Serum miRNA biomarker of type 2 diabetes mellitus and application thereof
A technology for diabetes and pre-diabetes, applied in the fields of biotechnology and medicine, can solve the problems of lack of effective means for type 2 diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Collection of clinical samples and arrangement of clinical data.
[0037] The inventor obtained a large number of serum samples from Zhejiang Hospital of Traditional Chinese Medicine from November 2011 to March 2013, and systematically collected complete sample demographic and clinical examination data. The collected clinical samples were divided into three groups: normal glucose tolerance (NGT), prediabetes (IFG / IGT) and type 2 diabetes (T2D) according to WHO (1999) diagnostic criteria for diabetes (see Table 1). Refer to the case classification criteria of the WHO (1999) diagnostic criteria for diabetes (Table 1).
Embodiment 2
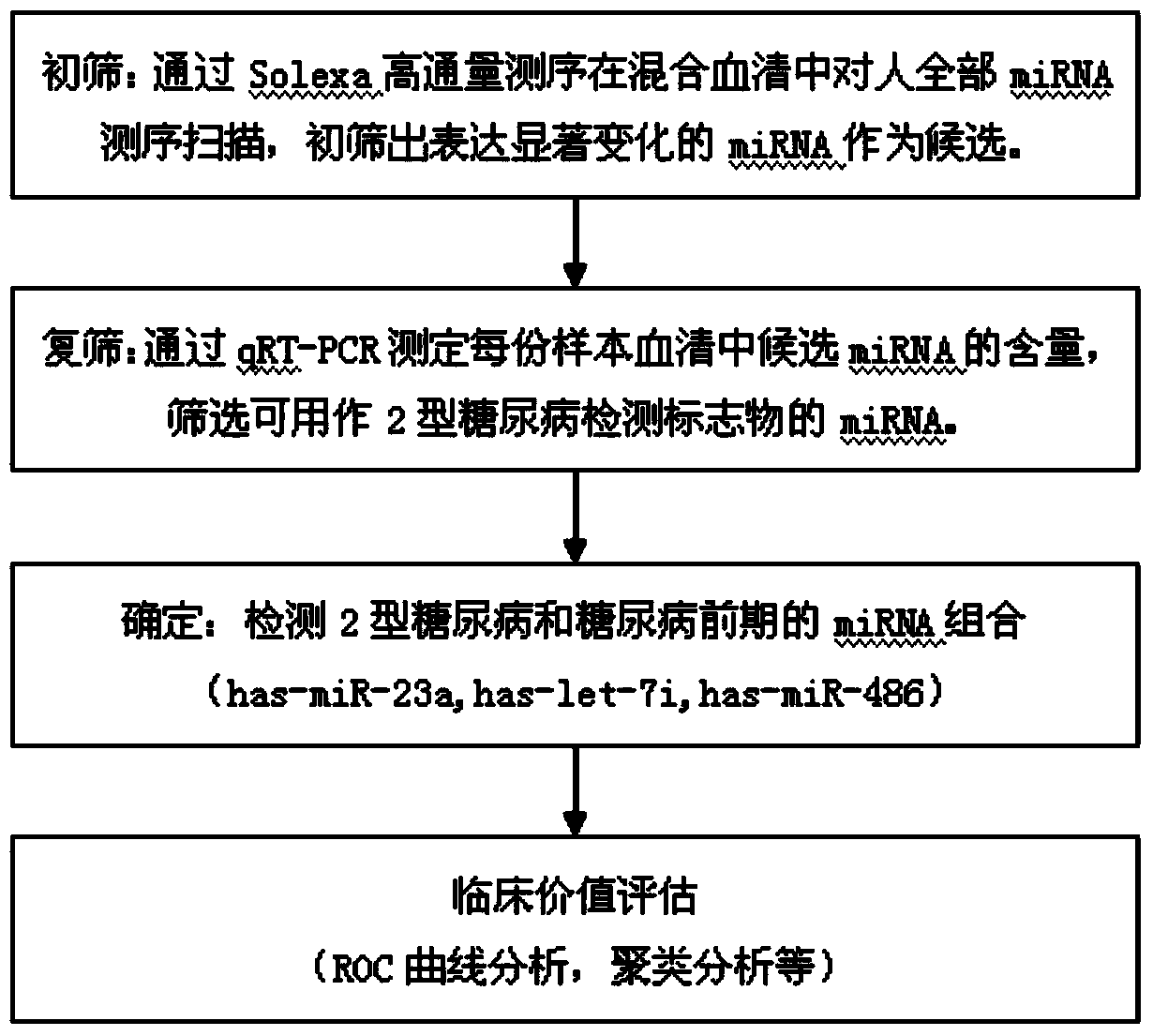
[0038] Example 2. Preliminary screening of type 2 diabetes serum-specific miRNA expression profile Solexa / Illumina high-throughput sequencing
[0039] (1) Screening of sequencing samples: By sorting out the sample data, the inventor selected 5 cases of type 2 diabetes (T2D) (50.60±5.13, age range 42-55, male: 3) from the collected clinical serum samples , female: 2) Serum samples were mixed thoroughly and used as mixed samples of type 2 diabetes case group (ie T2D group). In addition, 5 serum samples of normal glucose tolerance (NGT) (44.40±9.60, age range 34-58, male: 4, female: 1) were selected, mixed well, and used as the normal control group (NGT group) for Solexa / Experimental samples for primary screening by Illumina high-throughput sequencing.
[0040] (2) Use the Illumina TruSeq Small RNA Preparation kit kit to refer to the kit instructions Illumina’s TruSeq Small RNA Sample Preparation Guide to extract the total RNA of the T2D group and the NGT group respectively, an...
Embodiment 3
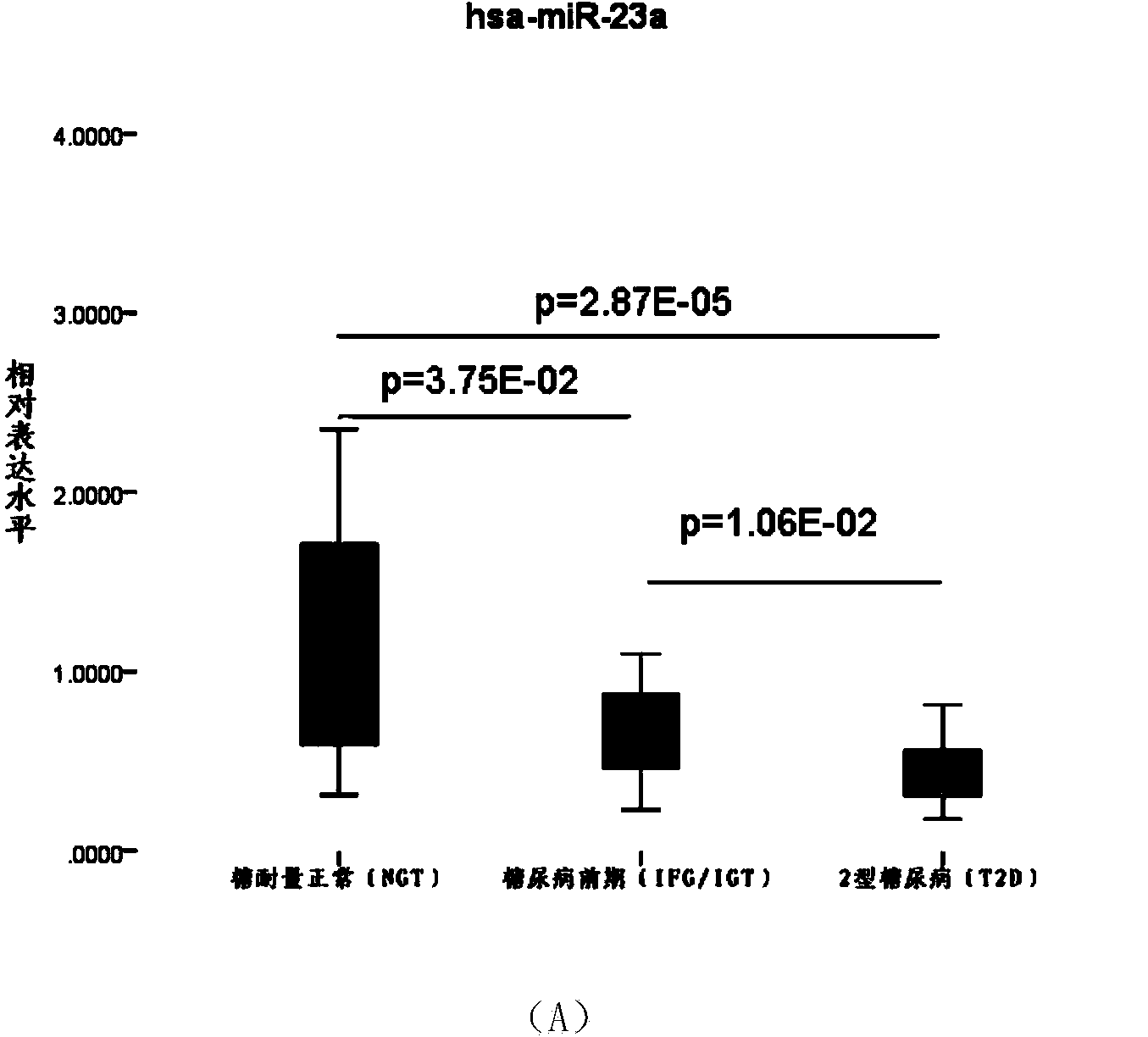
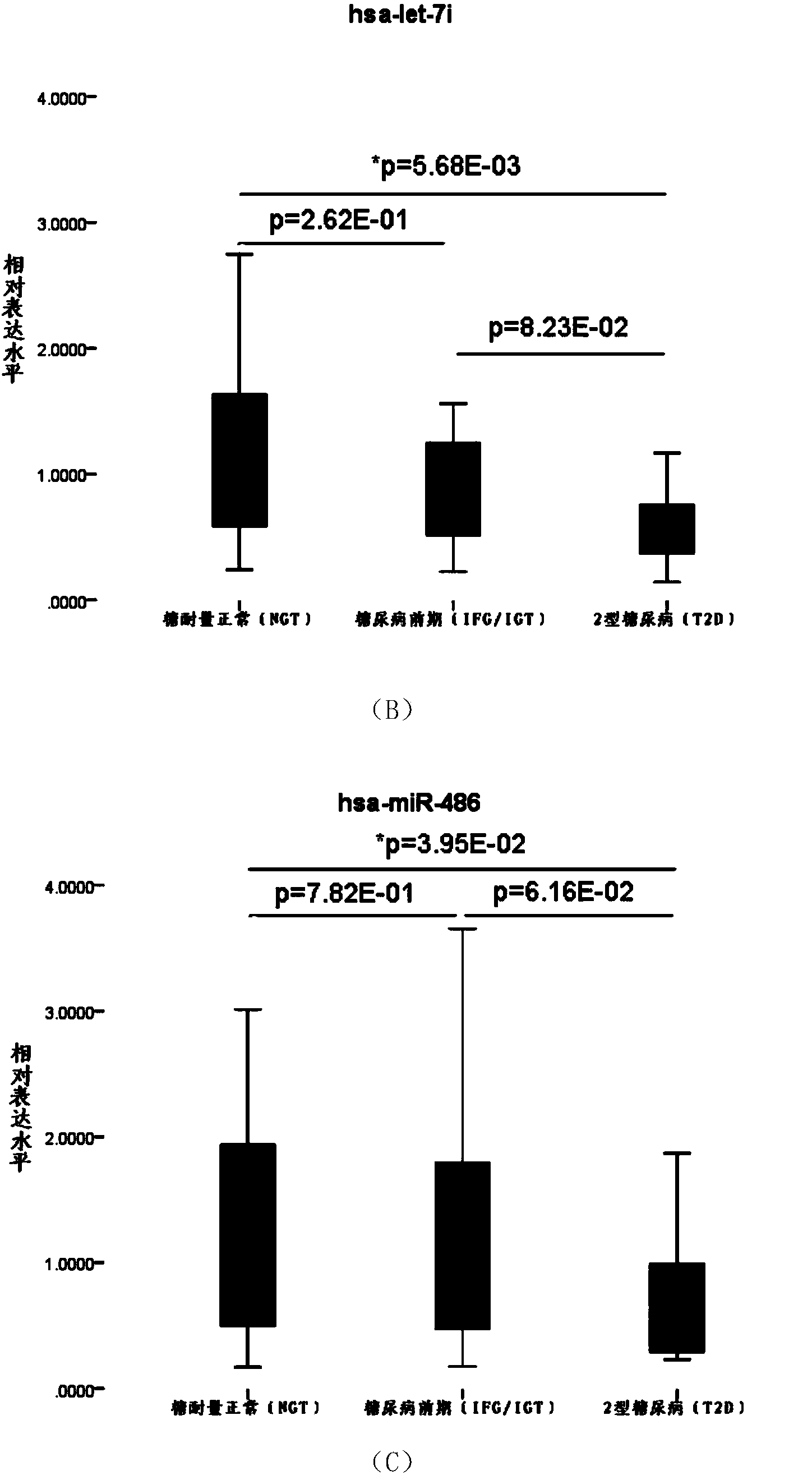
[0052] Example 3, Real-time fluorescence quantitative PCR method verification of type 2 diabetes serum candidate miRNA.
[0053] (1) Screening of samples: Through sorting out the sample data, the inventor selected 24 cases of type 2 diabetes (T2D) (51.13±9.21, age range 28-64, male: 16, Female: 8), 20 cases of prediabetes (IGT / FGT) (52.40±11.00, age span 31-65, male: 12, female: 8) and 20 cases of normal glucose tolerance (NGT) (46.65±16.18, age span 26 -75, male: 8, female: 12) as experimental samples for the verification of real-time fluorescent quantitative PCR method of serum candidate miRNA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com