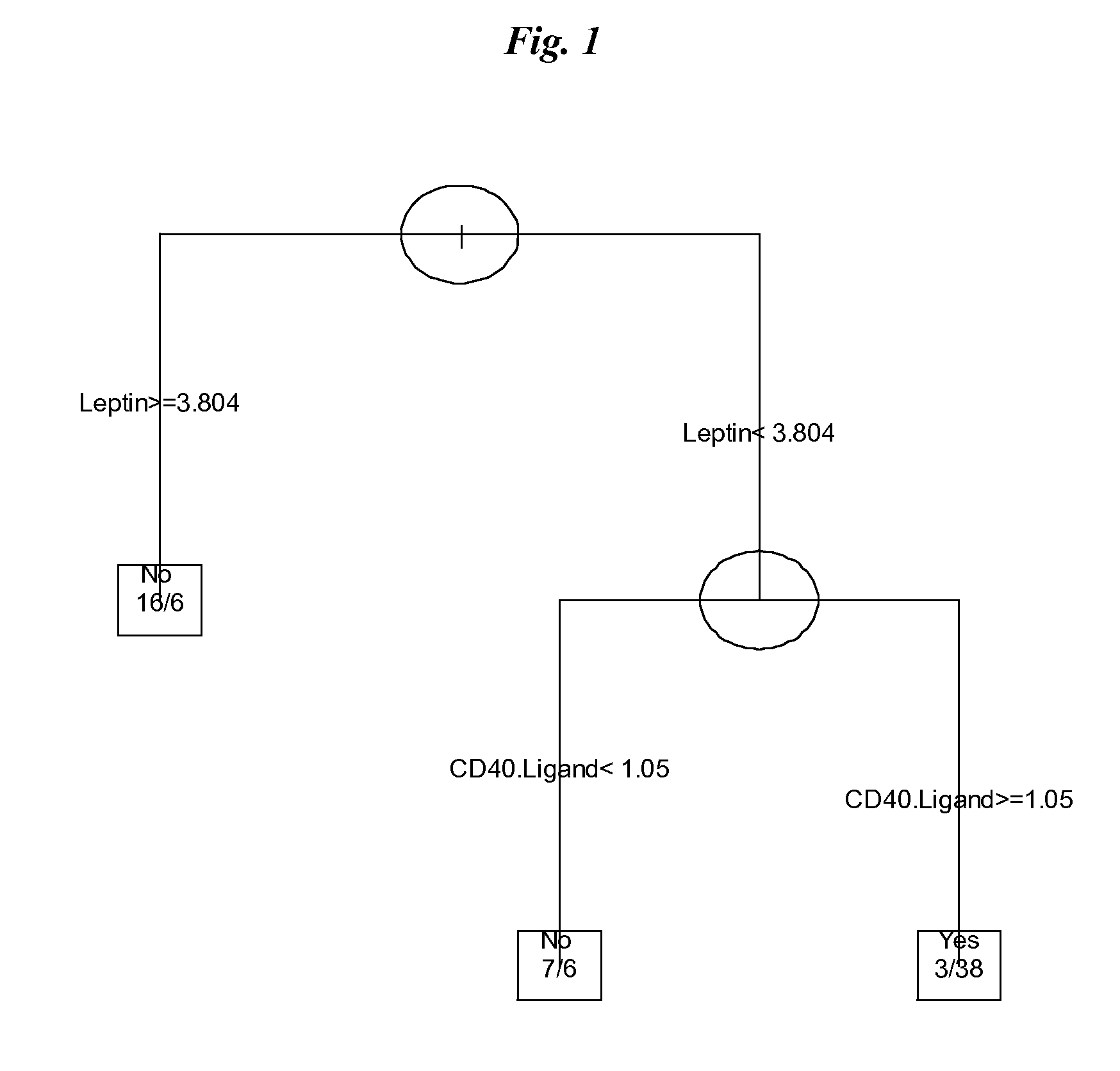
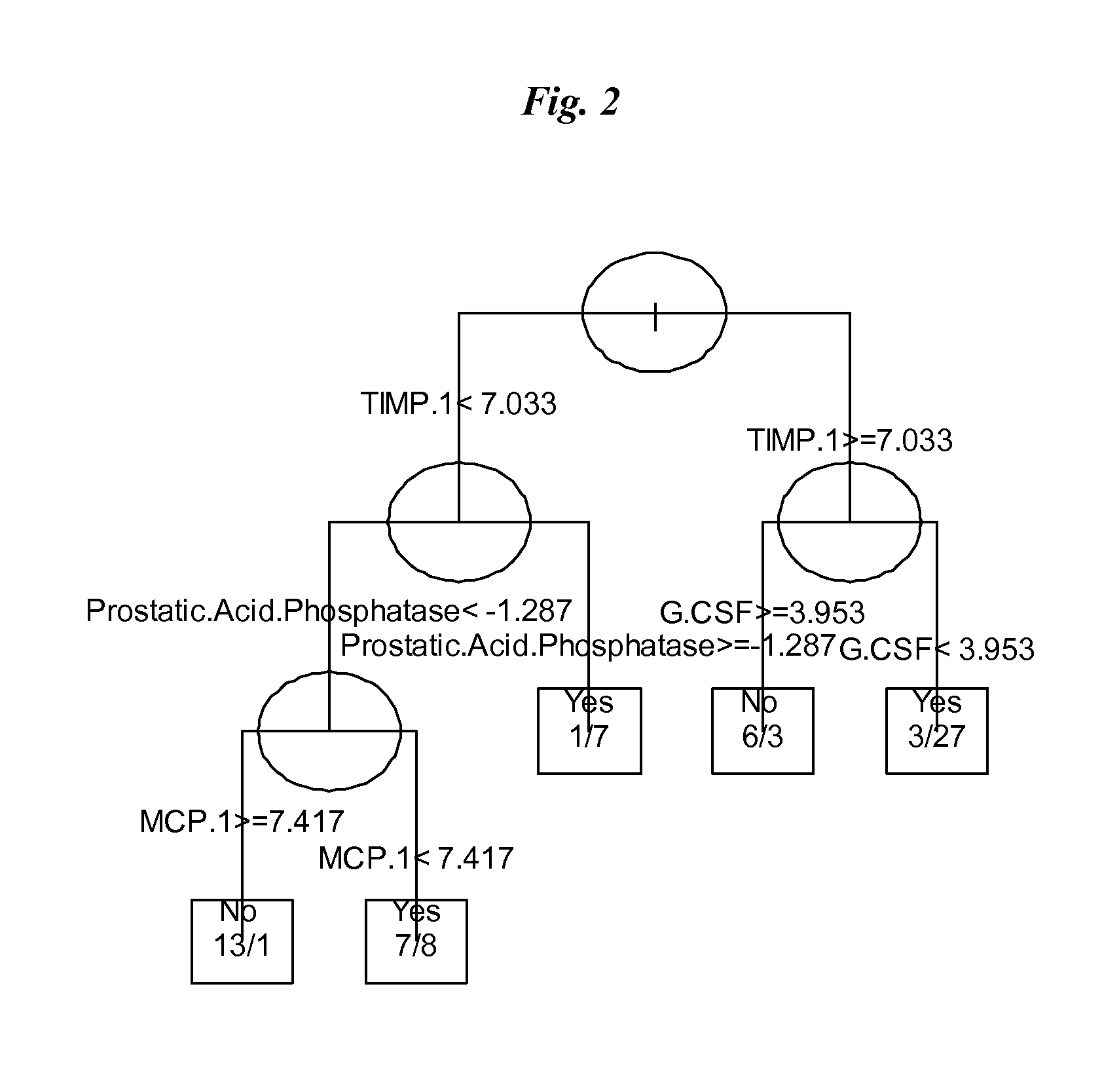
The invention provides tools for management of patients diagnosed with ankylosing spondylitis and prior to the initiation of therapy with an anti-TNFalpha agent. The tools are specific markers and algorithms of predicting response to therapy based on standard clinical primary and secondary end-points using serum marker concentrations. In one embodiment the baseline level of leptin or osteocalcin is used to predict the response at Week 14 after the initiation of therapy. In another embodiment, the change in a serum protein biomarker after 4 weeks of therapy is used such as complement component 3.
Owner:JANSSEN BIOTECH INC
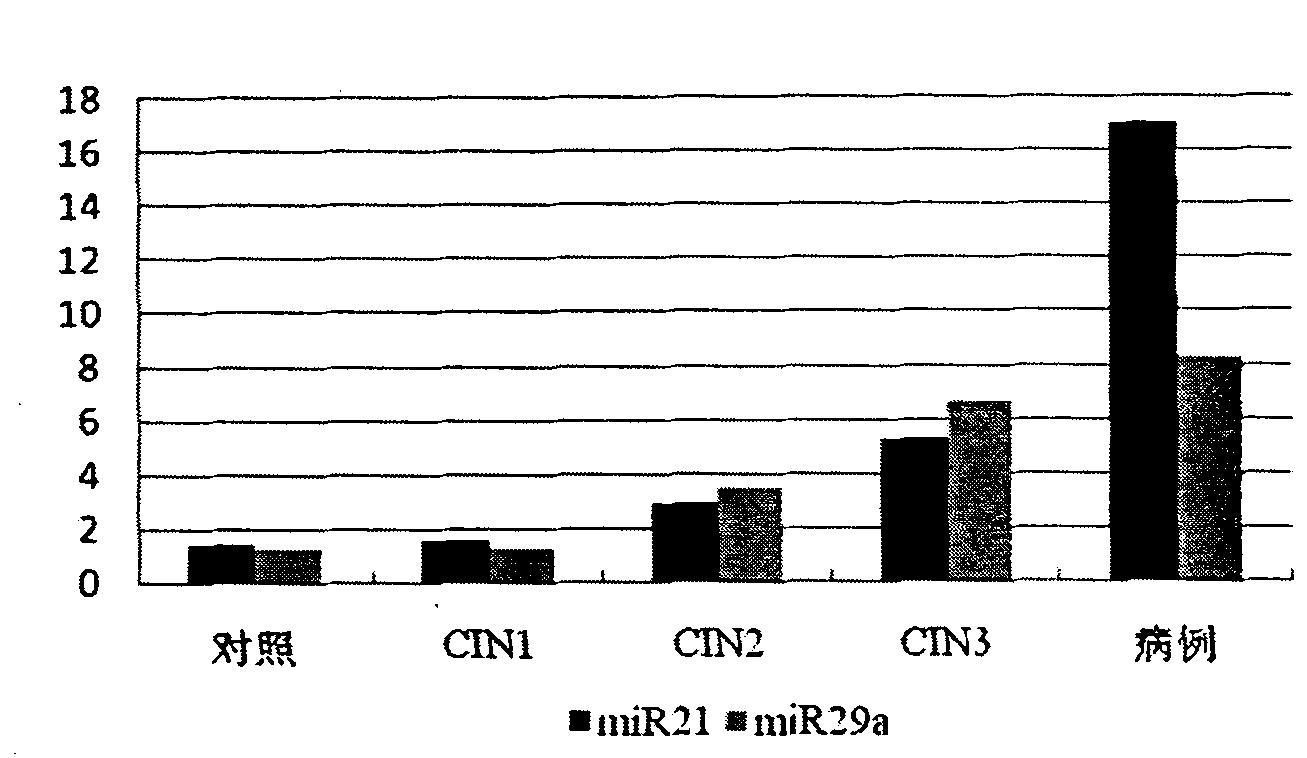
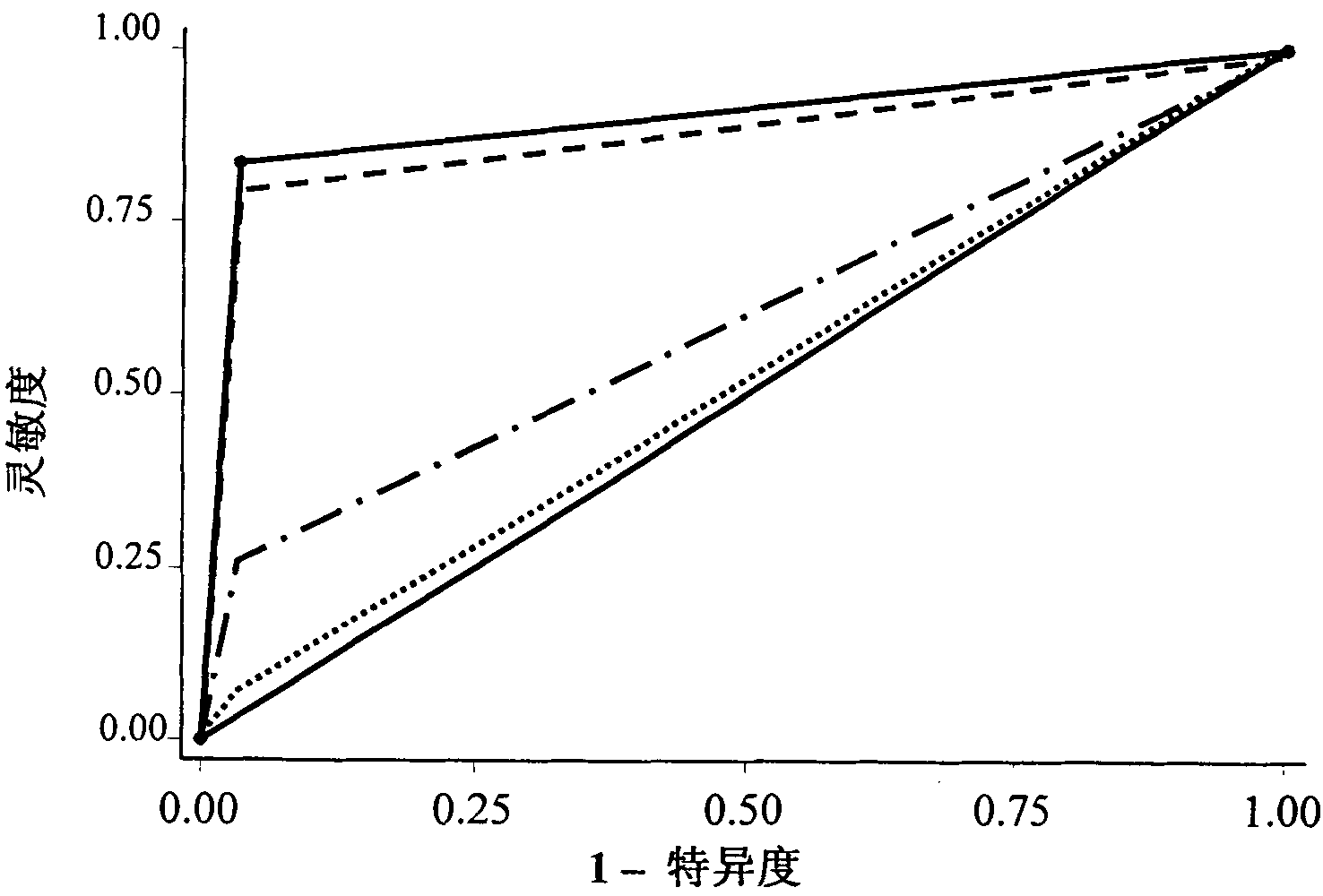
Serum/plasma miRNA serum marker related to cervical carcinoma and precancerous lesions thereof and application thereof
ActiveCN101921759AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationSerum markersBlood plasma
The invention belongs to the fields of gene engineering and oncology, and discloses a serum / plasma miRNA serum marker related to cervical carcinoma and precancerous lesions thereof and application thereof. The marker is single miR-21 or the combination of miR-21 and miR-29a. The marker and the primer thereof can be used for preparing diagnosis kits which are used for assistant early diagnosis of cervical carcinoma and precancerous lesions thereof.
Owner:NANJING MEDICAL UNIV
Liver disease blood serum specific protein and use thereof
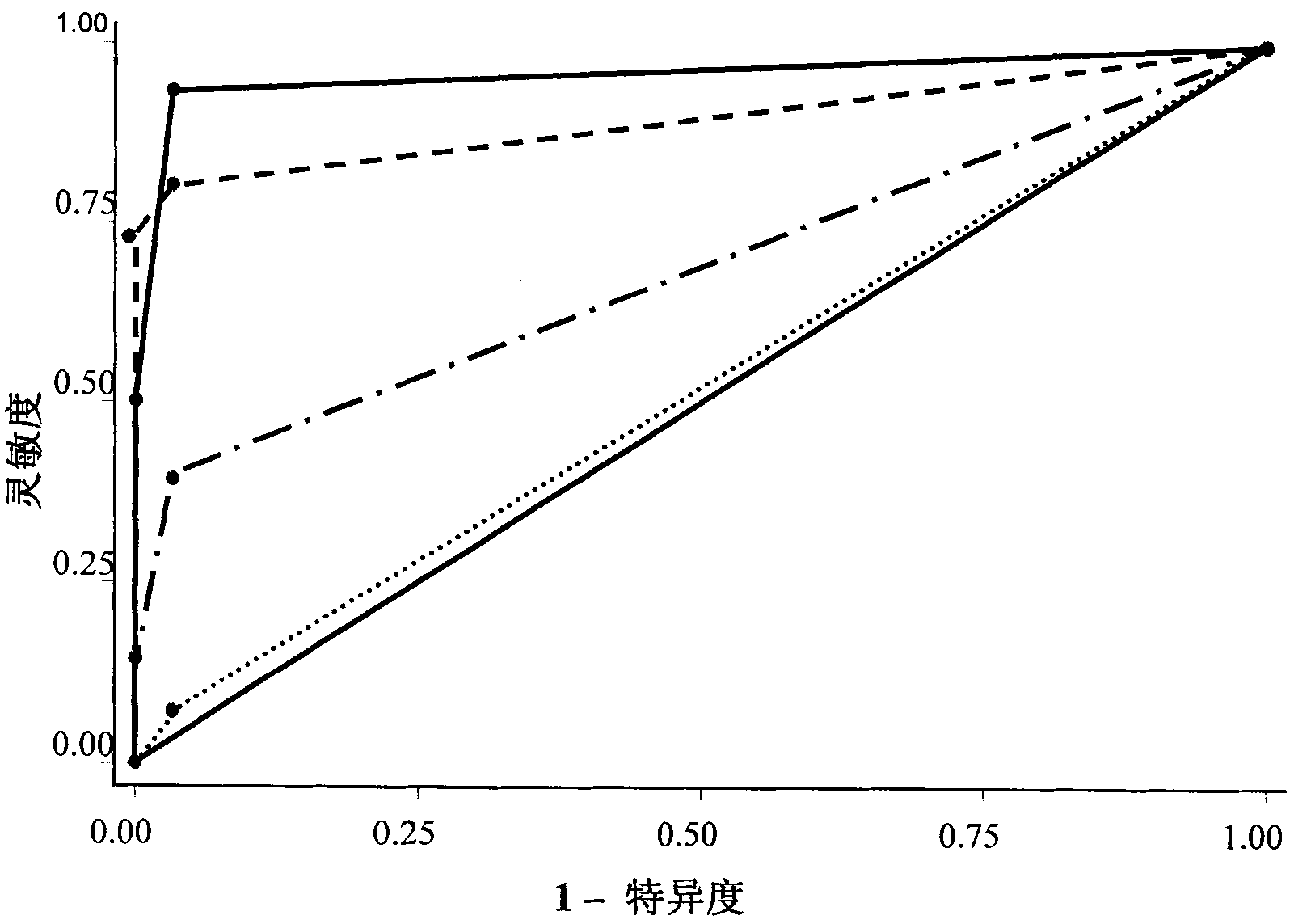
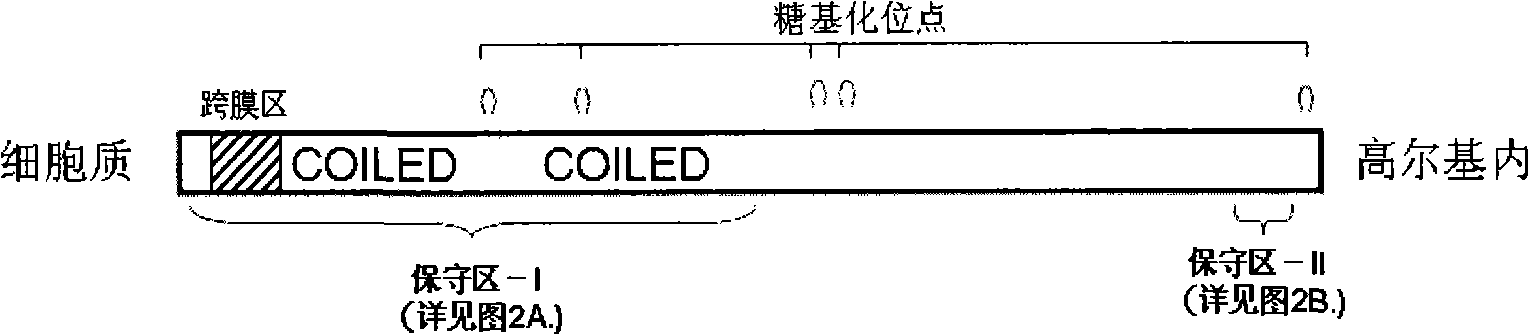
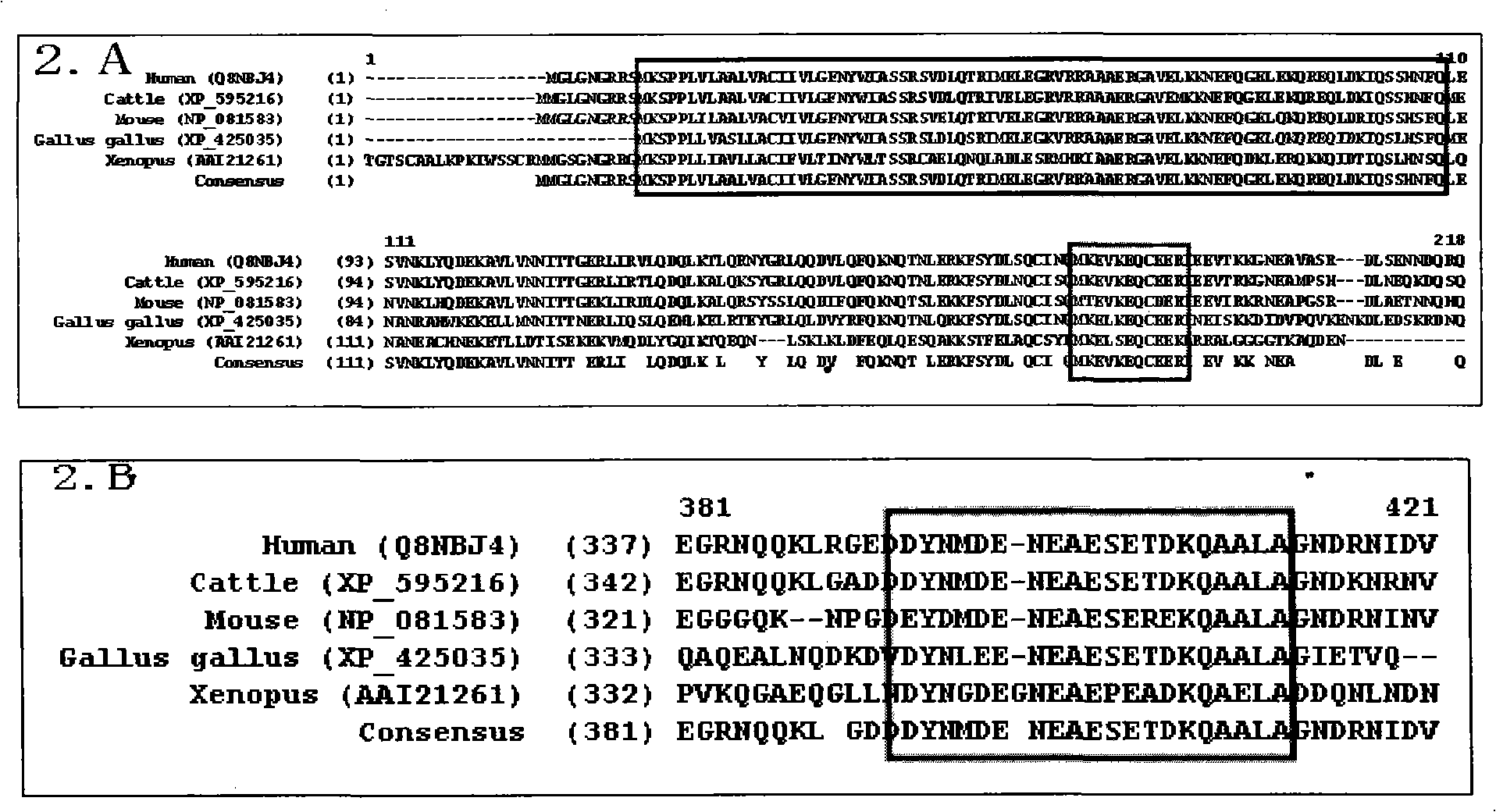
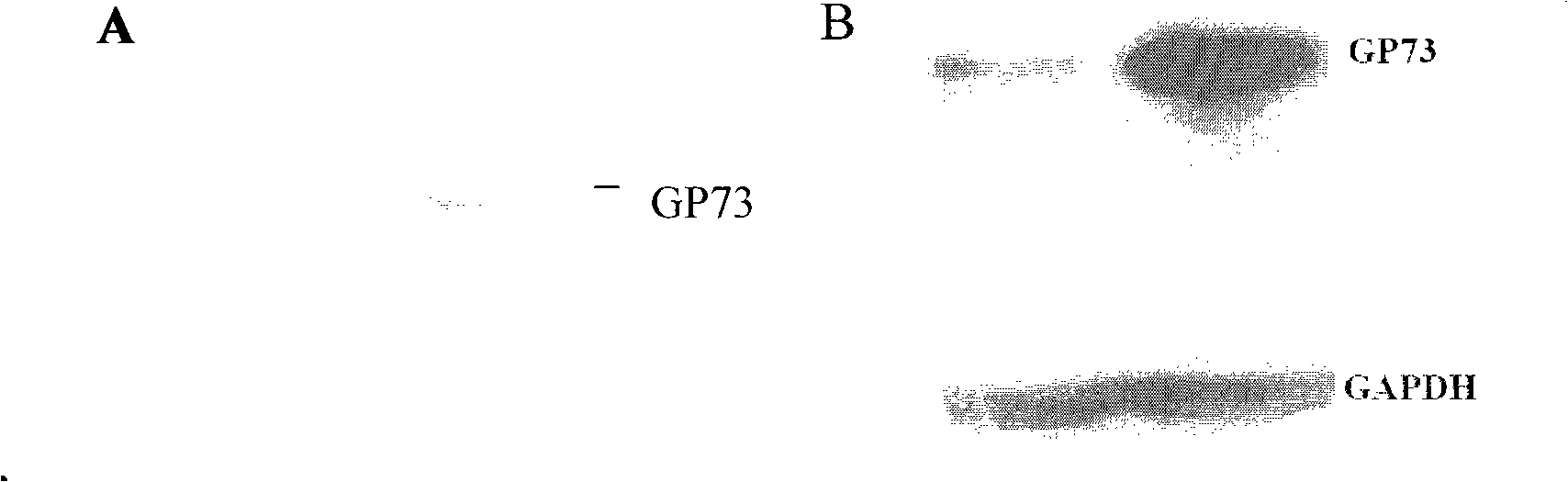
The invention relates to a novel pneumonosis serum marker, namely a Golgi protein gp73, which belongs to the biological monitoring technical field. The invention also discloses an application of the serum Golgi protein gp73 in diagnosing pneumonosis and provides a detection kit for the serum gp73.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Preparation method of microfluidic monolithic column chip and application of chip in raman detection
ActiveCN103508411APromote enrichmentReduce weightDecorative surface effectsRaman scatteringMicrosphereUltra sensitive
The invention provides a preparation method of a microfluidic monolithic column chip and an application of the chip in raman detection. The method comprises the steps that a polydimethylsiloxane (PDMS) microfluidic chip is prepared; a porous monolithic column solution is prepared; a monomer, a crosslinking agent, a pore-forming agent and a photoinitiator are subjected to ultrasonic mixing; nitrogen is supplied for deoxygenization; and finally the monolithic column solution is put in an ice bath for preservation. The solution is injected into a microfluidic chip pipeline, sealed and subjected to ultraviolet exposure, methanol washing is adopted for removing the pore-forming agent and the unreacted monomer, deionized water is used for washing sufficiently, a silver microsphere solution is injected into the microfluidic chip, ultra-sensitive raman real-time detection of a to-be-detected sample is achieved by utilizing silver microsphere enrichment and a surface raman enhancement effect, and a detection limit of the method can reach 10-12M. The method is convenient, flexible, low in cost, low in energy consumption, and convenient to popularize, and is widely applied to the fields of serum marker detection, gas marker detection, environmental monitoring, food safety and the like.
Owner:SHANGHAI JIAO TONG UNIV
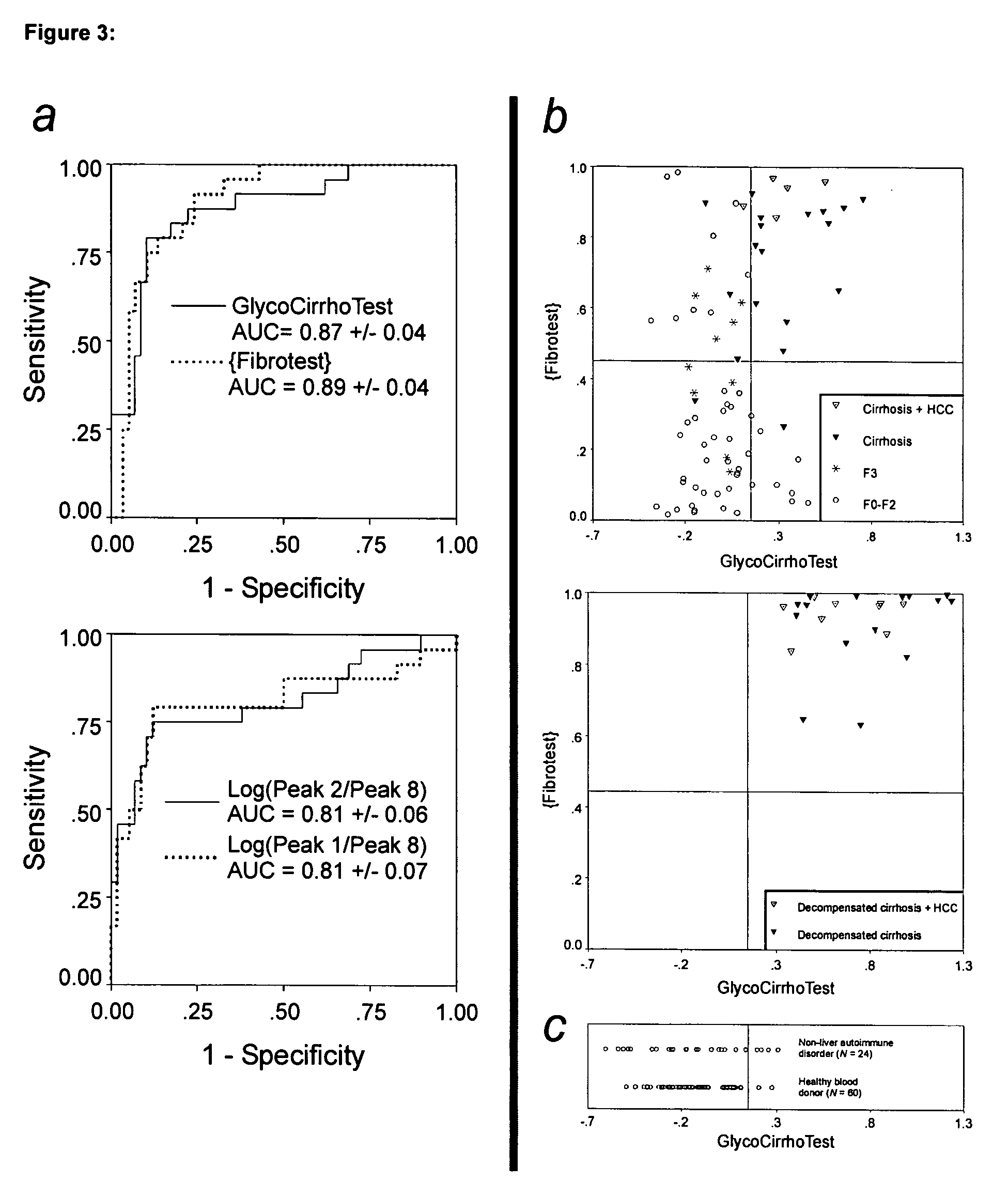
Method for predicting liver fibrosis and related pathologies
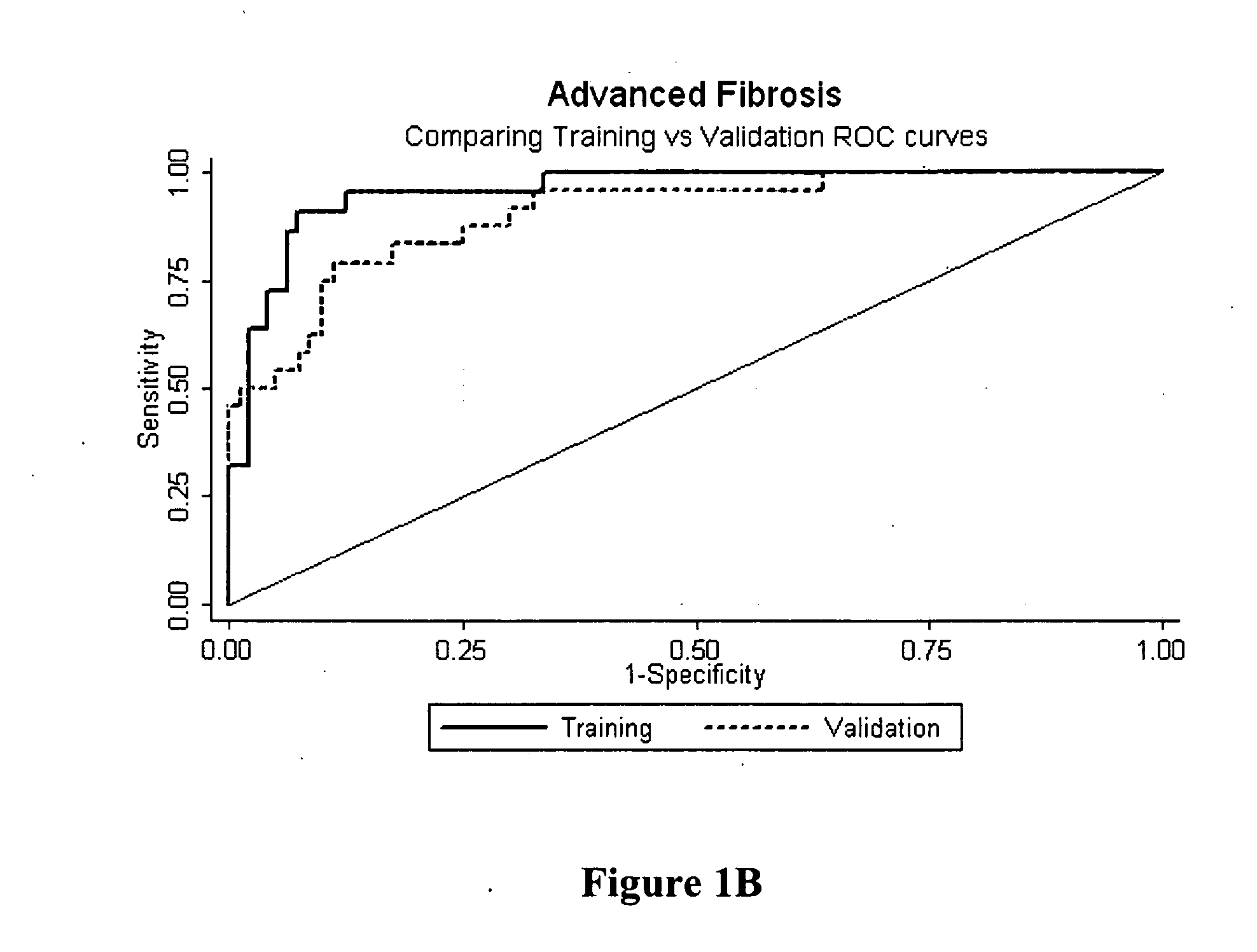
ActiveUS20070225919A1Microbiological testing/measurementDisease diagnosisSignificant fibrosisSerum markers
Provided herein are methods of detecting and staging liver fibrosis in an individual with liver disease. Also provided are methods of detecting necroinflammatory activity. Invention methods utilize four serum markers, age, and gender to determine an end value. The end value is compared to a cut-off value, in order to identify significant fibrosis (METAVIR stages F2 to F4), or an absence of advanced fibrosis (stages F3 and F4) or cirrhosis (stage F4). In particular aspects, progression or treatment of liver fibrosis can be monitored by invention methods. The end value is also used to distinguish between no to mild necroinflammatory activity (METAVIR grade A0 to A1) and moderate to severe necroinflammatory activity (grade A2 to A3).
Owner:WESTERN AUSTRALIA UNIV OF THE +1
Ovarian tumor serum marker
InactiveCN101832977ASimple methodAvoid simple repetitive competitionComponent separationSmall molecule metabolismBiomarker (petroleum)
The invention belongs to the technical field of biology, and particularly relates to an ovarian tumor serum marker. The serum of a patient is subjected to multi-level analysis with high flux and large scale by adopting color spectrum and mass spectrum technology so as to screen serum differential expression molecules, and the serum of the ovarian tumor patient is subjected to metabonomics and polypeptide spectrum analysis of different levels to determine a small molecular metabolism spectrum so as to find a plurality of groups of characteristic biomarkers related with benignant, borderline and malignant ovarian tumor. The invention is possible to provide a simple method for screening the ovarian tumor of different stages in large scale, and also can provide valuable reference for differential diagnosis of related clinical patients.
Owner:THE OBSTETRICS & GYNECOLOGY HOSPITAL OF FUDAN UNIV +5
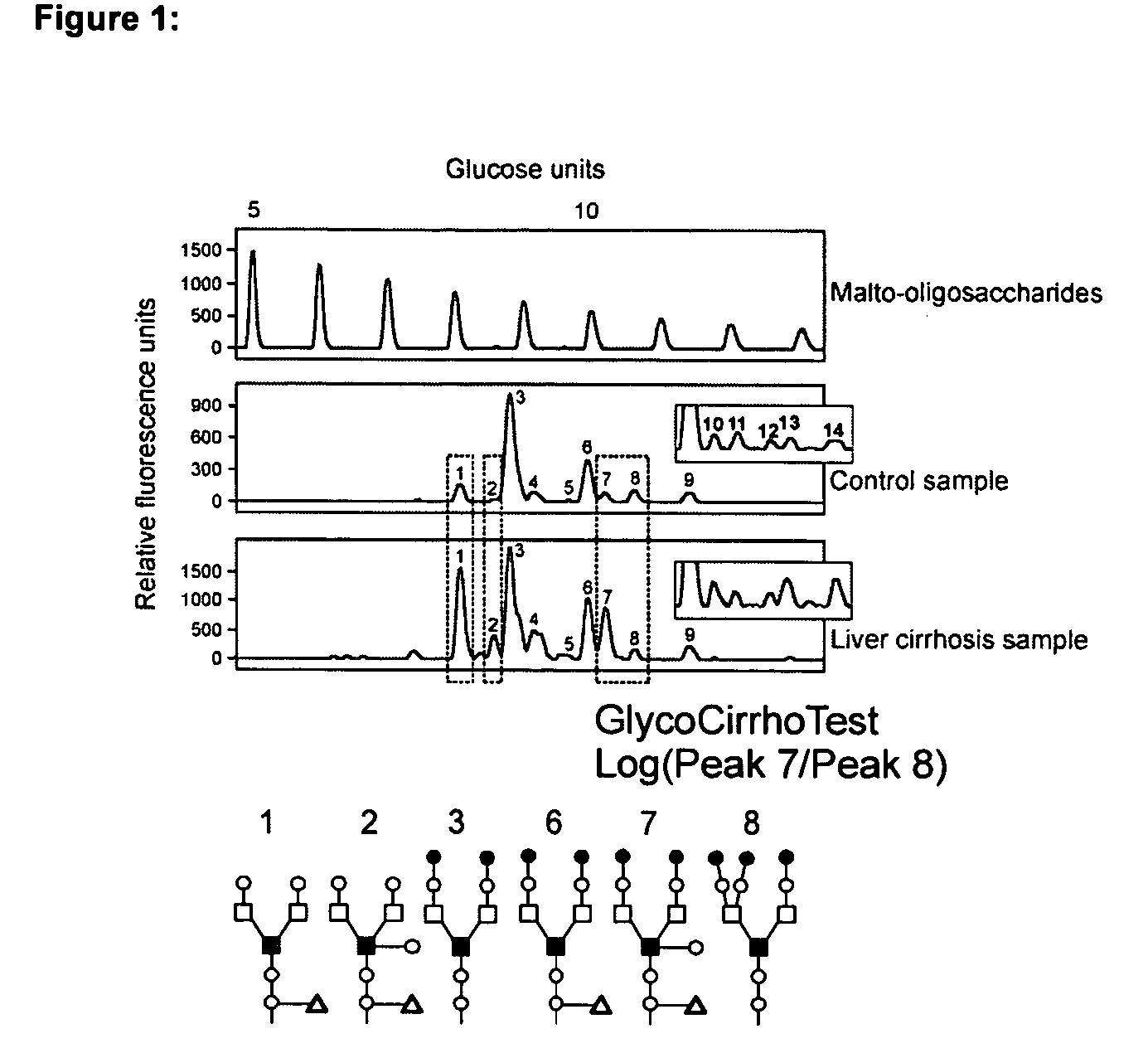
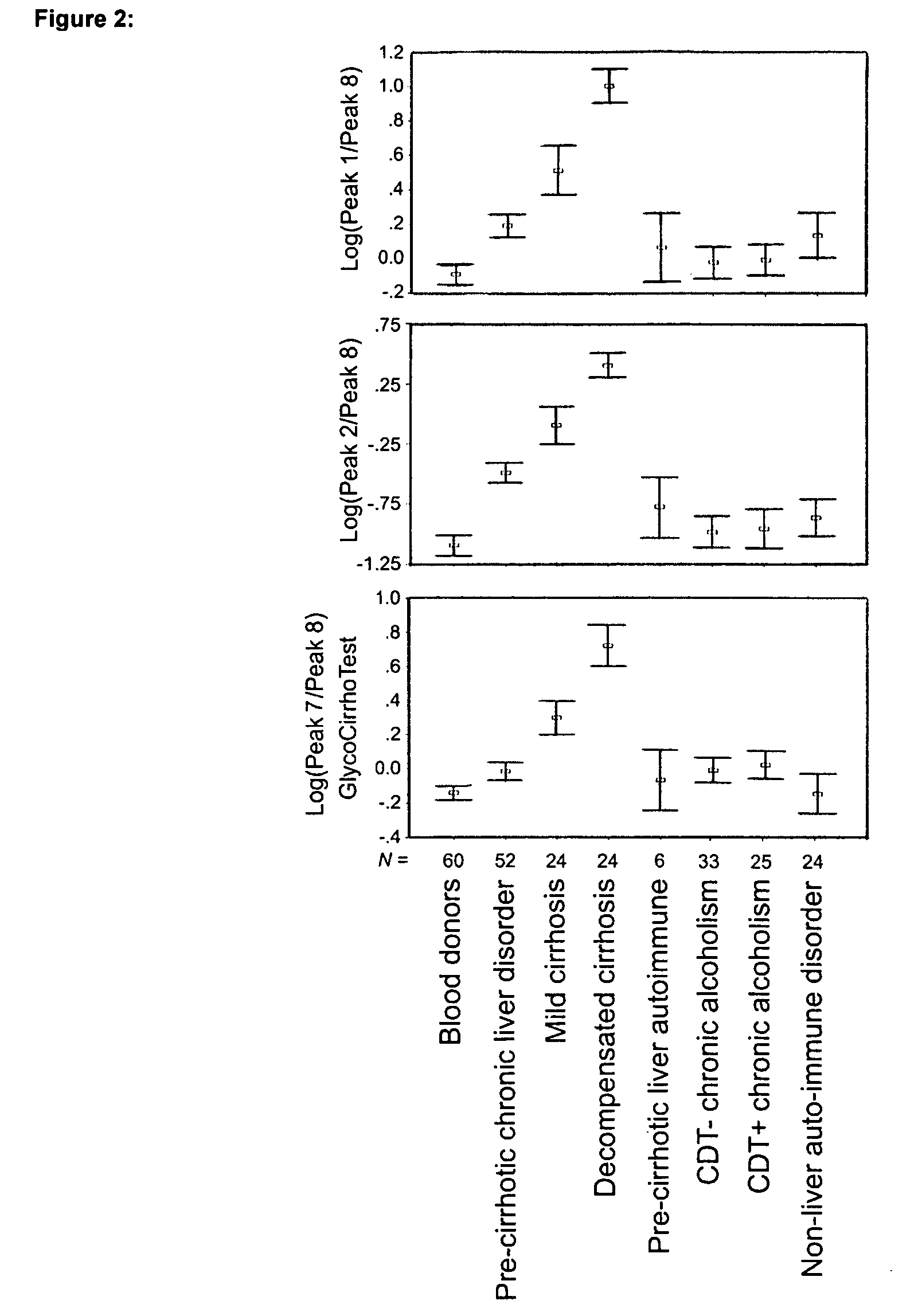
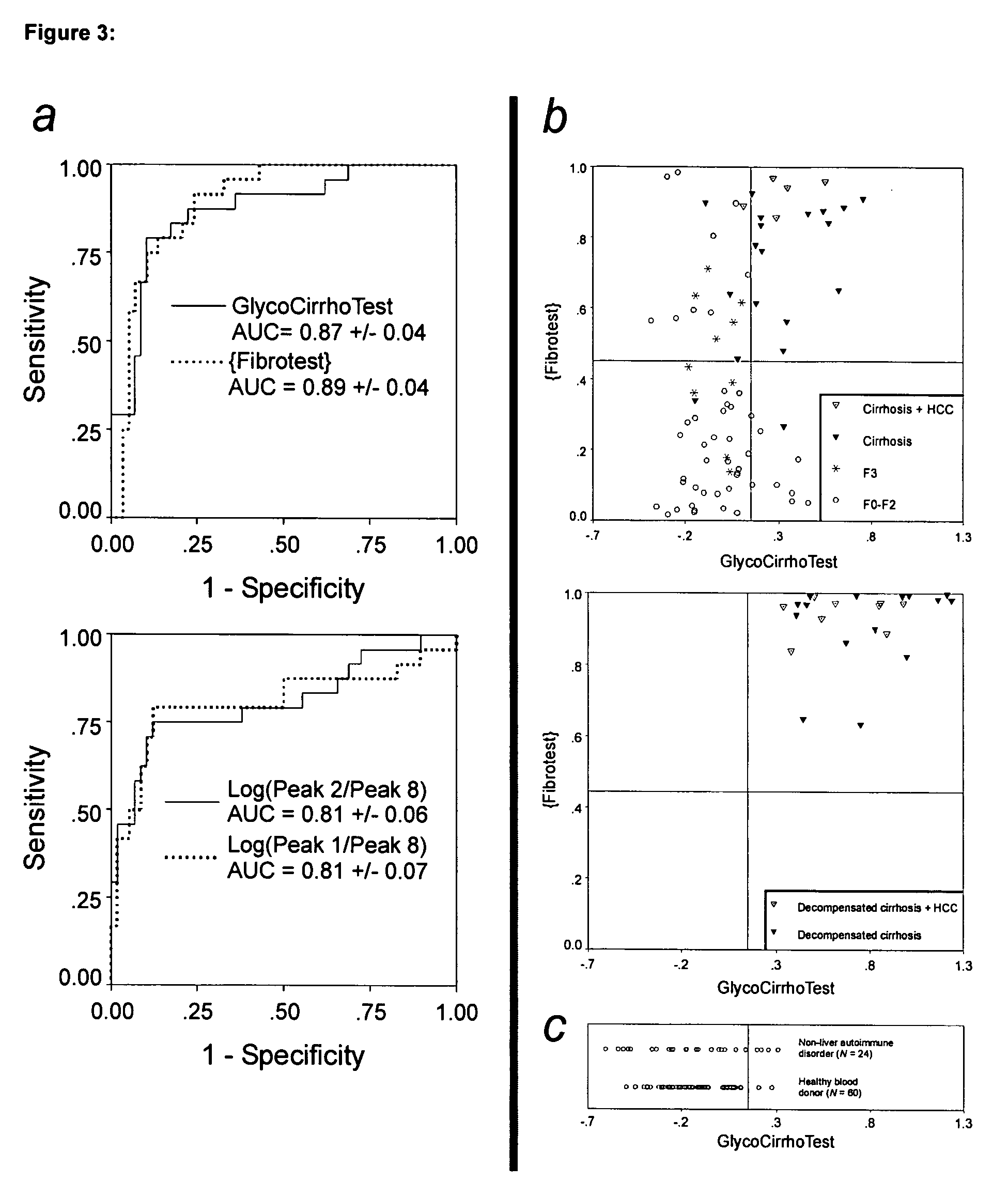
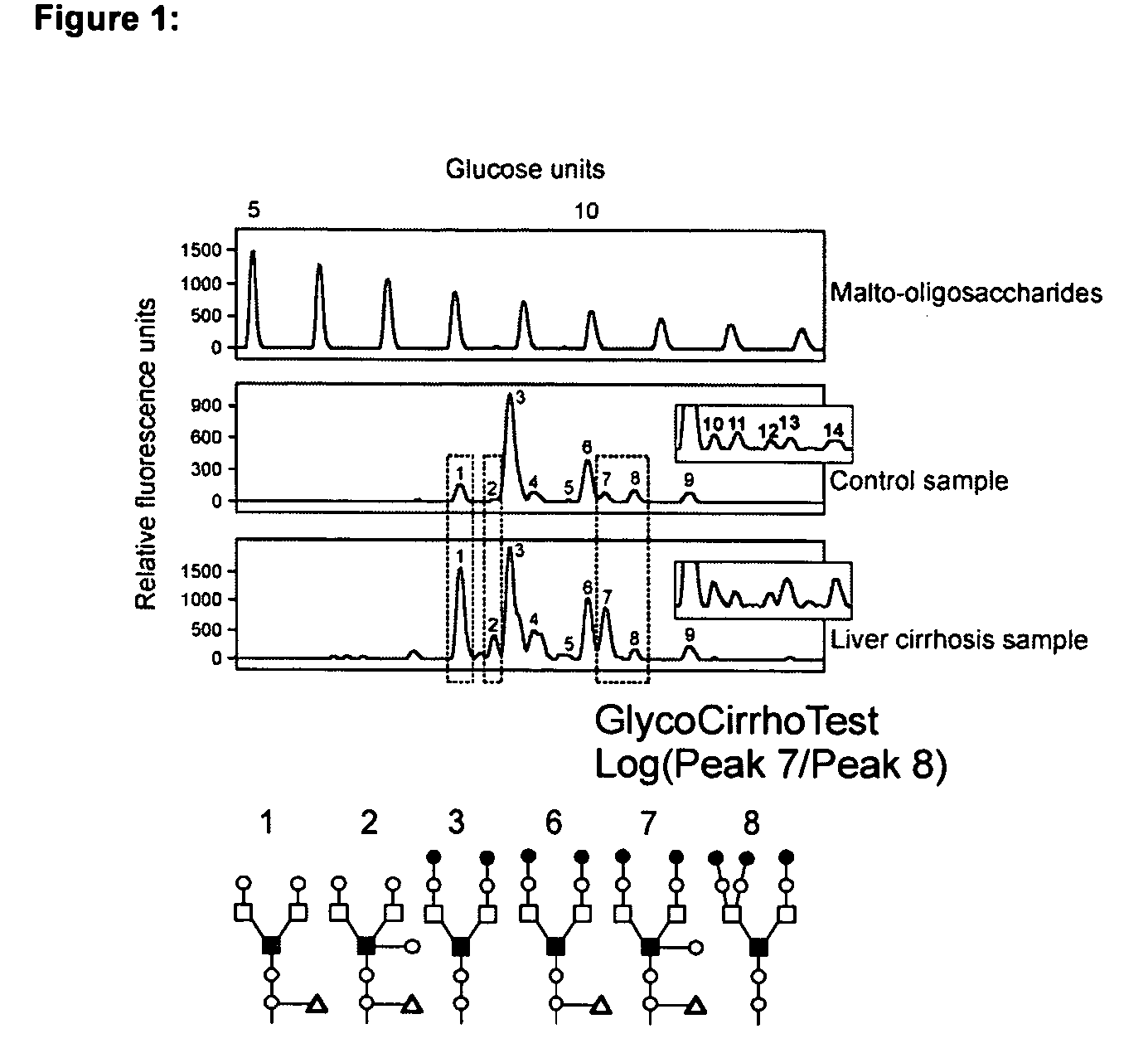
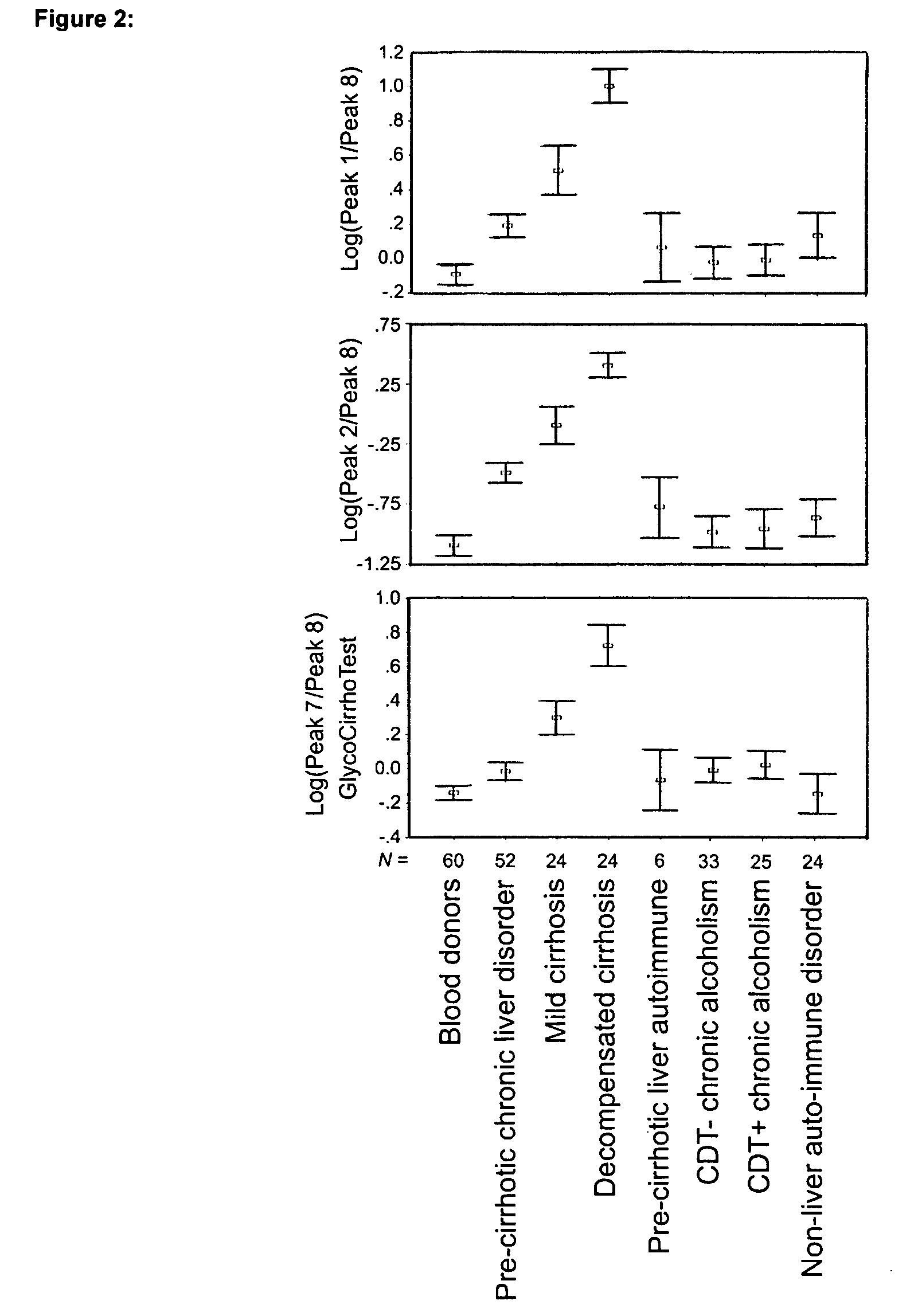
Serum marker for measuring liver fibrosis
The invention provides methods and kits to detect liver fibrosis or a change in the gradation of liver fibrosis in mammals. The diagnostic marker is based on the profiling and identification of diagnostic carbohydrates present in a body fluid such as blood serum.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
Screening method for hepatitis B specific serum marker
ActiveCN110057955AEffective early diagnosis targetComponent separationSerum markersMass spectrum analysis
The invention belongs to the technical field of medicine biology, and relates to a screening method for a specific serum marker in the body of a patient, in particular to a screening method for a hepatitis B specific serum marker. The screening method comprises the following steps of collection and storage of serum samples, processing methods of the serum samples, positive phase and reversed phasechromatographic technical conditions, mass spectrum data acquisition and analysis, non-targeted metabolome data processing, result screening with significance difference, and verification and application of a screening result.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Serum marker capable of evaluating cerebral hemorrhage risk before thrombolysis and application thereof
InactiveCN105203749AAccurate Risk PredictionAccurately draw risk predictionsMaterial analysisPathologyHuman body
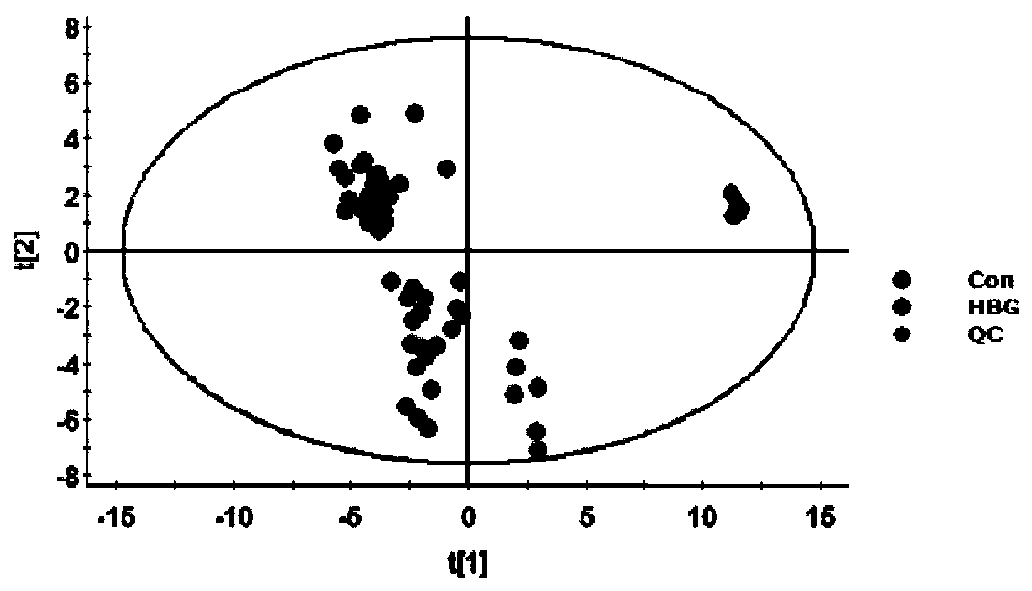
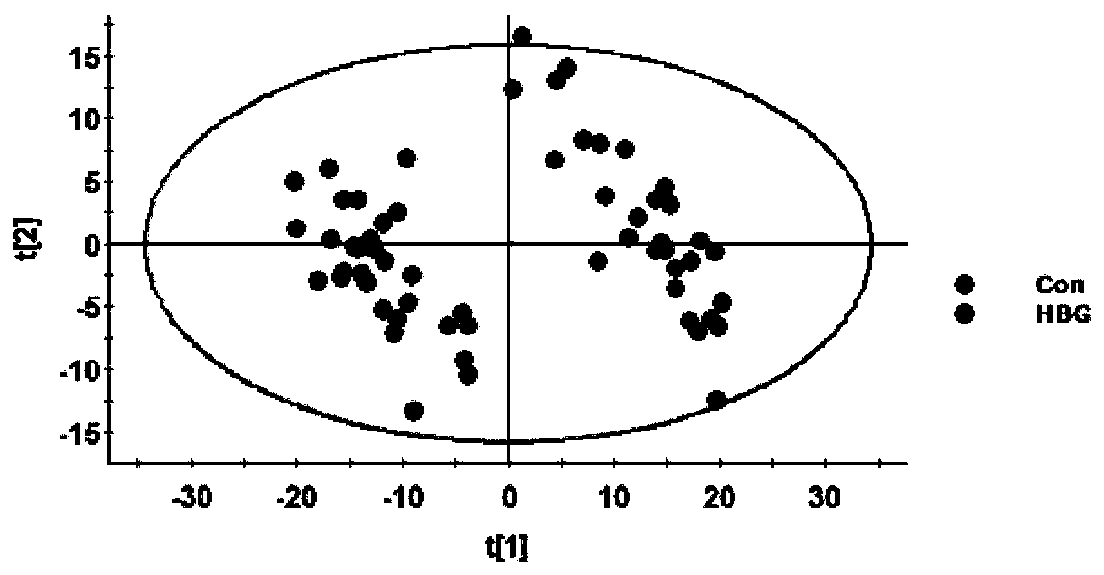
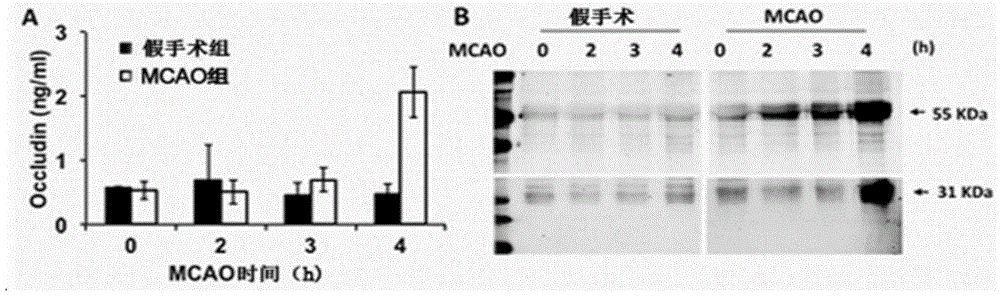
The invention relates to a serum marker capable of evaluating a cerebral hemorrhage risk before thrombolysis and application thereof and provides a kit used for detecting Occludin protein in the serum in the human body. When the kit comprising test strips is adopted, the kit has the advantages of being rapid, easy and convenient to use, accurate, low in cost, good in specificity and high in sensitivity, and the Occludin protein level result in peripheral blood before thrombolysis can be read within 5-15 min; when the kit comprising a 96-hole filter plate is adopted, the kit also has the advantage of being capable of rapidly testing the Occludin protein level in peripheral blood before thrombolysis. By adopting the kit for detecting the Occludin protein level in peripheral blood before thrombolysis (dosing), the risk that cerebral hemorrhage possibly occurs after thrombolysis can be effectively judged, a doctor is guided for medicine usage, and the great clinical significance is achieved.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Polypeptide composition for detecting serum marker of autoimmune disease patient and application of polypeptide composition
The invention discloses a polypeptide composition for detecting a serum marker of an autoimmune disease patient and an application of the polypeptide composition and belongs to the technical field of medicines. Polypeptide with bonding capability on the serum marker of an autoimmune disease is screened out from a phage random peptide library, and is applied to immunological detection of the serum marker through artificial synthesis. The polypeptide composition comprises all or partial sequences as shown in SEQID NO.1-19. In addition, the invention further discloses a chip comprising the polypeptide composition, an enzyme-linked immune response plate and a kit. The obtained polypeptide sequences have good bonding capability on the serum marker of the autoimmune disease patient, and can be applied to diagnosis and evaluation of the autoimmune disease.
Owner:HARBIN MEDICAL UNIVERSITY
Method for detecting cancer
The invention concerns methods and compositions that can be used for detecting cancer in mammals, particularly humans. The invention particularly concerns serum markers of cancers and their use in diagnostic procedures. The invention also concerns tools and / or kits that can be used for carrying out these methods (reagents, probes, primers, antibodies, chips, cells, etc., the preparation thereof and their use. The invention can be used for detecting the presence or the progression of a cancer in mammals, particularly breast cancer including during early stages.
Owner:BIOMERIEUX SA +1
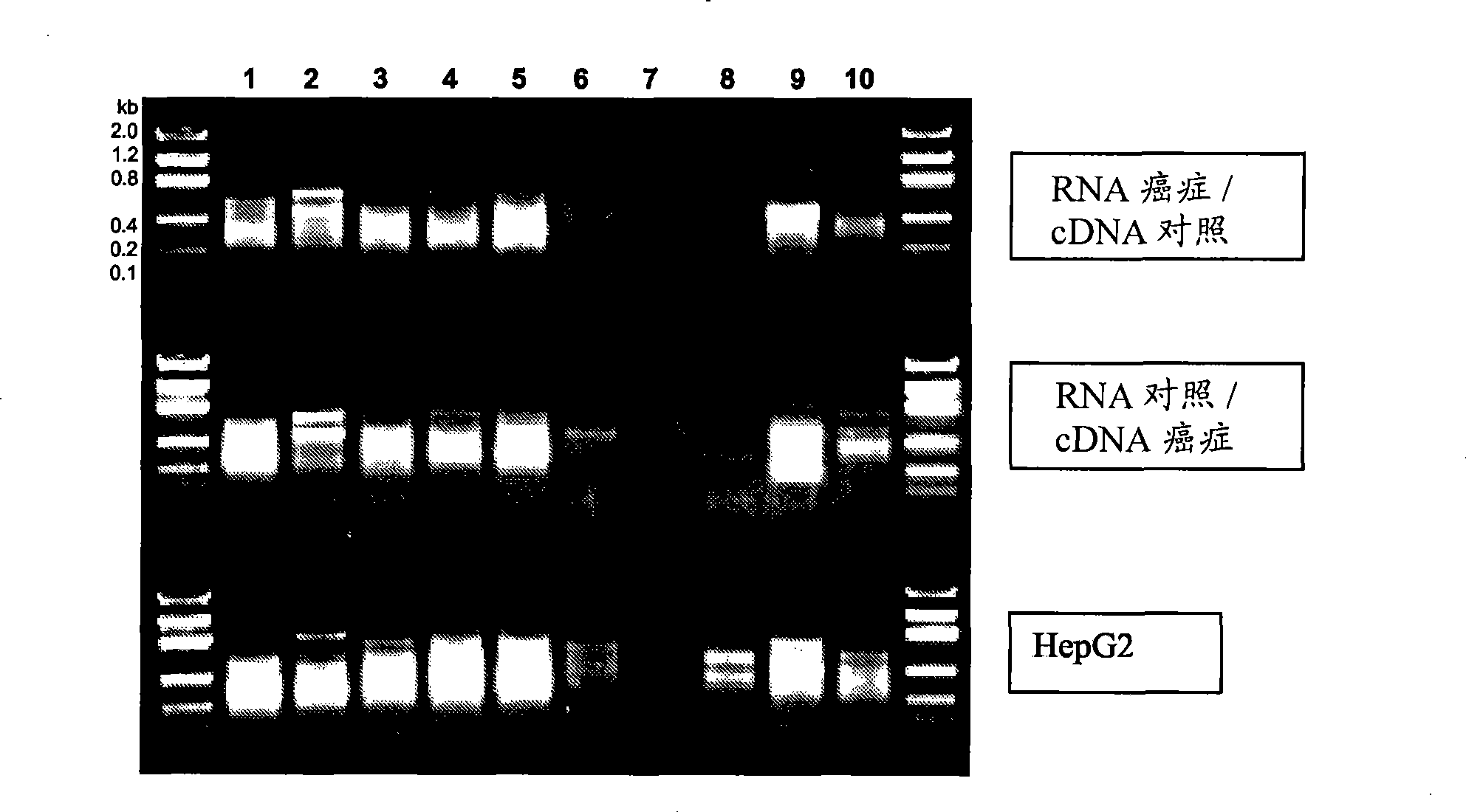
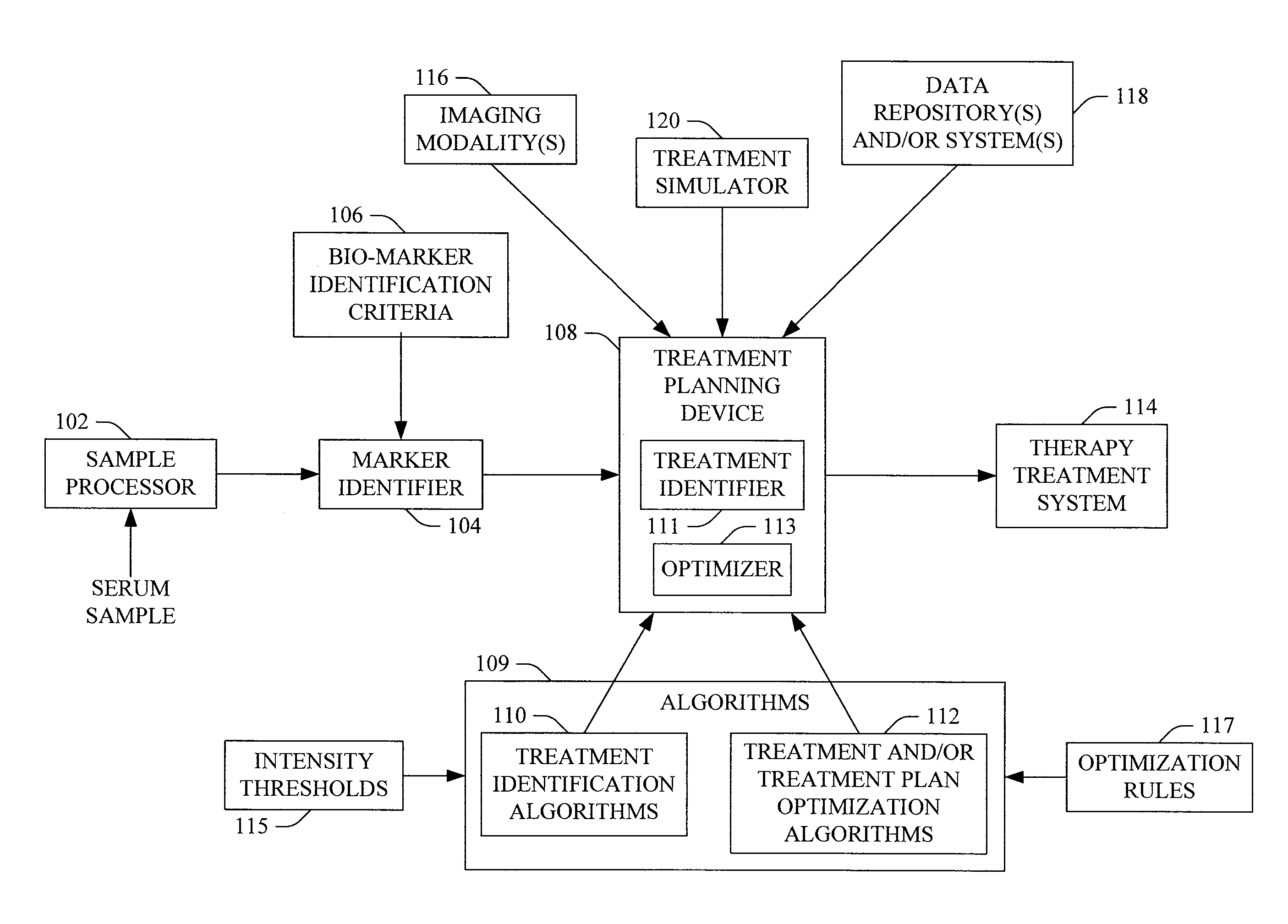
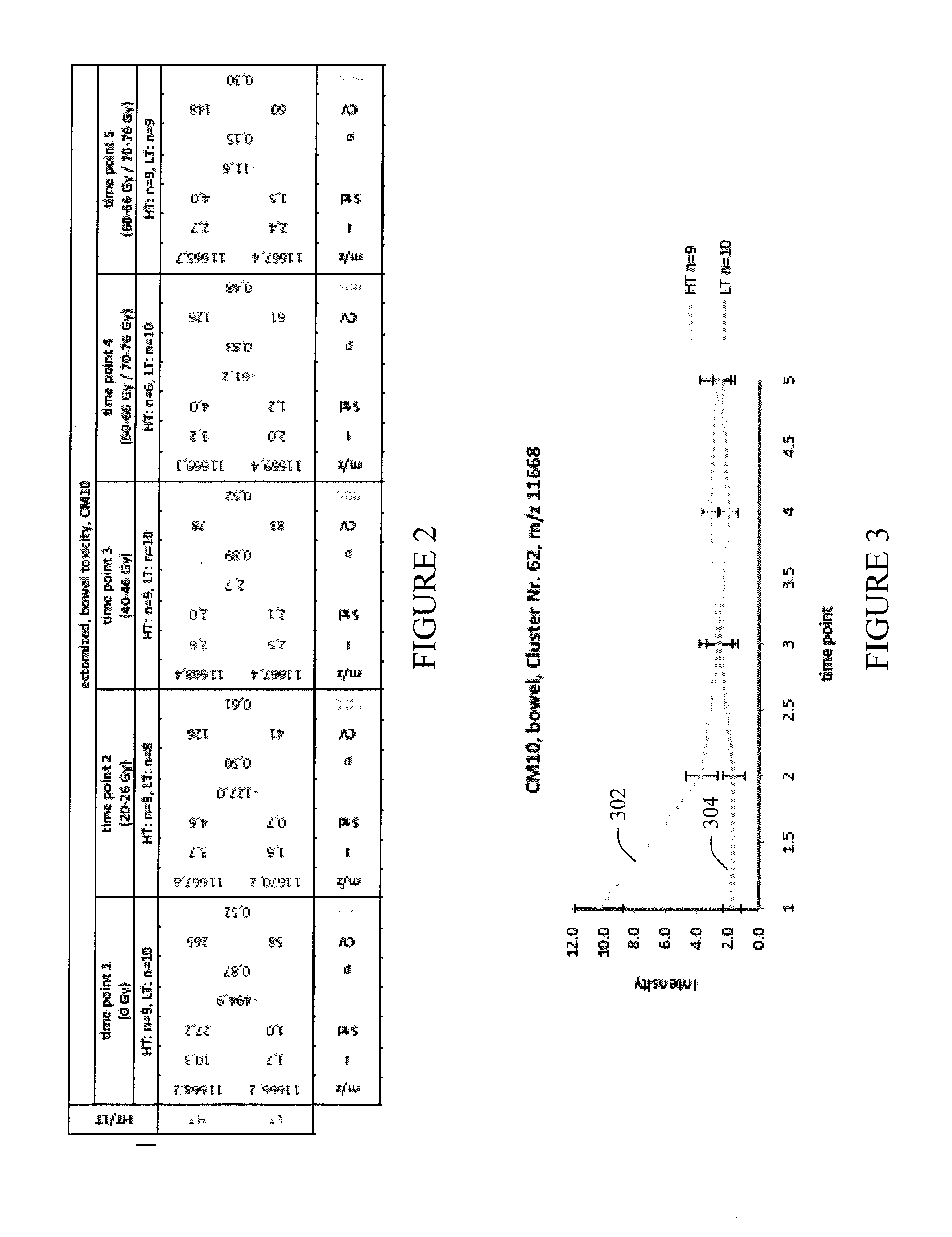
Treatment planning based on polypeptide radiotoxicity serum markers
InactiveUS20140113388A1High of radiotoxicityHigh riskMechanical/radiation/invasive therapiesDisease diagnosisSerum markersSerum ige
A method includes at least one of creating or adapting a treatment plan for a patient based on a set of serum polypeptides of the patient that are indicative of a radiotoxicity of the patient at least one of before or after at least one of a plurality of radiotherapy treatments of the treatment plan, wherein the radiotoxicity is induced by radiation exposure from the radiotherapy treatment. A system includes a treatment planning device (108) that facilitates at least one of creating or adapting a treatment plan for a patient based on amounts or concentrations of a set of serum polypeptides of the patient that indicate a high risk of or an early radiotoxicity of the patient to radiation from radiotherapy.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Side flow test paper strip for serum marker luminescence detection based on near infrared excitation and emission and preparation and use methods thereof
ActiveCN108872163AImprove luminositySmall volume requirementBiological material analysisAnalysis by material excitationPolyvinyl chlorideEngineering
The invention provides a side flow test paper strip for serum marker luminescence detection based on near infrared excitation and emission and preparation and use methods thereof, and relates to a side flow test paper strip and preparation and use methods thereof. The technical problems of heat damage of the existing detection material on biomolecules and high background signals can be solved. Theside flow test paper trip consists of a polyvinyl chloride support back plate, a detection pad, a combination pad, a sample pad and an absorption pad, wherein the combination pad is loaded with an upper conversion beta-NaYF4:Yb,Tm@NaXF4 nanometer crystal probe combined with antibodies and an upper conversion beta-NaYF4:Yb,Tm@NaXF4 nanometer crystal probe combined with mouse IgG. The preparation method comprises the following steps of 1, preparing a core; 2, coating a shell; 3, assembling a luminous probe; 4, assembling the side flow test paper strip. In the use process, the laser is used forirradiating the detection line to detect the emission spectrum; the detection range is 0.05 to 50ng / mL; the detection limit is 0.02ng / mL; the side flow test paper strip can be used in medical care detection.
Owner:HARBIN INST OF TECH
Micro-fluid control electrochemical biological sensing system for simultaneous detection on different serum markers of prostate cancer
ActiveCN103616427AReduce consumptionEasy to operateMaterial analysis by electric/magnetic meansFluid controlProstate cancer
The invention provides a micro-fluid control electrochemical biological sensing system for simultaneous detection on different serum markers of prostate cancer. The system comprises a continuous feeding unit, a micro-fluid control chip and a power system, wherein the continuous feeding unit is used for sequentially conveying a sample solution, a sample eluant, a signal probe solution, a signal probe eluant and an electrochemical detection buffer solution; the sample solution contains a protein marker and / or a miRNA marker for the prostate cancer; the micro-fluid control chip consists of one or more micro-channel networks; the micro-fluid control chip covers an electrode array so as to form a channel system; antibodies and / or capture probes which have interaction with the sample solution are fixed on the upper surface of the electrode array; the channel system is connected with the continuous feeding unit; the power system is used for providing power for the continuous feeding unit. The invention innovatively provides the micro-fluid control electrochemical biological sensing system which is capable of simultaneously detecting different serum markers closely related to prostate cancer diseases and is high in sensitivity and low in cost.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
Serum marker for measuring liver fibrosis
The invention provides methods and kits to detect liver fibrosis or a change in the gradation of liver fibrosis in mammals. The diagnostic marker is based on the profiling and identification of diagnostic carbohydrates present in a body fluid such as blood serum.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
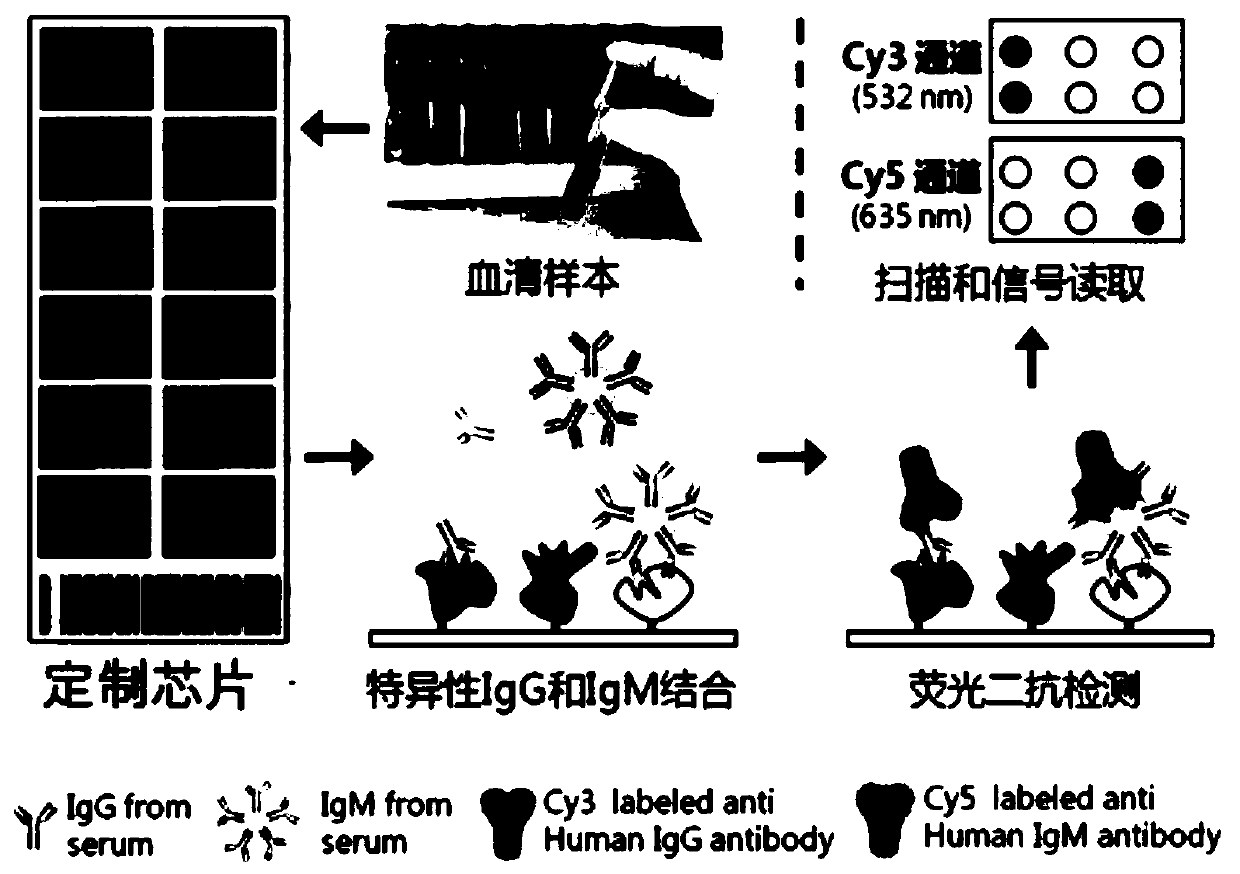
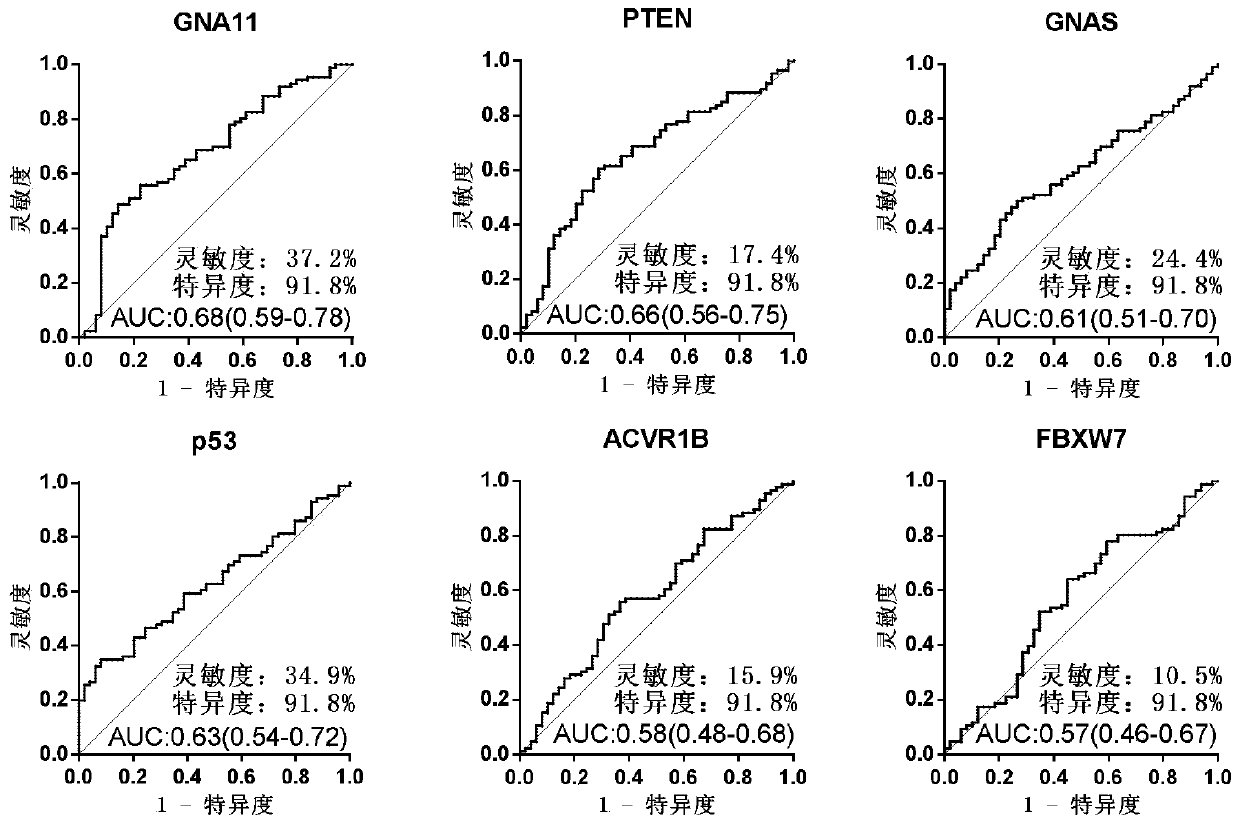
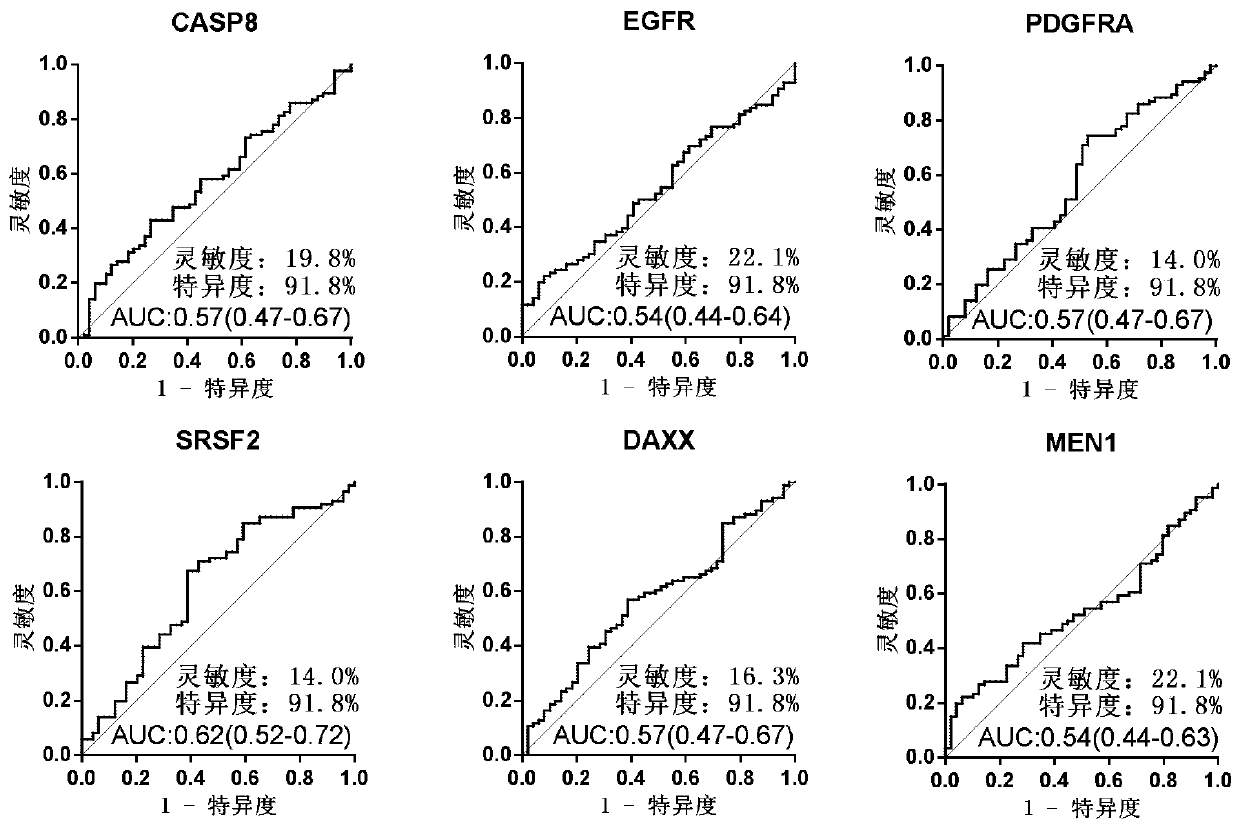
Serum protein marker for early screening and diagnosis of esophageal squamous carcinoma, kit and detection method
ActiveCN110716044AGuaranteed specificityGood reference valueColor/spectral properties measurementsOncologyBiomedicine
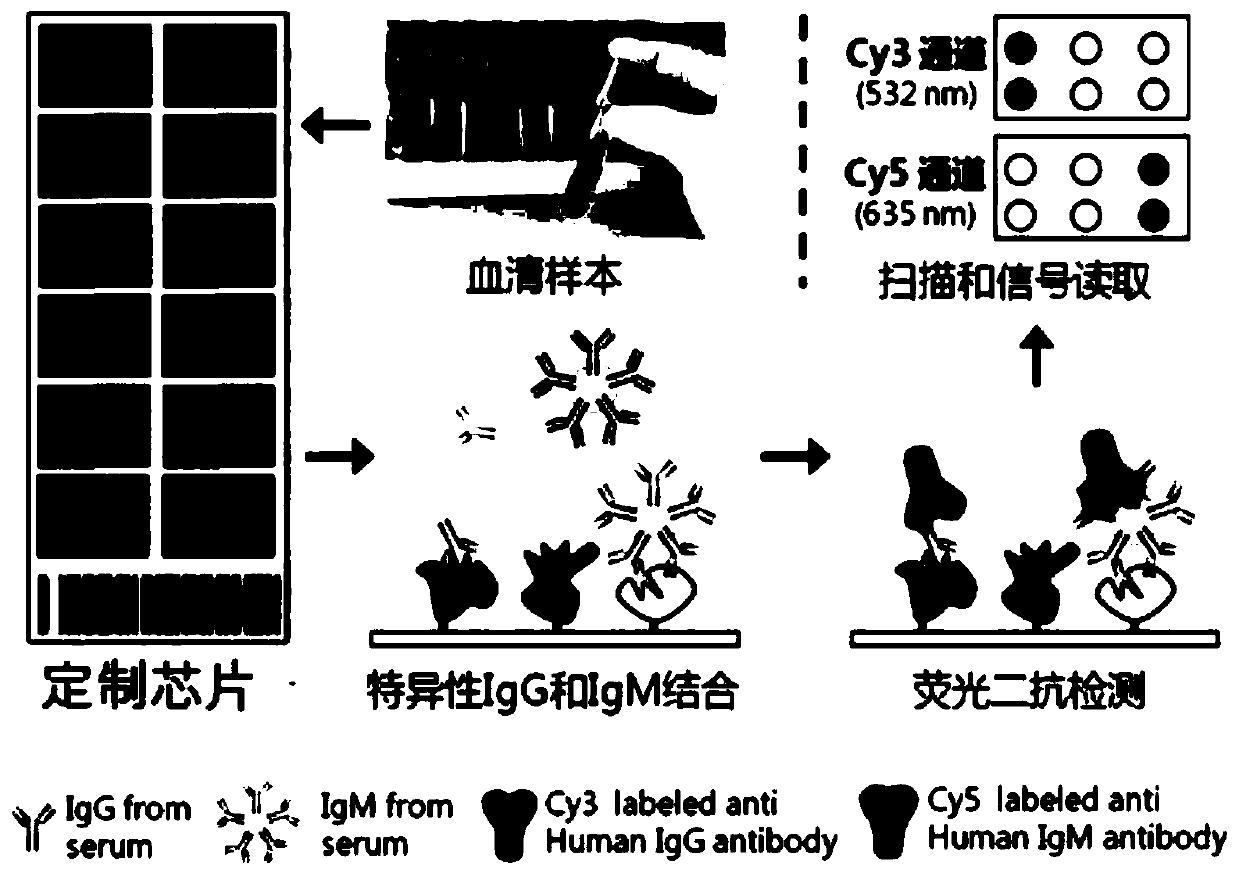
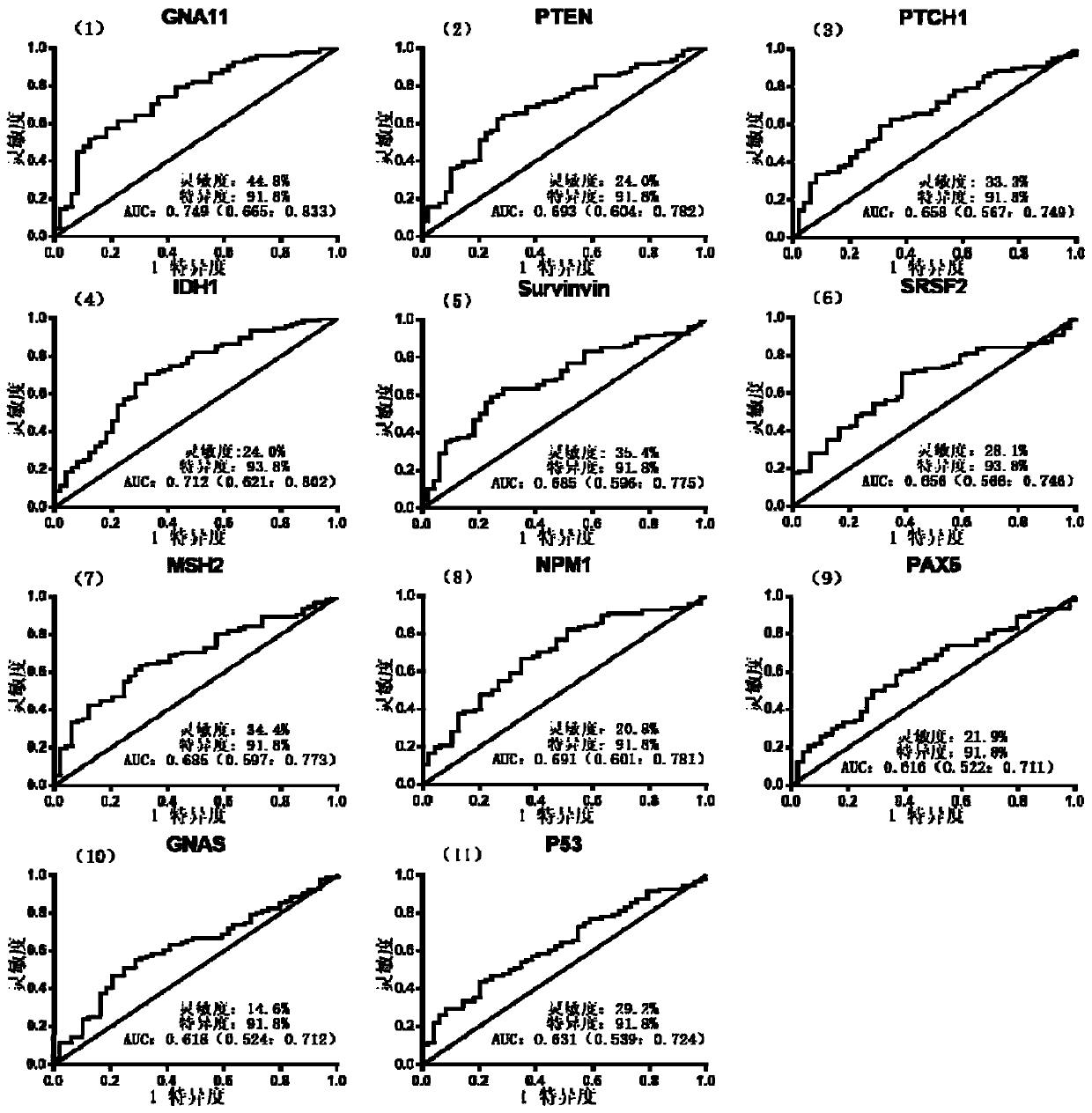
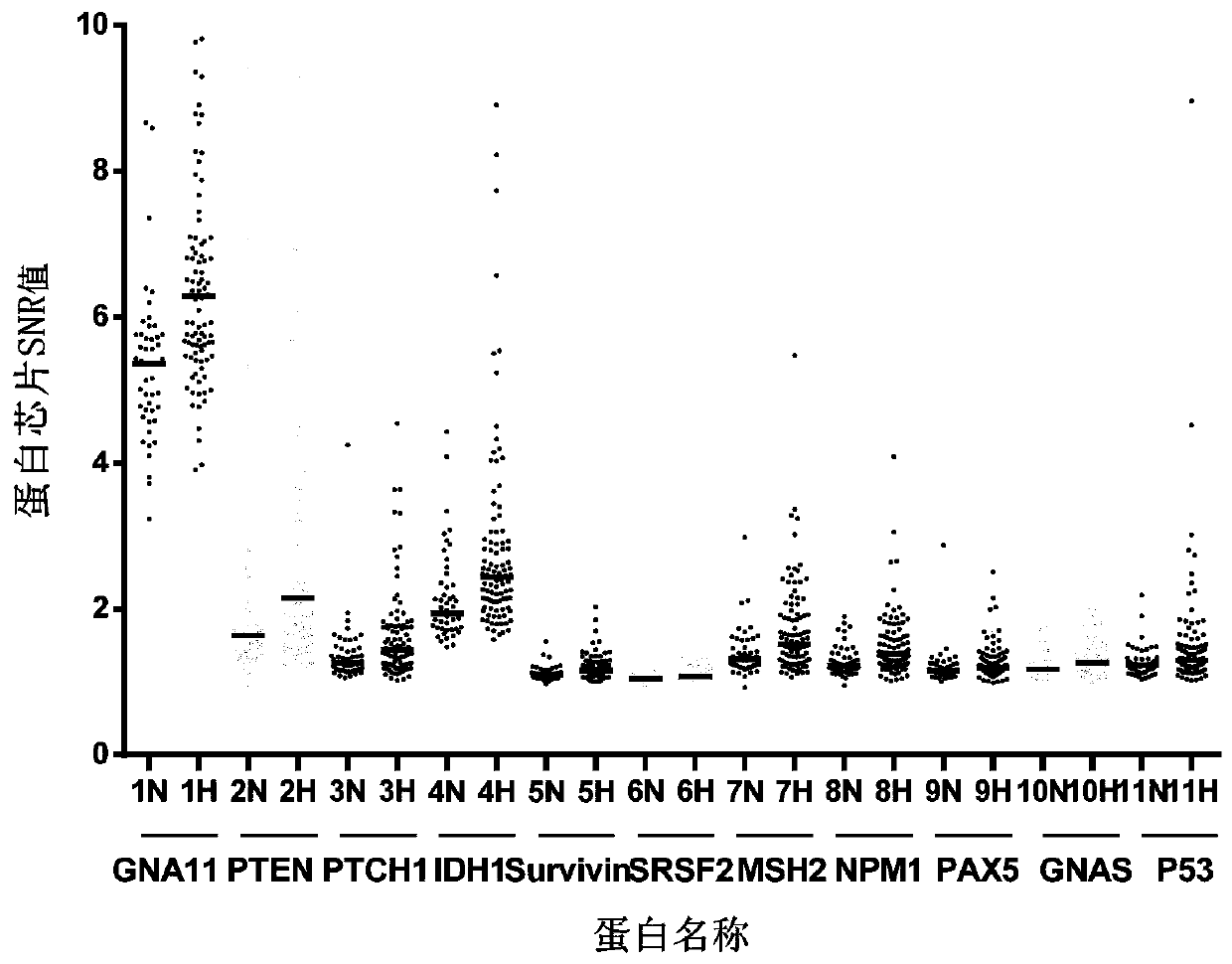
The invention discloses a serum protein marker for early screening and diagnosis of esophageal squamous carcinoma, and belongs to the field of biomedical technology. The serum protein marker is any one or a combination of two or more of proteins encoded by P53, GNA11, GNAS, PTEN, ACVR1B, FBXW7, EGFR, PDGFRA, SRSF2, MEN1, DAXX or CASP8 genes. Based on the role played by cancer driving genes in tumorigenesis and development, a human protein chip encoded by 138 cancer driving genes is customized, the human protein chip contains 180 human-derived recombinant proteins for screening out potential markers capable of diagnosing caners or other markers capable of characterizing cancers, early detection serum markers of the esophageal squamous carcinoma are primarily screened out by the protein chip, and then are verified by an ELISA indirect method experiment, a group of joint serum protein markers of esophageal squamous carcinoma for the early screening and diagnosis of the esophageal squamouscarcinoma is screened out at last for assisting the clinical diagnosis of the esophageal squamous carcinoma, so that the serum protein marker has better reference value.
Owner:ZHENGZHOU UNIV
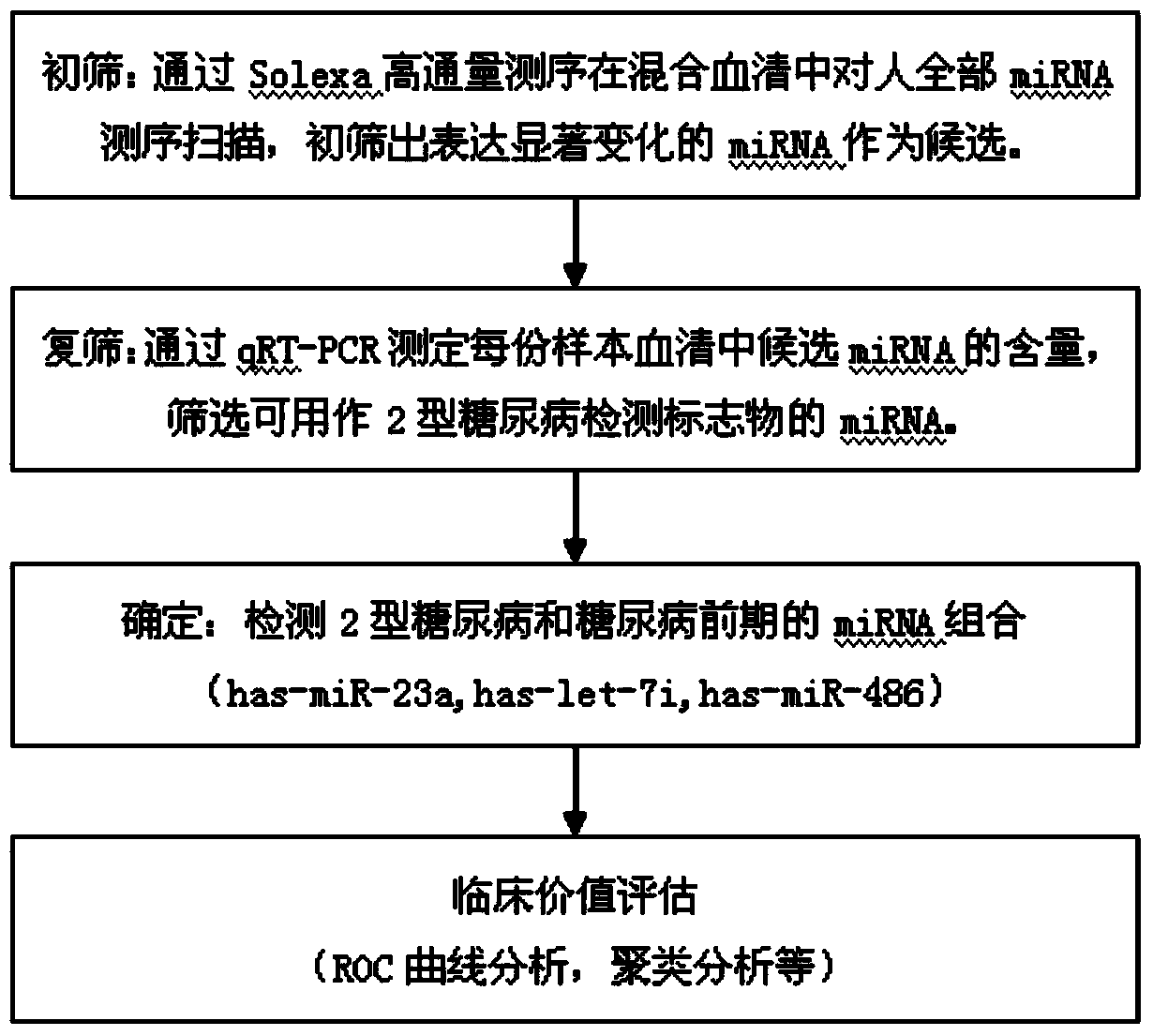
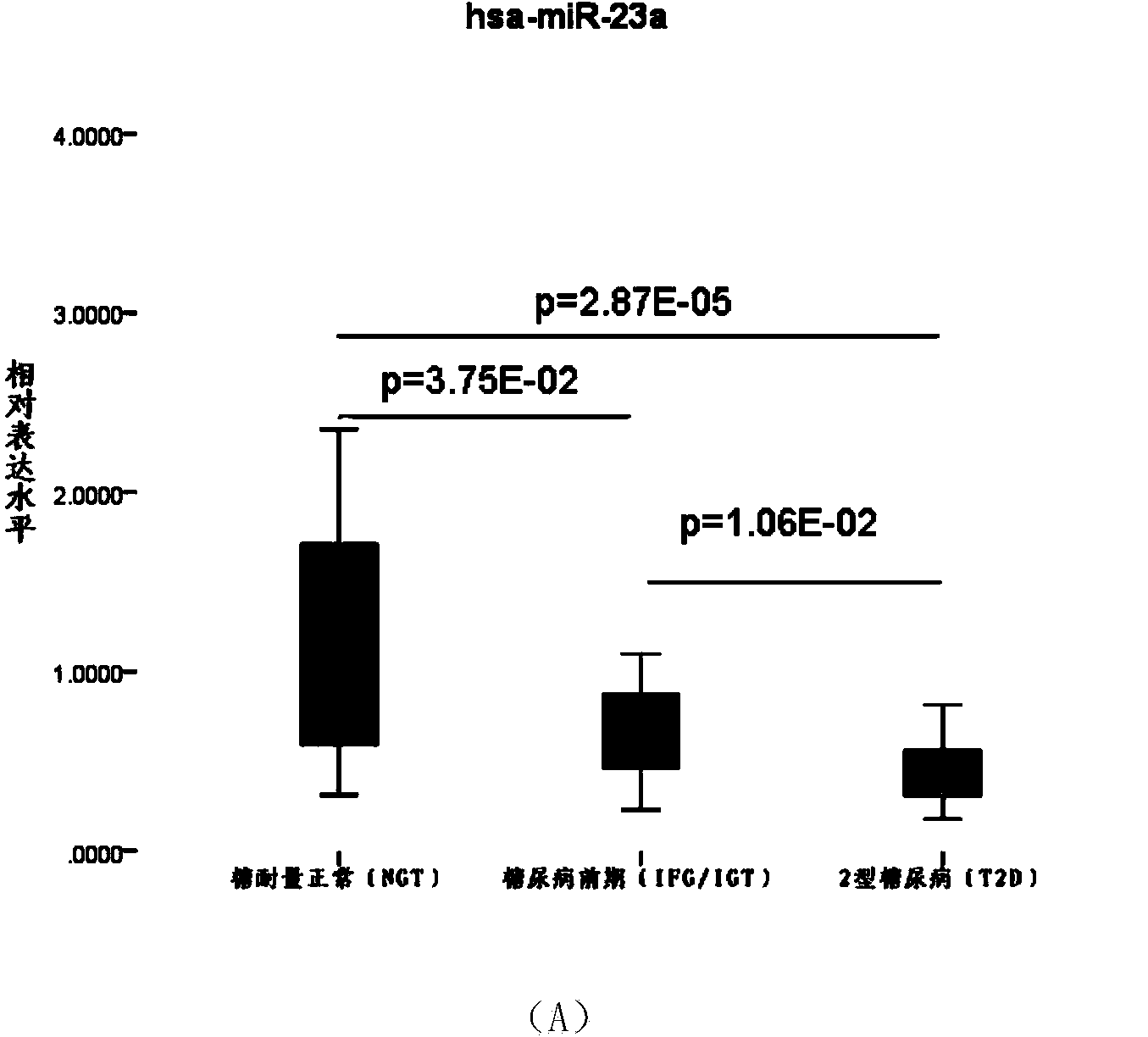
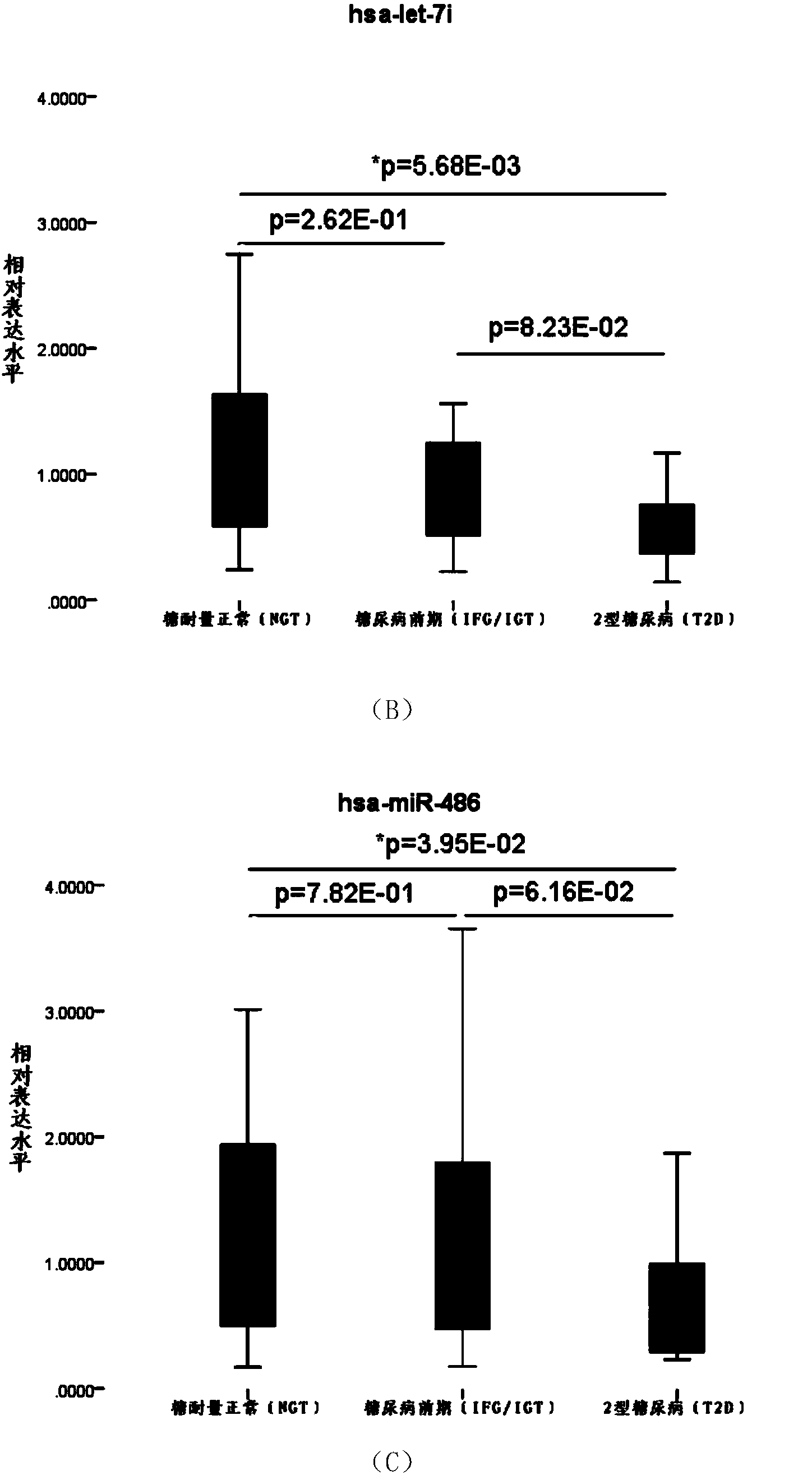
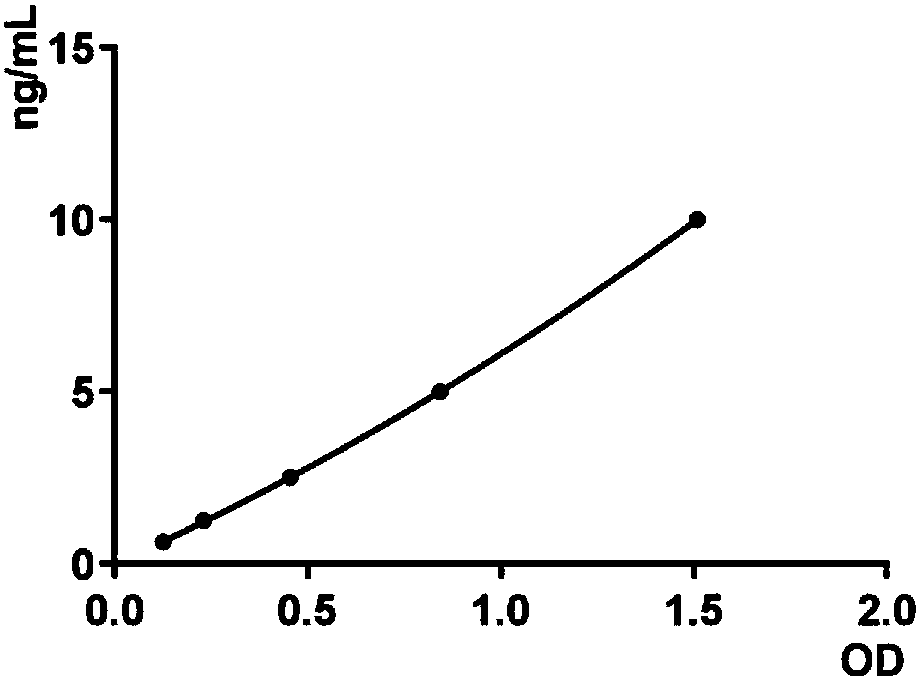
Serum miRNA biomarker of type 2 diabetes mellitus and application thereof
The invention discloses a serum miRNA composition, which is at least one of the following serum miRNAs: hsa-miR-23a, hsa-let-7i, and hsa-miR-486. The invention also simultaneously provides a fluorescent quantitative detection primer composition for detection of type 2 diabetes mellitus. The composition comprises an RT primer, an FW primer and an RV primer of each miRNA in hsa-miR-23a, hsa-let-7i, and hsa-miR-486. By adopting the serum miRNA marker for early diagnosis of type 2 diabetes mellitus and cancer risk evaluation provided by the invention, a serum miRNA biomarker can be used for diagnosing whether a subject has the type 2 diabetes mellitus or early diagnosis of the type 2 diabetes mellitus and cancer risk evaluation.
Owner:ZHEJIANG SCI-TECH UNIV +1
Serum marker MMP-7-based biliary atresia diagnosis kit
InactiveCN108267585AEasy to operateAid in early diagnosisDisease diagnosisPositive controlPRIMARY BILIARY ATRESIA
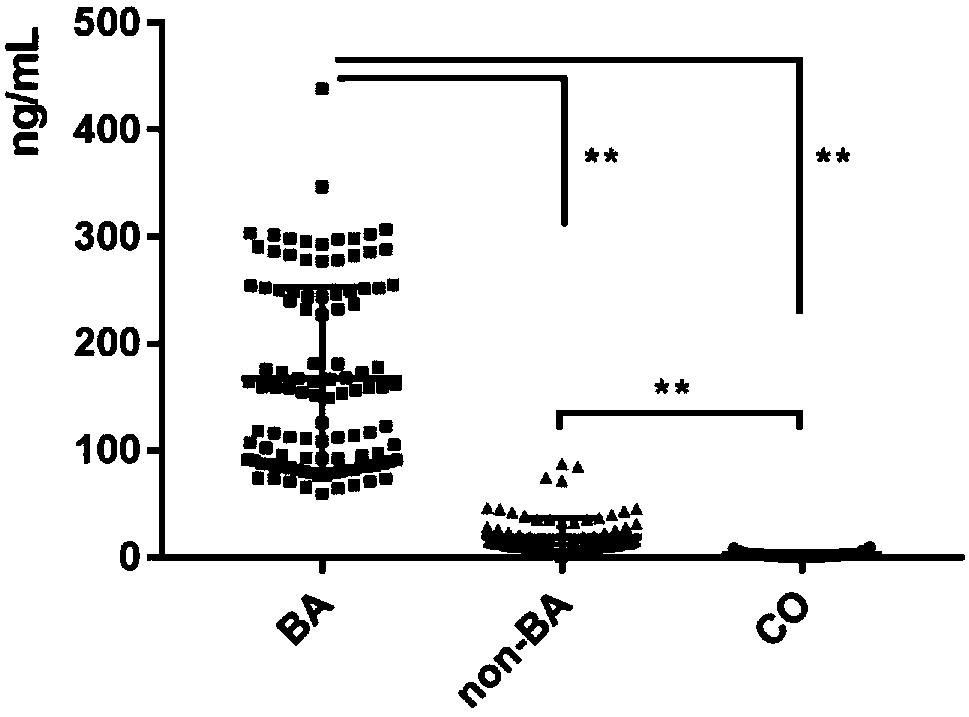
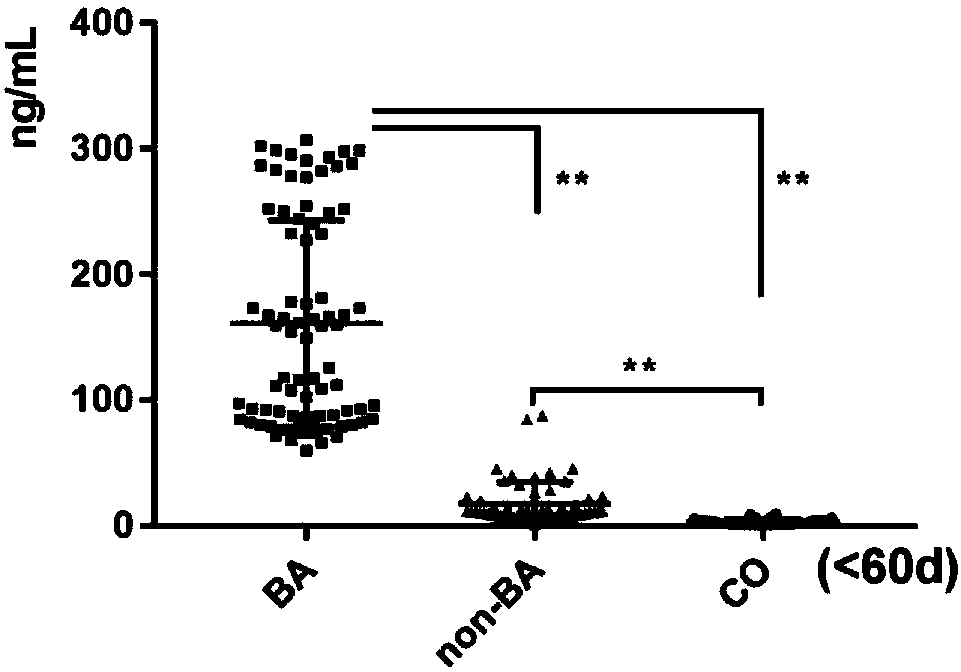
The invention relates to a serum marker MMP-7-based biliary atresia diagnosis kit. The diagnosis kit comprises an anti-human MMP-7 monoclonal antibody coated ELISA plate, a negative control solution,a positive control solution, an enzyme labeling reagent, an enzyme substrate solution, a blocking solution, a sample diluent, a washing solution and a stopping solution. The diagnosis kit is a new sensitive, safe, reliable and easily-operated commercial kit. Quantitative detection of the level of MMP-7 in human serum is helpful for early diagnosis of BA; the BA diagnosis sensitivity of the serum biomarker protein MMP-7 is 100%, and the BA diagnosis specificity is 95.6%, so the kit has the characteristics of high specificity and high sensitivity; and the kit improves the early diagnosis rate ofBA, reduces misdiagnosis, and improves the self liver survival rate of the BA.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Serum markers associated with early and other stages of breast cancer
Methods for identifying disease-specific markers, in particular breast cancer markers, by electrophoretically separating serum albumin complexes in a biological sample on a membrane are provided. Electrophoretic separation profiles representing different diseases or different cancer stages can be produced, and used in the diagnosis, prognosis and treatment of these diseases. Methods for identification of a cancer peptide fragment comprising a cancer peptide motif are provided. Also provided are breast cancer and other cancer markers and antibodies that specifically recognize these markers.
Owner:TEMPLE UNIVERSITY
Method for detecting serum marker of pancreatic cancer
InactiveCN101613748AEasy to detectIncreased sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceMalignancyAdvanced stage
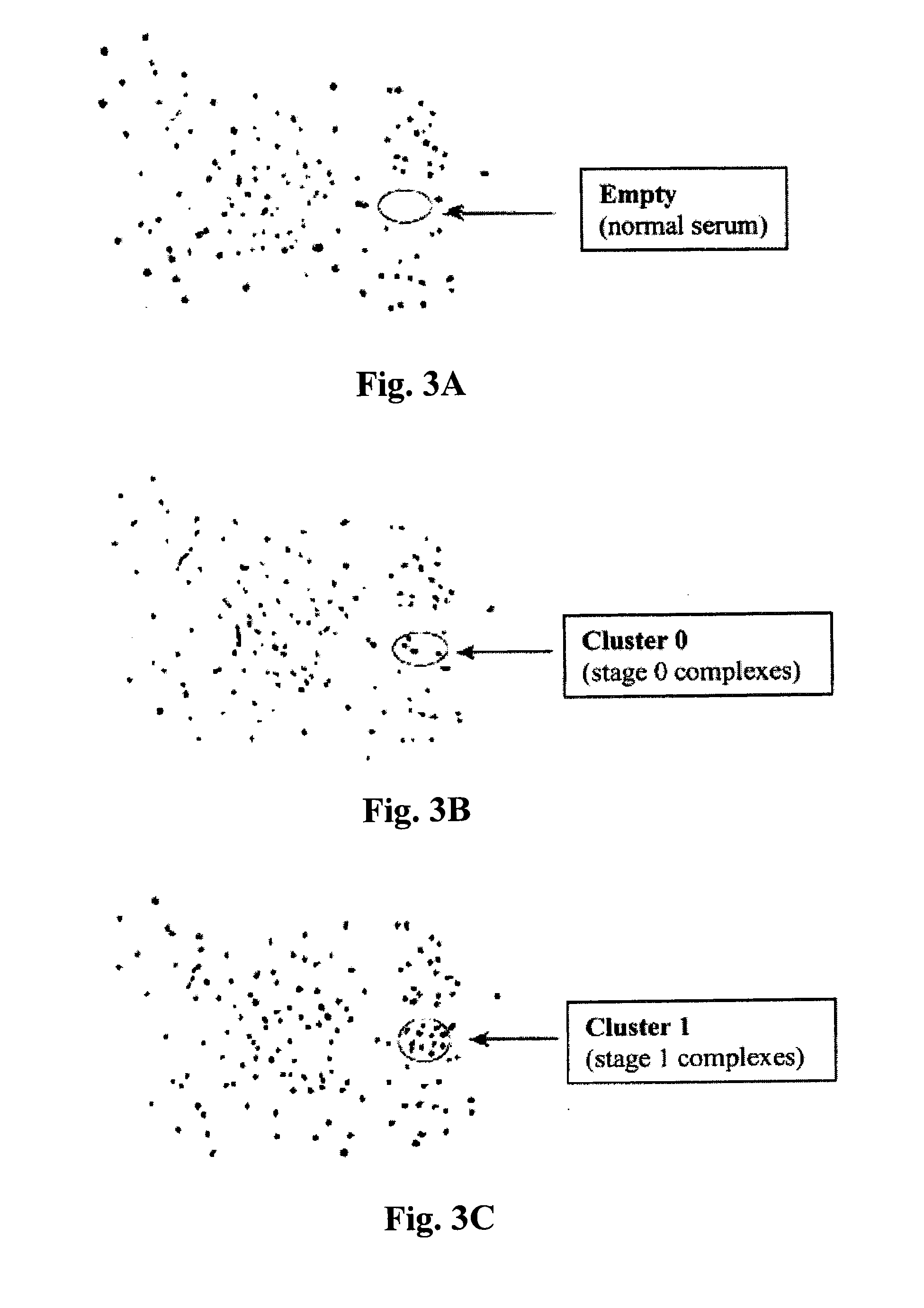
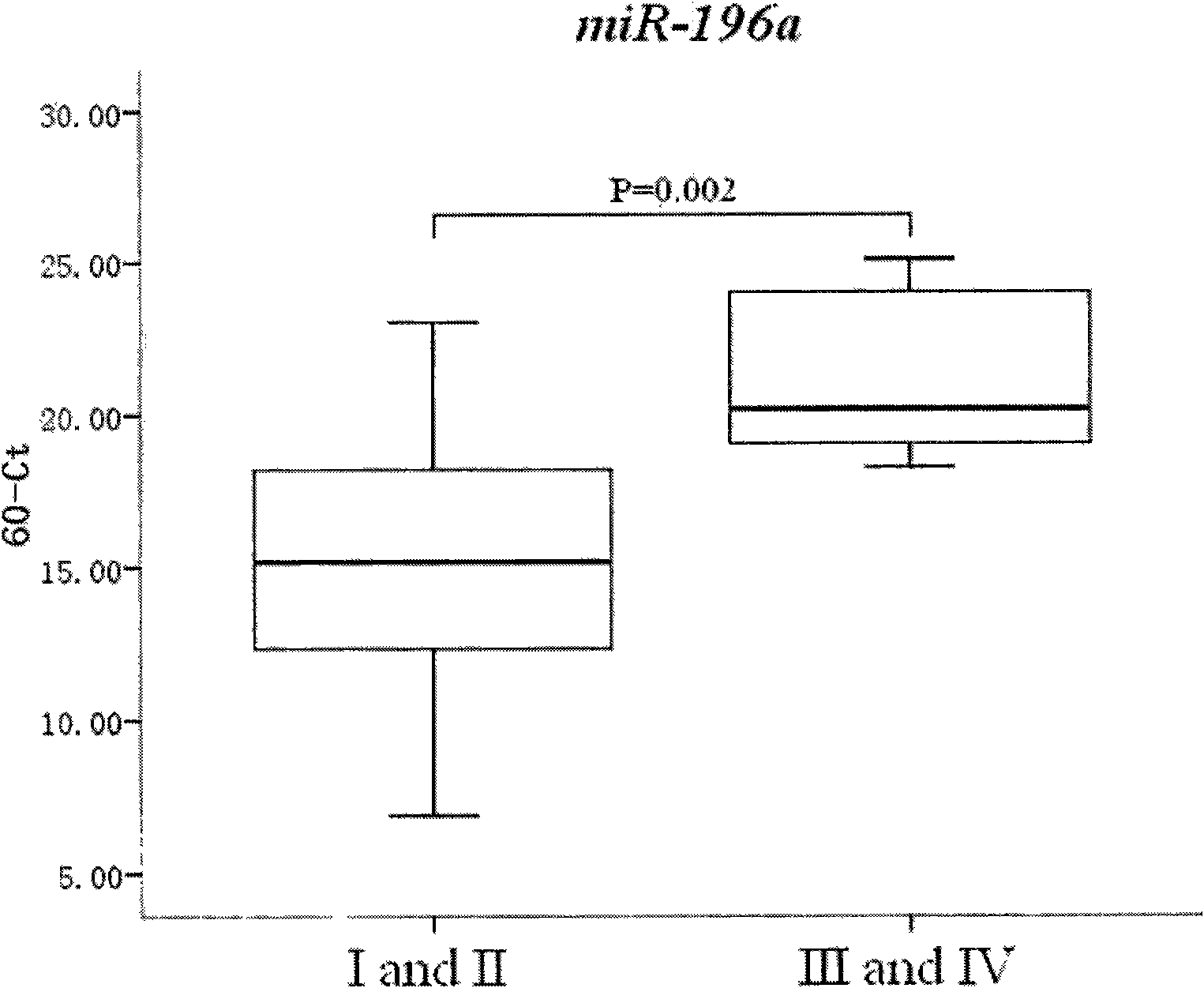
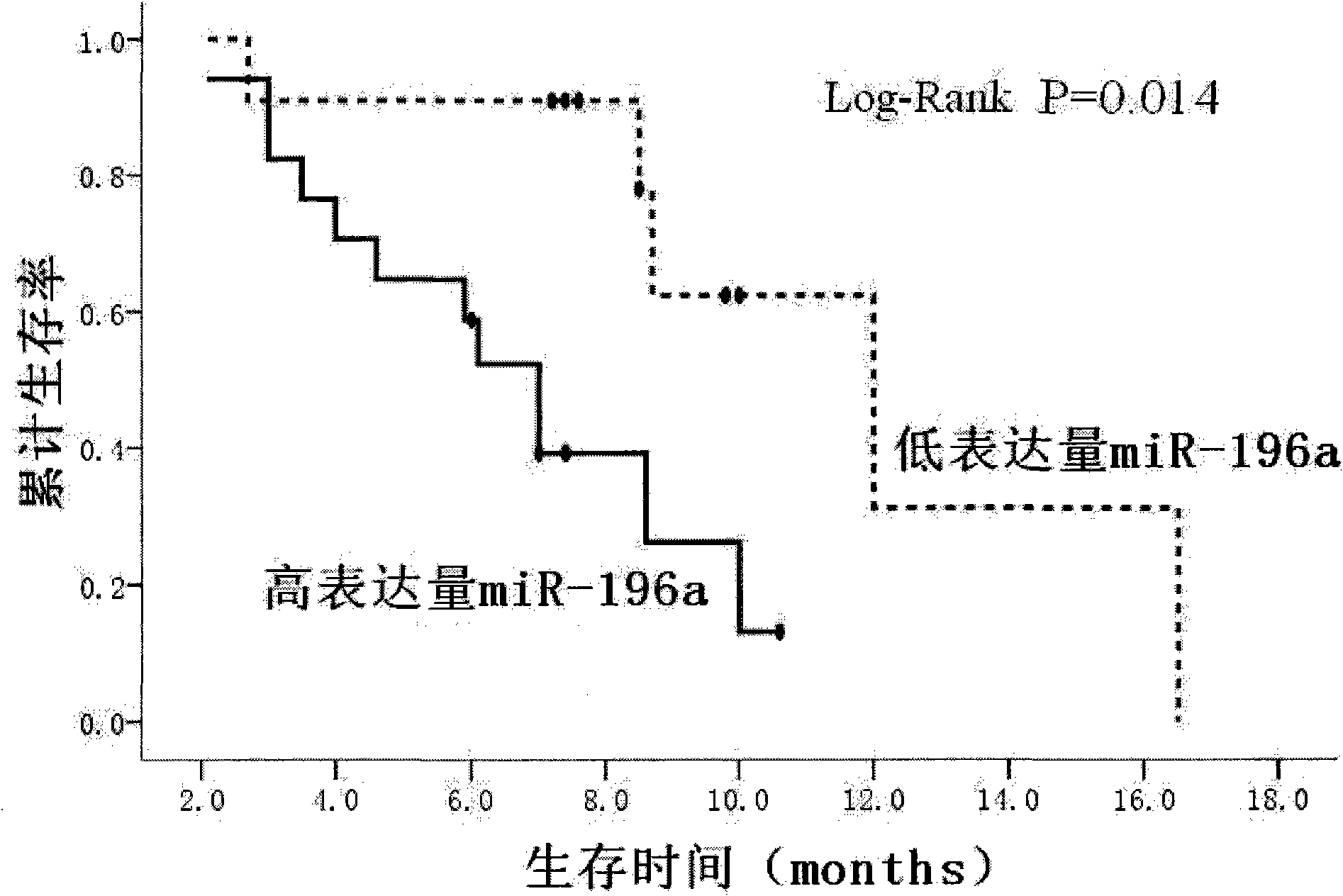
The invention relates to the technical field of medical molecular biology and provides a method for detecting the serum marker of pancreatic cancer. The pancreatic cancer has high grade malignancy, difficult early diagnosis and poor prognosis, and the pancreatic cancer also lacks really effective solution, with surgical resection as the only therapeutic method to prolong survival period. Unfortunately, most of pancreatic cancer patients are in the advanced stage (TNM belongs to III and IV stages) and miss the surgical option. The invention aims at providing a method for detecting the serum marker of pancreatic cancer and applying the method to early diagnosis of pancreatic cancer and clinical judgment of laparotomy indication. The study proves that in the invention, the expression level of miR-196a in the serum is closely related to the postoperative survival period of pancreatic cancer; the study later proves that the relative expression abundance of miR-196a can well distinguish resectable pancreatic cancer (TNM belongs to I and II stages) from pancreatic cancer in advanced stage (TNM belongs to III and IV stages), so the method in the invention can be used for detecting the serum marker microRNA-196a of pancreatic cancer, and the method further has the advantages of convenient detection, good sensitivity and high accuracy.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Reagent kit for detecting 2-diabetes serum mark in Chinese people
The invention relates to the reagent box to detect serum of II-type diabetes. Specially, it applies the serum of apolipoprotein A-I and corresponding express-level in patient to modify the box. It also relates to the application in diagnosis and treatment of apolipoprotein A-I.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Detection kit for schistosomiasis japonica blood serum designated object
InactiveCN101393217AImprove featuresIncreased sensitivityMaterial analysisPeptide antigenTherapeutic effect
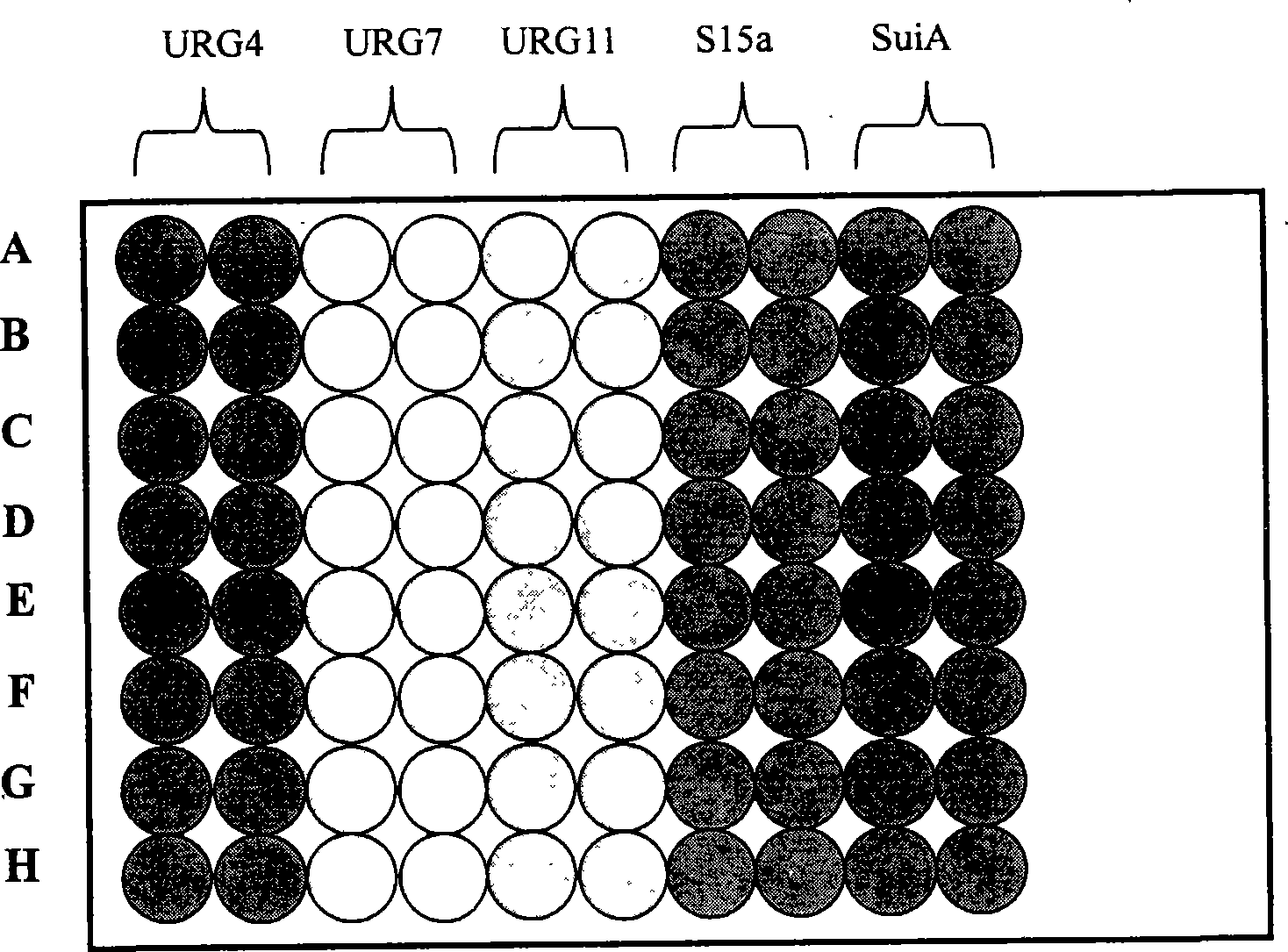
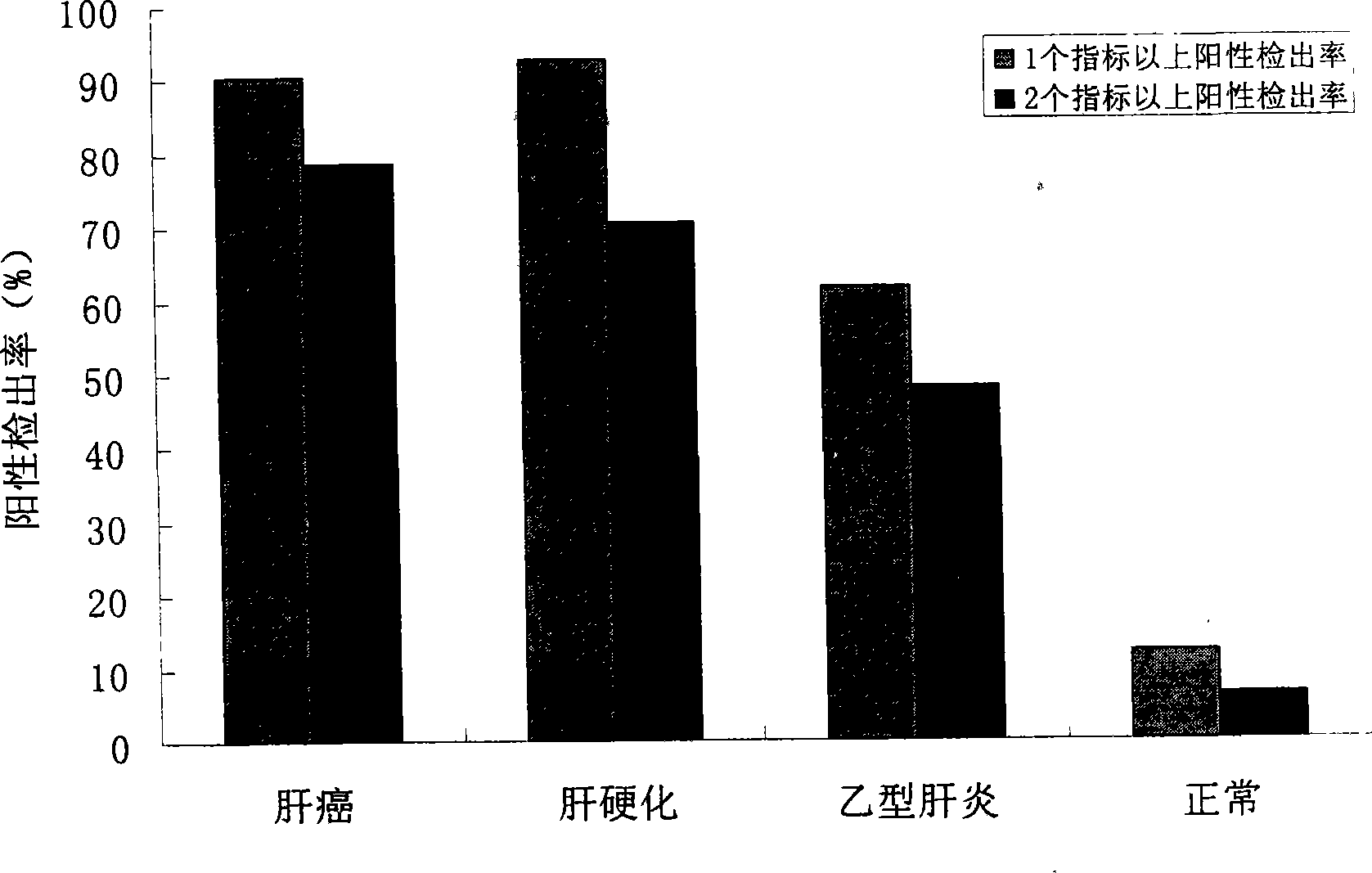
The invention relates to the technical field of biomedical diagnostics and discloses a reagent kit for detecting a serum marker of primary hepatic carcinoma, which consists of an ELISA plate enveloped by antigens, an enzyme-labeled antibody working solution, a sample diluent, a washing liquid, positive and negative control serum, a chromogenic solution and a stopping liquid. The reagent kit is characterized in that the envelope antigens of the ELISA plate are five groups of peptide antigens L4-A and L4-B; L7-B; L11-1, L11-3 and L11-4; L12-A and L12-B; and Sui1-A and Sui1-B, which correspond to five serum marker antibodies of the primary hepatic carcinoma; and Sui1-A and Sui1-B respectively. Except the L7B which is separately enveloped, each group of all the other groups of peptide antigens is enveloped by proportionally mixing polypeptides in the same group. Proven by evaluation experiments of the reagent kit and clinical trial, the reagent kit has good specificity and sensitivity, and can be used for early warning prompt or early diagnosis before the primary hepatic carcinoma appears or at early stage of the primary hepatic carcinoma, thereby improving the survival rate of carcinoma patients and evaluating treatment effect and disease outcome in time by observing the dynamic change of related indicators of the serum.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Joint destruction biomarkers for Anti-il-17a therapy of inflammatory joint disease
InactiveUS20100239590A1Low serum levelsAvoid destructionAntipyreticAnalgesicsSerum markersRANKL Protein
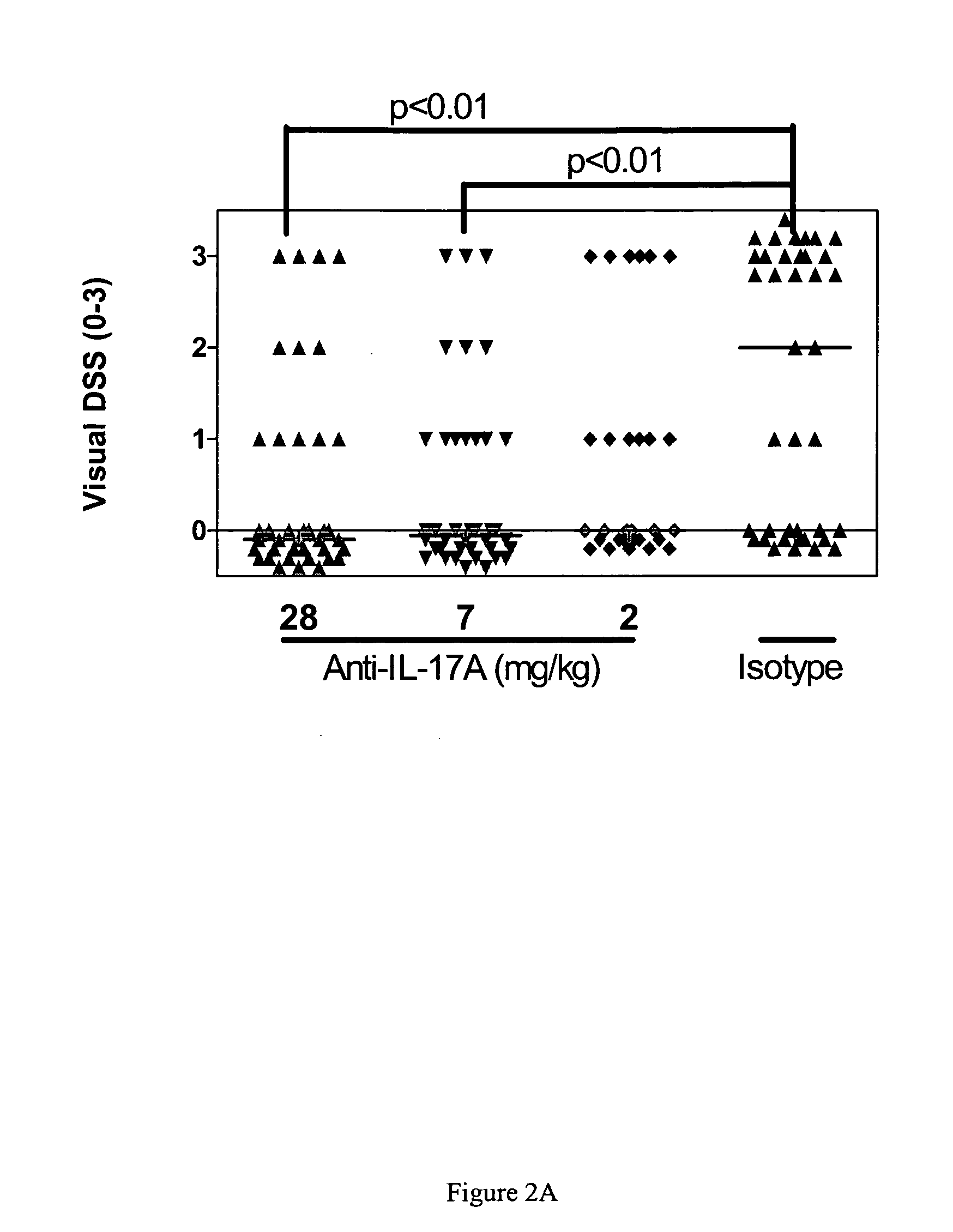
Novel methods and drug products for treating inflammatory joint diseases such as rheumatoid arthritis and associated arthritides are disclosed. The methods and products employ various serum markers of bone and cartilage metabolism or destruction, including cartilage oligomer matrix protein (COMP) and Receptor activator of NFB ligand (RANKL), as biomarkers to assess the effect of IL-17A antagonists on joint destruction in inflammatory joint diseases.
Owner:MERCK SHARP & DOHME CORP
Atherosclerosis-related serum miRNA (microribonucleic acid) marker group, and specific primers and application thereof
ActiveCN103160588AStrong complementarityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseNucleotide
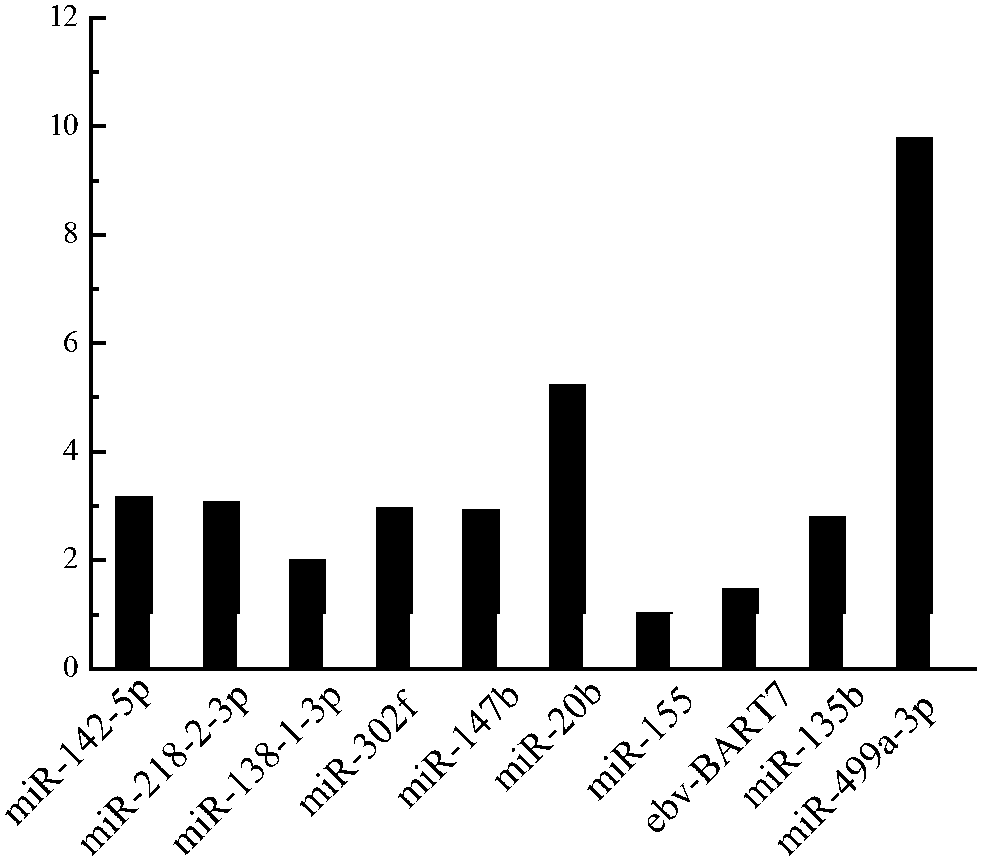
The invention relates to an atherosclerosis-related serum miRNA (microribonucleic acid) marker group, and specific primers and application thereof. The marker group comprises a miR-135b marker of which the nucleotide sequence is shown as SEQ ID NO.1 and a miR-499a-3p marker of which the nucleotide sequence is shown as SEQ ID NO.2. The invention also relates to specific primers and application of the miRNA marker group. The two markers screened out from the serum miRNA marker group provided by the invention have high complementarity, thereby ensuring that the detection is quantitatively precise and greatly improving the sensitivity and specificity of disease diagnosis.
Owner:SHANDONG UNIV
Triple test diagnostic kit for breast cancer
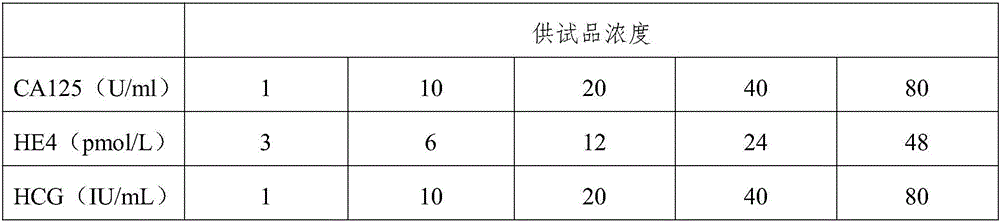
ActiveCN105866418AHigh sensitivityPlay the role of multi-stage amplificationMaterial analysisPositive controlEarly breast cancer
The invention belongs to the field of biological detection and specifically relates to a triple test diagnostic kit for breast cancer of tumor serum markers CA125, HE4 and P-her2. The triple test diagnostic kit for breast cancer provided by the invention mainly comprises an abzyme elisa plate, a biotin antibody enveloped plate, bovine serum albumin, serum diluent, horse radish peroxidase, tetramethyl benzidine, sulfuric acid, negative control liquid and positive control liquid. The triple test diagnostic kit for breast cancer provided by the invention can be used for detecting the expression levels of CA125, HE4 and P-her2 in the body of the patient after operative treatment or the relapsing and metastatic tumor patient; the triple test diagnostic kit for breast cancer has important significance in clinical diagnosis of breast cancer; the triple test diagnostic kit for breast cancer provided by the invention can reduce the interference of other matters in serum, can greatly increase the accuracy and stability of detection result and is beneficial to the early diagnosis and prognosis treatment of the patient suffering from breast cancer.
Owner:赛特斯(海南)生物医学有限公司
Circular RNA marker hsa_circ_0001788 and application thereof
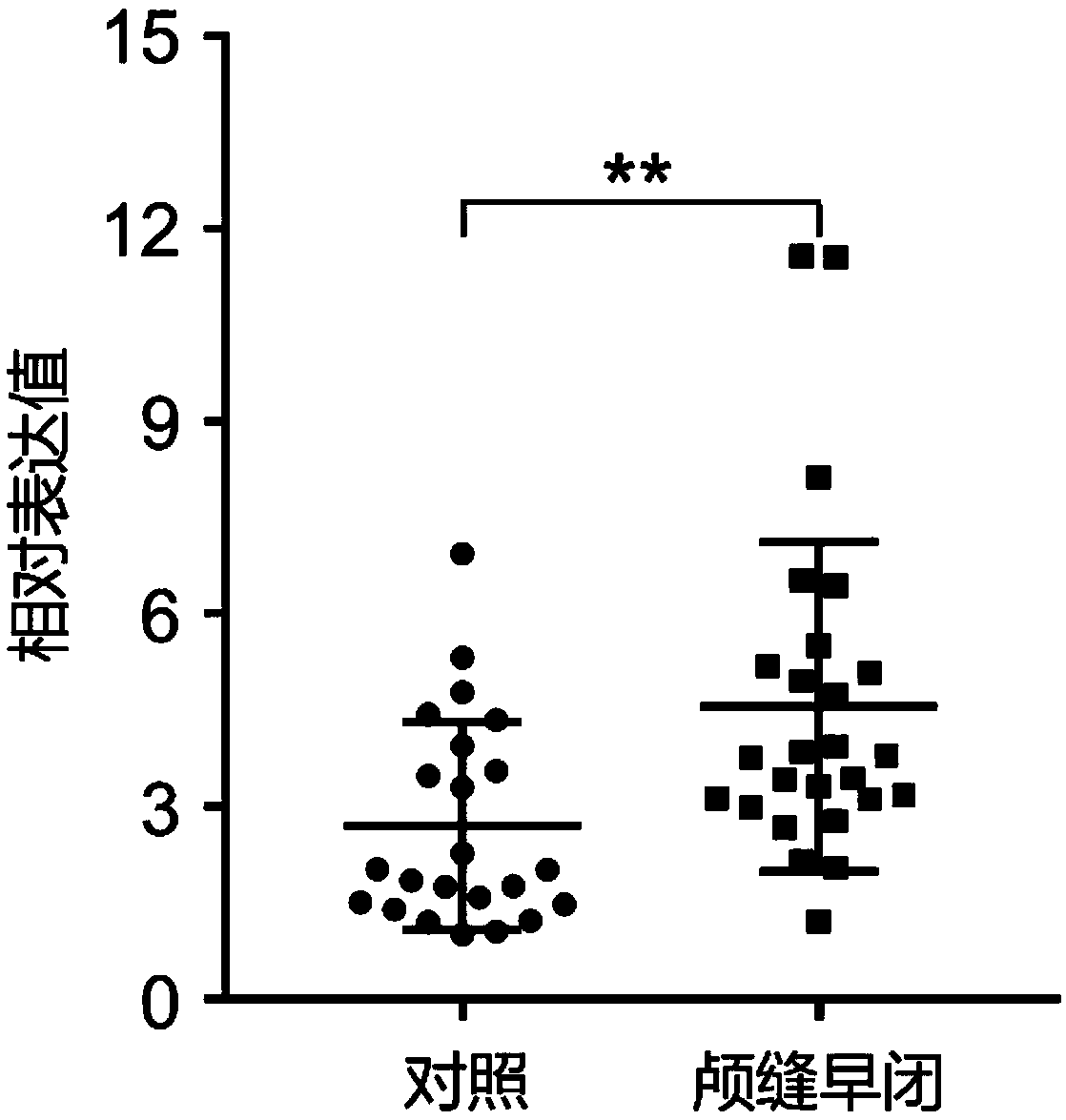
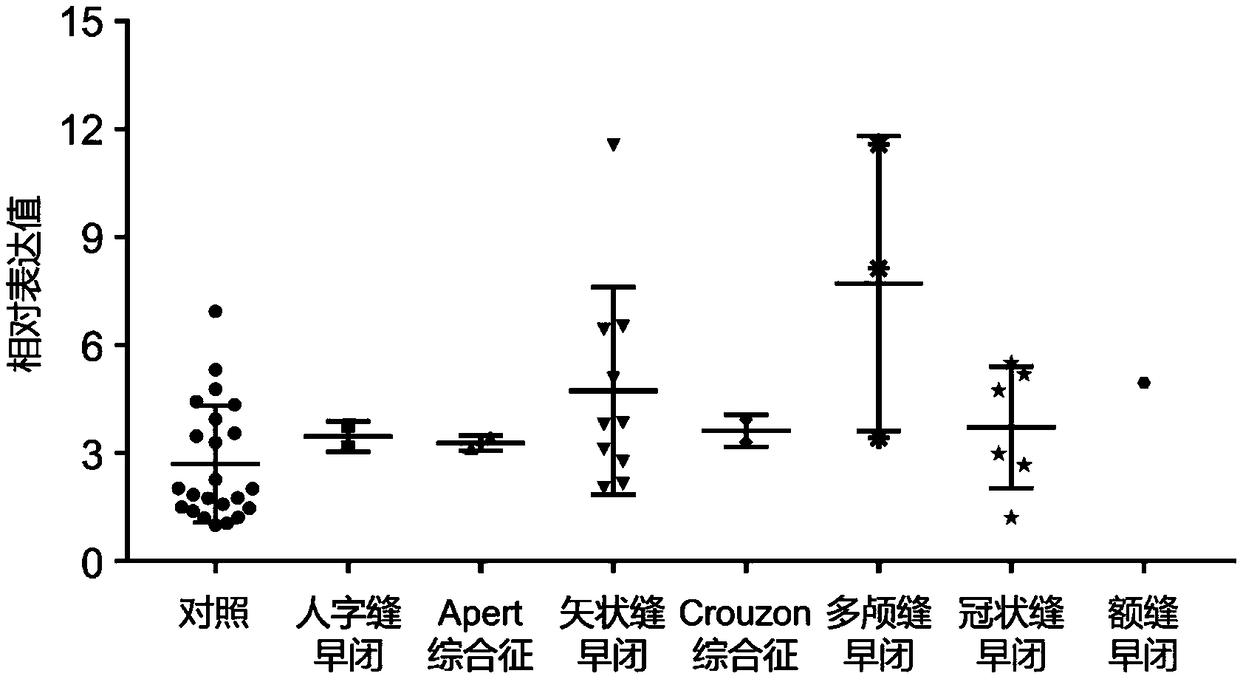
ActiveCN109295218AImprove stabilityEasy to degradeMicrobiological testing/measurementDNA/RNA fragmentationDiagnosis earlyCraniosynostosis
The invention establishes a circRNA expression profile for a patient with craniosynostosis, provides, provides serum circRNA marker hsa_circ_0001788 for the patient with craniosynostosis, discloses diagnostic value of hsa_circ_0001788 of serum for craniosynostosis, applies the marker herein to prepare a diagnostic kit for the patient with craniosynostosis, and provides supporting for clinical early discovery and treatment of the patient with craniosynostosis. Serum circRNA has good stability, degradation difficulty and the other advantage, has high value in auxiliary diagnosis, and is easy toclinically popularize and use. A circRNA chip is used herein to carry out detecting to obtain disease-specific and abnormally-expressed serum circRNA expression profiles; verifying is performed by means of qRT-PCR; a strict design and evaluation system is provided. It is discovered for the first time that the marker hsa_circ_0001788 in serum is an important biological detection index which causesminimal invasion and has low cost and which is applicable to clinical detection; the marker hsa_circ_0001788 is important to the clinical early diagnosis and differential diagnosis of craniosynostosis; a new idea is provided for the diagnosis of craniosynostosis in molecular level, and it is expected that early diagnostic rate of craniosynostosis is increased.
Owner:NANJING MEDICAL UNIV
Orrhology detection method and use of substrate metal protease MMP11
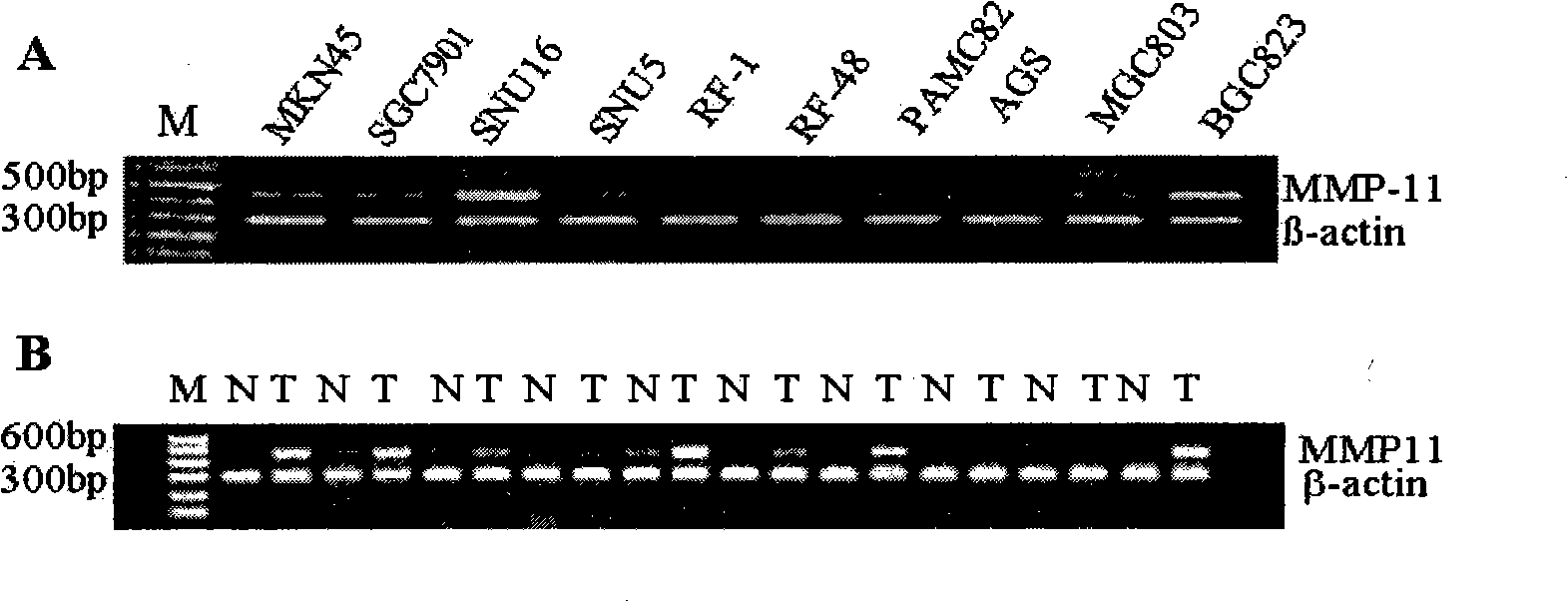
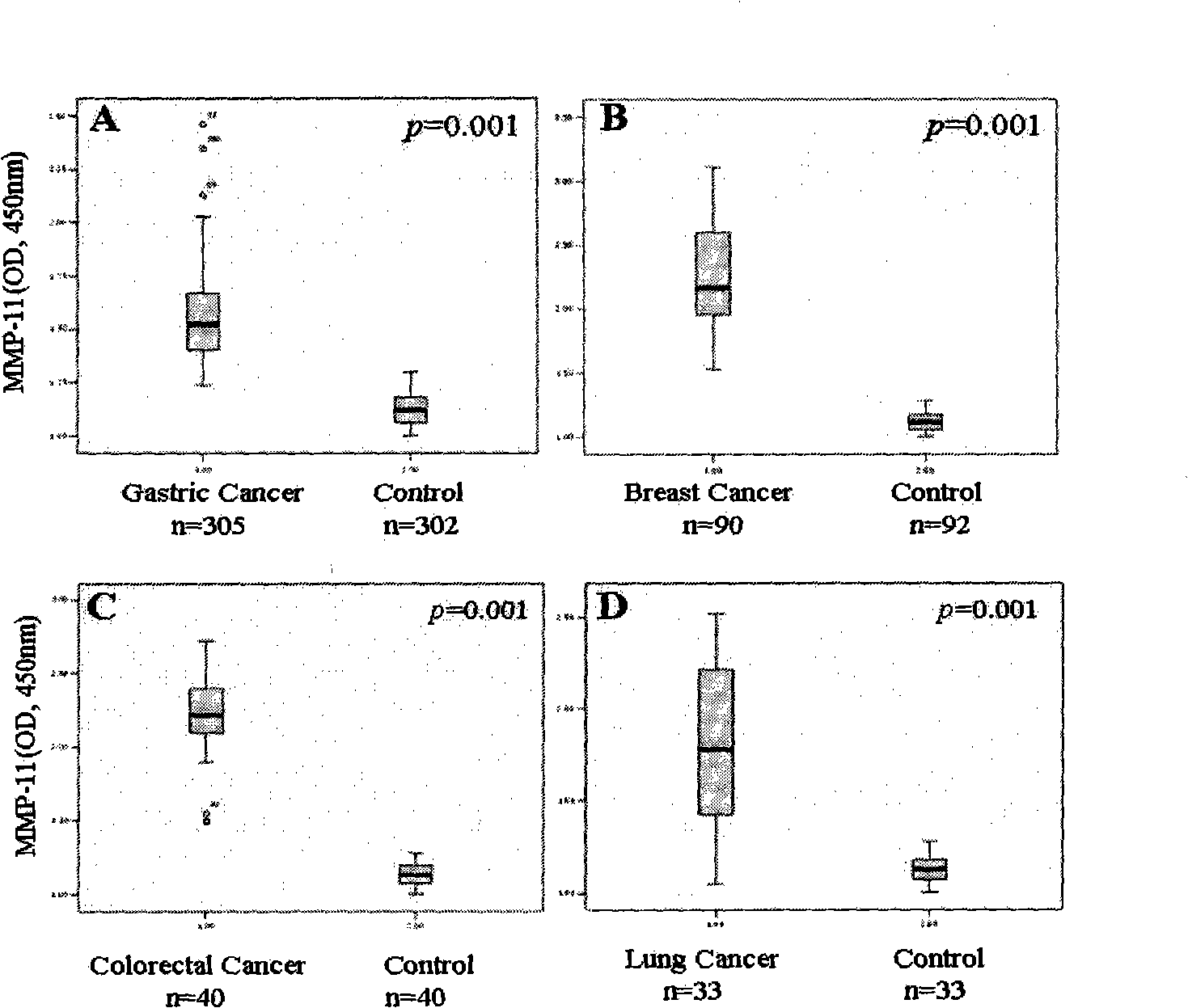
The invention discloses a method for serologically detecting Matrix metalloproteinase 11 (MMP11) and the application thereof. The gene chip technology and the bioinformatics method are adopted to obtain stomach cancer gene expression spectra, and by taking the MMP11 gene for example, the expression characteristics of the gene in the tumor cell lines and tissues are verified at the levels of mRNA and protein. At the same time, a detection kit for the MMP11serum is established by utilizing the technique of the enzyme linked immuno sorbent assay (ELISA), and the expression level of the MMP11 protein in the serum of stomach cancer patients is detected. The level of the MMP11 protein in the serum of stomach cancer patients is obviously higher than that of a non-cancer comparison group (p is lower than 0.001), and the same tendency is also found in the breast cancer, the colon cancer / rectum cancer and the lung cancer. In the serum detection to the stomach cancer patients, the sensitivity of the MMP11 is higher than that of a tumor molecule marker such as CEA, CA199, CA72.4, CA242, etc., and MMP11 and CA199 have good correlation (p is equal to 0.017). Through the analysis of the clinic pathological data, the level of the serum MMP11 has the obvious correlation (p is equal to 0.009) with the cancerometastasis state of the stomach cancer patients. The MMP11 is possible to become a novel tumor serum marker for the diagnosis and the prognostic judgement of the tumors.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Quantitative determination method of immune chromatography test strip
InactiveCN103954753AIncreased linear detection rangeRealize accurate quantitative detectionMaterial analysisHigh concentrationLinear regression
The invention discloses a quantitative determination method of an immune chromatography test strip. The quantitative determination method comprises the following steps: drawing a standard curve; dropwise adding a liquid to be detected to the immune chromatography test strip which comprises an internal reference line, at least one detection line and an excessive antigen detection line, detecting, and reading a detection signal capable of reflecting the intensity of antigen-antibody reaction to obtain a reading result; carrying out linear regression on the reading result of the nth detection line by utilizing the nth standard curve when the detection signals of the n-1 detection lines of at least one detection line exceed a threshold value according to the reading results, and calculating to obtain the concentration of the liquid to be detected. By adopting the mode, the quantitative detection method disclosed by the invention can solve the problem that the low-concentration measurement and the high-concentration measurement are simultaneously carried out, and can be used for the quantitative detection on biological macromolecules or micromolecules, such as serum markers, microbe antigens, virus particles and illicit drugs; the linear detection range of the test strip is enlarged, the detection accuracy is improved, and the accurate quantitative detection on a target detection object is realized.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Combined detection serum marker for early screening and diagnosis of liver cancer, kit and detection method
ActiveCN110286235AGood reference valueHigh sensitivityDisease diagnosisBiological testingWilms' tumorBiomedicine
The invention relates to a combined detection serum marker for the early screening and diagnosis of liver cancer, a kit containing the combined detection serum marker and a detection method and belongs to the technical field of biomedicine. According to the combined detection serum marker for the early screening and diagnosis of the liver cancer of the invention, a human protein chip coded by 138 cancer-driven genes is customized on the basis of a role played by cancer-driven genes in tumor generation and development; an early-stage detection serum marker for liver cancer is preliminarily screened out through the protein chip; and finally a set of liver cancer combined detection serum marker which can be used for the early screening and diagnosis of liver cancer is screened out through the verification of an ELISA experiment; and the liver cancer combined detection serum marker includes a PTEN encoded protein, a PTCH1 encoded protein, an IDH1 coded protein, an SRSF2 coded protein, an MSH2 coded protein and an NPM1 coded protein. The combined detection serum marker can assist in the clinical diagnosis of liver cancer, and has the advantages of high sensitivity, strong specificity, low cost and the like.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com