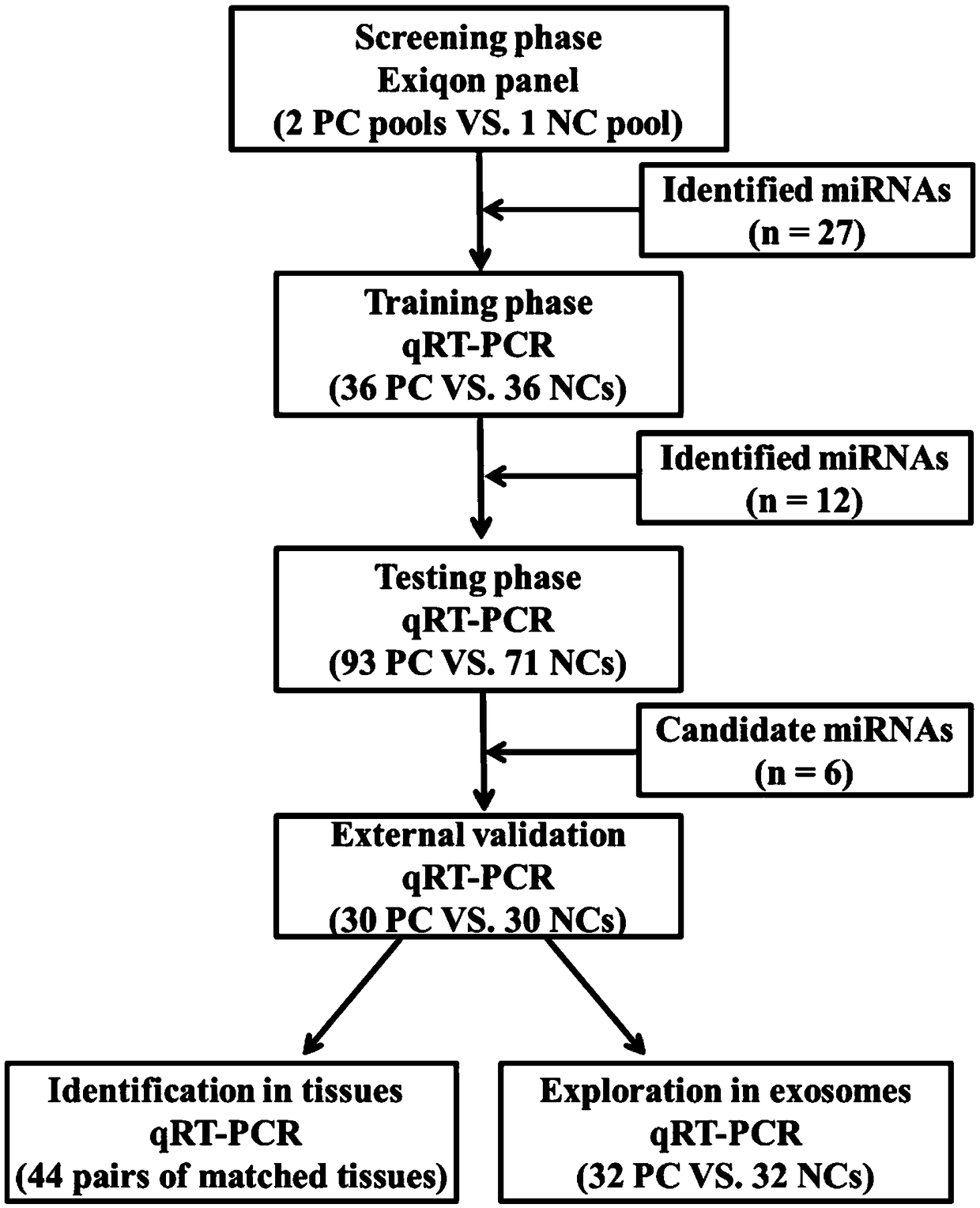
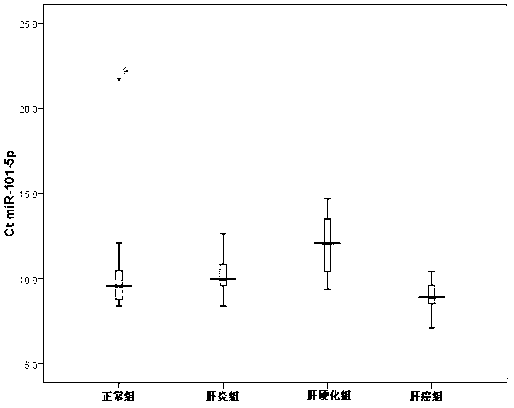Patents
Literature
113 results about "Serum mirna" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
miRNAs specific expression profile and diagnosis model for early colonic adenocarcinoma and rectal adenocarcinoma
ActiveCN103667516AImprove diagnostic efficiencyMicrobiological testing/measurementDNA/RNA fragmentationColonic adenocarcinomaGenetics
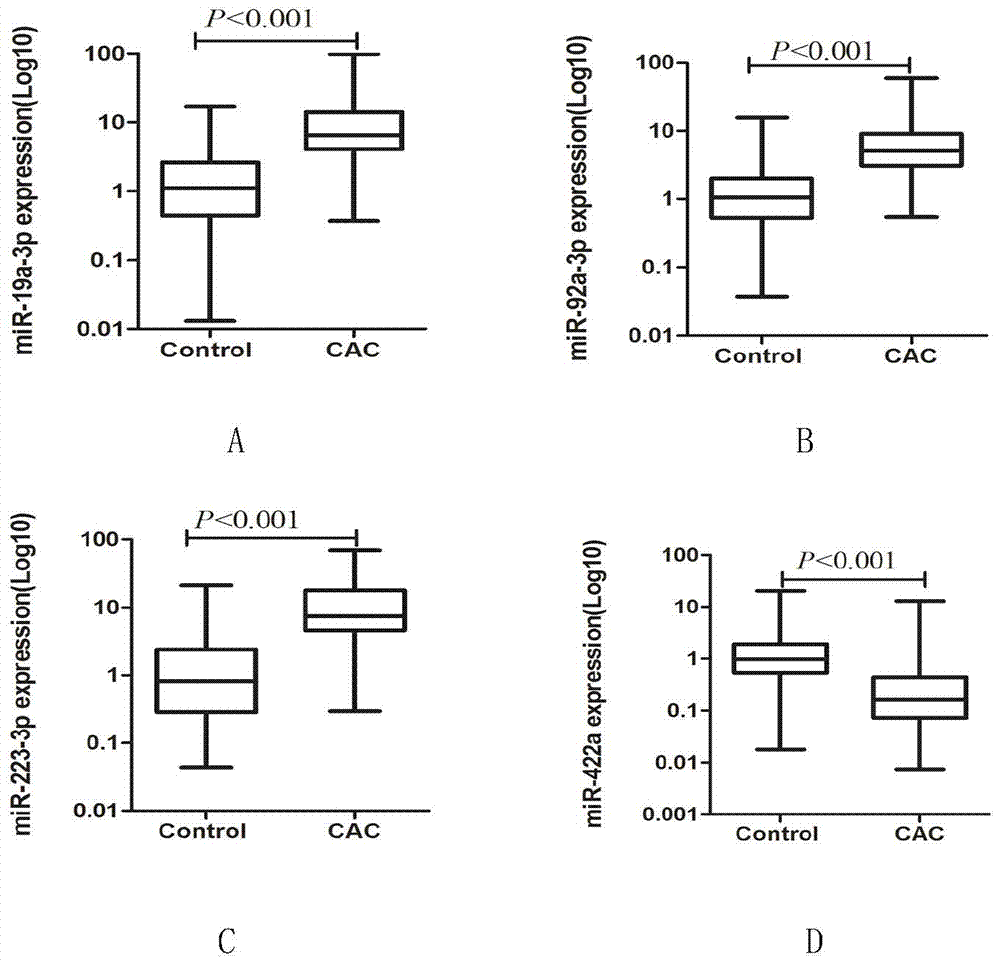
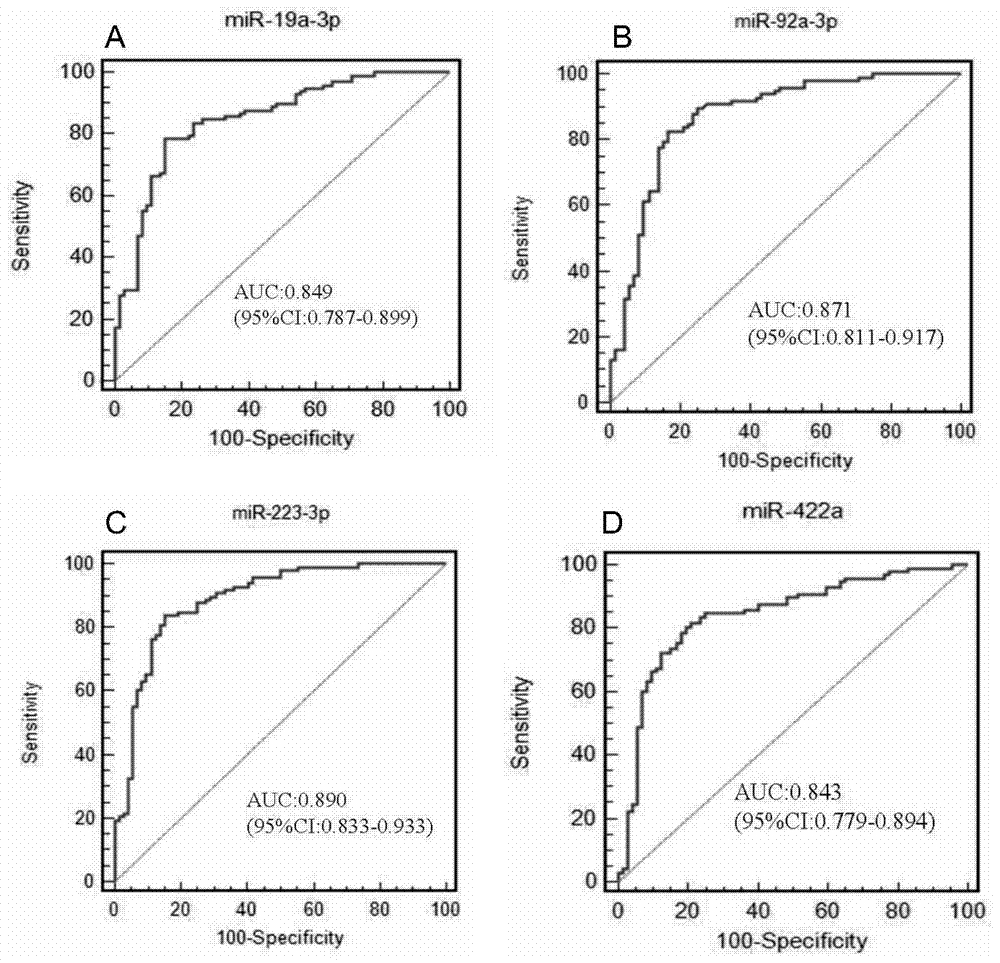
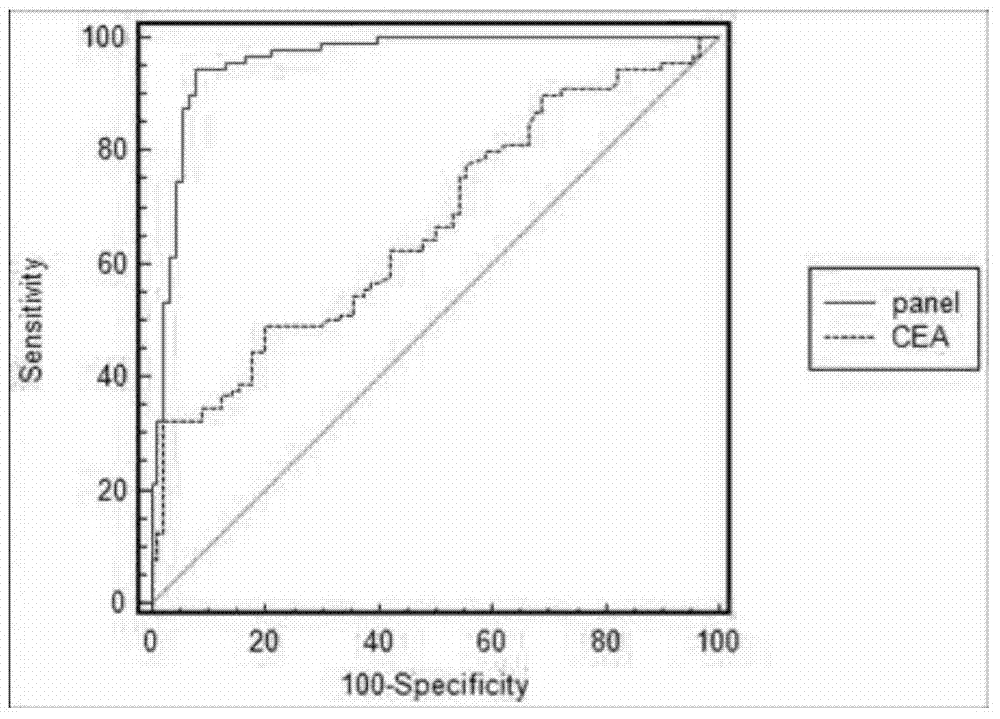
The invention discloses a serum miRNAs specific expression profile for early colonic adenocarcinoma and rectal adenocarcinoma, which is formed by up-regulated expressed miR-19a-3p, miR-92a-3p and miR223-3p and down-regulated expressed miR422a. A reverse transcription primer and a detection primer, which are shown as SEQ ID NO: 1-9, of the miRNAs are specifically expressed in the serum miRNAs specific expression profile for the early colonic adenocarcinoma and rectal adenocarcinoma. The invention also provides a serum miRNAs disgnosis model for the early colonic adenocarcinoma and rectal adenocarcinoma, the following formula of logit(P=CAC)=0.4424-0.0092*(miR-19a-3p)-0.0368*(miR-92a-3p)-0.0517*(miR-223-3p)+0.2439*(miR-422a) is used for calculation and for the diagnosis on the early colonic adenocarcinoma and rectal adenocarcinoma, the diagnosis efficiency of the serum miRNAs model on the colonic adenocarcinoma and rectal adenocarcinoma is higher and is higher than that of the traditional tumor marker CEA through ROC (Receiver Operating Characteristic) curve analysis, and the model can used for the diagnosis on the early colonic adenocarcinoma and rectal adenocarcinoma patient (TNM I / II period).
Owner:SHANDONG UNIV QILU HOSPITAL
miRNA (microribonucleic acid) biomarkers and detection kit for ovarian cancer diagnosis
InactiveCN105177173AImprove consistencyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMir 145 5pMir 193b
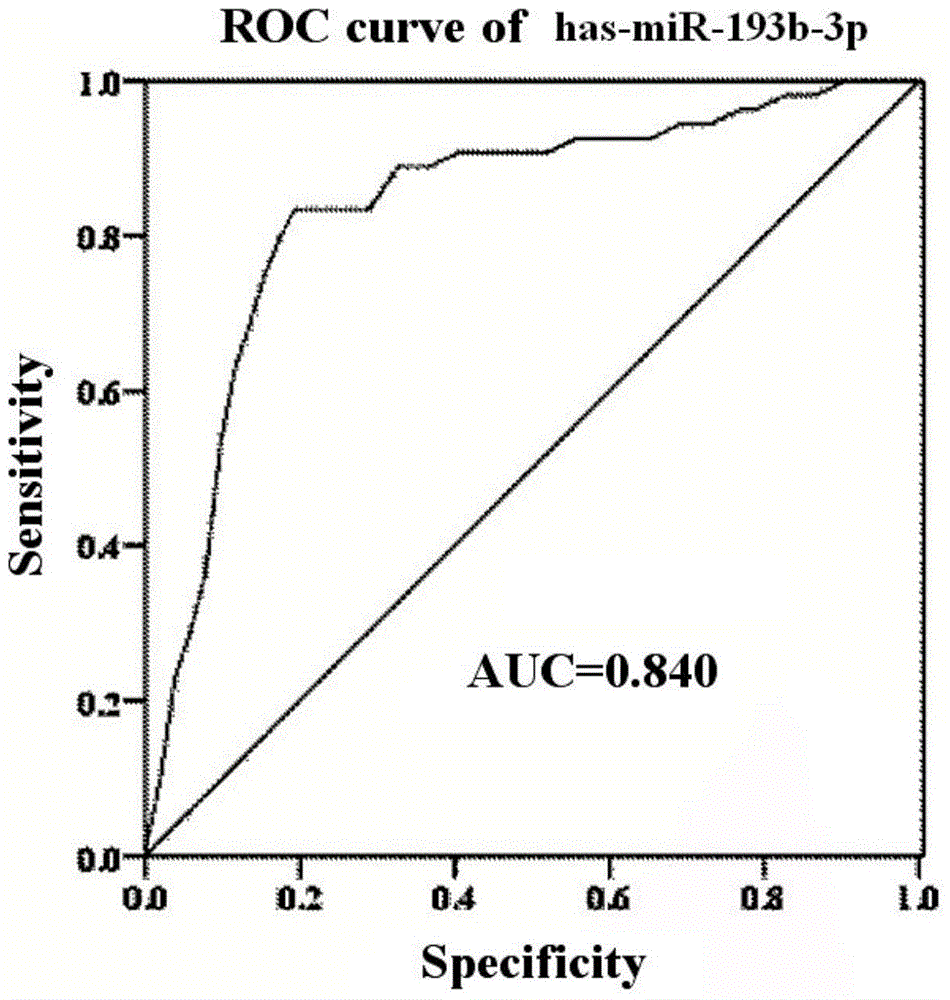
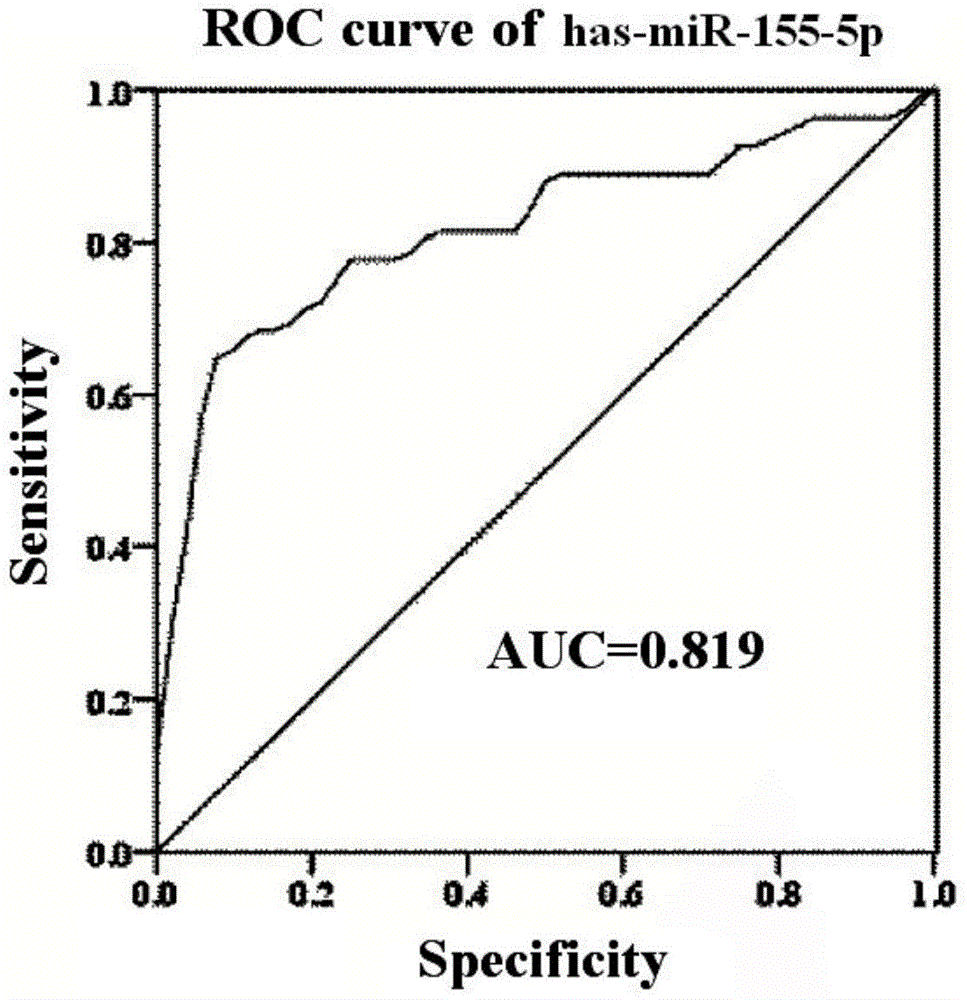
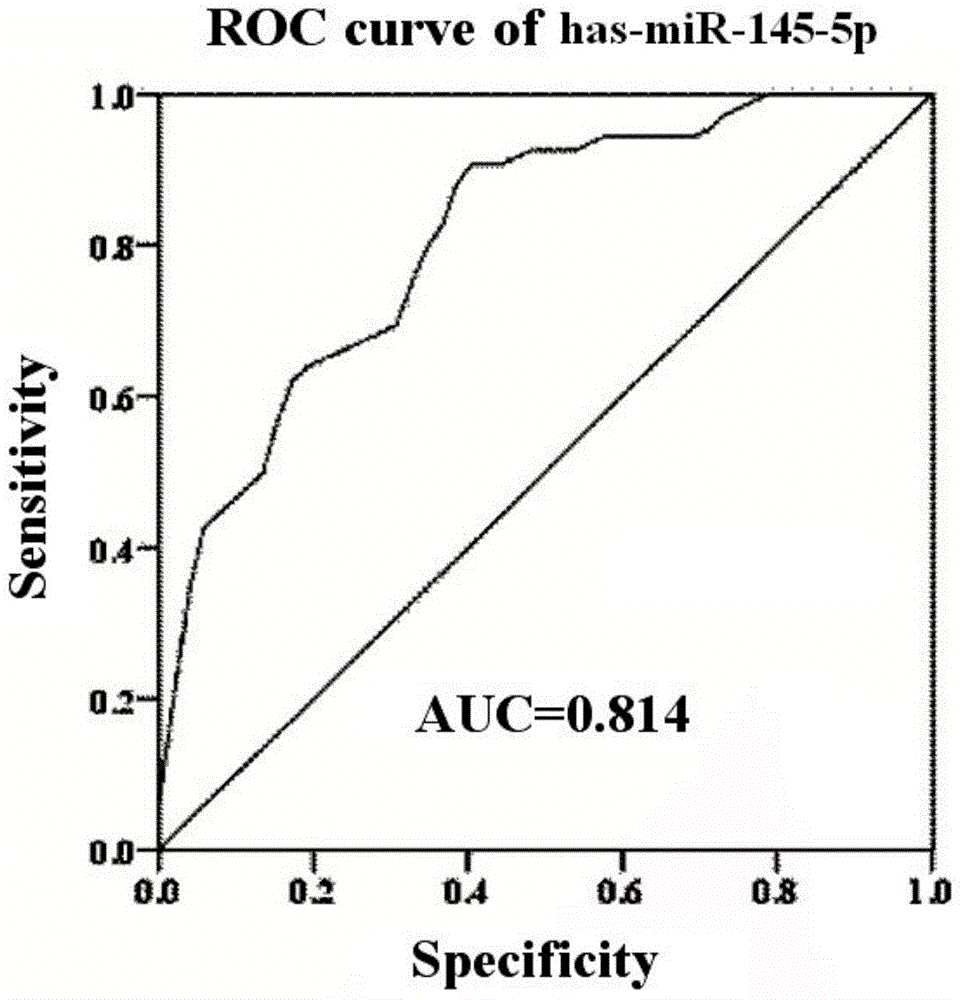
The invention discloses miRNA (microribonucleic acid) biomarkers and a detection kit for ovarian cancer diagnosis. The microRNA biomarkers are composed of the following microRNAs: has-miR-193b-3p, has-miR-155-5p, has-miR-145-5p, has-miR-132-3p and has-miR-143-3p. The serological expression analysis on the five miRNAs has-miR-193b-3p, has-miR-155-5p, has-miR-145-5p, has-miR-132-3p and has-miR-143-3p of ovarian cancer with obvious para-carcinoma tissue expression differences (the differential expression level is greater than 2 folds, and the CT value in RT-PCR (reverse transcription-polymerase chain reaction) is less than 30) indicates that the five miRNAs have stable expression in serum, the serum miRNA expression has favorable consistency with the tissue, the expressions of the has-miR-193b-3p, has-miR-155-5p and has-miR-145-5p are enhanced, and the expressions of the has-miR-132-3p and has-miR-143-3p are lowered. The five miRNAs can be used as biomarkers for ovarian cancer diagnosis, and the sensitivity and specificity of combined diagnosis are obviously higher than the sensitivity and specificity of single-miRNA diagnosis.
Owner:崔长友
Early bladder cancer serum miRNAs specific expression profile and diagnostic model
ActiveCN103993093AImprove diagnostic efficiencyMicrobiological testing/measurementDNA/RNA fragmentationBacteriuriaBladder cancer
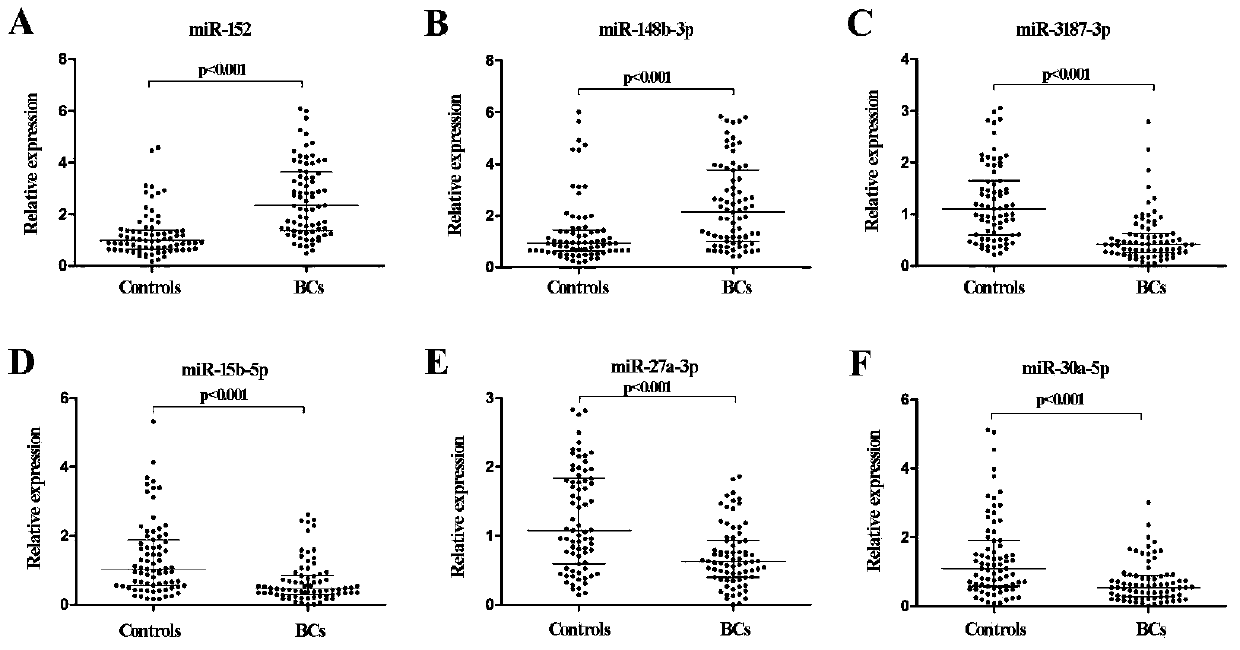
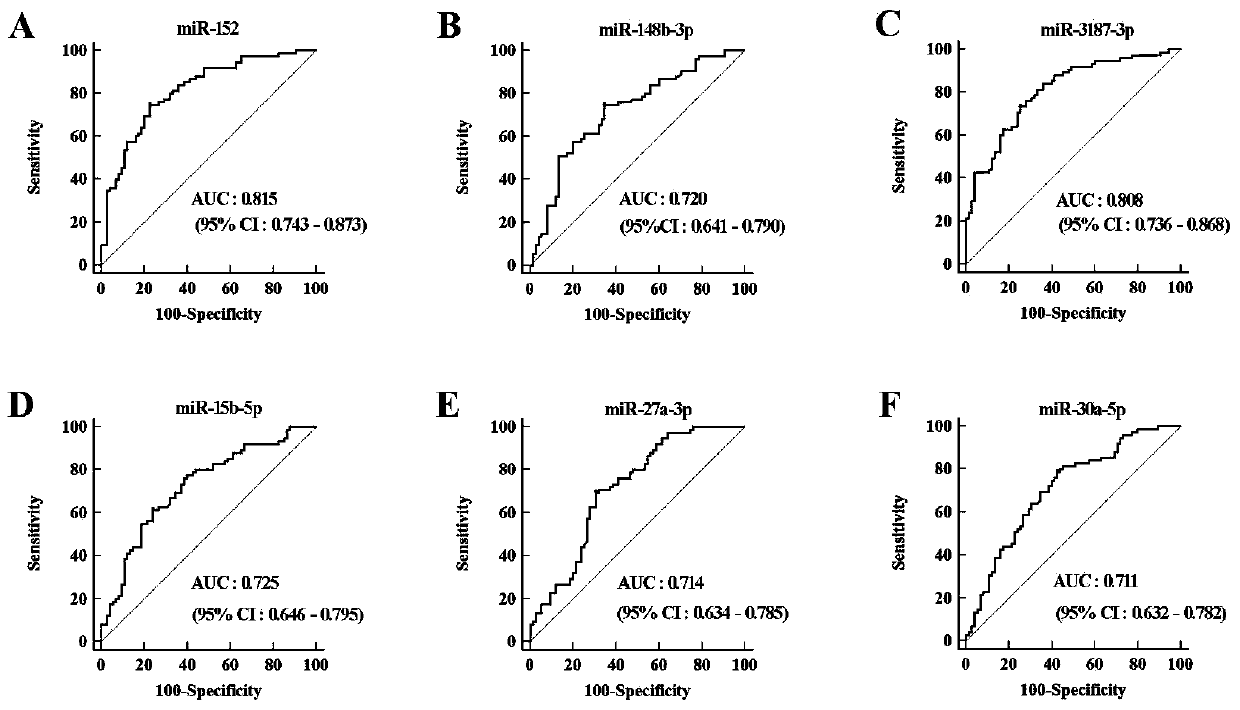
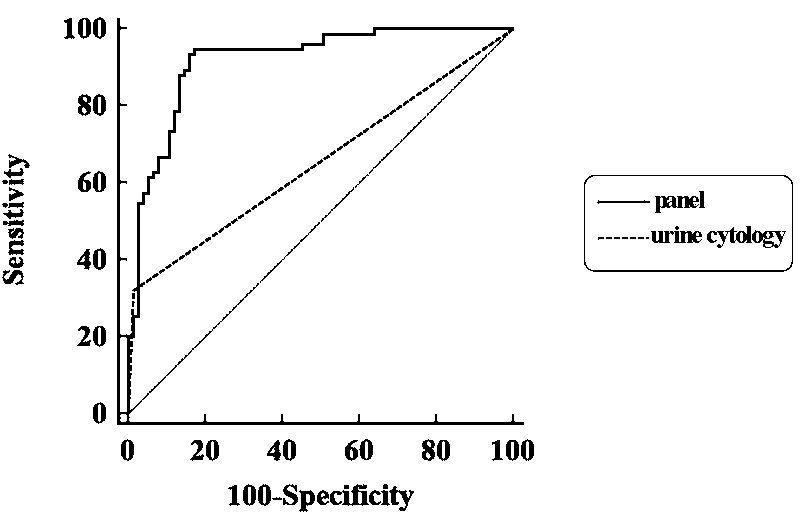
The invention discloses an early bladder cancer serum miRNAs specific expression profile. The early bladder cancer serum miRNAs specific expression profile is composed of miR-152 and miR-148b-3p which are used for expressing up regulation as well as miR-3187-3p, miR-15b-5p, miR-27a-3p and miR-30a-5p which are used for expressing down regulation. A reverse transcription primer and a detection primer which are used for specifically expressing miRNAs in the early bladder cancer serum miRNAs specific expression profile are shown in SEQIDNO:1-13. The invention also provides an early bladder cancer serum miRNAs diagnostic model. The early bladder cancer serum miRNAs diagnostic model is calculated by utilizing the following formula: logit(P=BC)=0.439+0.1775*(miR-152)+0.0767*(miR-148b-3p)-0.475*(miR-3187-3p)-0.2395*(miR-15b-5p)-0.625*(miR-27a-3p)-0.1693*(miR-30a-5p), can be used for early diagnosis of bladder cancer and has higher diagnosis efficiency to the bladder cancer as compared with the traditional urine exfoliative cytology through ROC (receiver operating characteristic) curve analysis, and the early bladder cancer serum miRNAs diagnostic model can diagnose patients with early bladder cancer (Ta and T1).
Owner:SHANDONG UNIV QILU HOSPITAL
Serum miRNA relevant to chronic heart failure and application of serum miRNA
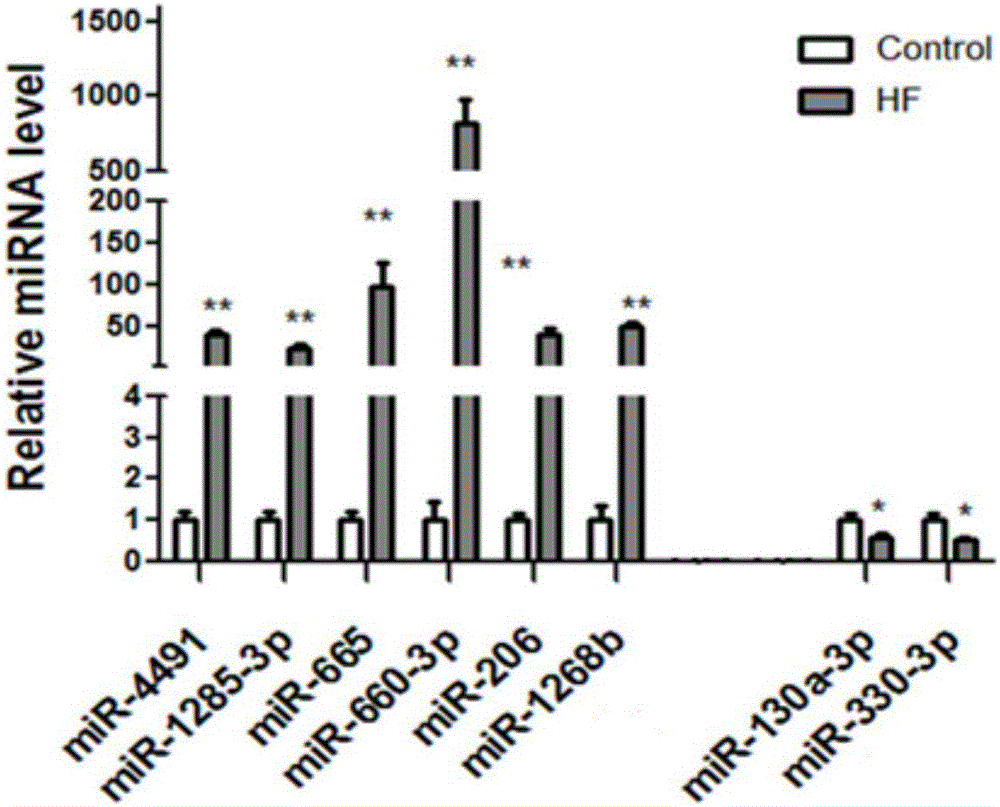
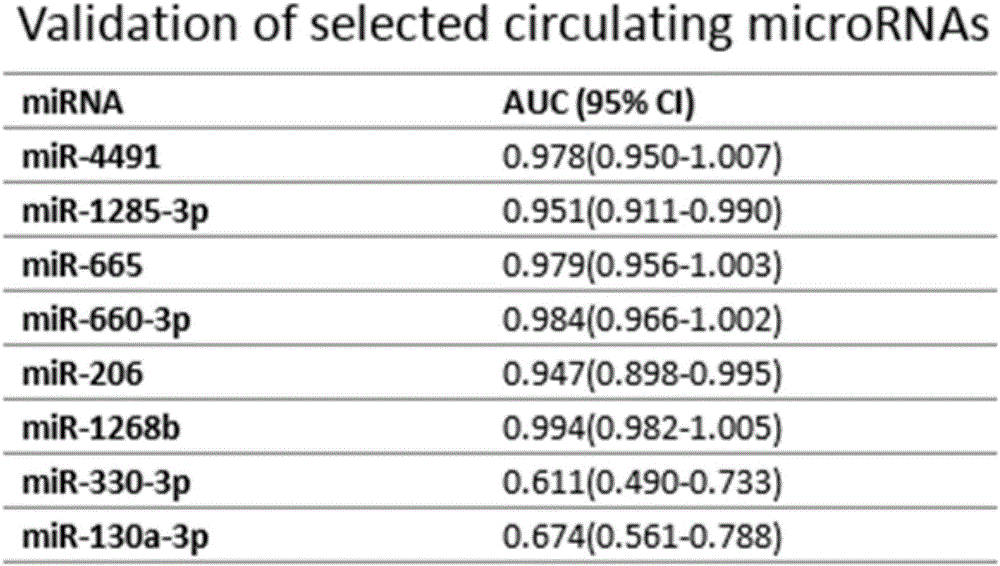
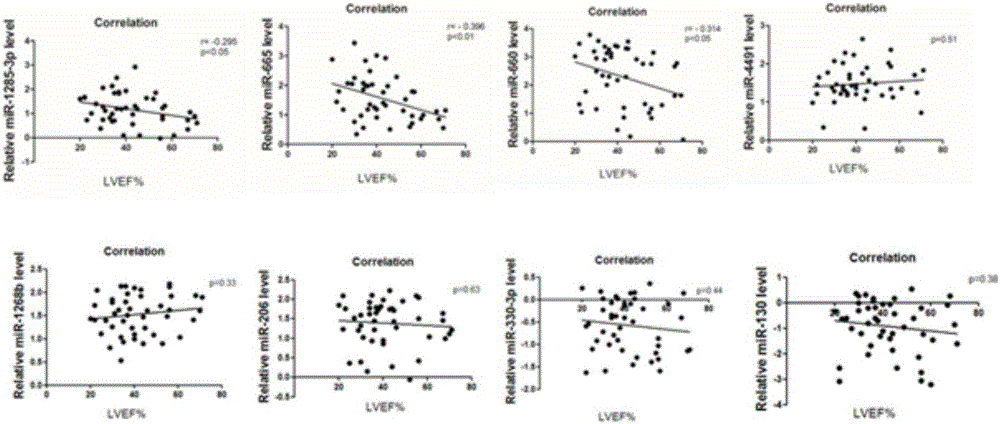
The invention provides serum miRNA relevant to a chronic heart failure and application of the serum miRNA. The invention provides application of peripheral blood miRNAs to the chronic heart failure as biomarker risk assessment / diagnosis and prognosis. Hsa-miR-4491, hsa-miR-1285-3p, hsa-miR-665, hsa-miR-660-3p, hsa-miR-206, hsa-miR-1268b, hsa-miR-130a-3p and hsa-miR-330-3p have differential expressions in peripheral blood of heart failure patients. It is proved that the peripheral blood hsa-miR-665 and the like can perform specific diagnosis on the chronic heart failure, the expression level of the hsa-miR-665 and the like is relevant to ejection fraction, and the hsa-miR-665 has an assessment and prognosis effect. Thus, by detecting the expression level of the miRNAs, the existing chronic heart failure can be predicted and coordinately diagnosed, or prognosis of the chronic heart failure can be estimated.
Owner:汪道文 +2
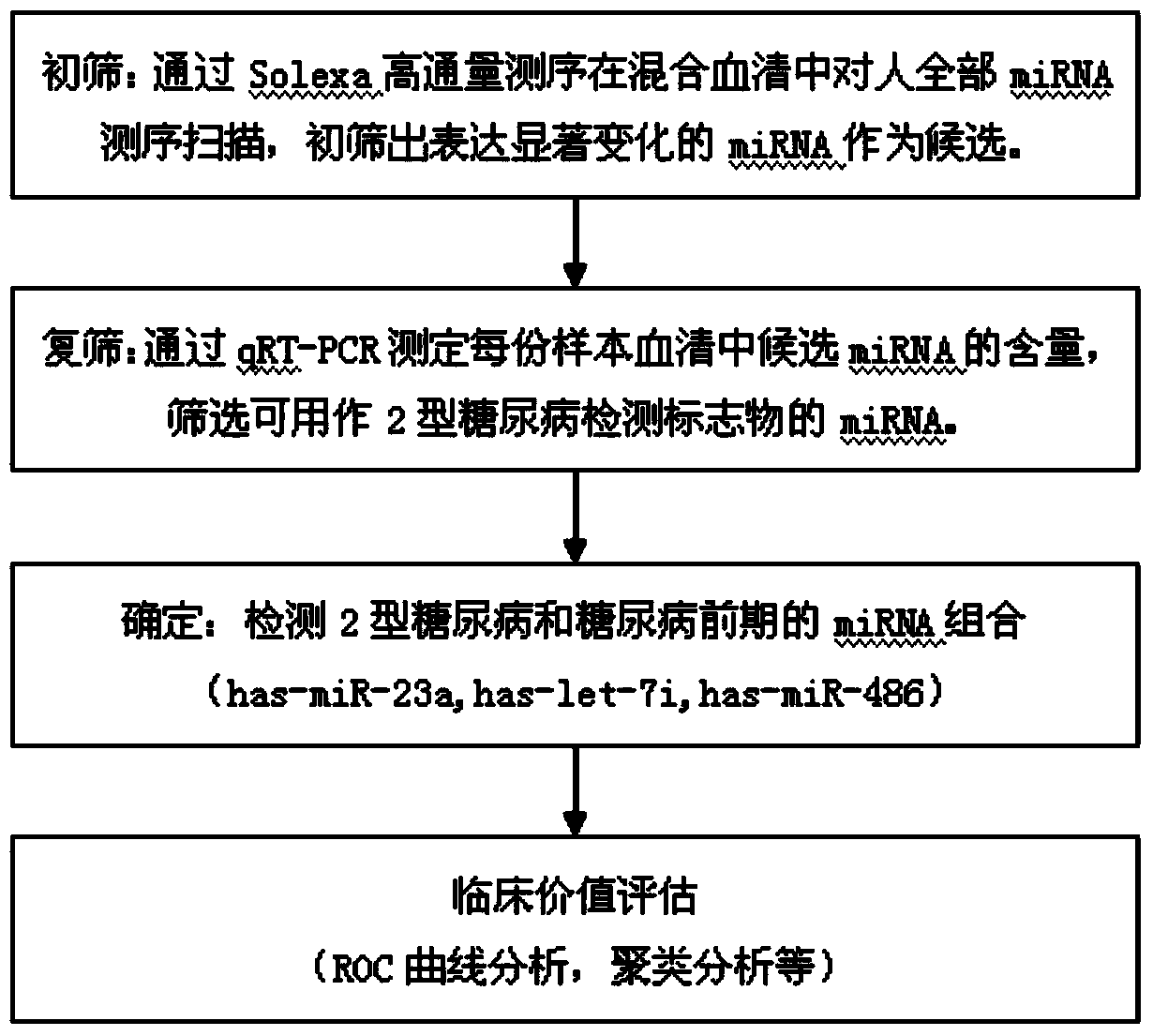
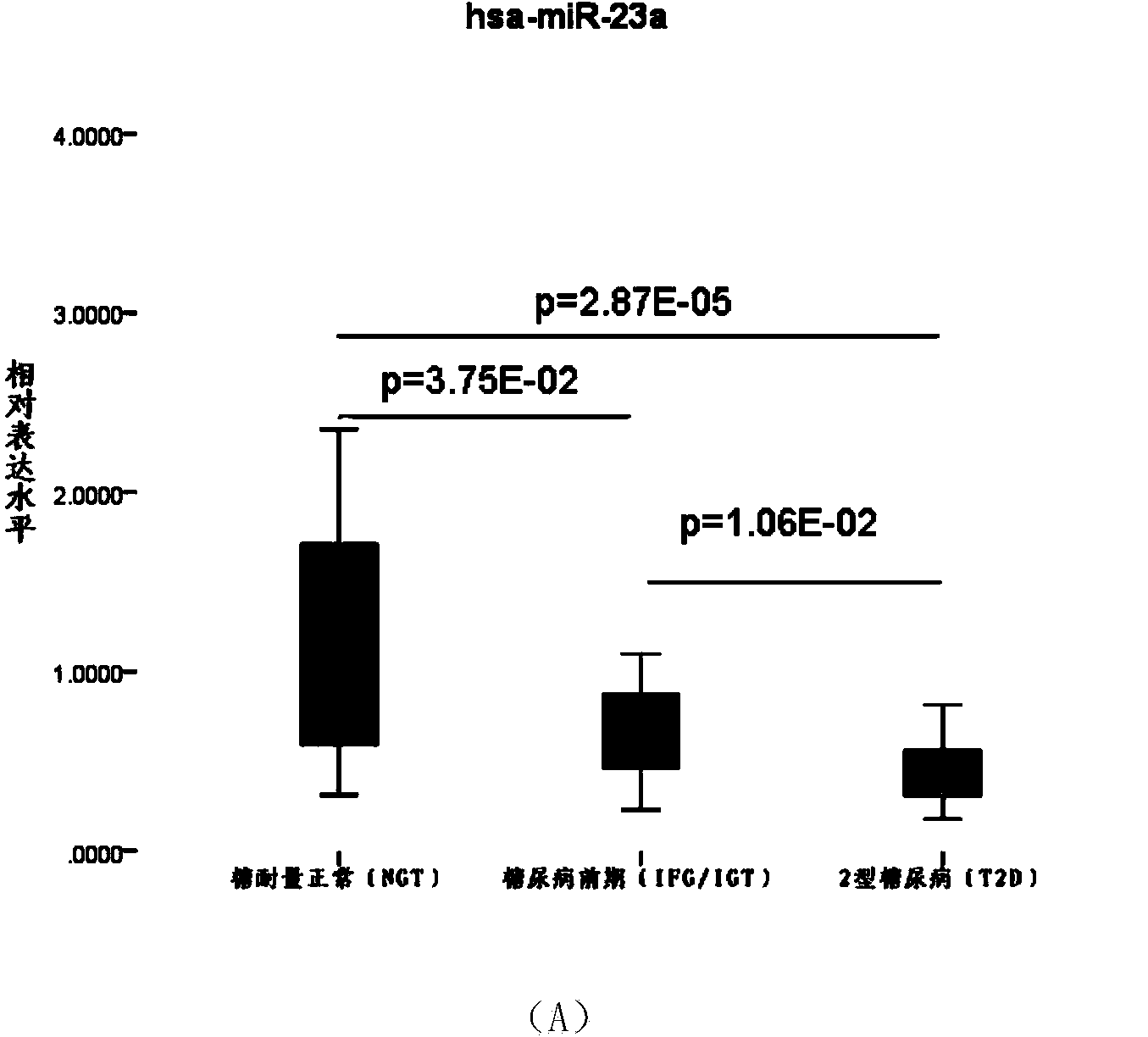
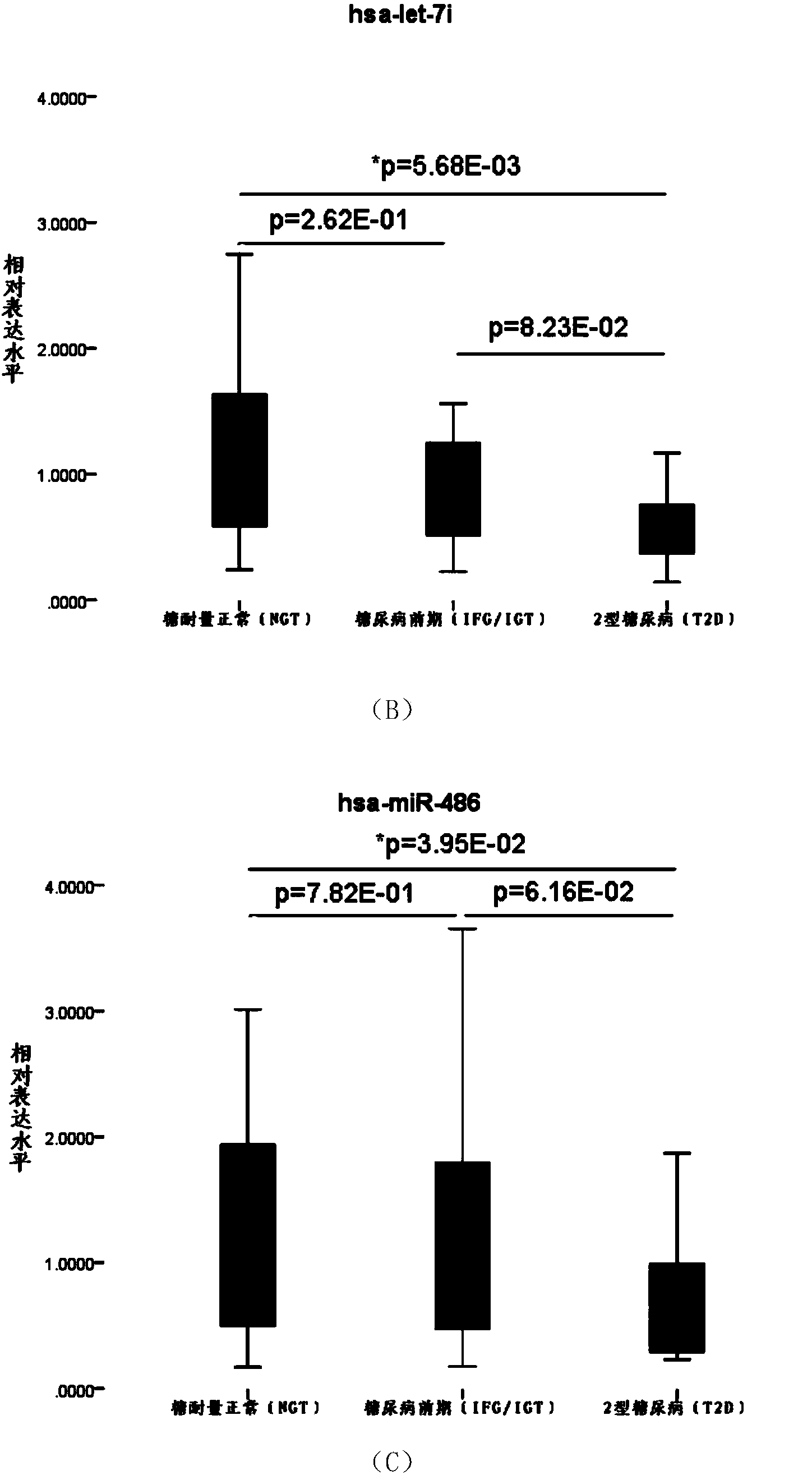
Serum miRNA biomarker of type 2 diabetes mellitus and application thereof
The invention discloses a serum miRNA composition, which is at least one of the following serum miRNAs: hsa-miR-23a, hsa-let-7i, and hsa-miR-486. The invention also simultaneously provides a fluorescent quantitative detection primer composition for detection of type 2 diabetes mellitus. The composition comprises an RT primer, an FW primer and an RV primer of each miRNA in hsa-miR-23a, hsa-let-7i, and hsa-miR-486. By adopting the serum miRNA marker for early diagnosis of type 2 diabetes mellitus and cancer risk evaluation provided by the invention, a serum miRNA biomarker can be used for diagnosing whether a subject has the type 2 diabetes mellitus or early diagnosis of the type 2 diabetes mellitus and cancer risk evaluation.
Owner:ZHEJIANG SCI-TECH UNIV +1
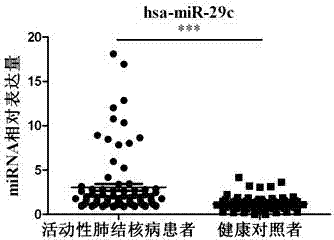
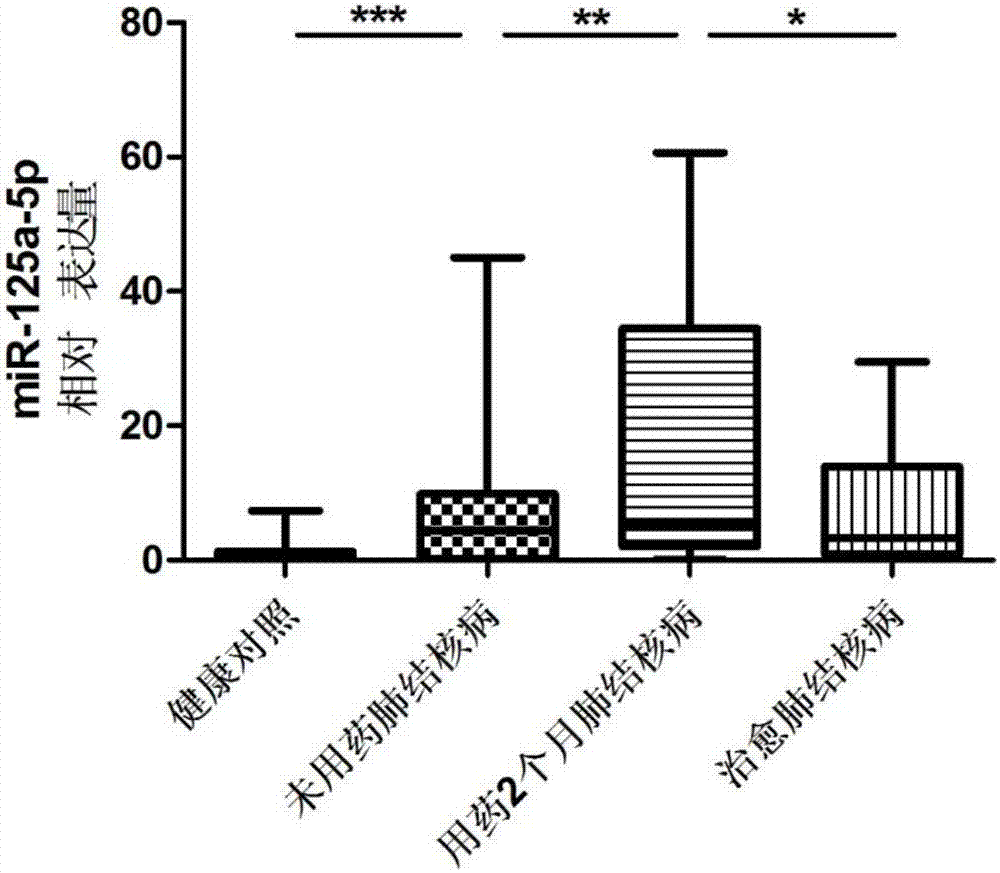
Kit for detecting active pulmonary tuberculosis
ActiveCN103045723AEarly Detection ImprovesEarly detection to improve the early diagnosis of active tuberculosisMicrobiological testing/measurementMicroorganism based processesSerum mirnaPulmonary tb
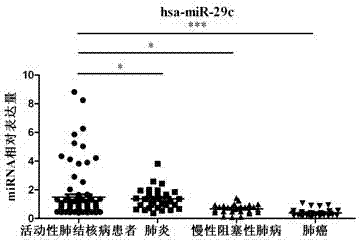
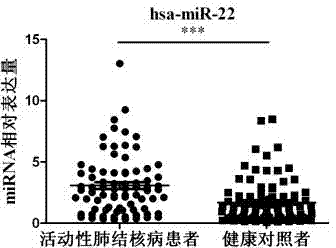
The invention provides a diagnostic kit for detecting active pulmonary tuberculosis. The kit consists of an RNA extract buffer solution, a specific serum miRNA composition for the active pulmonary tuberculosis, internal reference reverse transcription primers, polymerase chain reaction (PCR) primers and a fluorescent quantitative reverse transcription-polymerase chain reaction (RT-PCR) reaction solution, wherein the specific serum miRNA composition for the active pulmonary tuberculosis consists of four differential expression serum miRNAs, namely hsa-miR-29c, hsa-miR-22, hsa-miR-320b and hsa-miR-101. By using the specific serum miRNAs composition for the active pulmonary tuberculosis to detect the active pulmonary tuberculosis on the serum miRNAs level, the sensitivity is 90.2 percent, and the specificity is 75.0 percent; and the kit has early-stage diagnostic value for the active pulmonary tuberculosis, and can realize early warning and early diagnosis of the active pulmonary tuberculosis.
Owner:ZHEJIANG UNIV
Gout serum miRNAs biomarkers and method for detecting expression quantity thereof
ActiveCN104651513AMicrobiological testing/measurementDNA/RNA fragmentationSerum mirnaMolecular level
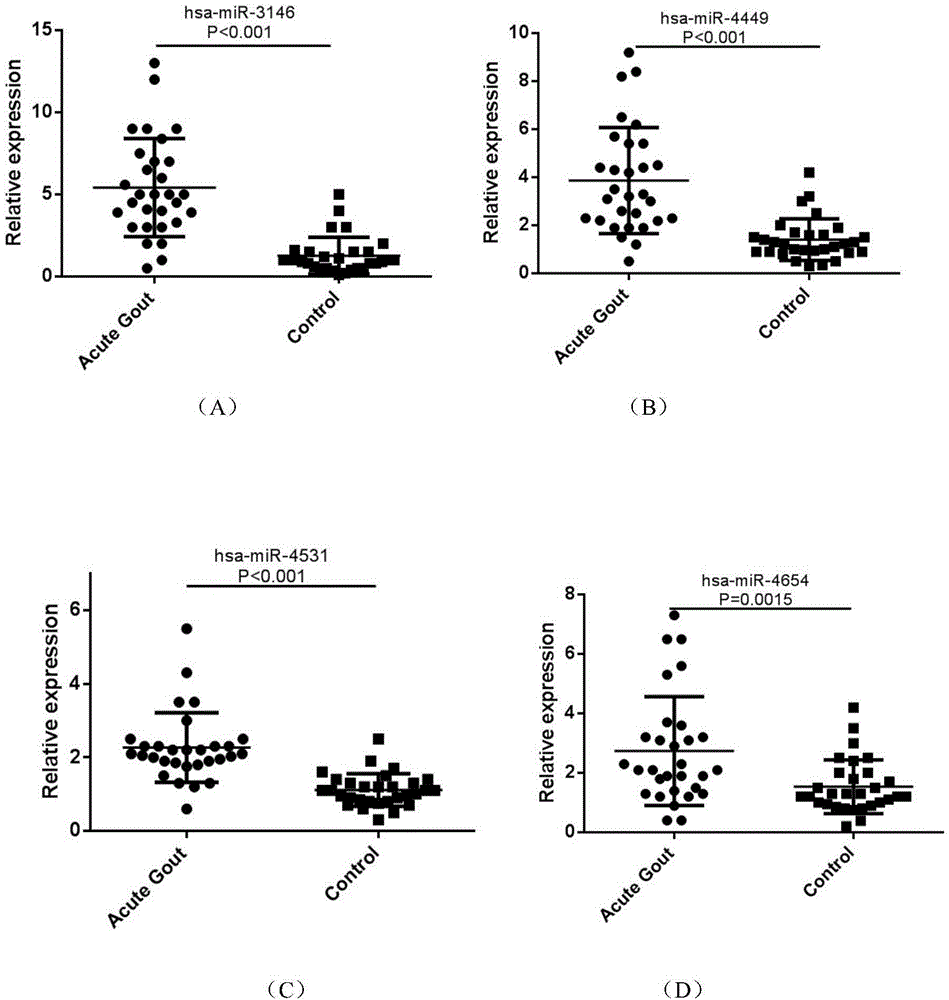
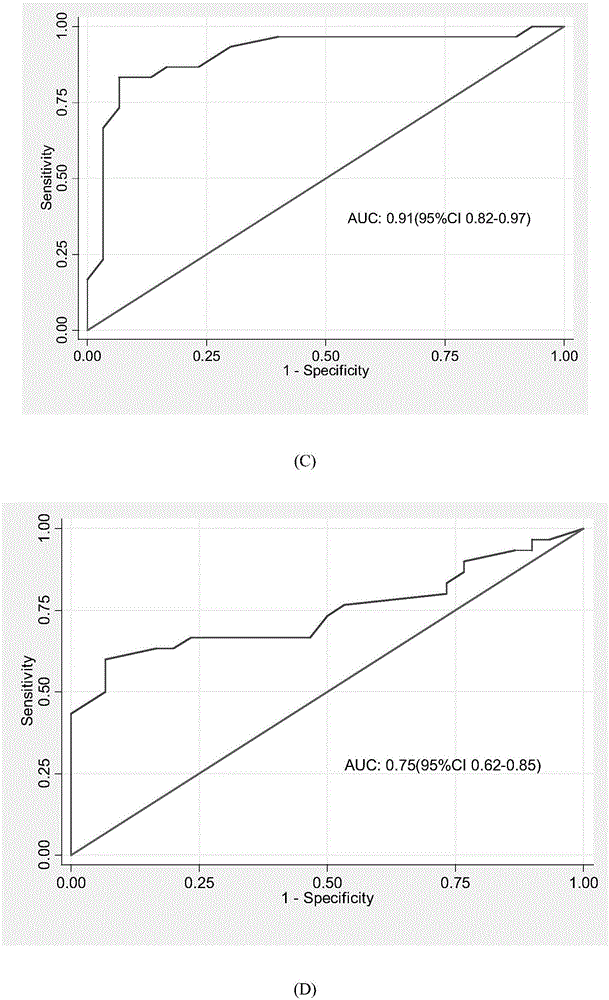
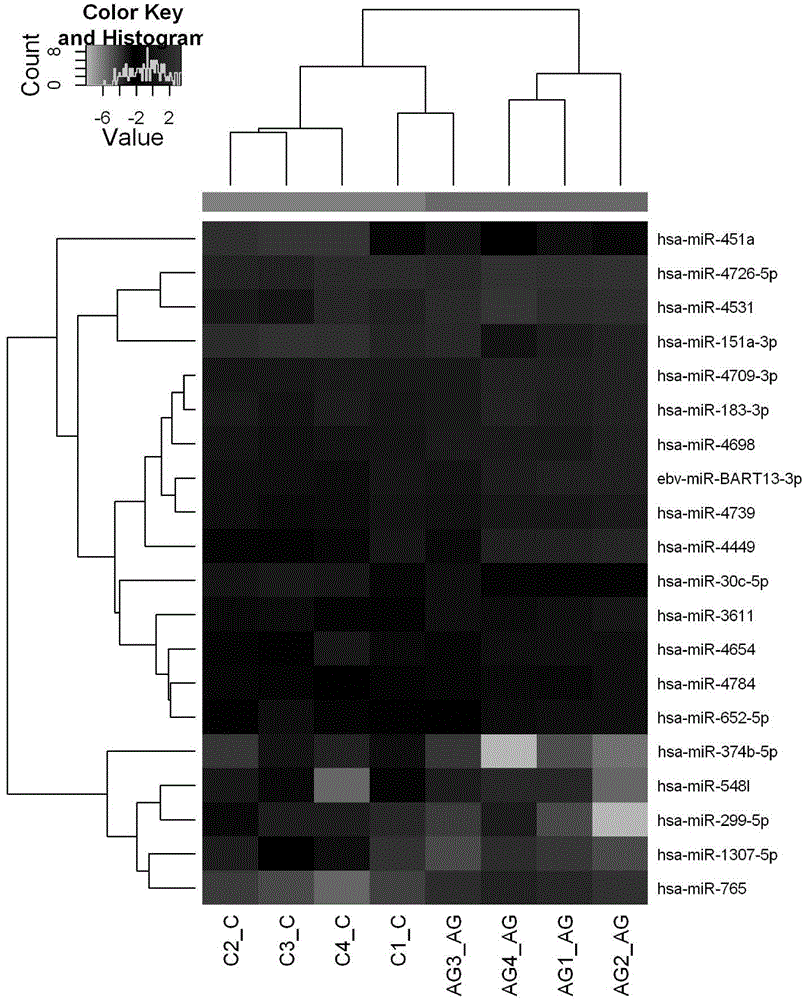
The invention belongs to the fields of biotechnology and medicine, and relates to gout serum miRNAs biomarkers and a method for detecting the expression quantity thereof. The gout serum miRNAs biomarkers comprise hsa-miR-3146, hsa-miR-4449, hsa-miR-4531, hsa-miR-451a, hsa-miR-223-3p, hsa-miR-589-5p and hsa-miR-885-5p. These markers serving as important biological detection indexes of serum can be used for preparing tools such as gout diagnosis reagents or bio-chips and the like, have important value in clinical early accurate diagnosis of gout, provide theoretical basis for molecular level diagnosis of gout and research of gout serum miRNAs later, and have important theoretical significance and potential practical value.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
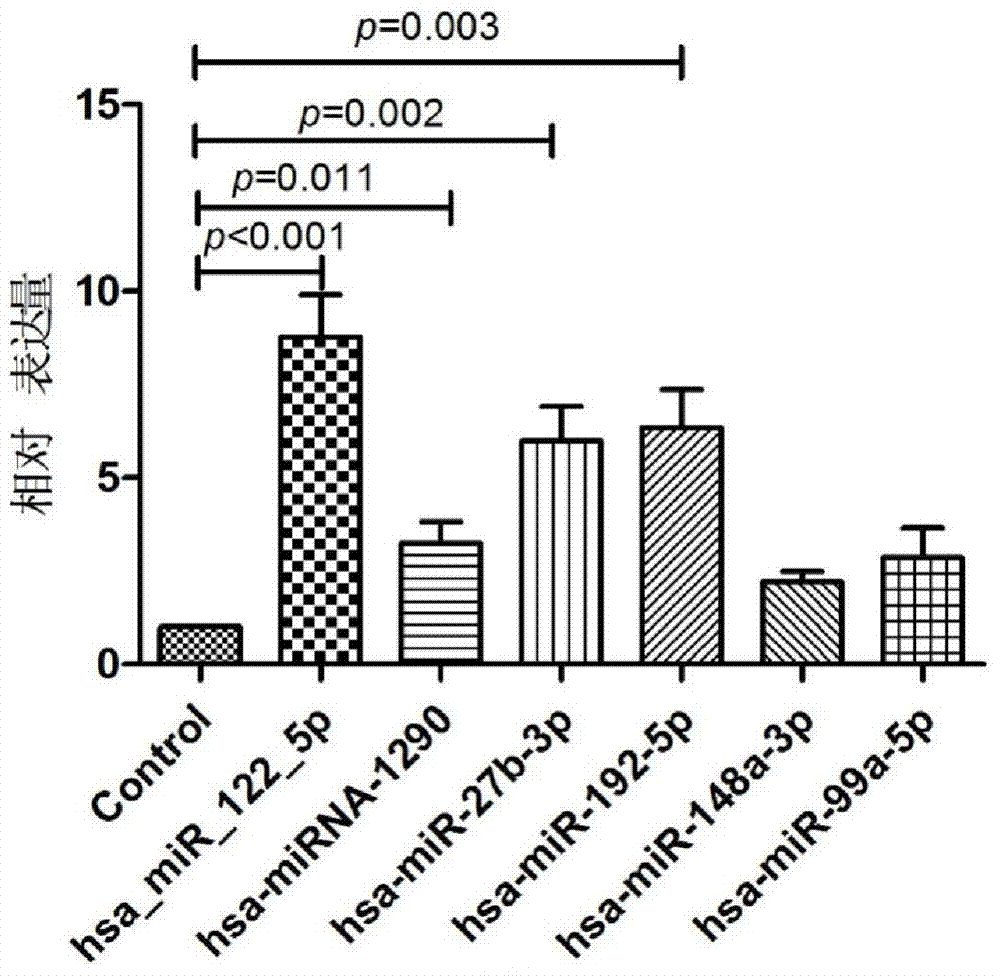
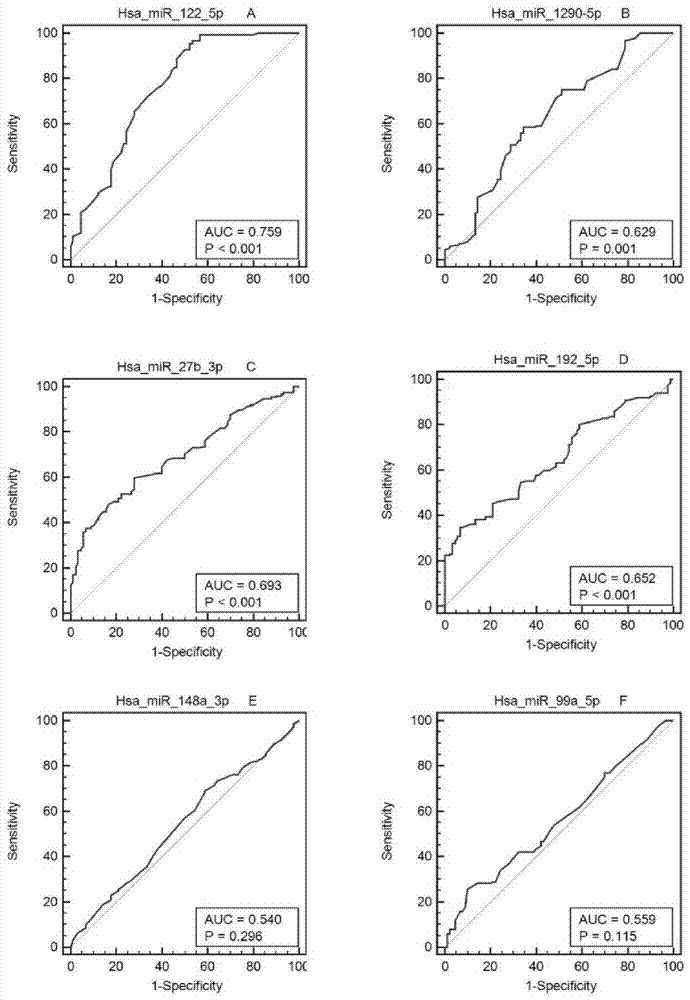
Serum miRNA maker assemblage for detecting nonalcoholic fatty liver, and its application
InactiveCN104293908AAchieve early detectionRapid non-invasive testingMicrobiological testing/measurementDNA/RNA fragmentationSerum igeMedicine
The invention belongs to the field of biotechnology, and relates to a serum miRNA maker assemblage for detecting nonalcoholic fatty liver, and its application. The serum miRNA maker assemblage for detecting nonalcoholic fatty liver includes the following four has-microRNAs: hsa-miR-122-5p, hsa-miR-1290, hsa-miR-27b-3p and hsa-miR-192- 5p. The serum miRNA maker assemblage can realize early detection and rapid noninvasive detection of the nonalcoholic fatty liver.
Owner:镇江市第三人民医院
Atherosclerosis-related serum miRNA (microribonucleic acid) marker group, and specific primers and application thereof
ActiveCN103160588AStrong complementarityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseNucleotide
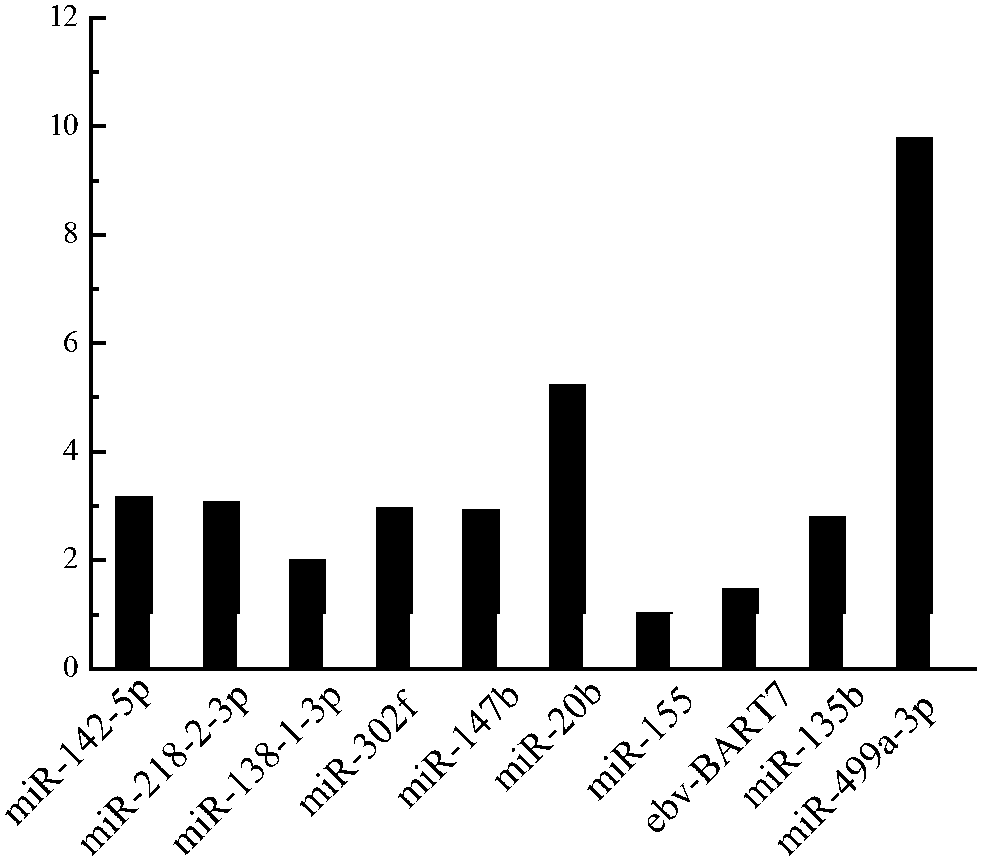
The invention relates to an atherosclerosis-related serum miRNA (microribonucleic acid) marker group, and specific primers and application thereof. The marker group comprises a miR-135b marker of which the nucleotide sequence is shown as SEQ ID NO.1 and a miR-499a-3p marker of which the nucleotide sequence is shown as SEQ ID NO.2. The invention also relates to specific primers and application of the miRNA marker group. The two markers screened out from the serum miRNA marker group provided by the invention have high complementarity, thereby ensuring that the detection is quantitatively precise and greatly improving the sensitivity and specificity of disease diagnosis.
Owner:SHANDONG UNIV
Serum micro ribonucleic acid (miRNA) biomarker of bladder cancer and detection method of expression quantity thereof
ActiveCN102925444AMicrobiological testing/measurementFluorescence/phosphorescenceSerum igeBladder cancer patient
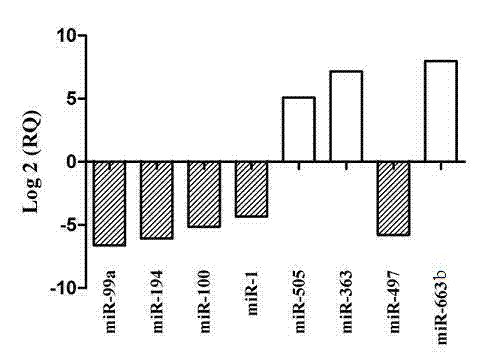
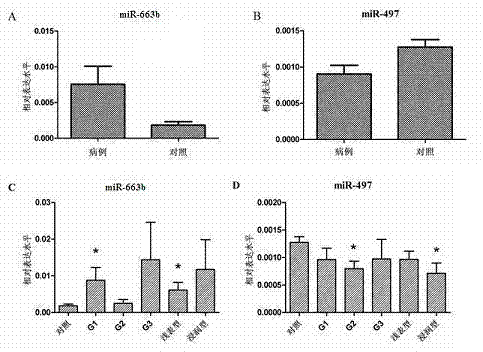
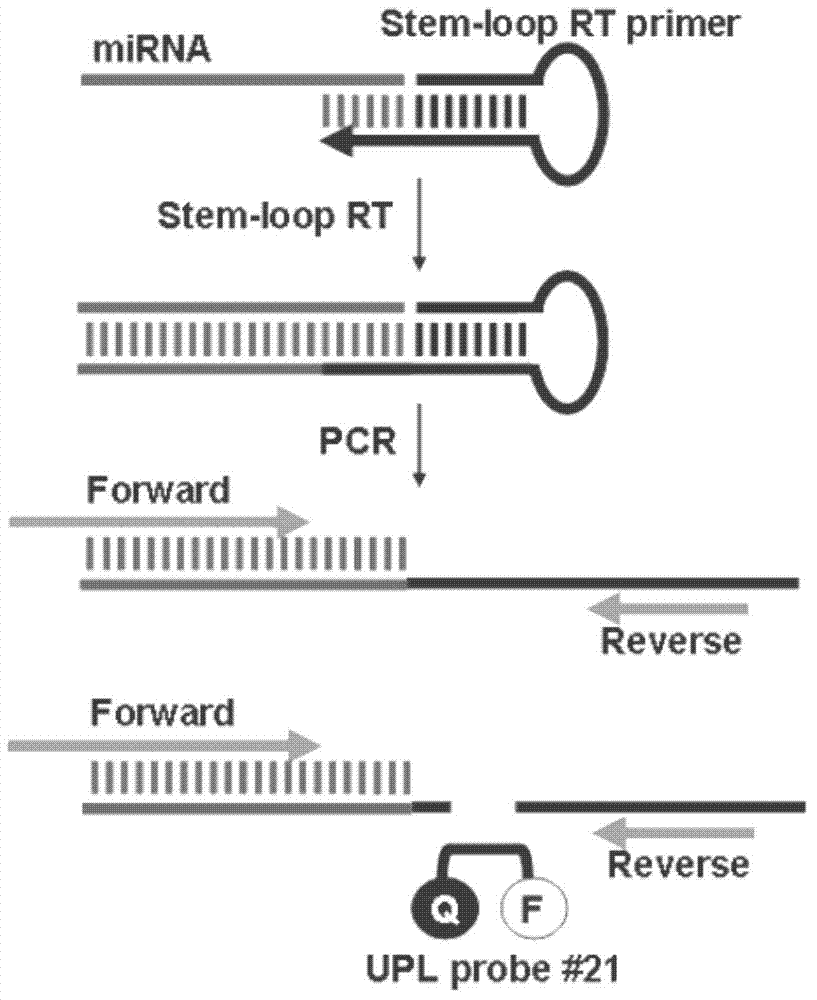
The invention relates to a serum micro ribonucleic acid (miRNA) biomarker used for detection of bladder cancer, overcomes the defects in the prior art, provides a miRNA marker circulating in serum of a patient having the bladder cancer, establishes a blood circulation miRNA expression profile of the patient having the bladder cancer, discloses the value of serum miRNAs in diagnosis of the bladder cancer, and provides support for early detection and early treatment of the patient having the bladder cancer clinically. According to the serum miRNA biomarker of the bladder cancer and a detection method of expression quantity of the serum miRNA biomarker, for the first time, the serum miRNAs, especially miR-663b and miR-497 can be used as an important indicator of biological detection and plays roles in clinical early diagnosis of the bladder cancer, differential diagnosis and effective observation, a theoretical basis is provided for research on the serum miRNAs of urinary system tumor in the future, novel ideas are provided for the diagnosis of the bladder cancer at the molecular level, and the serum miRNA biomarker of the bladder cancer and the detection method of expression quantity of the serum miRNA biomarker have great theoretical significance and potential practical value.
Owner:NANJING MEDICAL UNIV
MicroRNA biological markers for early lung cancer diagnosis and application thereof
ActiveCN103484550AHigh diagnostic valueMicrobiological testing/measurementDNA/RNA fragmentationSerum igeLung cancer early detection
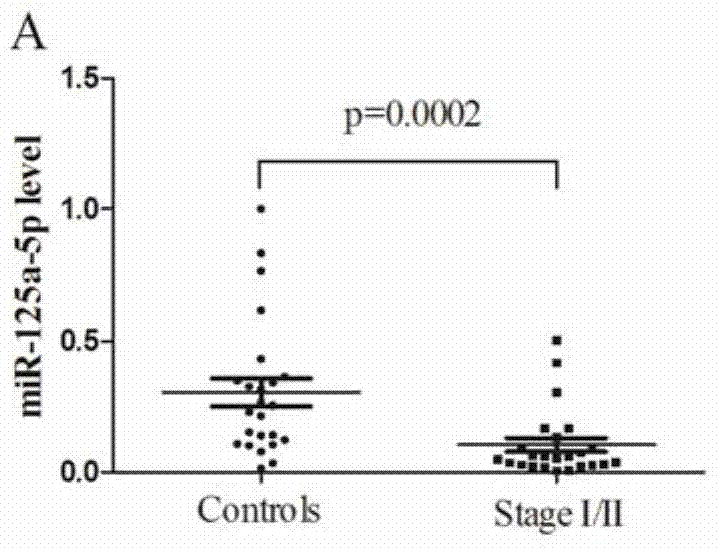
The invention relates to the field of biological detection, particularly to a group of microRNA biological markers for early lung cancer diagnosis and an application thereof. The microRNA biological marker comprises hsa-miR-125a-5p, hsa-miR-193a-5p, hsa-miR-25, hsa-miR-126 and hsa-miR-34a. The serum miRNA biological markers comprising the 5 miRNAs can not only be particularly applied to early lung cancer detection, but also be applied to advanced lung cancer detection.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Application of serum miRNA biomarker
ActiveCN104450707AEasy to useRelieve painMicrobiological testing/measurementDNA/RNA fragmentationForward primerGenetics
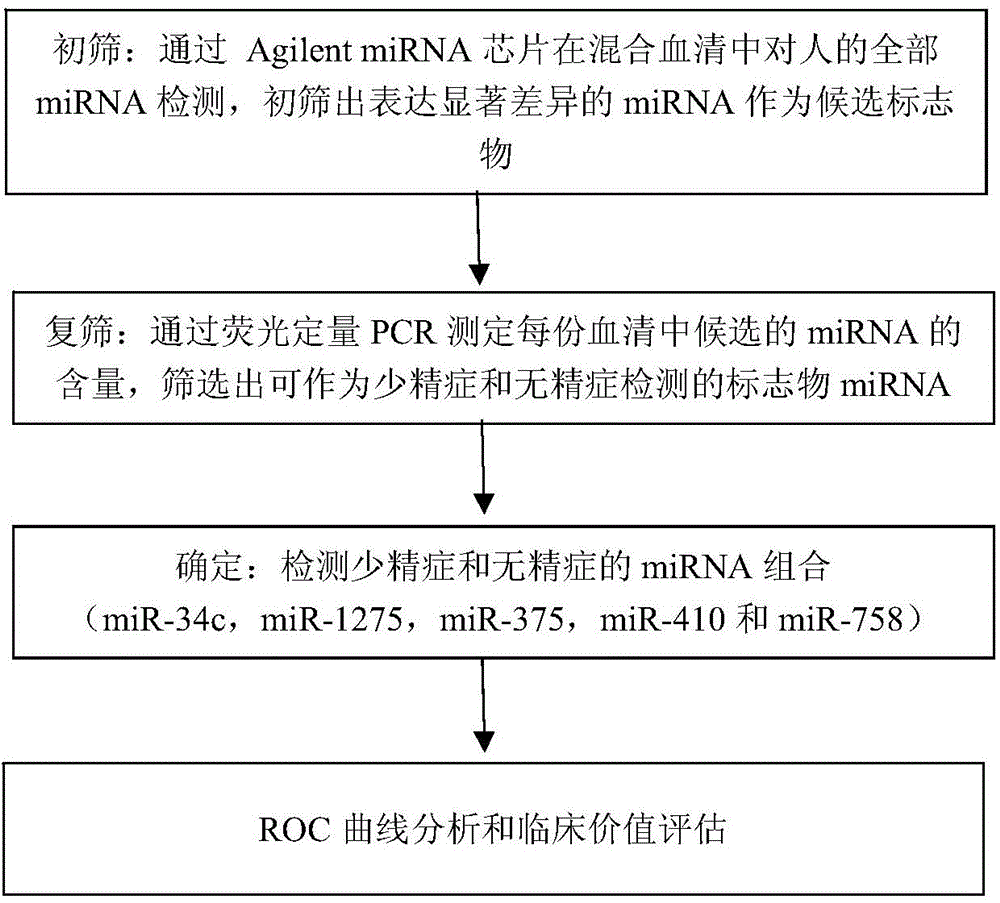
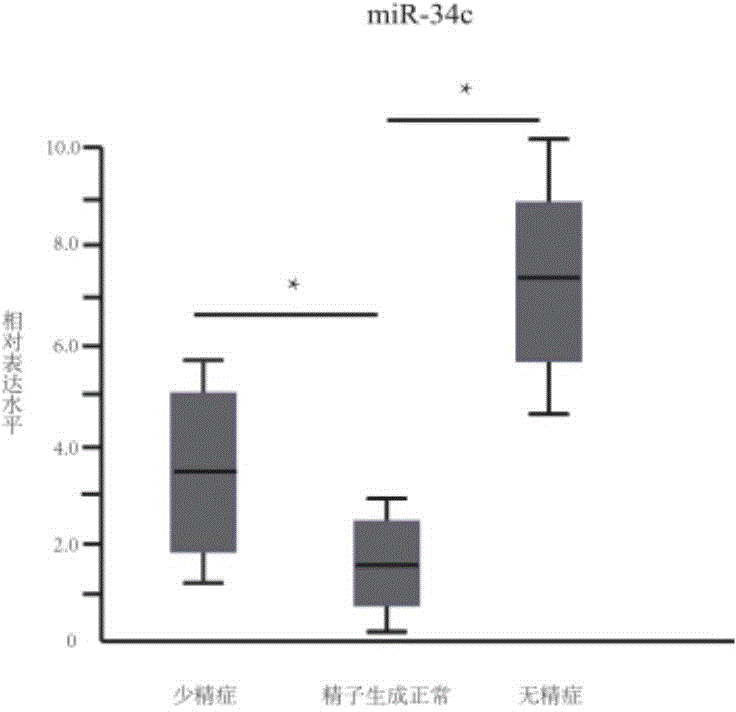
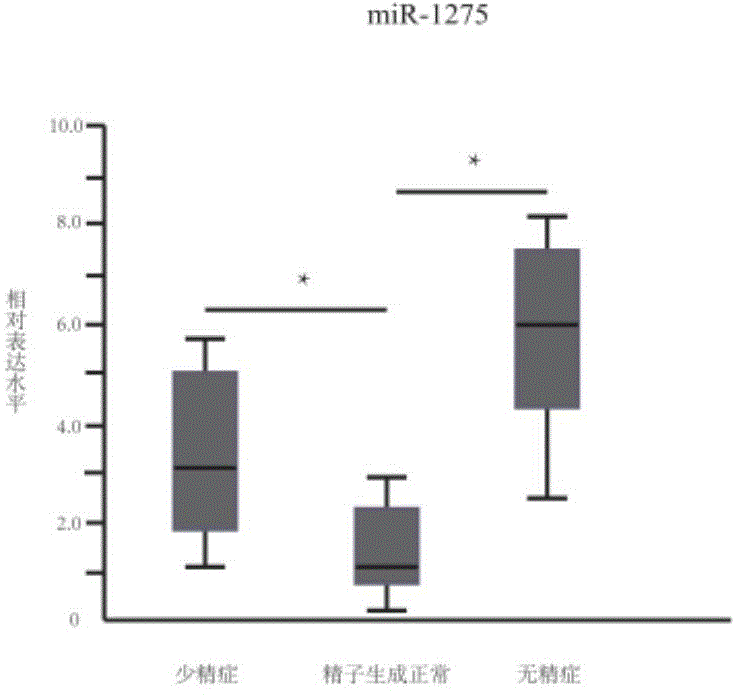
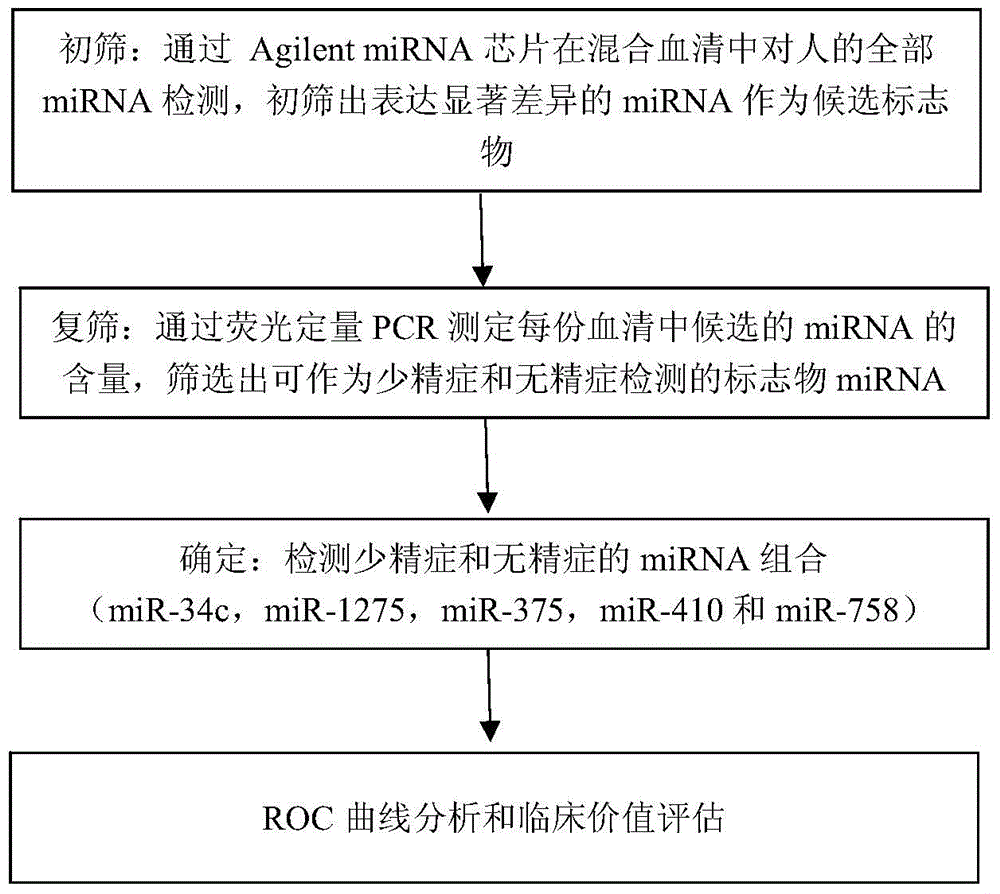
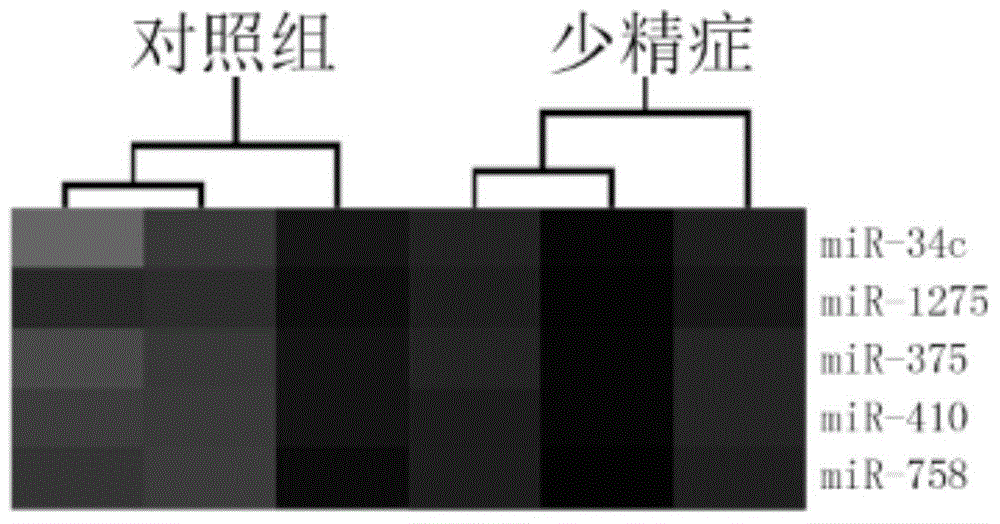
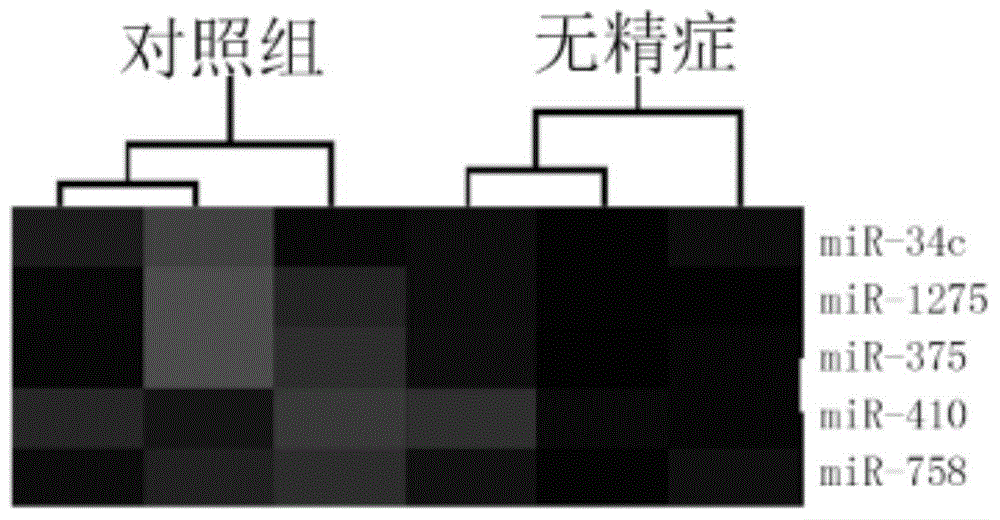
The invention provides application of a serum miRNA biomarker, and relates to biomarkers. The serum miRNA biomarker comprises at least one of miR-34c, miR-1275, miR-375, miR-410 and miR-758. The serum miRNA biomarker can be applied to preparation of a reagent for detecting oligospermia and azoospermia. A serum miRNA biomarker composition comprises at least one of the miRNA and sequences of reverse transcription primers, forward primers and reverse primers of each miRNA, and can be applied to preparation of a reagent for detecting oligospermia and azoospermia. The determined close correlation between the miRNA combination and indexes of oligospermia and azoospermia cases can be used as the biomarker to screen and diagnose patients with oligospermia and azoospermia, and an efficient basis is provided to clinical individual intervention treatment.
Owner:XIAMEN UNIV
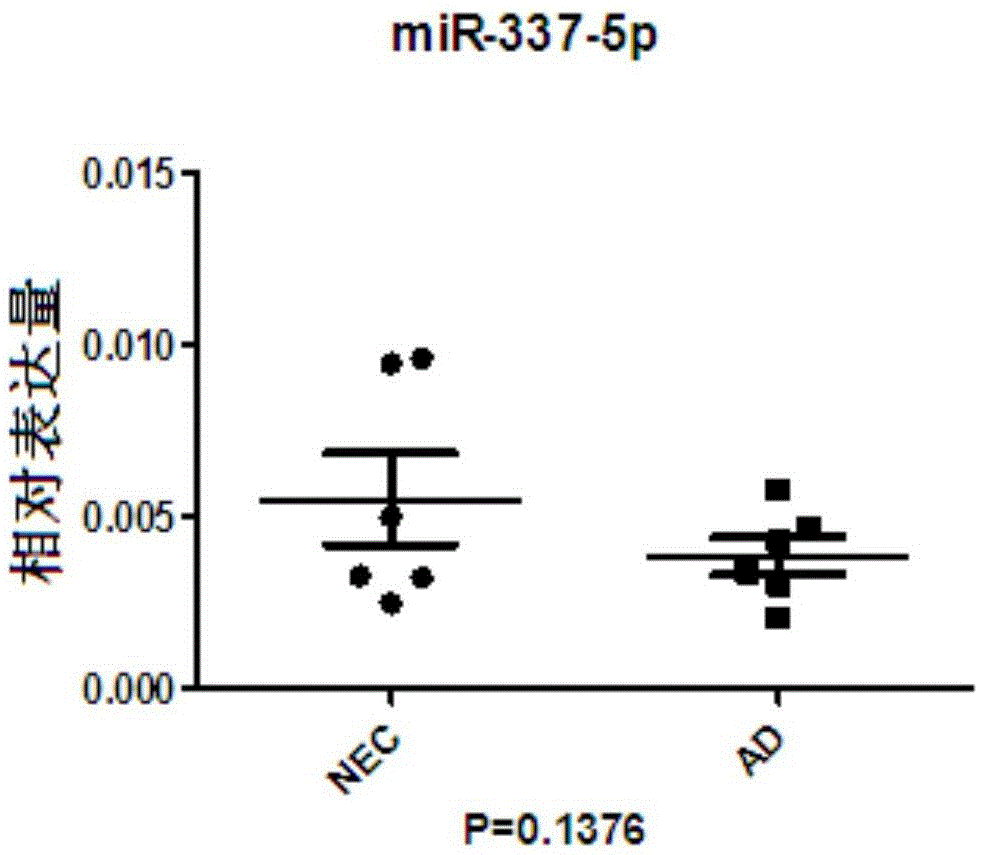
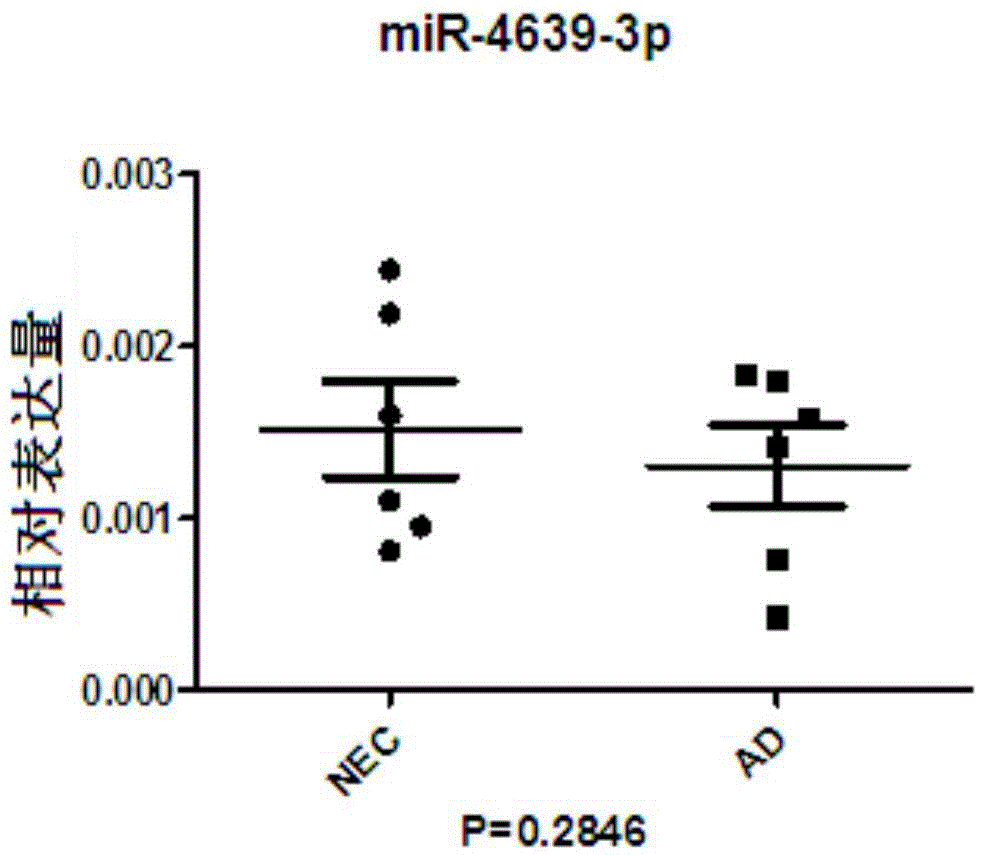
AD or MCI detection marker and detection method thereof
InactiveCN105648088AQuantitative test resultsThe test results are objectiveMicrobiological testing/measurementDNA/RNA fragmentationImage diagnosisSerum mirna
The invention discloses an AD or MCI detection marker and a detection method thereof. The AD or MCI detection marker is serum miRNA. The serum miRNA comprises hsa-miR-103a-3p, hsa-miR-15b-5p, hsa-let-7d-3p, hsa-let-7d-5p, hsa-miR-15a-5p and hsa-miR-150-5p. The AD or MCI detection marker has the advantages that the serum can be collected fast and simply, the AD or MCI detection marker is low in traumatic property and high in safety as compared with cerebrospinal fluid sampling, and the AD or MCI detection marker is free of radioactive damage to patients as compared to iconography manners such as PET; an miRCURYTM LNA Array gene chip is combined with the new S-Poly(T) method for miRNA detection, and method accuracy and sensitivity are increased; the detection of the expression quantity change of the miRNA has the advantage of quantitative detection as compared with scale detection and imaging diagnosis, subjectivity is lowered, and accuracy is achieved.
Owner:SHENZHEN UNIV
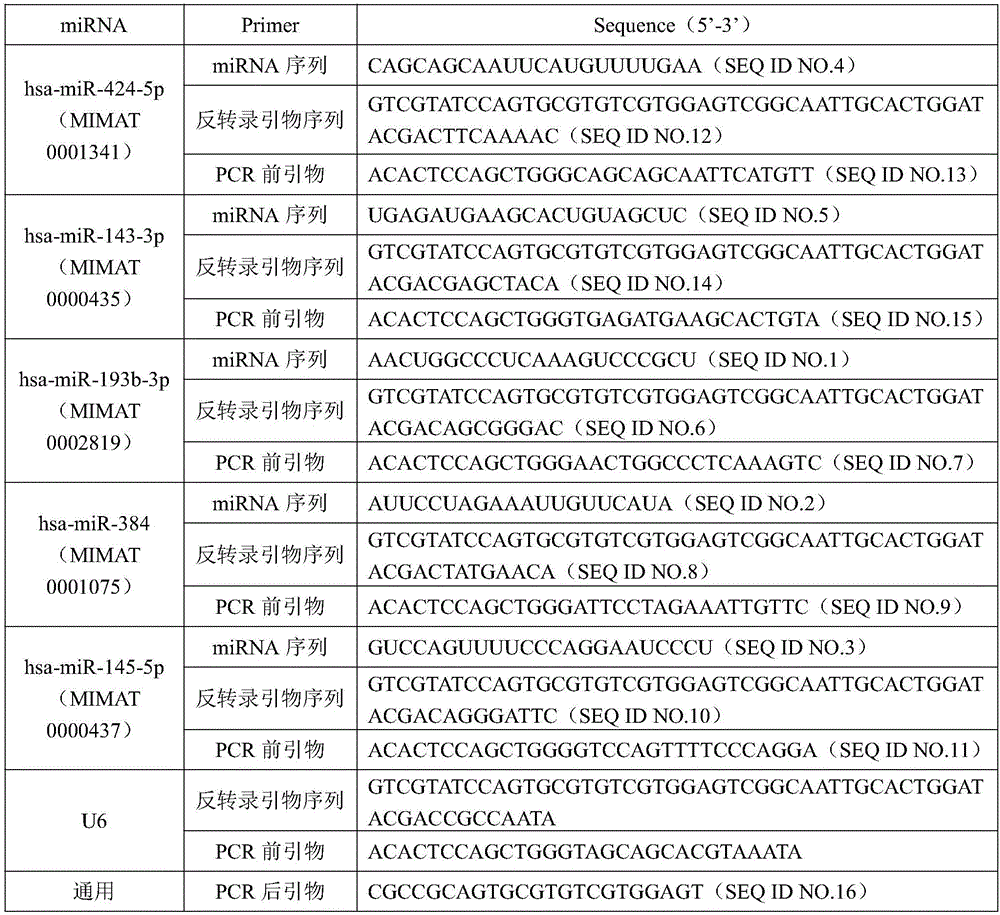
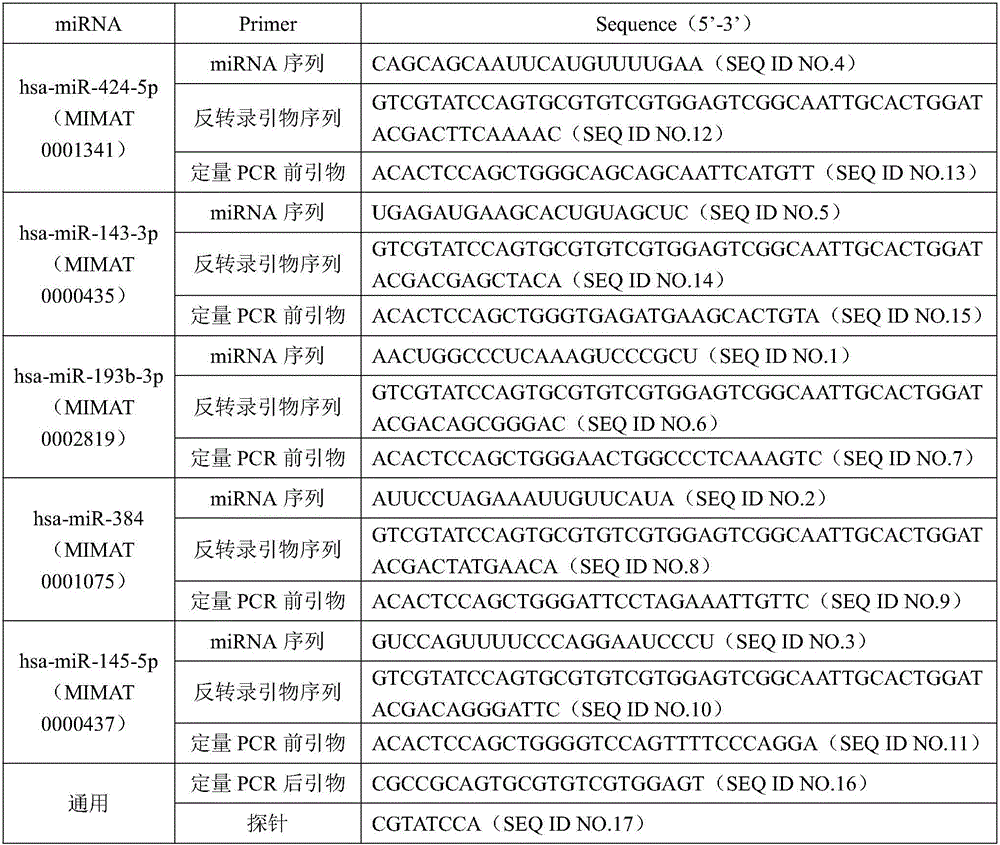
MiRNA biomarker and detection kit for thyroid cancer diagnosis
InactiveCN105950730AImprove consistencyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMir 145 5pMir 193b
The invention discloses an miRNA biomarker and a detection kit for thyroid cancer diagnosis. The miRNA biomarker comprises microRNAs, namely hsa-miR-193b-3p, hsa-miR-384, hsa-miR-145-5p, hsa-miR-424-5p and hsa-miR-143-3p. The five miRNAs, namely the hsa-miR-193b-3p, the hsa-miR-384, the hsa-miR-424-5p, the hsa-miR-145-5p and the hsa-miR-143-3p, in which expression differences between the thyroid cancer and para-carcinoma tissues are obvious (difference expression amount is larger than 2 folds, and the CT value in RT-PCR is smaller than 30), are subjected to serologic expression analysis; results show that the five miRNAs are expressed stably in serum, expression and organization of serum miRNAs have high uniformity, expression of the hsa-miR-193b-3p, the hsa-miR-384 and the hsa-miR-145-5p is regulated down, and expression of the hsa-miR-424-5p and the hsa-miR-143-3p is regulated up. The five microRNAs can serve as the miRNA biomarker for thyroid cancer diagnosis, and sensitivity and specificity of combined diagnosis are higher than those of single miRNA diagnosis remarkably.
Owner:崔学俊
Liver cancer prognosis related serum miRNA markers and application of detection kit thereof
ActiveCN105647923AHigh clinical application valueBreakthrough innovationMicrobiological testing/measurementDNA/RNA fragmentationIndividualized treatmentSerum mirna
The invention discloses liver cancer prognosis related serum miRNA markers and an application of a detection kit thereof. The tumor markers are serum miR-29a-3p and miR-192-5p which are related with human liver cancer patients. According to preliminary work, liver cancer related serum miRNA expression profiles containing miR-29a-3p and miR-192-5p and having obvious expression difference are screened out through high-throughput deep sequencing, and qPCR (quantitative polymerase chain reaction) proves and finally confirms that miR-29a-3p and miR-192-5p are liver cancer risk (prognosis) related tumor markers. The markers and detection reagents can be used for preparing the rapid-detection pPCR kit, and the kit has the characteristics of rapidness and convenience in detection, high accuracy rate, no wound and the like; a prognostic model built through combination of miR-29a-3p, miR-192-5p and BCLC stage (Barcelona clinic liver cancer stage) can be applied to classification of risk degrees of the liver cancer patients, thereby guiding individualized treatment of the liver cancer patients.
Owner:SUN YAT SEN UNIV CANCER CENT
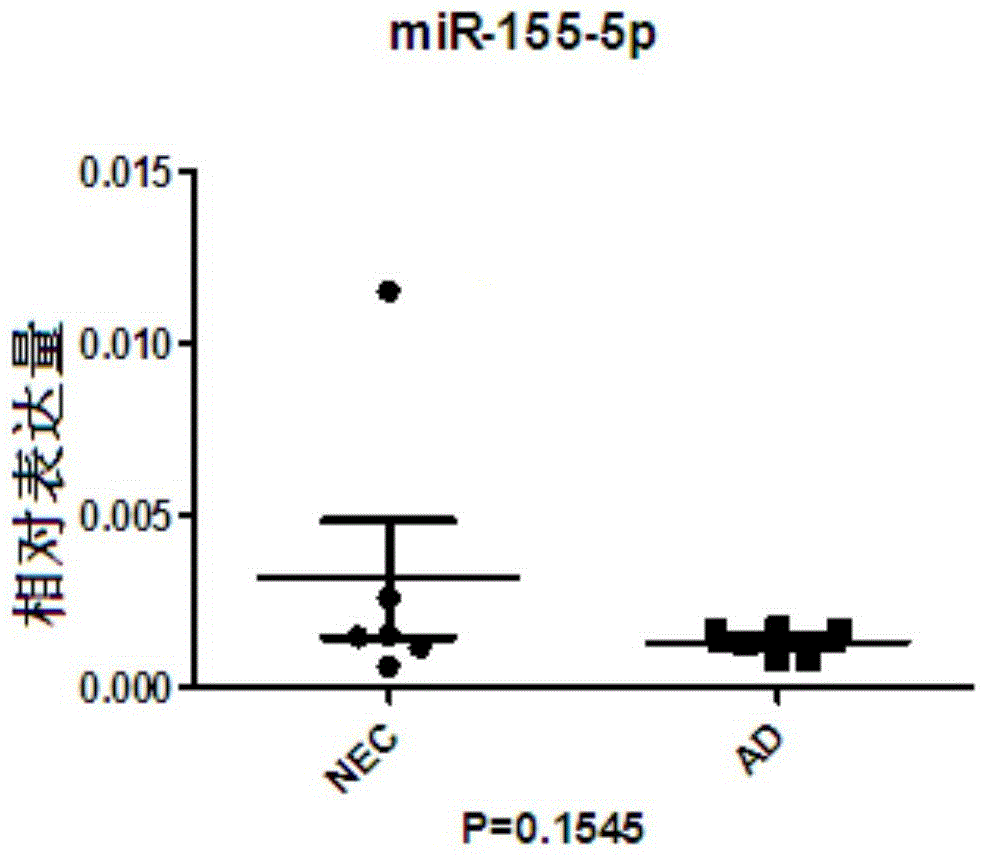
Serum miRNA marker and method thereof for detecting ionizing radiation injury by serum miRNA marker
ActiveCN109385472AStable extractionStable storageMicrobiological testing/measurementDNA/RNA fragmentationSerum igeSerum mirna
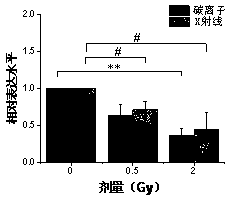
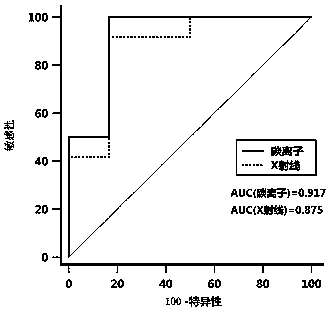
The invention relates to a serum miRNA marker. The miRNA marker is a combination of miR-134-5p and miR-155-5p. Meanwhile, the invention also discloses a method for detecting ionizing radiation injuryby the serum miRNA marker. Compared with a traditional chromosomal aberration and protein biomarker, the serum miRNA marker disclosed by the invention is more stable in the process of extraction and preservation, so that a detection result is more reliable and accurate; in addition, verified by a qRT-PCR method, the expression level and the radiation dose of the serum miRNA marker are also closelycorrelated, so that a reliable and accurate assessment basis can be provided for judging whether a test object is at risk of suffering from ionizing radiation.
Owner:INST OF MODERN PHYSICS CHINESE ACADEMY OF SCI
Type-1 diabetes markers and application thereof
InactiveCN108676868AGood diagnostic supportMake up for sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDemographic dataSerum mirna
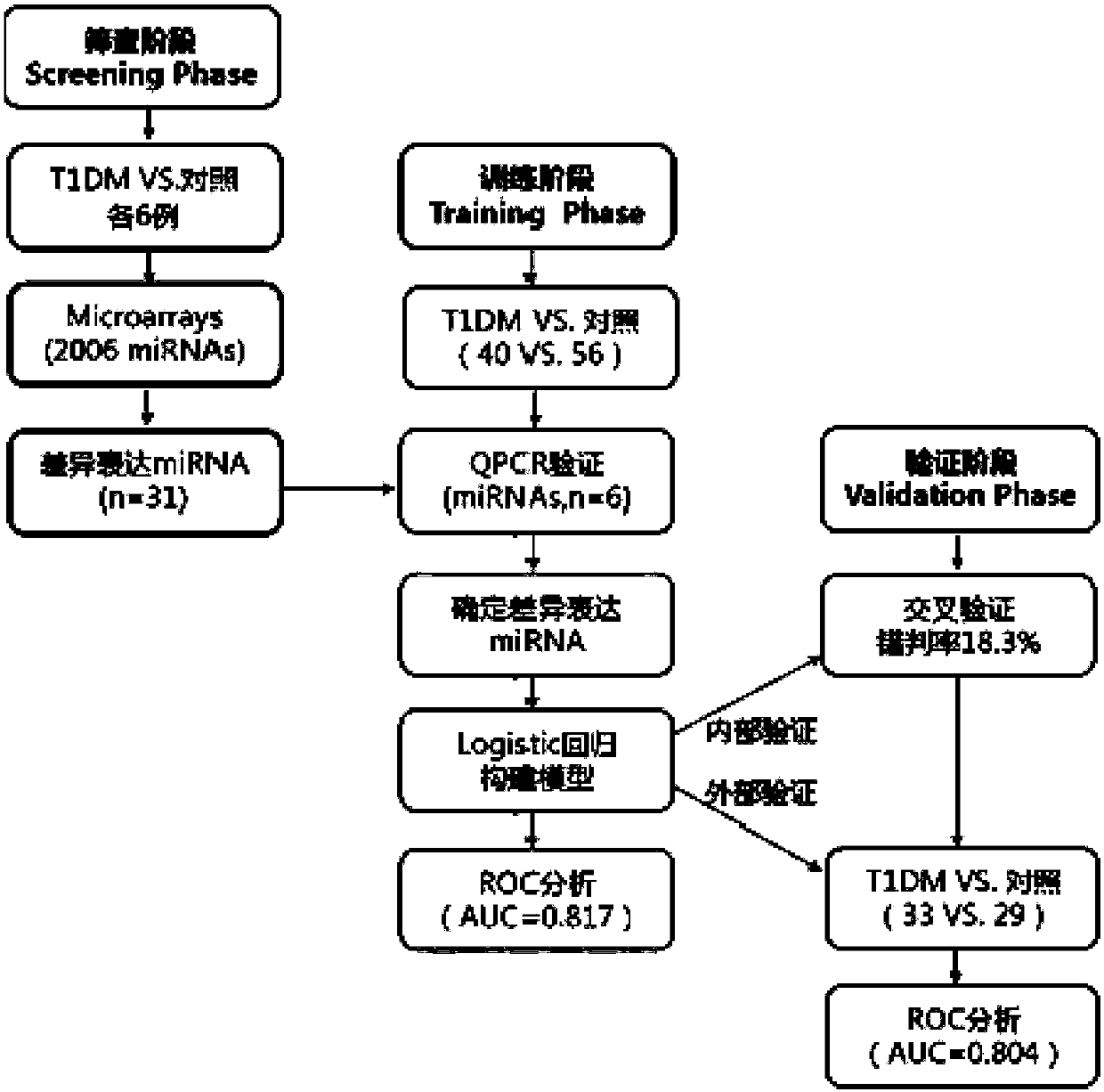
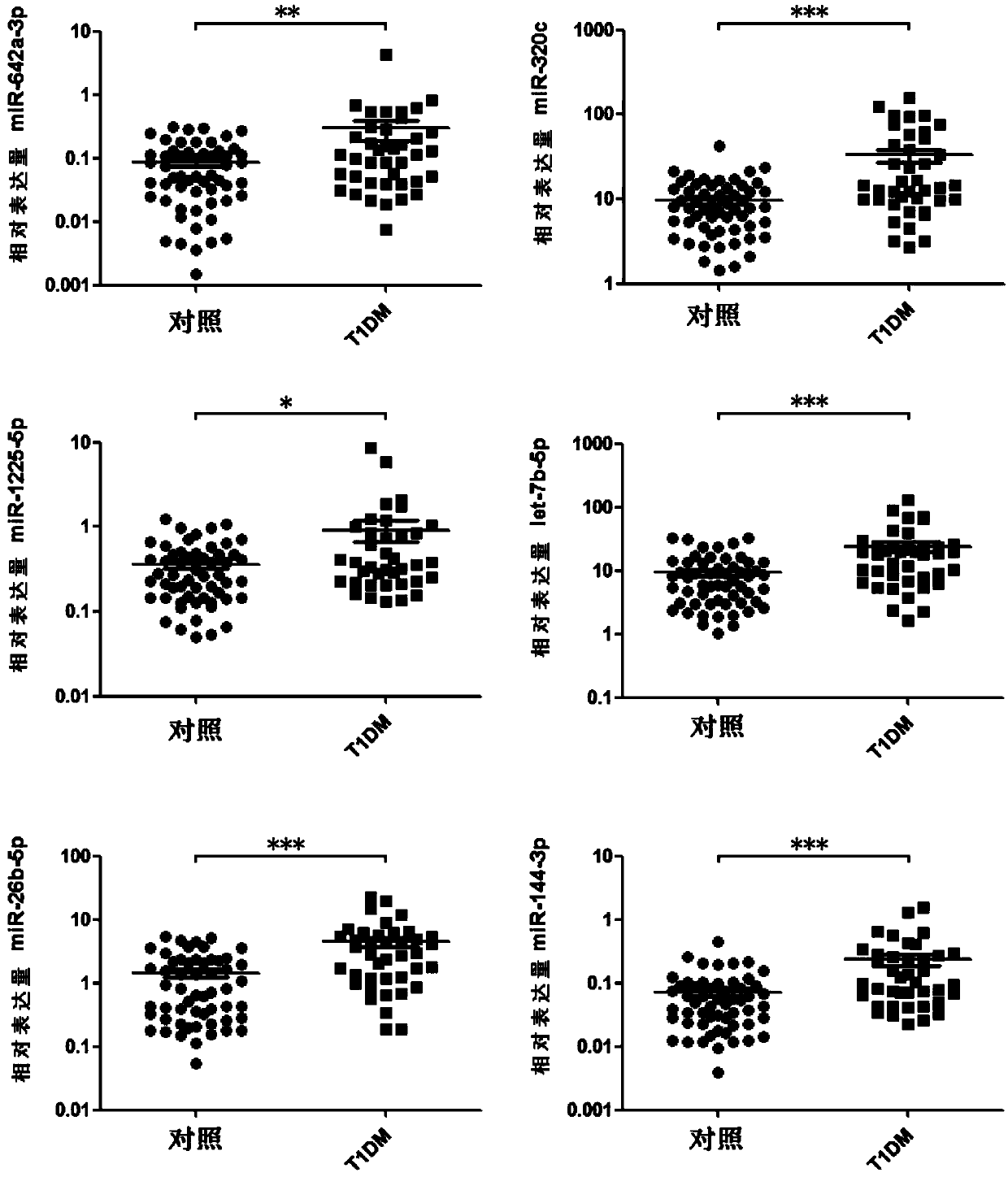
The invention discloses a set of type-1 diabetes markers, consisting of three miRNAs, including hsa-miR-642a-3p, hsa-miR-320c and hsa-miR-1225-5p; the invention also discloses the application of the markers in preparation of a type-1 diabetes diagnostic kit, and the kit contains reagents for quantifying the expression of the hsa-miR-642a-3p, hsa-miR-320c, hsa-miR-1225-5p. Compared with the known single biomarkers, utilizing a statistical method, and combining with multiple serum miRNAs and clinical demographic data to establish a type-1 diabetes serum miRNA-assisted diagnosis model, so the markers have better diagnostic support efficacy. The miRNA in circulation of a patient is utilized, the expression is stable, the storage and detection are easy, and clinical application is facilitated.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
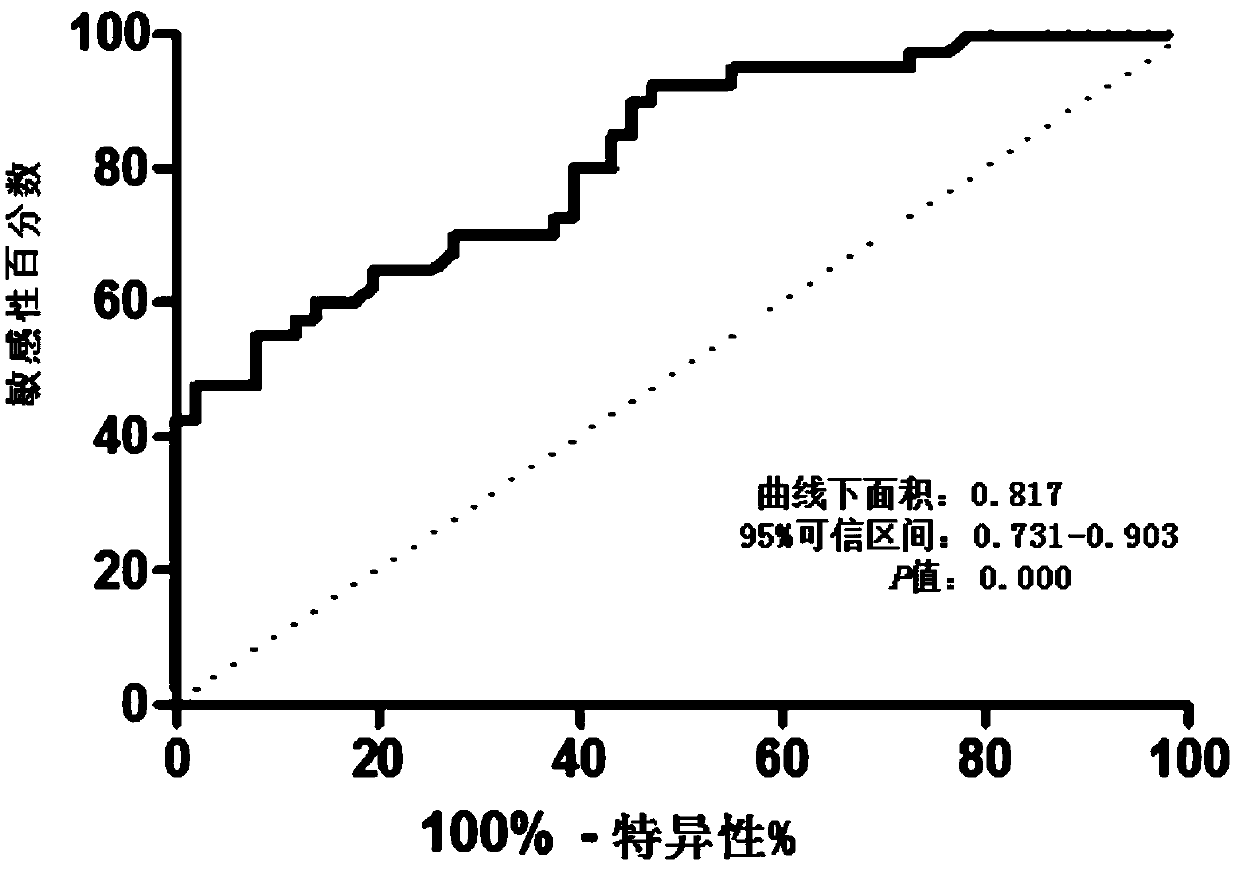
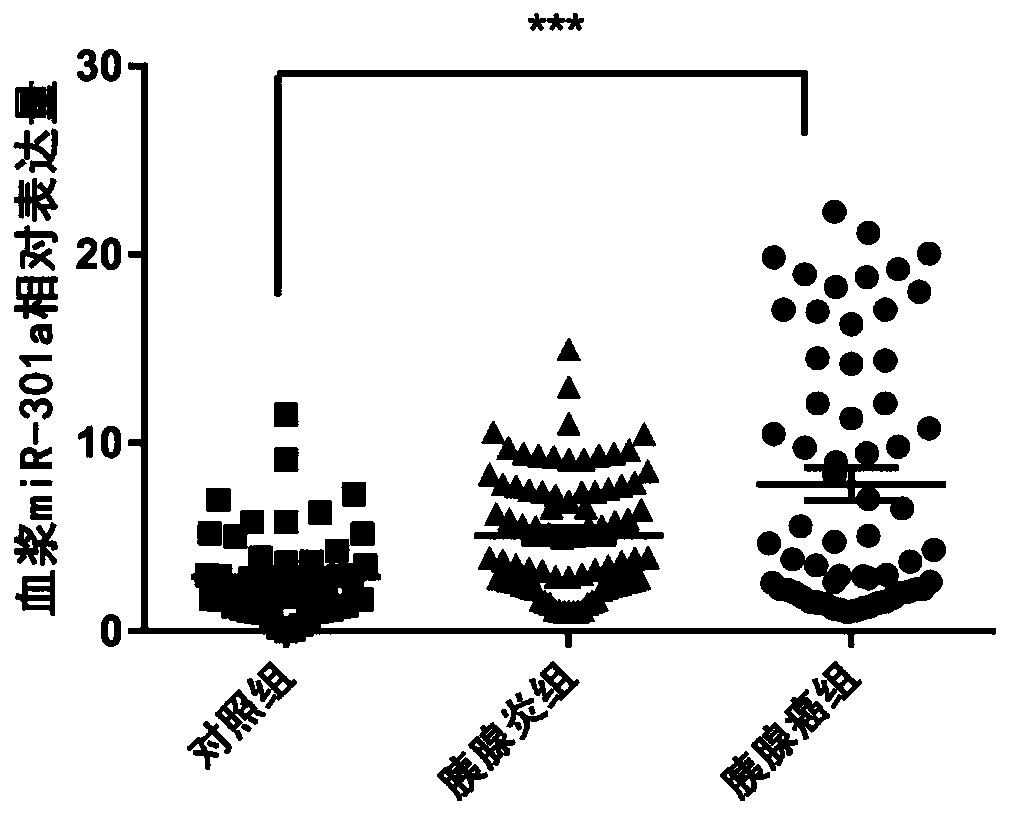
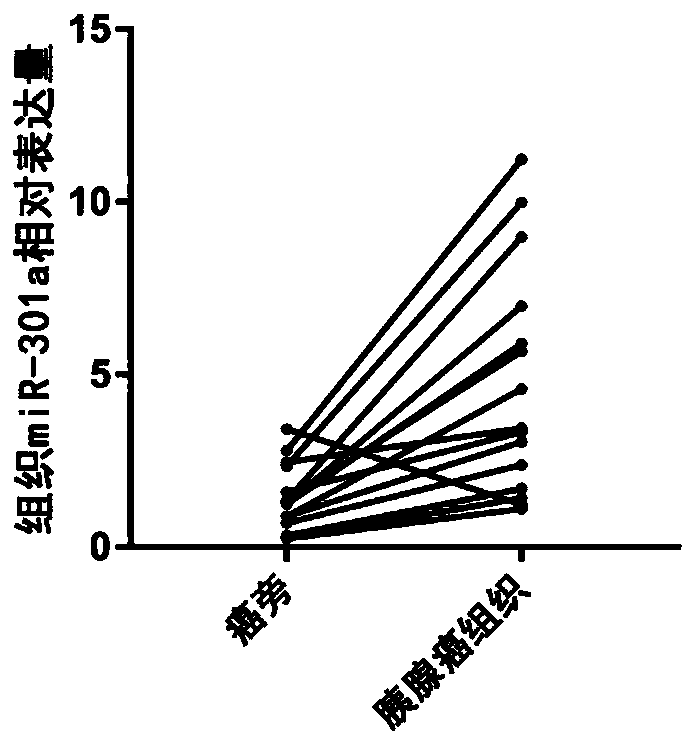
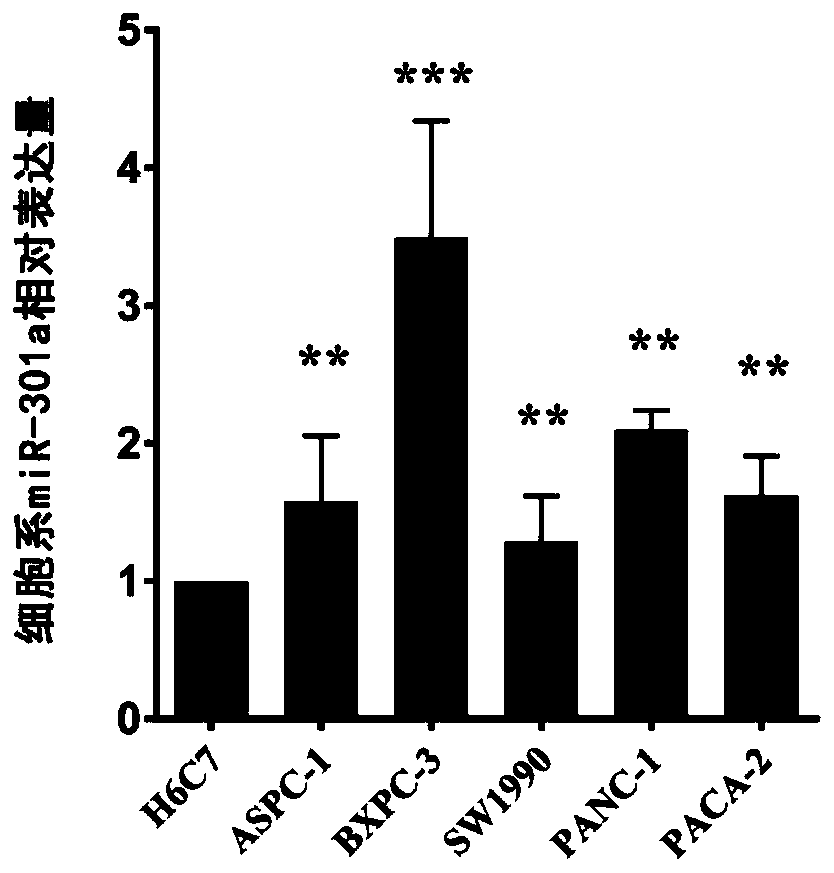
Serum miRNA marker and application of serum miRNA marker in early diagnosis for pancreatic cancer induced by pancreatitis
InactiveCN109897855AMicrobiological testing/measurementDNA/RNA fragmentationReceiver operating characteristicSerum mirna
The invention discloses a serum marker miR-301a with the sequence of SEQ ID NO. 1 and application of a kit prepared by adopting the miR-301a marker as a molecular marker in early diagnosis for pancreatic cancer induced by pancreatitis and curative effect monitoring. The kit prepared by adopting the miR-301a marker can conveniently and quickly detect the expression level of miRNA, it is not necessary to customize special primer sequences additionally, and by detecting the blood plasma, pancreatic cancer tissue and expression level of the miR-301a in a pancreatic cancer cell line of a patient suffering from pancreatitis and pancreatic cancer, the Pearson correlation test is utilized for analyzing the relationship between the miR-301a and a known traditional marker of pancreatitis and comparing the miR-301a with the known traditional marker of pancreatitis; through a receiver operating characteristic curve (ROC), the identification capacity of the miR-301a and joint detection by the miR-301a and CA199 in pancreatic cancer diagnosis and pancreatic cancer staging is evaluated, and the potential value of the miR-301a marker of the blood plasma in early diagnosis for pancreatic cancer induced by pancreatitis is investigated to provide a new theory and a new test basis for early diagnosis and targeted therapy of pancreatic cancer from the miRNA level.
Owner:THE CENT HOSPITAL OF WUHAN
Method and kit for diagnosing liver cancer by use of the ratio of change in serum miRNA quantity
InactiveCN104120185AImprove accuracyMicrobiological testing/measurementMedicineQuantitative determination
The invention relates to the technical field of immunology and molecular biology, and particularly relates to a method for diagnosing liver cancer by use of the ratio of change in serum miRNA quantity. The method comprises the following steps: collecting a blood sample; separating Mir-RNA; performing quantitative determination of two or more Mir-RNAs in blood; calculating the proportion of the two or more Mir-RNAs according to the determination result; and measuring the proportion range of multiple groups of Mir-RNAs to determine the index of Mir-RNA change as the diagnosis basis. In the method provided by the invention, the liver cancer is diagnosed by measuring two or more Mir-RNAs and the ratio thereof, and the accuracy in liver cancer diagnosis can be effectively increased.
Owner:常州百代生物科技股份有限公司
Serum miRNA combination-based pulmonary tuberculosis therapeutic effect evaluation kit and applications thereof
InactiveCN106884052AReliable resultsStrong specificityMicrobiological testing/measurementRNA extractionCurative effect
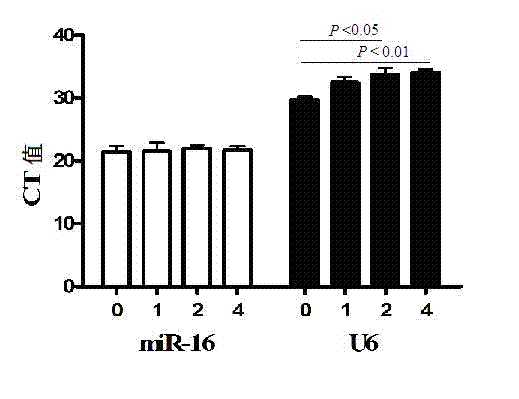
The invention discloses a serum miRNA combination-based pulmonary tuberculosis therapeutic effect evaluation kit and applications thereof. The kit comprises an RNA extraction buffering solution, a Poly (A)-containing reaction solution, a reverse transcription reaction solution, a serum miRNA upstream primer combination, an internal reference upstream primer, a universal downstream primer and a fluorescent quantitation RT-PCR reaction solution, wherein the serum miRNA upstream primer combination comprises four differentially expressed serum miRNA: hsa-miR-21-5p, hsa-miR-92a-3p, hsa-miR-125a-5p and hsa-miR-148b-3p, the sequences of which are respectively SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3 and SEQ ID NO:4; the internal reference is hsa-miR-16, the sequence of which is SEQ ID NO:5. The detection sensitivity of the kit on treatment of pulmonary tuberculosis is 65.38%, and the specificity is 80.77%, so that the specificity is strong and the sensitivity is high, and the kit has higher accuracy, and is simple and convenient to operate, high in efficiency, small in specimen state limit, and superior to the sputum culture currently clinically adopted, thus providing a new evaluation method for the evaluation on the therapeutic efficacy of pulmonary tuberculosis.
Owner:李继承
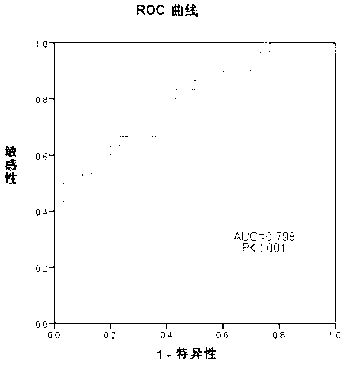
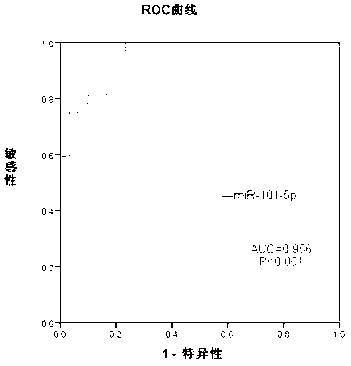
Method and composition based on tiny RNA for liver cirrhosis and early liver cancer diagnosis
The invention provides a method and a composition based on tiny RNA for liver cirrhosis and early liver cancer diagnosis. The invention also provides application of the tiny RNA and / or a detection primer of the tiny RNA in preparation of the composition for liver fibrosis / liver cirrhosis and liver cancer diagnosis. The invention discloses and proves that a serum miRNA-101-5p level can be used as a potential noninvasive index for early diagnosis of hepatitis B / liver cirrhosis related liver cancers for the first time and can also be used as an index related to liver fibrosis / liver cirrhosis diagnosis.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV
Plasma miRNA marker related to assistant diagnoses of pancreatic cancer and application thereof
ActiveCN109055557AImprove stabilityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSerum mirnaNon invasive
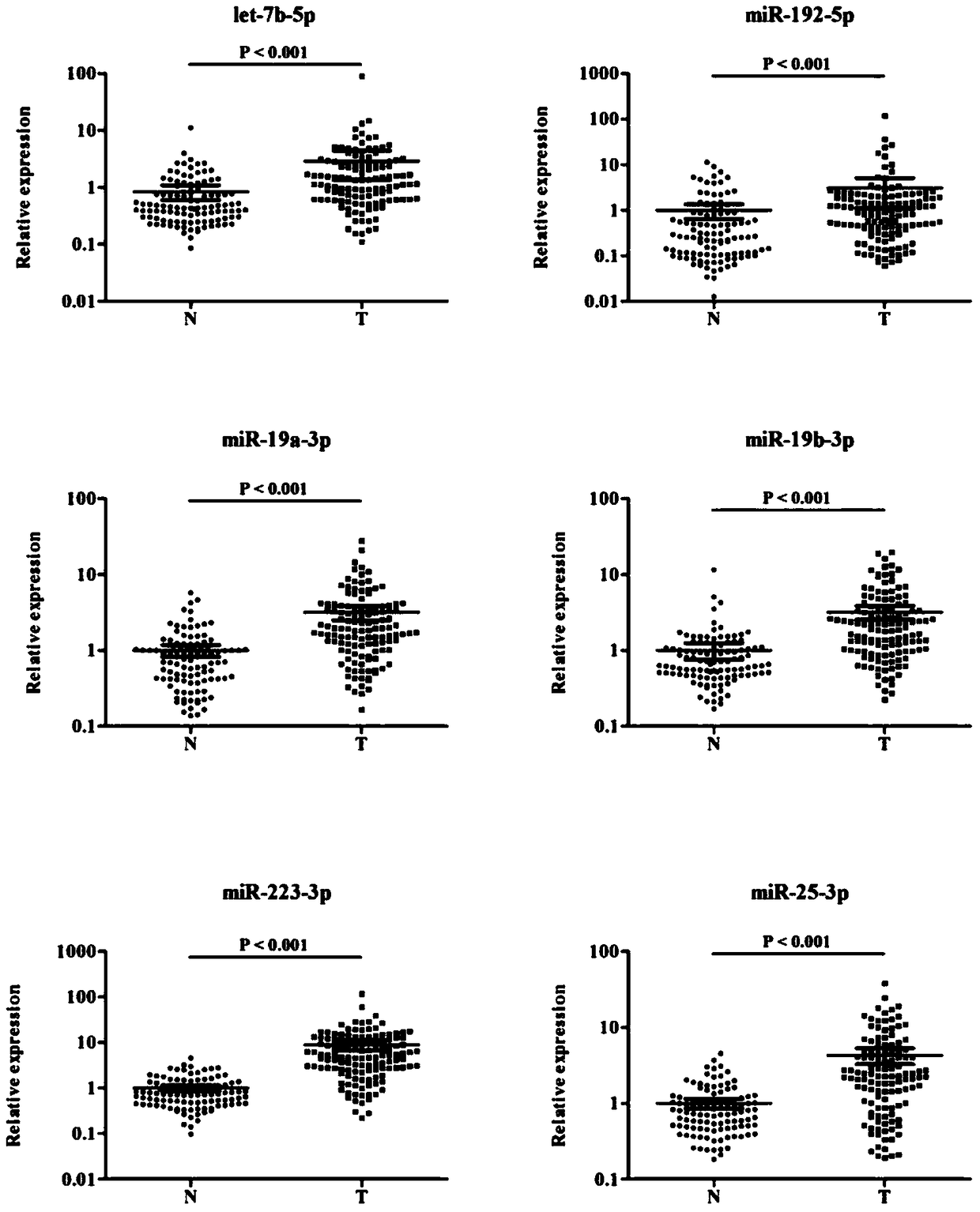
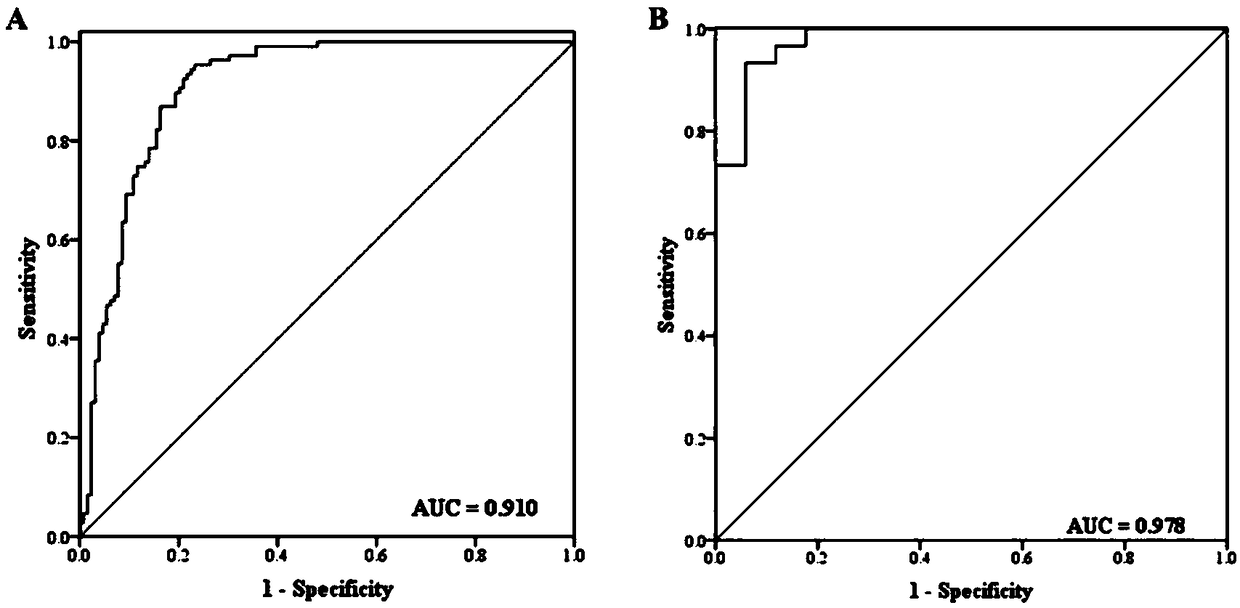
The invention discloses a plasma miRNA marker related to assistant diagnoses of pancreatic cancer and application thereof. The marker is one or more of let-7b-5p, miR-192-5p, miR-19a-3p, miR-19b-3p, miR-223-3p, and miR-25-3p. Plasma miRNA as a new biomarker has the characteristics of good stability, high sensitivity and specificity, and is minimally invasive and can be easily obtained. The development and utilization of such molecular markers can provide a new direction for the diagnosis and further treatment of various diseases comprising tumors. The research will be more targeted to obtain apancreatic cancer serum miRNA marker with clinical diagnostic potentials. The research confirms the reliability and reproducibility of this group of miRNAs as a non-invasive marker for the diagnosisof pancreatic cancer.
Owner:朱伟
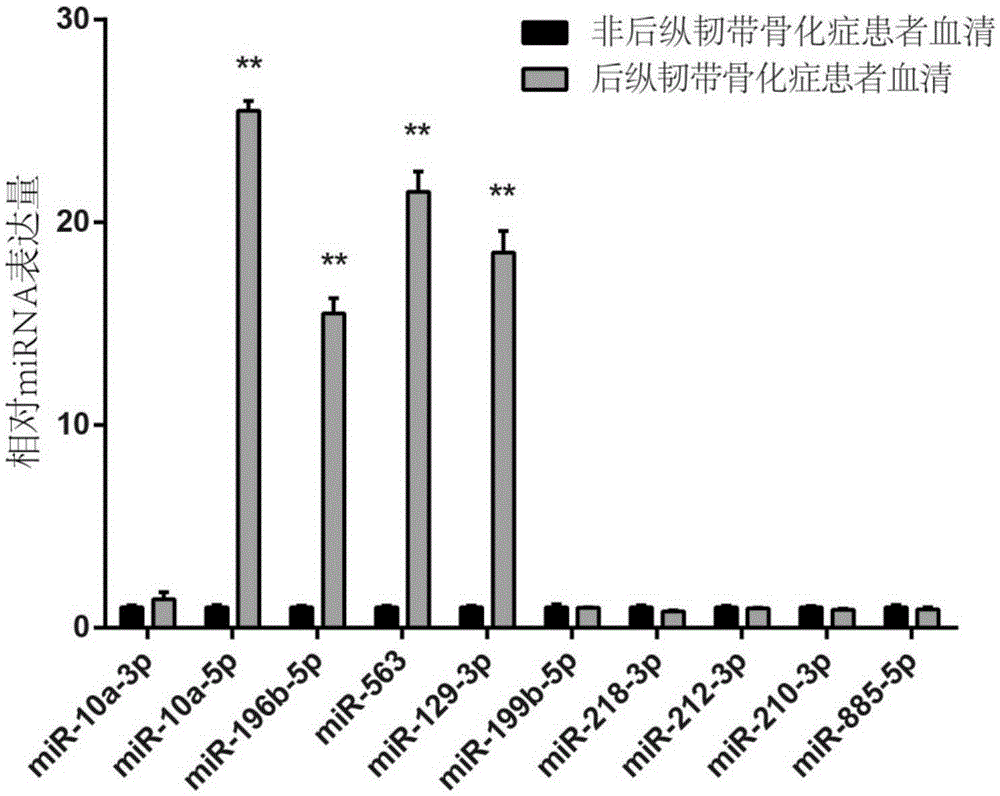
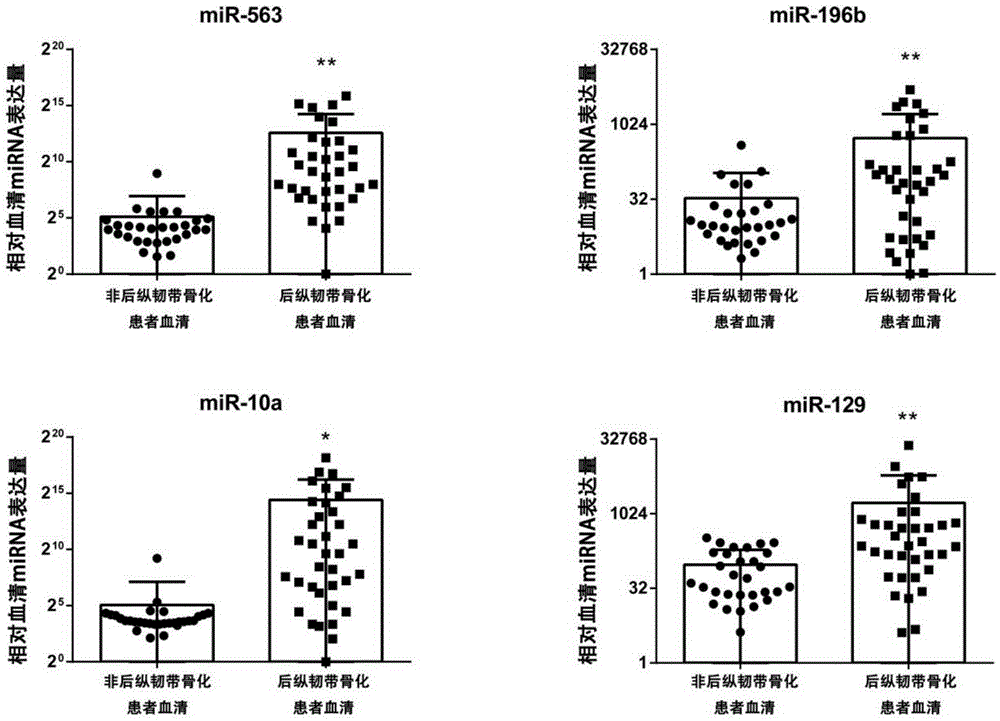
Serum miRNA markers suitable for diagnosis of ossification of posterior longitudinal ligament and application thereof
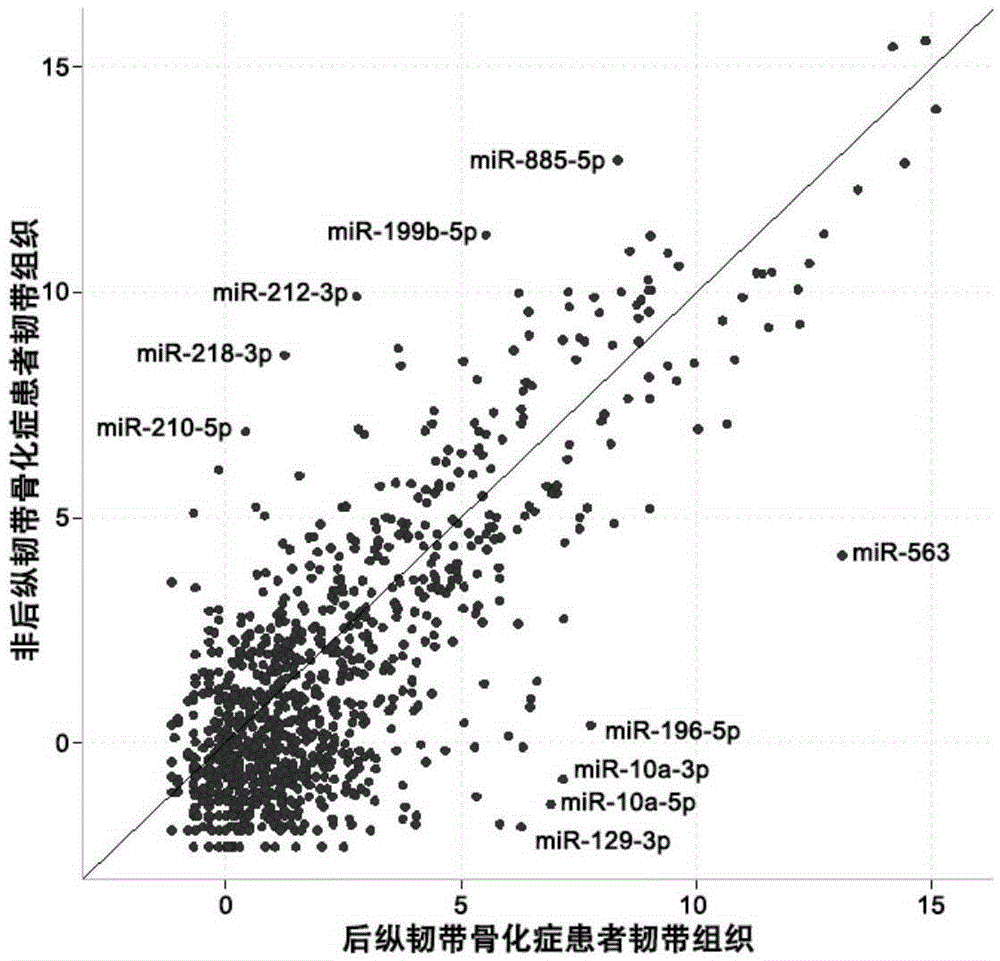
ActiveCN105400882ADelay disease progressionReduce morbidityMicrobiological testing/measurementSerum mirnaDrug target
The invention relates to the technical field of medical biological detection, and particularly relates to serum miRNA markers suitable for early screening and diagnosis of ossification of the posterior longitudinal ligament and an application thereof in preparation of a diagnostic reagent or kit for ossification of the posterior longitudinal ligament. The biological markers miRNA-563, miRNA-196b, miRNA-10a and miRNA-129 having relatively high diagnosis value on ossification of the posterior longitudinal ligament are found out for the first time. Through development and application of the serum miRNA markers and the diagnostic kit, early screening of ossification of the posterior longitudinal ligament can be more convenient and easier to implement, the foundation is laid for clinicians to quickly and accurately grasp patient conditions and for improving the clinical treatment effect, and help is provided for finding out novel small-molecular drug targets having potential therapeutic value.
Owner:SHANGHAI YUHUA LIFE SCI & TECH DEV CO LTD
Serum miRNA biomarker composition and application thereof
ActiveCN104450702AEasy to useRelieve painMicrobiological testing/measurementDNA/RNA fragmentationForward primerAzoospermia
The invention relates to a biomarker, and particularly relates to a serum miRNA biomarker composition and an application thereof. The serum miRNA biomarker composition comprises at least one miRNA and sequences of reverse transcription primers, forward primers and reverse primers of all miRNAs. The serum miRNA biomarker composition can be used for preparing oligozoospermia and azoospermia detection reagents. An oligozoospermia and azoospermia detection kit comprises the serum miRNA biomarker composition, Taq enzyme, dNTP, MgCl2 and a PCR buffer solution. The serum miRNA biomarker composition provided by the invention has the advantages of high sensitivity, low sample consumption, wide detection range and wide linear quantitative range.
Owner:XIAMEN UNIV
Serum miRNA marker related to auxiliary diagnosis for squamous lung cell carcinoma and application thereof
ActiveCN106350582AImprove stabilityMinimally invasive and easy to obtainMicrobiological testing/measurementDNA/RNA fragmentationDiseaseSerum mirna
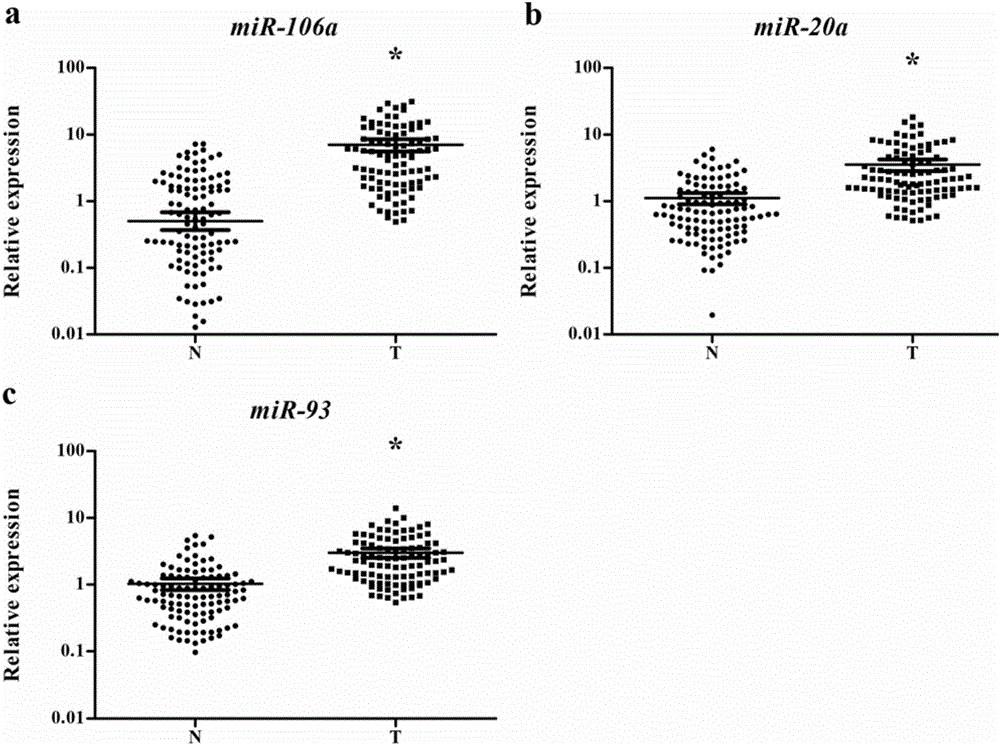
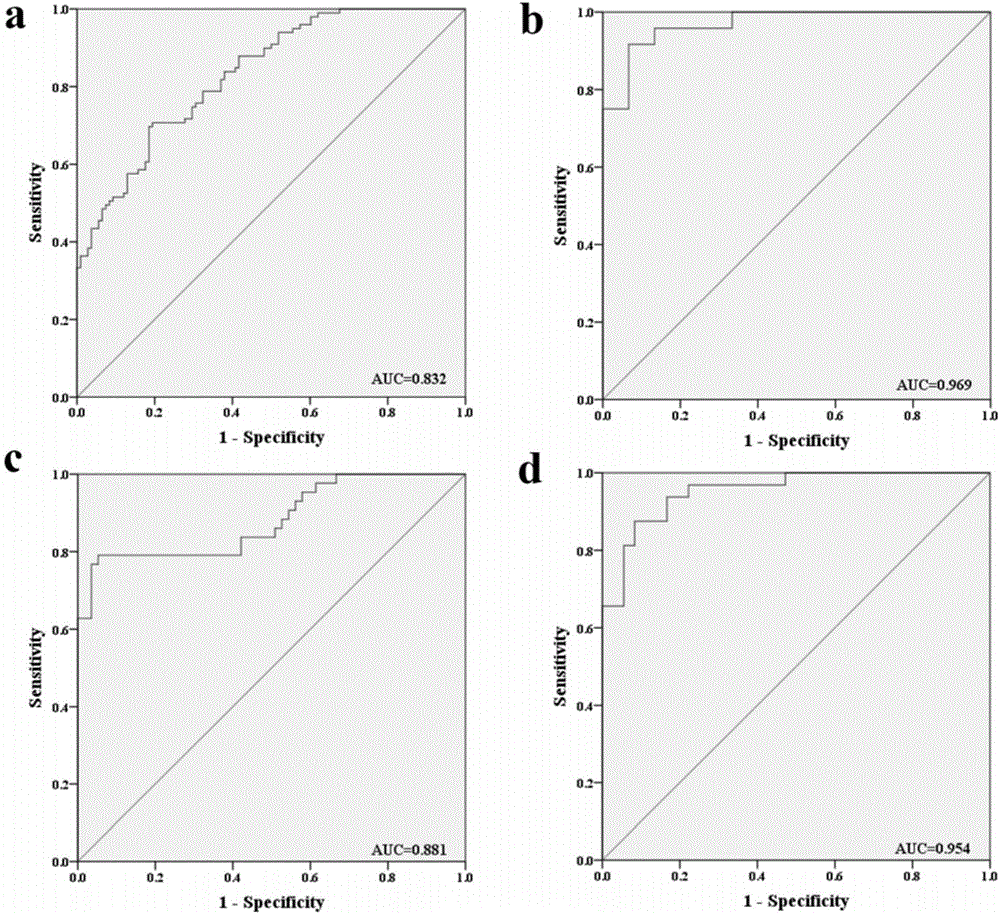
The invention discloses a serum miRNA marker related to auxiliary diagnosis for squamous lung cell carcinoma and an application thereof. The marker is one or more of miR-106a-5p, miR-20a-5p and miR-93-5p. As a novel biomarker, the serum miRNA has the characteristics of high stability, minimal invasion, easiness in acquiring, sensitivity and specificity. Such a molecular marker can be developed and utilized to supply a new direction for diagnosing and further treating various diseases including tumor. According to the research, the serum miRNA marker for squamous lung cell carcinoma with clinical diagnosis potential can be more specifically acquired. The research proves that the miRNA as a noninvasive marker for diagnosing the squamous lung cell carcinoma has reliability and repeatability.
Owner:朱伟
Method used for detecting serum miRNA of patients with cancer based on short nucleotide chain connection
ActiveCN107099612AImprove throughputSolve the medical examinationMicrobiological testing/measurementNucleotideSerum mirna
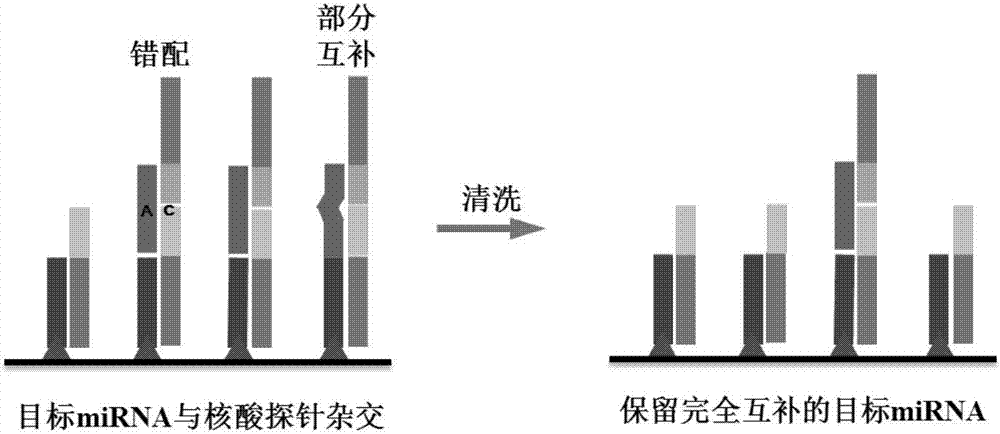
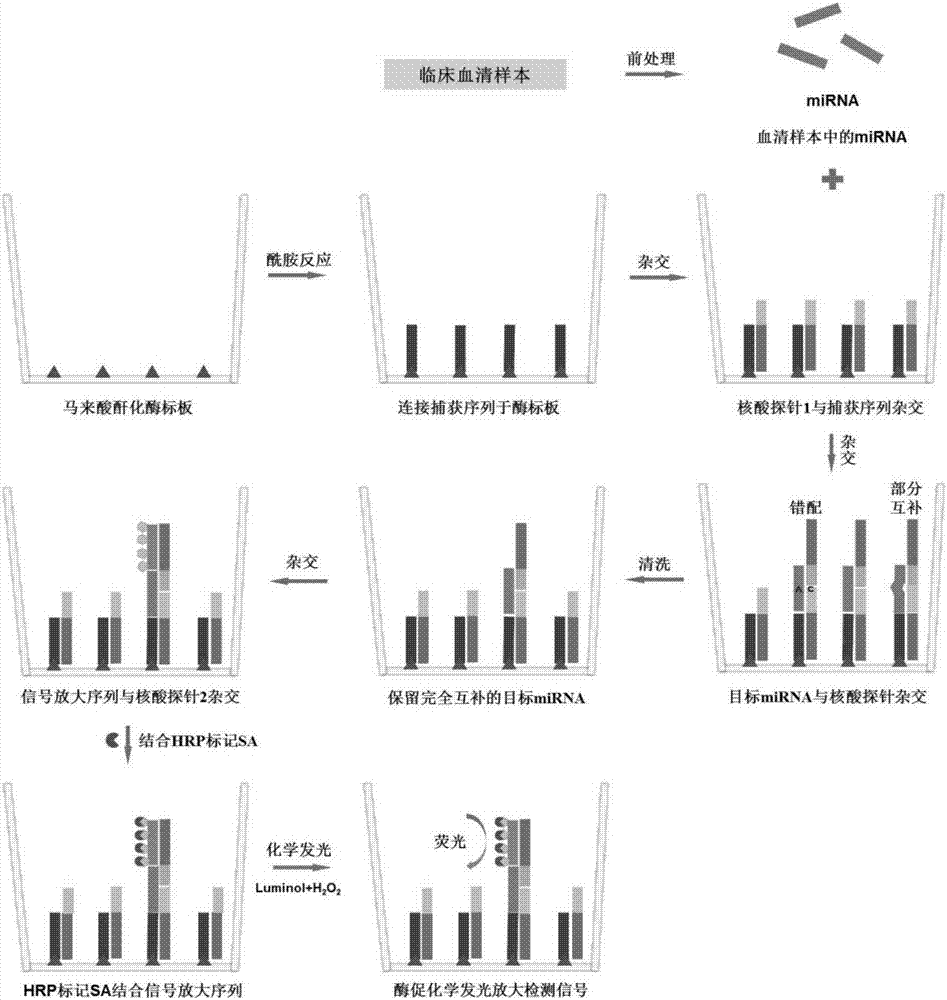
The invention discloses a method used for detecting serum miRNA of patients with cancer based on short nucleotide chain connection. According to the method, specific recognition and cutting of unpaired single strand nucleic acids and double-stranded nucleic acids with mismatched bases are carried out, mismatching and partial complementation of hybridization of a nucleic acid probe with target miRNA are detected, fluorescence is generated via enzymatic reaction of horse radish peroxidase combined on the nucleic acid probe with a chemiluminescent substrate, rapid amplification of detection signals is realized, and the target miRNA content is determined based on fluorescence intensity. The detection results are accurate; operation is simple and convenient; cost is low; detection throughput is high; and nucleic acid extraction or amplification is not needed.
Owner:深圳市展行生物有限公司
miRNA biomarker and detection kit used for renal cancer diagnosis
InactiveCN105779640AImprove consistencyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationKidney cancerSerum mirna
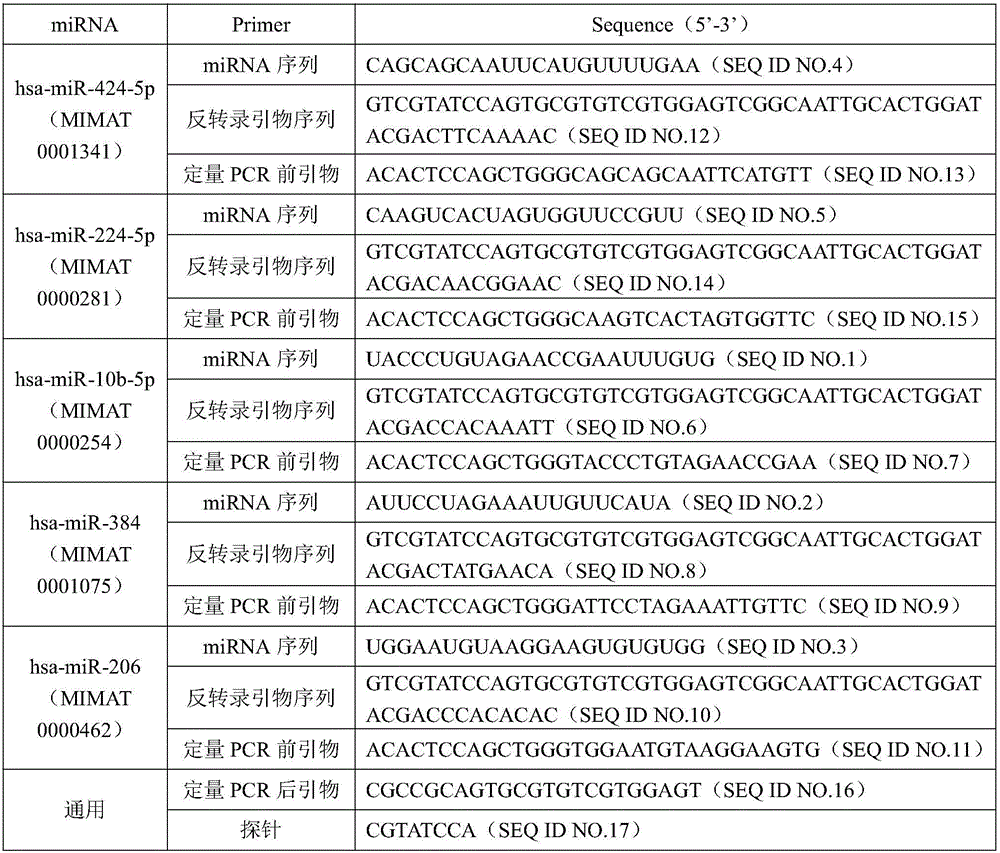
The invention discloses a miRNA biomarker and a detection kit which are used for renal cancer diagnosis. The microRNA biomarker is formed by the following microRNAs: hsa miR 10b 5p, hsa miR 384, hsa miR 206, hsa miR 424 5p and hsa miR 224 5p. According to the invention, serology expression analysis is carried out on five miRNAs obviously in differential expression of renal cancer and para-carcinoma tissues (differential expression quantity is more than 2 folds, and CT value in RT PCR is smaller than 30) hsa miR 10b 5p, hsa miR 384, hsa miR 424 5p, hsa miR 206 and hsa miR 224 5p, results show that the five miRNAs are stably expressed in serum, consistency between expression of serum miRNA and tissues is good, hsa miR 10b 5p, has miR 384 and has miR 206 are in down-regulated expression, and has miR 424 5p and has miR 224 5p are in up-regulated expression. The five miRNAs can serve as a biomarker used for the renal cancer diagnosis, and the sensitivity and specificity of combined diagnosis are obviously higher than those of single miRNA diagnosis.
Owner:崔学俊
Application of miRNA marker hsa-miR-486-5p
InactiveCN104164500AIncreased sensitivityImprove featuresMicrobiological testing/measurementSerum mirnaDistinctin
The invention provides an application of miRNA marker hsa-miR-486-5p. A serum micro ribonucleic acid marker provided by the invention as a CWP early diagnosis marker has the advantages that serum miRNAs are novel biomarkers, are different from traditional biomarkers, and have the advantages of being stable, minimally invasive, easy to detect, accurate to quantify and the like, the sensitivity and the specificity of disease diagnosis are greatly improved, a new situation is created for early diagnosis of CWP by successful development of micromolecular RNA biomarkers, and reference is provided for development of other disease biomarkers.
Owner:NANJING MEDICAL UNIV
Early embryo diapause villus tissue miRNAs biomarkers and detection method of expression quantity thereof
ActiveCN108642175ASignificant practical valueMicrobiological testing/measurementDNA/RNA fragmentationDiapauseSerum mirna
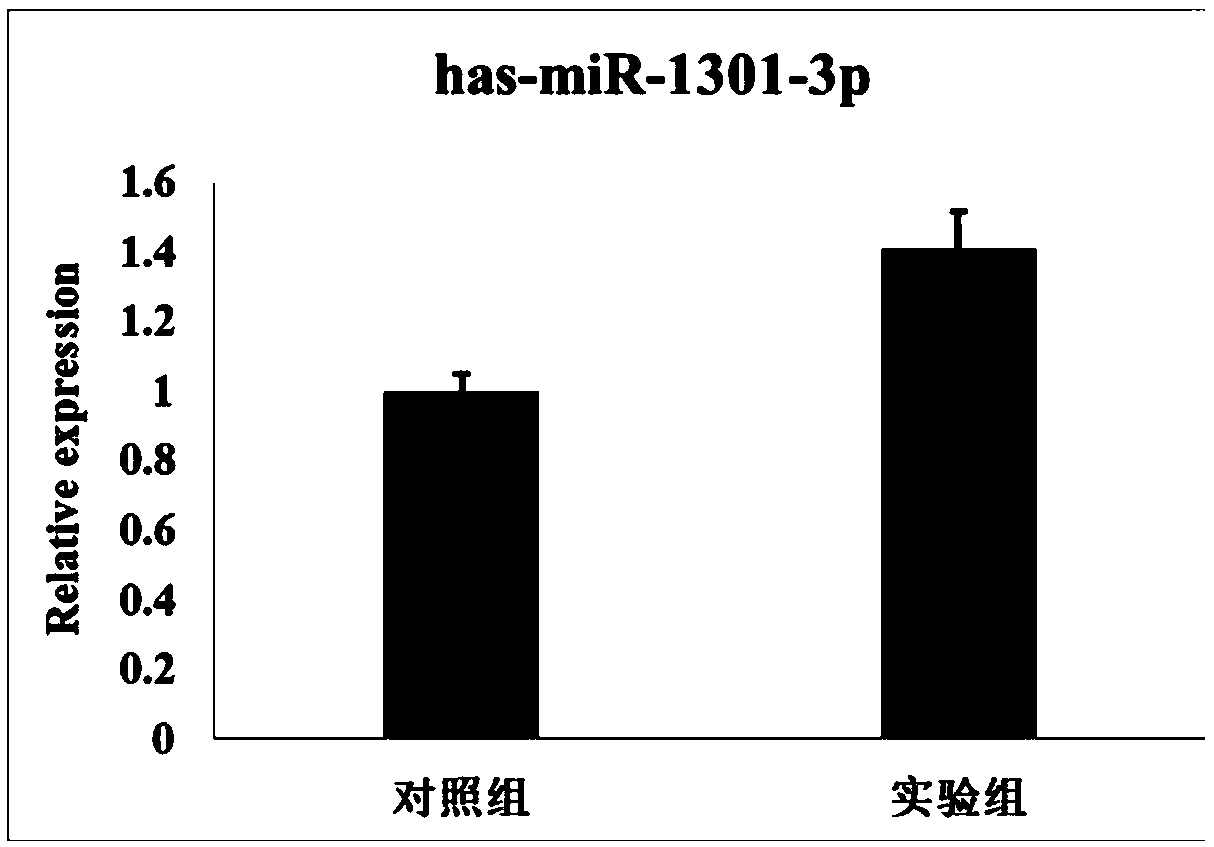
The invention discloses early embryo diapause villus tissue miRNAs biomarkers related to early embryo diapause degree,which includes hsa-miR-136-5p,hsa-miR-1301-3p,hsa-miR-135b-5p,hsa-141-3p,hsa-miR-19b-3p and hsa-iR-486-5p. Accordingly,the inventor also designs the corresponding reverse transcription or real-time fluorescent PCR primers aiming at the miRNAs biomarkers. The biomarkers can be usedas biomarkers of a fluff sample for detecting early embryo diapause,or used for preparing a diagnostic reagent or a biochip and the like,and has important potential value and application prospect on the diagnosis and treatment of early embryo diapause,can provide a theoretical basis for the later diagnosis of early embryo diapause from the molecular level and research of tissue and serum miRNAs,and has important theoretical significance and potential practical value.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV +1
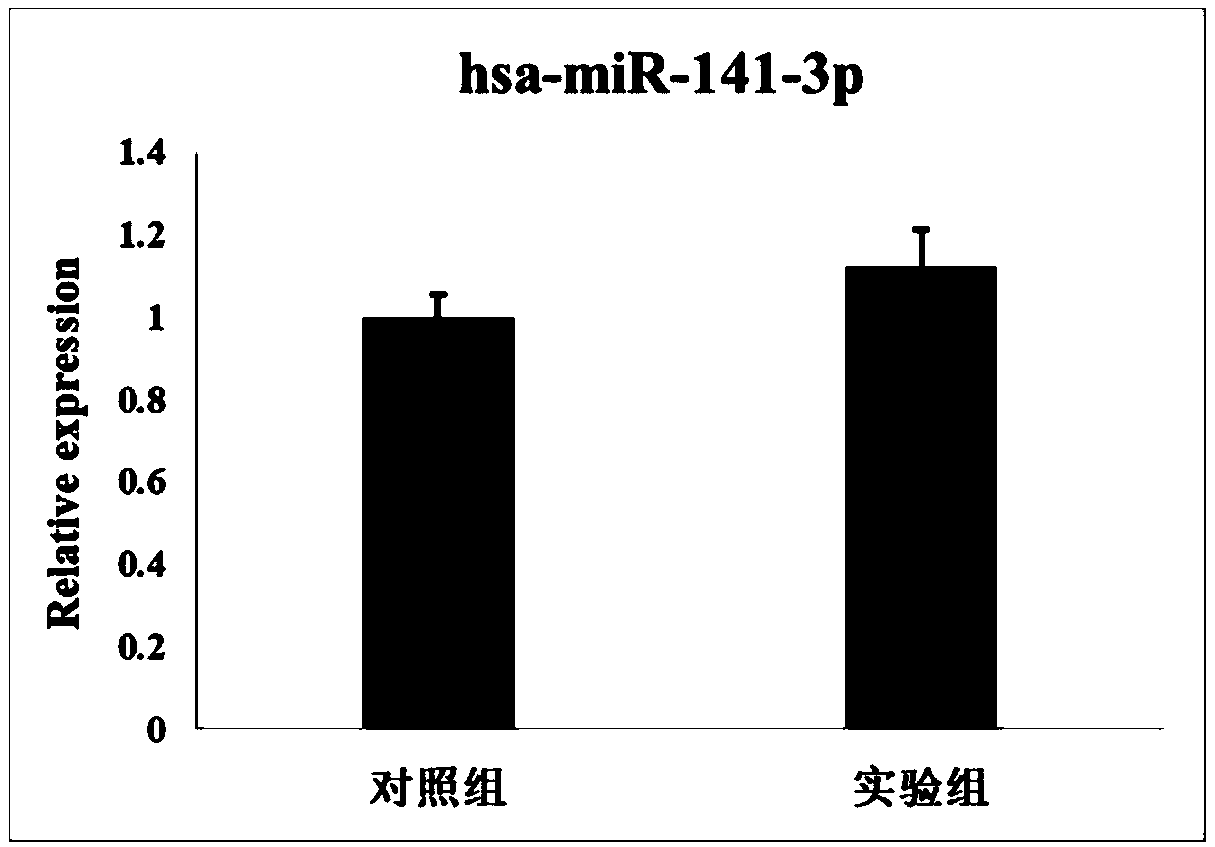
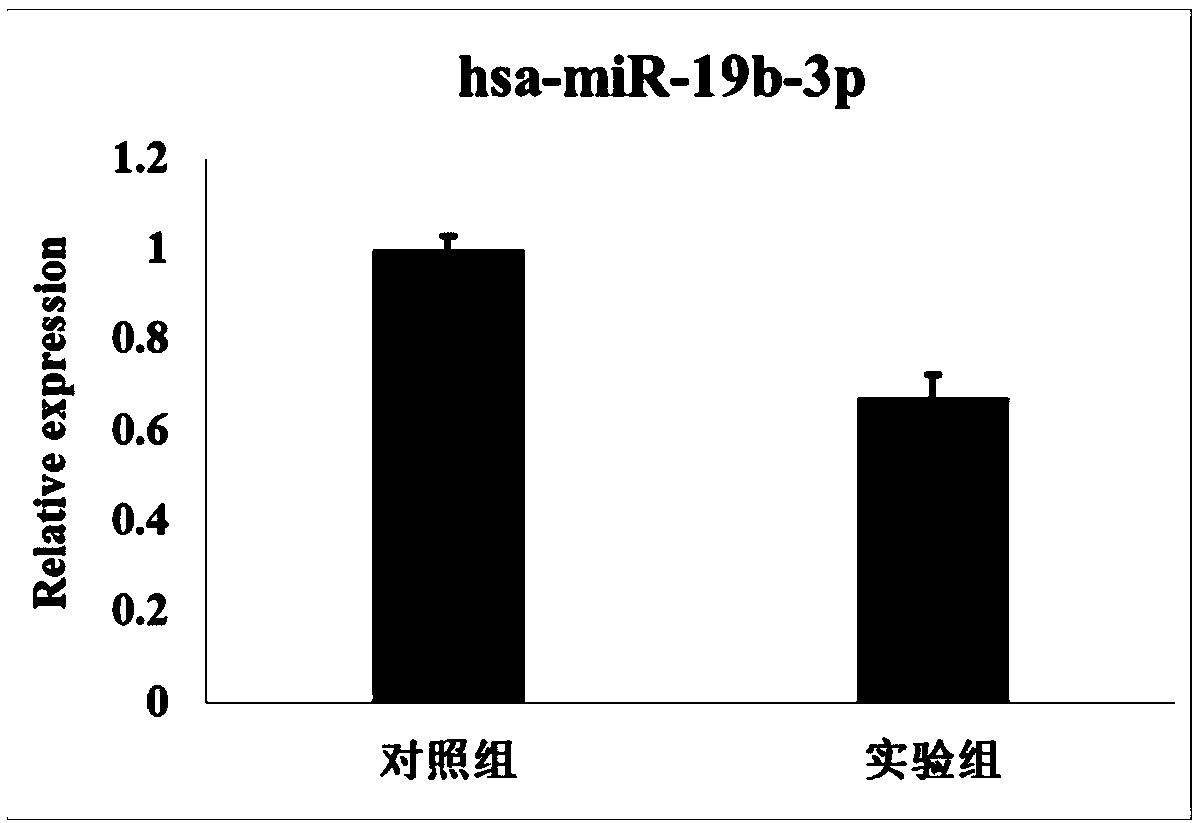
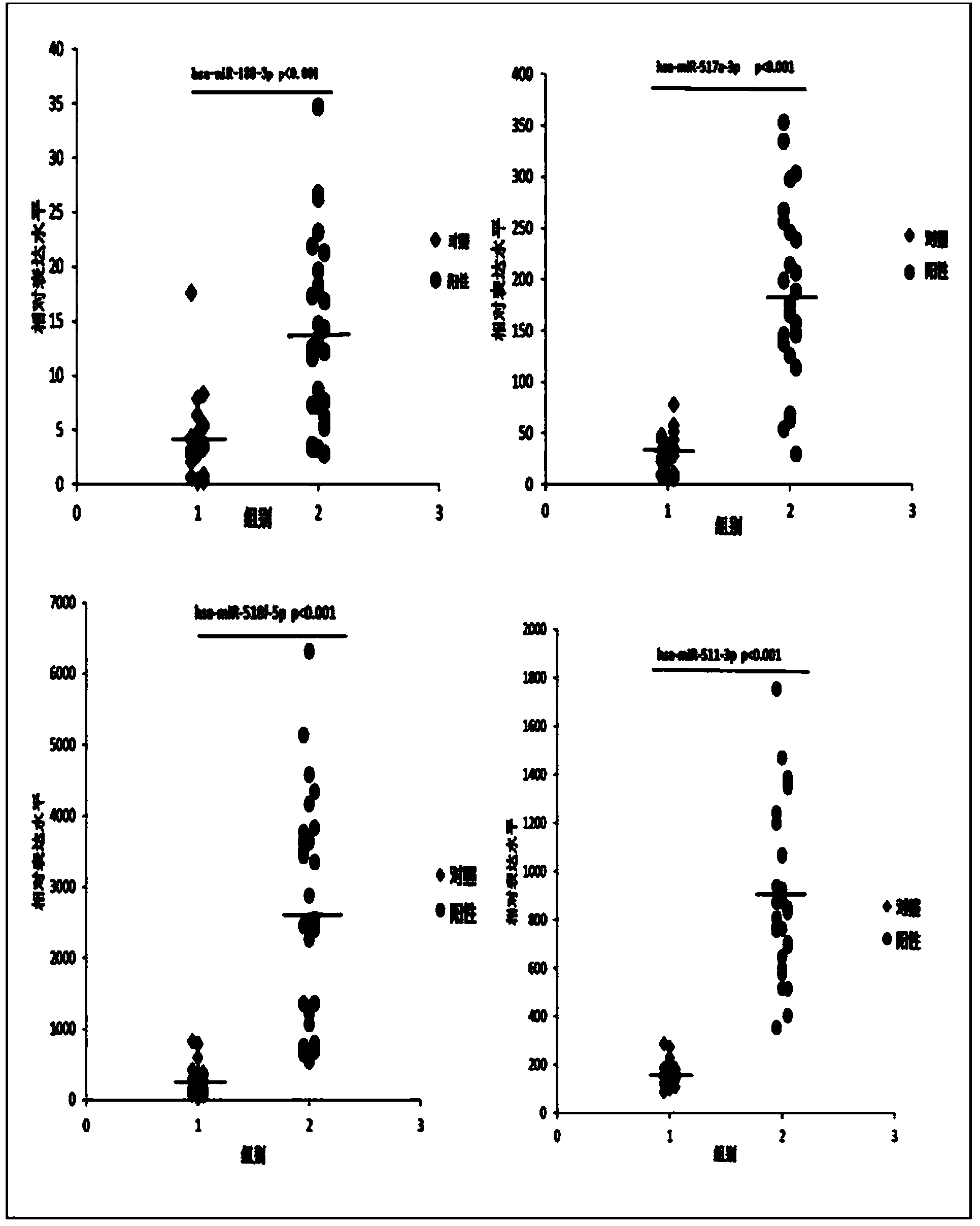
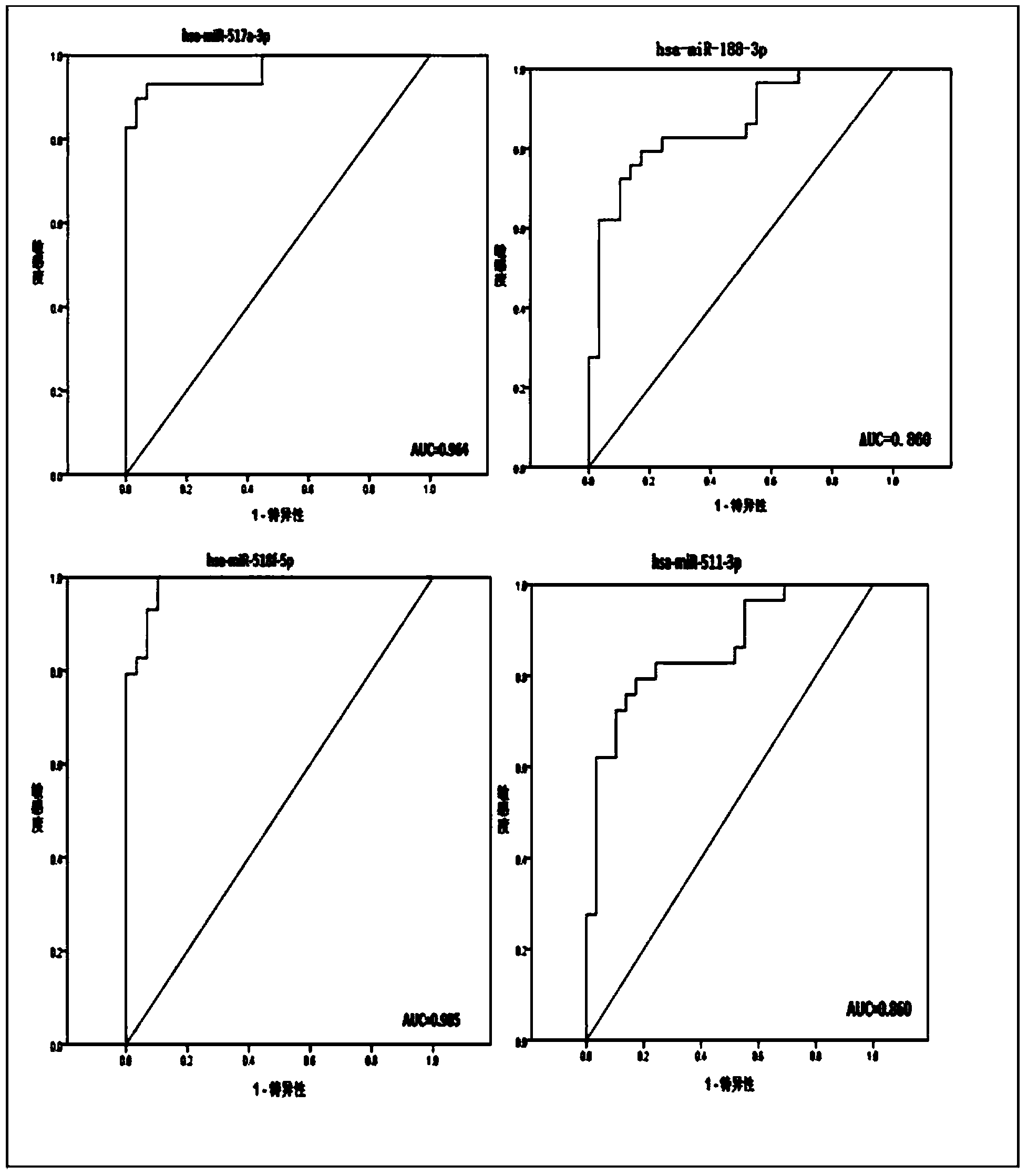
Kit based on serum miRNA as well as use method and application of kit
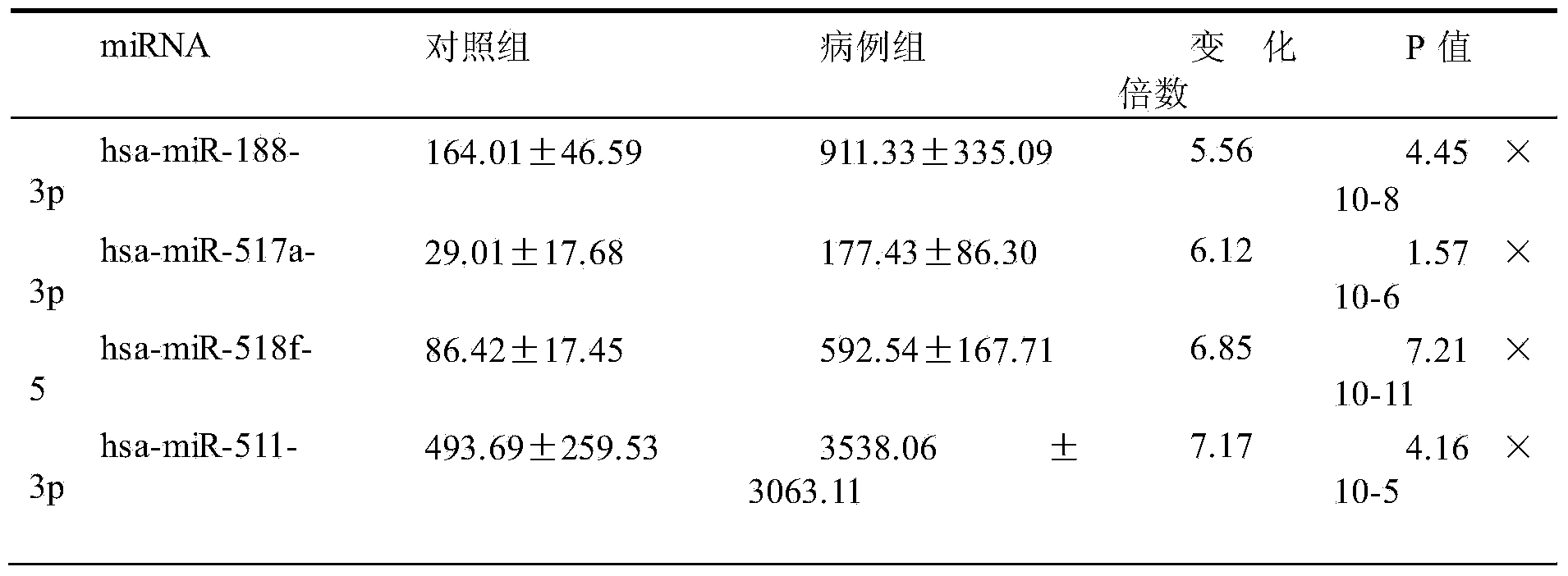
The invention belongs to the technical field of biology and in particular relates to a kit based on serum miRNA as well as a use method of the kit and application of the kit to early infection detection of fever with thrombocytopenia syndrome viruses. MiRNA molecules comprise hsa-miR-188-3p, hsa-miR-517a-3p, hsa-miR-518f-5p and hsa-miR-511-3p. The expression of the four miRNAs in early attack of the fever with thrombocytopenia syndrome viruses is specifically and remarkably increased through identification by adopting a Taqman low-density chip and a qRT-PCR (quantitative reverse transcription polymerase chain reaction) method. The four serum miRNAs are used as molecular targets, and a quick, sensitive and specific detection method for acute infection of the fever with thrombocytopenia syndrome viruses is built. The hsa-miR-188-3p, the hsa-miR-517a-3p, the hsa-miR-518f-5p and the hsa-miR-511-3p are applied to early diagnosis of fever with thrombocytopenia syndrome virus infection, and the kit is used for detecting the hsa-miR-188-3p, the hsa-miR-517a-3p, the hsa-miR-518f-5p and the hsa-miR-511-3p.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com