Serum miRNA combination-based pulmonary tuberculosis therapeutic effect evaluation kit and applications thereof
A tuberculosis and curative effect evaluation technology, which is applied in the field of disease curative effect evaluation, can solve problems such as easy misdiagnosis, long time-consuming sputum culture inspection, and poor discrimination efficacy, and achieve reliable results, high sensitivity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: Composition of the tuberculosis curative effect evaluation kit based on serum miRNA composition
[0075] In this embodiment, the pulmonary tuberculosis curative effect evaluation kit includes RNA extraction buffer, poly(A) reaction solution, reverse transcription reaction solution, serum miRNA upstream primer combination, internal reference upstream primer, universal downstream primer and fluorescent quantitative RT-PCR reaction solution; wherein, the serum miRNA composition is composed of 4 differentially expressed serum miRNAs, and the names and sequences of these 4 serum miRNAs are as follows:
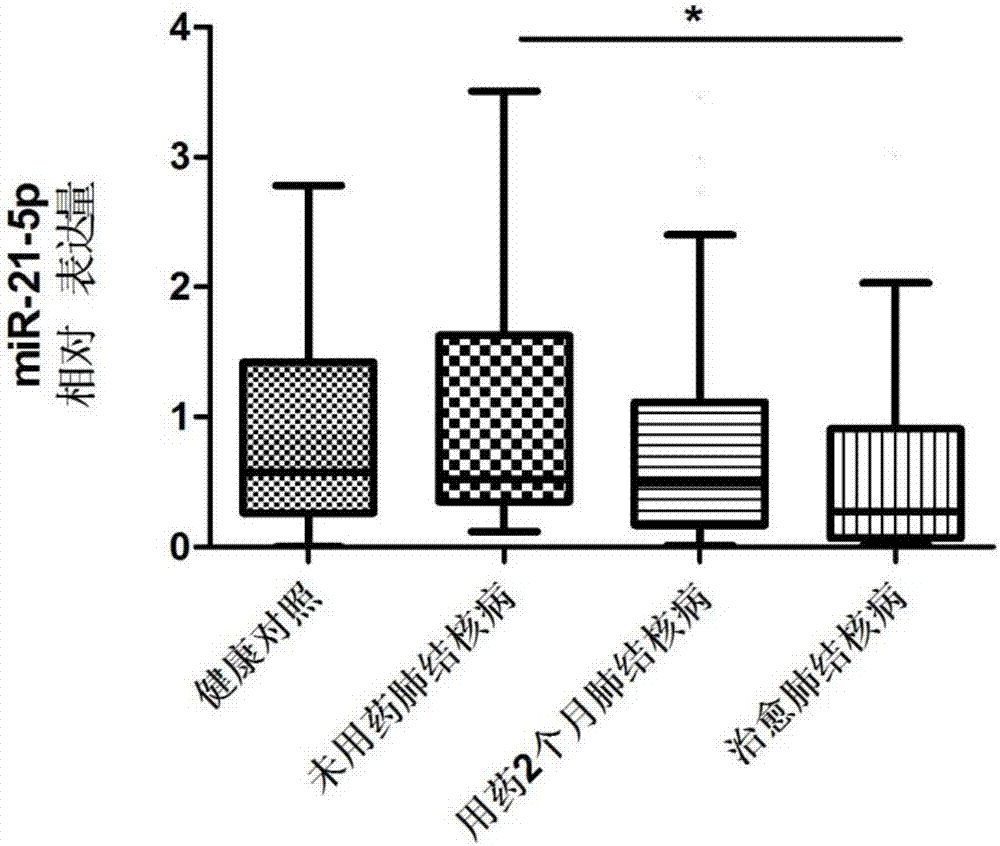
[0076] hsa-miR-21-5p: UAGCUUAUCAGACUGAUGUUGA, which is the sequence of SEQ ID NO:1;
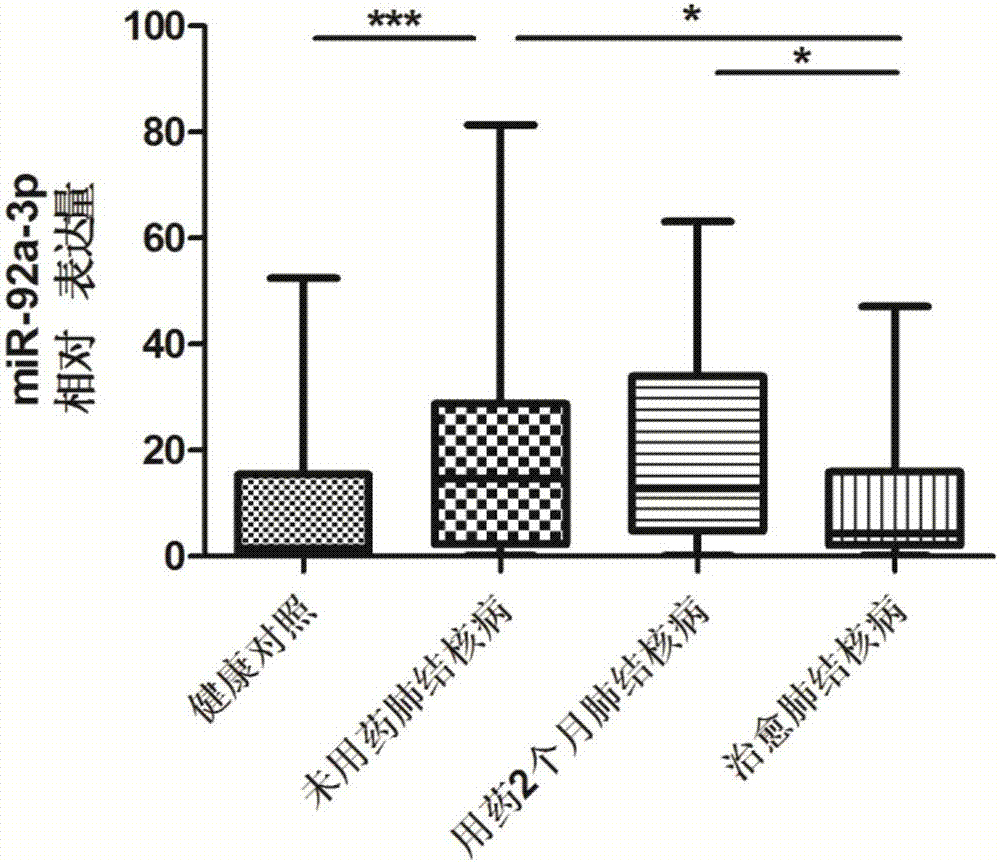
[0077] hsa-miR-92a-3p: UAUUGCACUUGUCCCGGCCUGU, which is the sequence of SEQ ID NO:2;
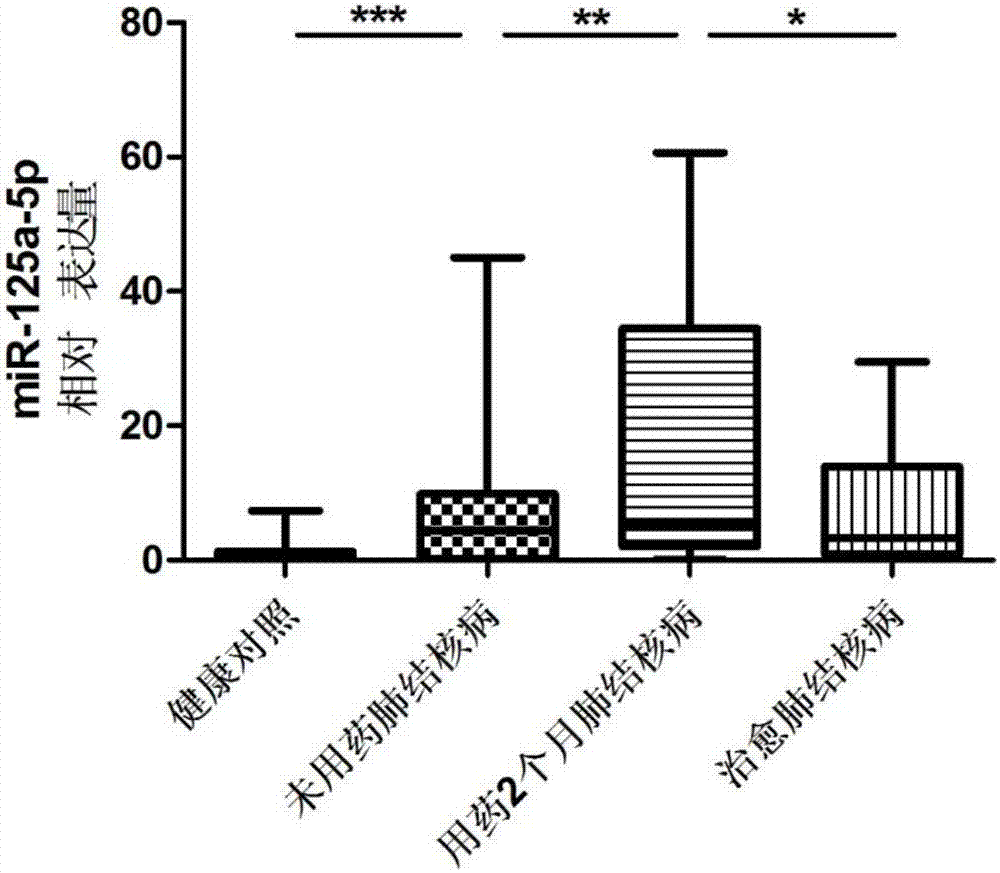
[0078] hsa-miR-125a-5p: UCCCUGAGACCCUUUAACCUGUGA, which is the sequence of SEQ ID NO:3;
[0079] hsa-miR-148b-3p: UCAGUGCAUCACAGAACUUUGU, which is the sequence of SEQ ID NO:,4;
[0080] Th...
Embodiment 2
[0091] Example 2: Establishment of the Logistic stepwise regression model of the pulmonary tuberculosis curative effect evaluation kit
[0092] Tuberculosis curative effect evaluation kit was used to detect hsa-miR-21-5p, hsa-miR-92a-3p, hsa-miR-125a-5p, hsa-miR-125a-5p, Expression levels of hsa-miR-148b-3p.
[0093] (1) Sample collection:
[0094] According to the diagnostic criteria of tuberculosis issued by the Ministry of Health of China, 53 cases of tuberculosis patients without medication, 2 months of treatment and cured tuberculosis patients were collected. During the same period, 53 cases of healthy controls were collected. All HIV tests were negative, no hepatitis B, no diabetes, no asthma, no history of tuberculosis, normal liver and kidney functions, no other congenital diseases, and other diseases such as chronic inflammation and autoimmune diseases were excluded. There was no other operation history, and there was no significant difference in age and gender.
...
Embodiment 3
[0143] Example 3: Verification and application of the detection effect of the pulmonary tuberculosis curative effect evaluation kit
[0144] Leave-one-out cross-validation: take serum samples from cured, 2-month-medicated, drug-naïve pulmonary tuberculosis patients, and healthy controls. Sample collection and detection methods are the same as those described in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com